Abstract
Following herpes simplex virus type 1 (HSV-1) ocular infection of C57BL/6 mice, activated CD8+ T cells specific for an immunodominant epitope on HSV-1 glycoprotein B (gB-CD8 cells) establish a stable memory population in HSV-1 latently infected trigeminal ganglia (TG), whereas non-HSV-specific CD8+ T cells are lost over time. The retention and activation of gB-CD8 cells appear to be influenced by persistent viral antigenic exposure within the latently infected TG. We hypothesized that the low-level expression of gB from its native promoter before viral DNA synthesis is critical for the retention and activation of gB-CD8 cells in the TG during HSV-1 latency and for their ability to block HSV-1 reactivation from latency. To test this, we created a recombinant HSV-1 in which gB is expressed only after viral DNA synthesis from the true late gC promoter (gCp-gB). Despite minor growth differences compared to its rescuant in infected corneas, gCp-gB was significantly growth impaired in the TG and produced a reduced latent genome load. The gCp-gB- and rescuant-infected mice mounted similar gB-CD8 effector responses, but the size and activation phenotypes of the memory gB-CD8 cells were diminished in gCp-gB latently infected TG, suggesting that the stimulation of gB-CD8 cells requires gB expression before viral DNA synthesis. Surprisingly, late gB expression did not compromise the capacity of gB-CD8 cells to inhibit HSV-1 reactivation from latency in ex vivo TG cultures, suggesting that gB-CD8 cells can block HSV-1 reactivation at a very late stage in the viral life cycle. These data have implications for designing better immunogens for vaccines to prevent HSV-1 reactivation.
Herpes simplex virus type 1 (HSV-1) is a ubiquitous human pathogen that is responsible for repeated corneal infections that can induce blinding keratitis. The murine model of ocular HSV-1 infection has elucidated the role of host immunity in the establishment and maintenance of viral latency in trigeminal ganglia (TG). HSV-1 infection of a scarified mouse cornea leads to a short-lived epithelial lesion caused by acute viral replication in and destruction of corneal epithelial cells. During replication in culture, viral genes are expressed in a tightly regulated temporal cascade characterized by the sequential expression of immediate-early (IE) (α) genes and early (β) genes before viral DNA synthesis. The late γ genes are maximally expressed after viral DNA replication and can be subdivided into γ1 genes, which are expressed in small amounts before viral DNA replication, and γ2 genes, which are absolutely dependent on DNA replication for expression (7). Confirmation of the expression kinetics of HSV-1 genes in vivo has proven to be difficult, as uniform infections cannot be established. Previous studies seeking to address viral replication kinetics in neurons have given rise to controversial conclusions (31). However, our group has previously demonstrated that during reactivation, the γ1 gene promoter of glycoprotein B (gB) is active before the γ2 gene promoter of glycoprotein C (gC), suggesting that the expression kinetics of the γ1 and γ2 genes are similar during both lytic replication and reactivation (24). gB is a multifunctional structural glycoprotein that contains an immunodominant epitope spanning amino acids 498 to 505 (gB498-505) recognized by a majority of CD8+ T cells (gB-CD8 cells) in C57BL/6 mice within 2 h of target cell infection (9, 19, 35).
Replicating HSV-1 in the corneal epithelium accesses the termini of interdigitating sensory neurons and travels via retrograde axonal transport to the neuronal soma in the TG. The viral genome is maintained in sensory neurons in a latent state in which no infectious virus is produced. Latency is characterized by the repression of most viral lytic cycle genes and the abundant expression of viral RNAs known as latency-associated transcripts (LATs) with no known protein products (11, 13). The repression of viral protein synthesis during latency has led to the prevalent view of latency as being a quiescent and antigenically silent infection that is ignored by host immunity. However, very low levels of gene transcripts and proteins from all kinetic classes have been detected in latently infected murine TG (4, 12). Furthermore, the findings of recent immunological studies of HSV-1 latency in mice are inconsistent with the notion that latent virus is ignored by the host immune system. CD8+ T cells infiltrate the TG during acute HSV-1 infection, with peak accumulation occurring coincident with the elimination of replicating virus and the establishment of latency (9, 35). In C57BL/6 mice, gB-CD8 cells represent about half of the CD8+ T-cell infiltrate in the TG (9). Most if not all of the remaining CD8+ T cells in infected TG appear to recognize as-yet-undefined HSV-1 proteins (27). The effector CD8+ T-cell population in the acutely infected TG undergoes contraction as latency is established, giving rise to a small but stable memory population with the same 50:50 ratio of gB-specific to non-gB-specific cells (9).
In both mice and humans, CD8+ T cells are found in the HSV-1 latently infected TG in direct apposition to neurons (9, 10, 32). In humans, HSV-1-specific CD8+ T cells surrounding HSV-1 latently infected neurons demonstrate an activated and effector memory phenotype (34). Using tetramers that bind to the T-cell receptor of gB-CD8 cells in C57BL/6 mice, we have demonstrated that these cells form an immunological synapse with neurons in latently infected TG and even release lytic granules into the synapse. Thus, CD8+ T cells can detect and respond to latent virus during immunosurveillance (9, 10). The TG-resident gB-CD8 cell population exhibits a more activated phenotype (CD69 and granzyme B expression) and is less dependent on homeostatic proliferation signals than its counterparts in noninfected tissue, such as the lungs and spleen (27). gB-CD8 cells can employ the cytokine gamma interferon (IFN-γ) and lytic granules to prevent HSV-1 reactivation from latency in vivo and in ex vivo TG cultures without inducing neuronal apoptosis (3, 10).
The HSV-1 protein ICP47 inhibits CD8+ T-cell recognition of infected targets by impairing the transport of peptides into the endoplasmic reticulum for loading onto major histocompatibility complex (MHC) class I molecules and transport to the cell surface (1). ICP47 more efficiently inhibits human transporter associated with antigen processing (TAP) transporters than their mouse counterparts. This might lead to a less efficient recognition of viral epitopes by CD8+ T cells in human TG and contribute to the frequent HSV-1 reactivation events that are observed for some humans but are absent or very infrequent in mice. Indeed, HSV-1 strains that incorporate murine cytomegalovirus (CMV) immune evasion molecules do show spontaneous reactivation in mice (21). Despite more-efficient immune evasion in humans, latently infected neurons in human TG are surrounded by contiguous CD8+ T cells that exhibit an activation phenotype similar to that seen in mice (9, 34). In both humans and mice, stimuli known to impair CD8+ T-cell function (stress, immunosuppressive drugs, and exposure to UV irradiation) lead to HSV-1 reactivation from latency (5, 14, 25, 26, 28). These findings are consistent with the notion that in both mouse and human TG, CD8+ T cells provide immunosurveillance of latently infected neurons with low-level and likely intermittent T-cell receptor (TCR) recognition of viral epitopes during partial reactivation events.
The importance of appropriate kinetic expression of HSV-1 genes for virulence and the generation of host immunity has not been explored. Here we constructed a recombinant HSV-1 that expresses the γ1 gB gene as a γ2 gene, eliminating low-level expression prior to viral DNA synthesis. We investigated whether this kinetic change of gB expression would influence HSV-1 virulence, the generation and homing of gB-CD8 cells to the TG, and the ability of gB-CD8 cells to inhibit HSV-1 reactivation from latency.
MATERIALS AND METHODS
Cells and viruses.
Vero cells (ATCC, Manassas, VA) were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% fetal bovine serum (FBS), 5% Serum Plus (SAFC Biosciences, Lenexa, KS), penicillin G (100 units/ml), streptomycin (100 mg/ml), and amphotericin B (Fungizone) (250 mg/ml). The RE strain of HSV-1 (18) was used as the basis for all studies.
Construction and analysis of recombinant HSV-1.
The recombinant virus containing gB under the late glycoprotein C (gC) promoter (HSV-1 gCp-gB) in the HSV-1 RE background and its corresponding rescuant (HSV-rescue) were constructed as shown in Fig. 1. The sequences of gB and its promoter were PCR amplified from bp 54817 to 56640 in two sections: gB was amplified by using primers gB-E (5′-AGCAAGCTTGTAGAAGCCGTCGACCTGCTTGAA [the HindIII site is underlined]) and gB-S (5′-GAGGGATCCCCGCCATGCGCCAGGGCGCC [the BamHI site is underlined]), and the promoter was amplified by using primers 28-E (5′-CGGGATCCGCACGCTAGCTGGCGCATGGCGGGACTACGG [the NheI/BamHI sites are underlined]) and 28-S (5′-GAGAATTCTGACGAAGCGGTCGTTGGCCAGCC [the EcoRI site is underlined]).
FIG. 1.
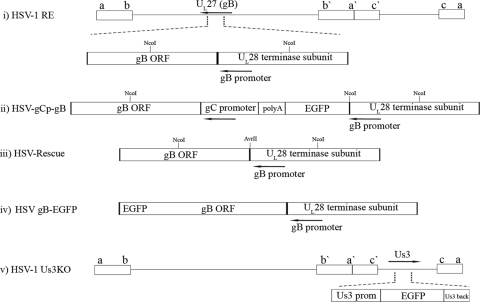
Construction of HSV gCp-gB, HSV-rescue, and HSV-1 US3KO viruses. (i) Representation of the HSV-1 genome showing the UL27/UL28 locus, which contains the gB promoter (arrow) in the UL28 coding sequences upstream of the gB ORF. The restriction sites used to identify the insertion, as detailed in the text, are indicated on the gene locus at their approximate positions. (ii) Expansion of the corresponding region in the recombinant HSV-1 gCp-gB virus showing that in place of its native promoter, gB is driven by the well-characterized γ2 gC promoter in gCp-gB so that the protein is expressed only following DNA replication. The strategy required the maintenance of the gB promoter in the genome, because it is concurrent with the upstream UL28 ORF encoding an essential terminase subunit. As such, HSV-1 gCp-gB had the gB promoter driving EGFP followed by a polyadenylation motif to terminate RNA made from the gB promoter. (iii) Representative structure of the HSV-rescue virus showing that gB expression is restored to its native promoter but that a unique noncoding AvrII site distinguishes it from the parental strain. (iv) Representative structure of the HSV gB-EGFP virus showing that the gB protein is expressed as a fusion with EGFP at its C terminus. (v) Representation of the genome showing the position of the US3 locus, which was modified such that the US3 promoter drives the expression of EGFP, followed by an untranslated portion (amino acids 170 to 481) of the US3 ORF. If spurious transcription/translation allowed the expression of the remaining part of the US3 reading frame, that part would not be kinase functional, since it lacks the critical ATP binding domain and the first part of the catalytic domains.
All DNAs were amplified from the RE genomic template by using Expand proofreading polymerase (Roche) under hot-start conditions and in reaction mixtures containing 5% dimethyl sulfoxide (DMSO). Each PCR fragment was cut with the terminal engineered restriction sites and triple ligated into vector pUC19 as a HindIII-BamHI-EcoRI fragment to generate plasmid pK1968, which contained a unique BamHI and NheI site just upstream of the gB ATG start codon. A portion (508 bp) of the region immediately upstream of the gC initiating ATG (bp 96227 to 95820) containing the gC promoter (17) was similarly PCR amplified by using primers gCp-S (5′-CGGGATCCGCCCGACGCCTCCCCCTCGCGA [the BamHI site is underlined]) and gCp-B (5′-GCGCGCTAGCAGATCTCTTAAGGCAGGTCATCAACCTCGGGTT [the Nhe-BglII-AflII site is underlined]). The PCR product was then cut with BamHI and NheI inserted into the corresponding sites engineered upstream of gB in pK1968. The resulting construct was digested with NheI and AflII, and an NheI-AflII fragment of pEGFP-C1 containing enhanced green fluorescent protein (EGFP) and its polyadenylation signal were inserted to be upstream of the gC promoter and downstream of and driven by the gB promoter. A plasmid derivative for the rescuant was developed by PCR amplifying a portion of the gB gene and the promoter/UL28 region using primers 54810F-EcoRI (5′-GGCCGAATTCGCGCGCGTAGAAGCCGTCGACC [the EcoRI site is underlined]) and 55812R AvrII (5′-GTCCTCCAGCACCTCGCCCCTAGGCTACCTGACG) and primer 55812F AvrII (5′-GTCAGGTAGCCTAGGGGCGAGGTGGAGGAC) with 56801R HindIII (GCCCAAGCTTACGACGGGGACCGTGTCGCCGT) (mutations are in boldface type). Each PCR fragment was digested with the terminal-end restriction sites, combined, and triple ligated into EcoRI- and HindIII-digested pUC19. The single mutation resulted in the insertion of a silent noncoding unique AvrII restriction site. All DNA inserts were sequenced for integrity.
HSV gCp-gB was derived by the cotransfection of infectious wild-type (WT) HSV-1 RE viral DNA with plasmids linearized with SspI into Vero cells by using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA). GFP-positive virus plaques were picked and plaque purified to homogeneity, as detailed previously (24). The rescuant (HSV-rescue virus) was derived by cotransfecting viral DNA from the EGFP-positive gCp-gB virus with the promoter-gB plasmid containing the novel AvrII site. Rescuant plaques were selected and picked based on the loss of fluorescence followed by the subsequent titration of the mixed progeny from the transfection (Fig. 1i to iii). The recombinants were positively identified for inserted DNA sequences (gCp-gB) or for the inserted AvrII site (rescue virus) by Southern blot analysis of restricted viral DNA. Viral DNAs from both viruses were cut with NcoI, AvrII, and NcoI-AvrII double digests and probed with 5′ 32P-radiolabeled oligonucleotides derived from the gB coding sequence or the UL28 coding sequence (data not shown).
A recombinant with US3 deleted in the HSV-1 RE background was also derived for this study. Sequences of part of the US3 protein were PCR amplified from the HSV-1 RE genomic template by using the GC-Rich PCR system (Roche) under hot-start conditions and primers US3.5F (5′-GGG AAT TCA TGT ACG GAA ACC AGG ACT AC-3′) and US3.5R (5′-GGA AGA TCT TCA TTT CTG TTG AAA CAG CGG CAA-3′). The PCR product was digested with BglII and EcoRI and ligated into pEGFP-C2 digested with BamHI and EcoRI. The collapse of this construct with XhoI resulted in the removal of the N-terminal part of the US3 coding sequence encoding residues 1 to 169. The remaining part lacks ATG, is out of frame with respect to EGFP, and lacks domains required for kinase activity. DNA containing the promoter sequence upstream of the US3 gene was PCR amplified by using primers US3PF (5′-GCG CCC TAG GGC TAG CTC GCC GCA CCG TGA GTG CCA-3′) and US3PR (5′-GCC ATT AAT ATT AAT GCC GCG AAC GGC GAT CAG AGG GTC AGT-3′). This PCR product was digested with AvrII and AseI and used to replace the CMV IE promoter. The resulting construct contained EGFP flanked by the US3 promoter and the distal end of US3 (Fig. 1v). Viruses were selected from cotransfections of HSV-1 RE viral DNA with the linearized plasmid and were identified and plaque purified based on the gain of EGFP fluorescence. The insertion of GFP and the deletion of the N termini of the US3 coding sequences were confirmed by Southern blot analysis of viral DNA (data not shown).
A recombinant HSV-1 in which the gB protein is fused to EGFP was also used for this study. HSV gB-EGFP (gB-EGFP) was generated on the RE backbone (Fig 1iv). Briefly, a plasmid was developed in which the initiating methionine of EGFP was placed in frame with the last residue of the gB coding sequence, followed by the sequences immediately downstream of gB. A portion of the gB sequence was PCR amplified with primers 5′-GACCGAATTCGACAACGTGATCGTCCAAAACTC and 5′-GCCGAGATCTTCACCCTAGGAGGTCGTCCTCGGTCGG and cloned into pGEM-T Easy using TA cloning. The construct was sequenced and verified for integrity. A portion of the sequences immediately downstream of the gB stop codon containing the ORF26 coding sequences was then generated by PCR using primers 5′-GCCG AGATCT GAC GCG GAC GAC CTG TGA TG and ACGCAAGCTTGGACTACCCGTACTA digested with HindIII and BglII and cloned downstream of the first gB fragment. The resulting construct was then digested with BglII and AvrII, and an NheI-BglII fragment derived from pEGFP C1 was inserted into EGFP in frame with the last residue of the gB coding sequence. This plasmid was linearized and cotransfected with infectious HSV-1 RE DNA, and recombinant viruses were picked and purified based on the gain of EGFP fluorescence when visualized by blue UV light. All viruses were carefully picked to reduce UV exposure to minimal levels. DNAs were verified by Southern blotting, and the fusion of EGFP to gB was determined by Western blot analyses (data not shown). Growth curves for Vero cells established that this virus grew to levels deemed not significantly different from those of wild-type RE (data not shown).
Analysis of HSV-1 protein expression.
Subconfluent Vero cell monolayers were infected with either HSV-1 RE, gCp-gB, or rescue virus at a multiplicity of infection (MOI) of 10 PFU/cell for 1 h at room temperature (25°C) in medium lacking or containing 350 μg/ml phosphonoacetic acid (PAA; Lancaster Synthesis, Pelham, NH). Following medium replacement under the same conditions, the monolayers were incubated at 37°C, and cells were harvested at the indicated time points. Cells were washed in ice-cold phosphate-buffered saline (PBS) and then directly solubilized in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) buffer, boiled, and separated by SDS-PAGE. Proteins were transferred onto Immobilon-P membranes (Millipore, Billerica, MA) for immunoblot analyses. Antibodies to gB and gC (pooled monoclonal antibodies, a kind gift of W. Goins, University of Pittsburgh) were detected by using horseradish peroxidase and West Dura chemiluminescent reagents (Pierce Biotechnology Inc., Rockford, IL).
Multistep in vitro growth kinetics.
Vero cells were infected at an MOI of 0.01 PFU/cell and incubated for 4, 24, and 48 h. Cells and supernatants were harvested, and infectious virus that was released following three freeze-thaw cycles was detected by a plaque assay on confluent Vero cells.
Mice and ocular infections.
Three- to five-week-old female wild-type C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) were anesthetized by the intraperitoneal injection of 2.0 mg of ketamine hydrochloride and 0.04 mg of xylazine (Phoenix Scientific, St. Joseph, MO) in 0.2 ml of Hanks buffered salt solution (BioWhittaker, Walkersville, MD). Both corneas were scarified and infected topically with HSV-1 viruses at the indicated PFU/eye in 3 μl of RPMI medium (BioWhittaker). All viruses were Percoll gradient purified as detailed previously (24). All animal studies were carried out under University of Pittsburgh IACUC-approved protocols and in accordance with the ethical treatment of animals, as defined by the Association for Research in Vision and Ophthalmology for the use of animals in ophthalmic and vision research.
Viral replication and spread.
Viral replication in the cornea was determined with tear film samples obtained by using a foam-tipped applicator (Kettenbach, Germany). The foam tips were transferred into DMEM and vortexed, and virus was titrated on Vero cells by a standard plaque assay. To determine viral titers in the ganglia, TG were surgically excised at 4, 5, and 8 days postinfection (p.i.) from euthanized animals, homogenized, and subjected to three freeze-thaw cycles prior to titration on Vero cells.
Flow cytometry.
At the indicated times after infection, mice were euthanized by exsanguination. TG were excised and resuspended in DMEM containing 10% FBS and 400 U/ml collagenase type 1 (Sigma-Aldrich) per TG for 1 h at 37°C. Cells were then dissociated into single-cell suspensions by trituration. Draining lymph nodes (DLN) were excised, mechanically dispersed with the use of a nylon filter (BD Pharmingen, San Diego, CA), and treated with red blood cell lysis buffer (0.16 M NH4Cl, 0.17 M Tris in distilled water [dH2O] [pH 7.2]) prior to staining. Single-cell TG or DLN suspensions were stained for flow cytometric analysis as previously described (5). Fluorochrome-conjugated antibodies against CD8α (clone 53-6.7) and CD45 (clone 30-F11) and the proper isotype control antibodies were purchased from BD Pharmingen. Antibodies against granzyme B (clone GB11) and the respective isotype controls were purchased from Caltag. Phycoerythrin (PE)-conjugated H-2Kb dimers (BD Pharmingen) were incubated with the SSIEFARL peptide at 37°C overnight prior to use to identify the H-2Kb-restricted HSV-1 gB498-505-specific CD8+ T-cell population.
Quantitative real-time PCR.
The HSV-1 genome copy number in HSV-1-infected TG was determined by quantitative real-time PCR as previously described by using primers that recognize the sequences of the gH gene (5).
Ex vivo TG cultures.
Latently infected TG suspensions (34 days p.i.) were depleted of endogenous CD8α T cells by antibody/complement-mediated lysis using Low-Tox M rabbit complement (Cedarlane). The efficiency of depletion was assessed by flow cytometry. Single-cell TG suspensions were plated at one-fifth TG equivalents per well in 48-well culture plates in 400 μl of DMEM containing 10% FBS, 10 mM HEPES buffer (Gibco), 10 U/ml recombinant murine interleukin-2 (IL-2) (R&D Systems), and 50 μM 2-mercaptoethanol. Where indicated, cultures were supplemented with exogenous gB-CD8+ T cells at 2 × 104 CD8+ T cells/well as determined previously (10). TG cultures were monitored for reactivation by testing culture supernatant fluid for live virus by standard viral plaque assays as previously described (16). Supernatants were tested every 2 days for a total of 8 days in culture. Data are represented as the percentage of wells that were positive for viral reactivation.
RESULTS
Construction and characterization of recombinant HSV-1.
We created HSV-1 gCp-gB, which exhibits gB expression with true late kinetics and complete dependence on viral DNA replication. The construction of the virus and its rescuant (rescue virus) is depicted in Fig. 1. In place of its native promoter, gB is driven by a γ2 gC promoter in gCp-gB so that the protein is expressed only following DNA replication. The strategy required the maintenance of the gB promoter in the genome, because it is contiguous with the upstream UL28 essential open reading frame (ORF) encoding a terminase subunit. As such, HSV-1 gCp-gB was engineered so that the gB promoter drove the expression of EGFP followed by a polyadenylation motif to terminate RNA made from the gB promoter (Fig. 1ii). The rescuant (rescue virus) engineered on the gCp-gB background restored gB expression back to its native promoter and could be differentiated from the parental virus (HSV-1 RE) by the insertion of a silent and novel AvrII restriction site (Fig. 1iii).
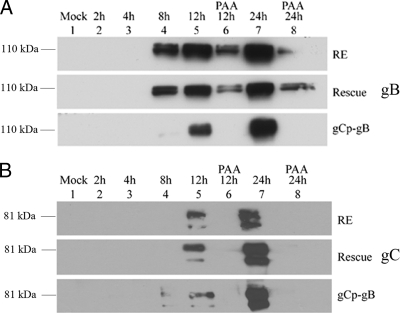
An immunoblot analysis performed at 8 h p.i. revealed an abundant expression of gB in cells infected with wild-type HSV-1 RE (WT RE) and rescue virus but only trace expression in cells infected with gCp-gB virus (Fig. 2A). This was similar to the low levels of the gC protein expressed by all the viruses (Fig. 2B). By 12 h p.i., similar levels of gB expression were detected in WT RE, rescue, and gCp-gB viruses. As expected, the presence of the HSV-1 DNA replication inhibitor phosphonoacetic acid (PAA) blocked the expression of gC in all viruses and of the gB protein in gCp-gB (Fig. 2A and B, lane 6) while permitting the low-level expression of gB from its native promoter in WT RE and the rescue virus (Fig. 2A, lane 6). These data confirm that gB expression kinetics in gCp-gB are regulated as a γ2 late gene, eliminating the low-level gB protein expression prior to DNA replication observed when expression is regulated by its native promoter.
FIG. 2.
HSV gCp-gB expresses gB with true late kinetics. Confluent monolayers of Vero cells were infected with HSV-1 RE, gCp-gB, or rescuant at an MOI of 10 with or without the addition of 350 μg/ml of phosphonoacetic acid (PAA). Total SDS-PAGE-separated proteins were analyzed by immunoblotting for gB (A) or gC (B) using pools of monoclonal antibodies. The times of harvest are shown above each figure and lane designation in hours. The top of each figure represents infection with HSV-1 RE, the middle represents infection with HSV-rescue virus, and the bottom represents infection with HSV gCp-gB. Only the regions corresponding to the main glycoprotein products are shown.
Viral replication of gCp-gB is impaired in vivo but not in culture.
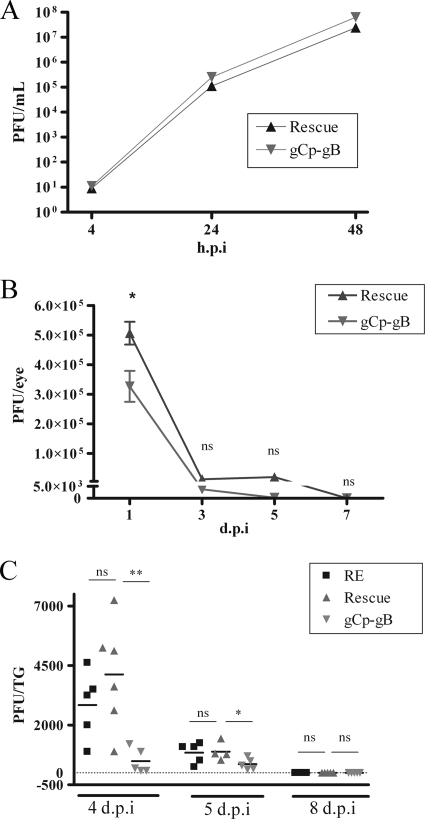
Following the infection of Vero cell monolayers at a low multiplicity of infection (MOI of 0.01 PFU/cell), gCp-gB exhibited a growth curve that was nearly identical to those of the rescuant (rescue virus), demonstrating normal replication efficiency for gCp-gB in vitro (Fig. 3A). However, the gCp-gB virus showed some growth impairment in vivo in mice. Following corneal infection of C57BL/6 mice with wild-type HSV-1, viral titers peaked at 1 day p.i., followed by clearance by 7 days p.i. (24). Here we observed a significant (approximately 2-fold) decrease in tear film titers for gCP-gB compared to those for its rescuant at 1 day p.i. (Fig. 3B), but titers equalized thereafter. By 7 days p.i., infectious virus could not be detected in the tear film of mice infected with either virus, demonstrating that the gCp-gB virus is cleared with normal kinetics in the cornea. Virus replication was assessed in the TG at 4, 5, and 8 days p.i. based on previous findings of peak replication at 4 days p.i. and viral clearance and the uniform establishment of latency by 8 days p.i. (24). The replication of the rescue virus was comparable to that of our parent wild-type strain RE at 4 and 5 days p.i., and replicating virus of both strains was cleared by 8 days p.i. (Fig. 3C). Having established similar replication kinetics of wild-type and rescue viruses in the cornea and TG, only rescue virus was used in subsequent experiments as the appropriate control. The replication of gCp-gB was detectable but severely impaired at 4 and 5 days p.i. compared to those of the rescue and wild-type viruses and was also completely cleared by 8 days p.i. (Fig. 3C). Thus, HSV-1 virulence is compromised in the cornea and more profoundly in the TG when gB expression prior to viral DNA replication is prevented.
FIG. 3.
Viral replication titers are reduced in corneal tear films and TG of gCp-gB-infected mice but not in culture. (A) Vero cell monolayers were infected at an MOI of 0.01 with gCp-gB or rescue viruses. Cells and supernatants were collected at the designated hours p.i. and subjected to three freeze-thaw cycles, and PFU/ml of HSV-1 were measured on Vero cells. The difference between the viral titers of gCp-gB and rescue was not significantly different at any time tested (P > 0.01). The experiment was repeated two independent times, with similar results. (B) Mice were infected with rescue or gCp-gB virus at 1 × 105 PFU/eye. Eye swabs were performed at the indicated days p.i., and the titers of HSV-1 were determined on Vero cells. The viral titers are shown as means ± standard errors of the means (SEM). An asterisk indicates that titers were significantly different as assessed by a Student's t test (P < 0.05). (C) Mice were infected with RE, rescue, or gCp-gB virus at 1 × 105 PFU/eye. Infected TG were excised, homogenized, and subjected to three freeze-thaw cycles, and HSV-1 titers were determined on Vero cells. Each data point represents the mean viral titer from a single TG as determined by plaque assay. The data shown are combined data from two independent experiments. The significance of differences in TG titers was assessed by a Student's t test (**, P = 0.0008; *, P = 0.0573). At 8 days p.i., no infectious virus could be detected from TG infected with either virus. ns, not significant.
HSV-1 gCp-gB establishes a reduced latent viral load in the TG.
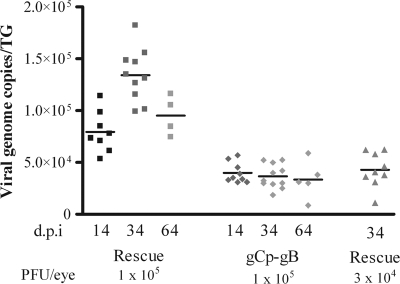
We next determined if gCp-gB induced a reduced latent viral load in the TG, as shown previously for other HSV-1 strains that exhibited impaired replication in the TG (9). Corneal infection of mice with 1 × 105 PFU/eye of gCp-gB indeed resulted in a reduced latent viral load in the TG compared to its rescuant when assessed at 14, 34, and 64 days p.i. (Fig. 4). Unexpectedly, we observed an increase in viral genome copy numbers in rescue virus-infected mice from 14 to 34 days p.i. The cause of this increase is currently unknown and has not previously been observed for wild-type HSV-1 RE. However, we were able to equalize the latent loads of gCp-gB and its rescuant by reducing the infectious dose of the rescue virus by 3-fold (Fig. 4).
FIG. 4.
HSV gCp-gB establishes latency with fewer genome copies than the rescue virus. Corneas of mice were infected at an infectious dose of 1 × 105 or 3 × 104 PFU/eye. TG were excised at 14, 34, and 64 days p.i., and genome copy numbers were determined by real-time PCR. Each data point represents the viral genome copy numbers from a single TG. The data shown are combined data from two independent experiments. At a similar infectious dose (1 × 105 PFU), the rescue virus induced a significantly higher (P < 0.05) latent viral load than the gCp-gB virus at all times tested. Reducing the infectious dose of rescue virus 3-fold relative to that of gCp-gB virus resulted in latent viral loads that were not significantly different (P > 0.05). Data were analyzed by a Student's t test.
Expansion of gB498-505-specific CD8+ T cells in the lymph nodes and their accumulation and retention in infected TG.
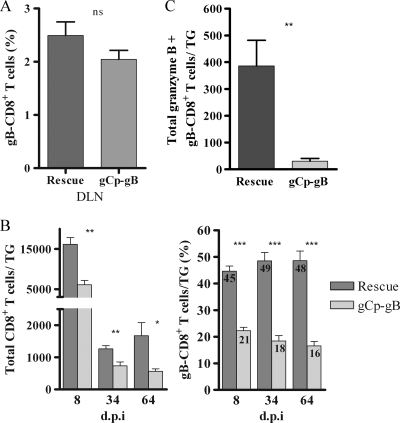
We determined if the altered kinetics of expression of gB would influence the initial expansion of gB-CD8 cells in the draining lymph nodes following corneal infection. At 8 days p.i., a similar total number (not shown) and a similar frequency of gB-CD8 cells (Fig. 5A) were observed for lymph nodes of mice infected with gCp-gB and the rescuant, indicating that delayed gB expression did not impair the priming of naïve CD8+ T cells. The total CD8+ T-cell population in TG infected with both gCp-gB and rescue virus exhibited the expected expansion (8 days p.i.), contraction (8 to 30 days p.i.), and stable memory (>30 days p.i.), but the size of the CD8+ T-cell population in gCp-gB-infected TG was significantly reduced at all times tested (Fig. 5B). The frequency of gB-CD8 cells in TG latently infected (34 and 64 days p.i.) with rescue virus was approximately 50% (Fig. 5B), as previously reported for TG latently infected with wild-type RE (9). Interestingly, the delayed expression of gB by gCp-gB was associated with a dramatically reduced frequency of gB-CD8 cells in both acutely and latently infected TG (Fig. 5B). The reduced frequency of gB-CD8 cells in gCp-gB-infected TG was not related to the latent genome copy number, since infection with a reduced infectious dose of rescue virus dramatically reduced the latent genome copy number at 34 days p.i. (Fig. 4) but did not alter the frequency of gB-CD8 cells in the TG (data not shown).
FIG. 5.
HSV gCp-gB-infected mice contain fewer gB498-505-specific CD8+ T cells in their TG but not lymph nodes, and fewer gB498-505-specific CD8+ T cells in the TG of gCp-gB-infected mice are activated. TG or lymph nodes were excised; dispersed into single-cell suspensions; stained for CD45, CD8, gB498-505 T-cell receptor or intracellular granzyme B expression; and analyzed by flow cytometry. The data are represented as the means ± SEM. (A) Graph representing the percentage of gB-CD8+ T cells in draining lymph nodes (DLN) of mice infected with 1 × 105 PFU/eye of either rescue or gCp-gB virus at 8 days p.i. The data shown are combined data from two independent experiments. The mean percentages of gB-CD8+ T cells are not significantly different between rescue and gCp-gB viruses as assessed by a Student's t test (P > 0.05). (B) Total number of CD8+ T cells (left) and percentage of gB-CD8+ T cells (right) per TG from mice infected with 1 × 105 PFU/eye HSV. The mean for the percentage of gB-CD8+ T cells is shown within each bar graph. The data are combined data from three independent experiments. The mean percentages of total and gB498-505-specific CD8+ T cells between rescue and gCp-gB viruses are significantly different at all time points tested as assessed by a Student's t test (P < 0.05). (C) Graph representing the absolute number of gB-CD8+ T cells in infected TG expressing intracellular granzyme B at 34 days p.i. from mice infected with 3 × 104 PFU/eye rescue virus and 1 × 105 PFU/eye gCp-gB virus to yield equal genome loads. The data are combined data from two independent experiments. The differences between the absolute numbers are significantly different as assessed by a Student's t test (P = 0.0016).
Late expression of gB contributes to the diminished gB-CD8 cell population in gCp-gB latently infected TG.
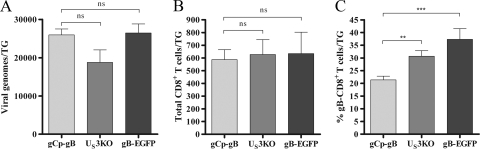
To determine if the altered gB-CD8 cell frequency in gCp-gB-infected TG during latency resulted from impaired virus replication during acute infection, we compared the absolute numbers and frequencies of gB-CD8 in TG that were latently infected with gCp-gB or two other recombinant HSV-1 strains with similarly impaired TG replication. The first virus contains a disruption of the US3 kinase gene (US3KO) (Fig. 1v), and the second virus expresses a gB protein fused to EGFP (gB-EGFP) (Fig. 1iv). Both US3KO and gB-EGFP viruses express gB under the control of its native promoter, and kinetic studies revealed that both viruses expressed gB at low levels prior to viral DNA synthesis and in the presence of DNA replication inhibitors, as seen for RE (data not shown). The gCp-gB, US3KO, and gB-EGFP viruses induced similar loads of latent virus in the TG at 34 days p.i. (Fig. 6A) and similar overall CD8+ T-cell infiltrates into the TG at 34 days p.i. (Fig. 6B). Thus, the reduced sizes of the CD8+ T-cell populations in TG latently infected with gCp-gB, US3KO, and gB-EGFP relative to that observed for TG latently infected with rescue virus (Fig. 5B) appear to reflect the reduced load of latent virus. Importantly, TG harboring the latent US3KO or gB-EGFP virus exhibited a significantly higher frequency of gB-CD8 cells (33% and 37%, respectively) than did those harboring gCp-gB (∼20%) (Fig. 6), suggesting that delayed gB expression and not the latent viral load resulted in the reduced frequency of gB-CD8 cells in gCp-gB latently infected TG.
FIG. 6.
Delaying gB contributes to the diminished gB-CD8+ T-cell response in the TG. Mice were infected at 1 × 105 PFU/eye with gCp-gB or two other TG replication-impaired HSV-1 strains, one lacking the US3 kinase (US3KO) and the other expressing gB as a fusion protein with EGFP (gB-EGFP). The data shown are combined data from two independent experiments. (A) Viral genome copy numbers were determined by real-time PCR at 34 days p.i. The data are represented as the means ± SEM. The differences between gCp-gB and US3KO genome copy numbers and gCp-gB and gB-EGFP genome copy numbers are not significantly different as assessed by a Student's t test (P > 0.05). (B and C) Single-cell suspensions of TG infected for 34 days were stained for CD45, CD8, and gB498-505 TCR expression and analyzed by flow cytometry. The data are presented as the means ± SEM. (B) The total number of CD8+ T cells retained in TG of mice infected with gCp-gB or US3KO and gCp-gB or gB-EGFP is not significantly different as assessed by a Student's t test (P > 0/05). (C) The percentage of gB498-505-specific CD8+ T cells is significantly different between gCp-gB and US3KO (P = 0.0013) and gCp-gB and gB-EGFP (P = 0.0003) viruses at all time points tested as assessed by a Student's t test.
gCp-gB fails to activate gB-CD8 cells during latency.
We previously demonstrated that non-HSV-specific CD8+ T cells are lost from HSV-1 latently infected TG during latency and that gB-CD8 cells form an immunological synapse with neurons within latently infected TG and maintain an activation phenotype, including granzyme B expression (9,10, 27). These observations suggest that antigenic exposure maintains gB-CD8 cells in an activated state during latency. The reduced frequency of gB-CD8 cells in TG harboring latent gCp-gB suggested reduced antigenic exposure, and we hypothesized that this would also result in reduced activation, as assessed by intracellular granzyme B expression. Indeed, the absolute number (Fig. 5C) and frequency (not shown) of granzyme B-positive gB-CD8 cells were significantly reduced in gCp-gB-infected TG at 34 days p.i.
gB-CD8 cells are capable of blocking reactivation of gCp-gB.
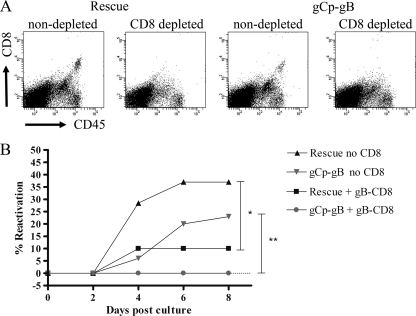
The early expression of gB before viral DNA synthesis might provide a requisite window of opportunity for gB-CD8 cells to prevent full reactivation and virion formation. However, it was of interest to ask if gB-CD8 cells are still able to block reactivation if they encounter a neuron late in reactivation after DNA synthesis is initiated. To address this, cultures of dispersed TG harboring similar loads of latent gCp-gB or rescuant virus were depleted of endogenous CD8+ T cells, reconstituted or not with purified memory gB-CD8 cells that were previously expanded from latently infected TG (10), and monitored for HSV-1 reactivation based on the recovery of infectious virus from culture supernatants. Figure 7A verifies that the TG cell suspensions were effectively depleted of endogenous CD8+ T cells. In the absence of added gB-CD8+ T cells, reactivation was observed in neurons harboring both gCp-gB and the rescuant, although the reactivation frequency was slightly lower in neurons harboring gCp-gB (Fig. 6B). Notably, late gB expression did not impair the ability of the gB-CD8+ T cells to block reactivation. Thus, gB-CD8 cells can act very late in the viral life cycle to block the full reactivation and formation of infectious virus.
FIG. 7.
gB498-505-specific CD8 T cells can block gCp-gB reactivation. Mice were infected with 3 × 104 PFU/eye rescue virus and 1 × 105 PFU/eye gCp-gB virus to establish equal numbers of genome copies during latency. TG were excised at 34 days p.i., and single-cell suspensions were depleted of CD8 as described in Materials and Methods. Depleted TG were plated as one-fifth TG cultures, half of the cultures received an add-back of gB498-505-specific CD8 T cells (gB-CD8 T cells), and the other half did not. Reactivation was monitored by sampling supernatants for infectious virus via plaque assay. (A) Representative dot plots before and after CD8 depletion, showing depletion efficacy. (B) Representative graph showing reactivation frequencies of rescue and gCp-gB viruses with or without gB-CD8 cells added back. For both viruses, the difference in reactivation frequencies between cultures with gB-CD8 cells added back and without CD8+ T cells is statistically significant (*, P = 0.0114; **, P = 0.0017), as assessed by a survival curve analysis (log rank test). The experiment was repeated three independent times, with similar results.
DISCUSSION
We investigated the influence of gB gene expression kinetics on HSV-1 virulence and targeting by the host immune system. To test this, we created a recombinant virus (gCp-gB) that expresses gB as a late protein from the γ2-regulated gC promoter, thus abrogating the expression of the gB protein prior to DNA synthesis. This should not influence the level of gB expression since gB and gC are expressed at similar levels at late times postinfection (29). We observed that gCp-gB exhibited reduced virulence in the mouse cornea and particularly in the TG. HSV-1 gB is required for viral entry into cells by mediating the fusion of the viral envelope to the host cell membrane (6, 33). However, several recent studies have demonstrated other functions for gB during the viral life cycle, and it is possible that delaying the expression of gB might impair replication by modulating one or more of these functions. For instance, a delay of gB expression could delay gB binding to the cellular stress sensor PKR-like endoplasmic reticulum kinase (PERK) and the resulting augmentation of viral protein translation (20). In our studies we observed some growth impairment of the gCp-gB virus in the cornea, consistent with our recent observation of gCp-gB virus growth impairment and increased phosphorylation of the alpha subunit of eukaryotic initiation factor 2 (eIF2α) in primary corneal fibroblasts (our unpublished observations) but not in Vero cells in vitro. More severe gCp-gB growth impairment was observed in the TG, suggesting that the activity on this pathway might be particularly important for TG neurons. The transport of gCp-gB capsids from the cornea to the nerve body might also be hindered, since the phosphorylation of gB is needed for the efficient egress of nucleocapsids from the inner nuclear membrane (36). HSV-1 gB is also a highly glycosylated protein, and delayed expression kinetics could also contribute to altered glycosylation patterns, which could negatively affect protein function. Our findings emphasize the selective importance of the kinetics of gB expression for viral growth in certain cell types. The mechanisms underlying the growth impairment of gCp-gB are currently under investigation.
To our knowledge this is the first study to assess the influence of viral gene expression kinetics on host immunity. The expansion of naïve HSV-specific CD8+ T cells in the lymphoid organs appears to result from a cross-presentation of viral antigens rather than by direct presentation by infected antigen-presenting cells (2). Accordingly, any of the kinetic classes of HSV-1 proteins could theoretically be presented, and a delayed expression of gB should not greatly influence the initial expansion of gB-CD8+ T cells in mice infected with gCp-gB. This was in fact observed, as quantitatively similar expansions of gB-CD8 cells were observed for draining lymph nodes of mice infected with gCp-gB and rescuant viruses. Thus, the immunogenic properties of gB are not influenced by altering the kinetics of expression. In contrast, a delay in gB expression kinetics could impact gB-CD8 cell cognate recognition of gB epitopes in latently infected neurons and their capacity to prevent full reactivation and virion formation. The sequential expression of HSV-1 genes during lytic infection has been appreciated for some time, but little is known about the importance of expression kinetics for the targeting of viral proteins by host immunity. By use of classical molecular approaches, gB was shown previously to be produced early in the viral life cycle before viral DNA replication, leading to its classification as a leaky late γ1 gene (22, 23). Very low levels of protein synthesis are required to sensitize cells for recognition by CD8+ T cells (30). Accordingly, while gB is hardly detectable by immunoblotting at very early stages of infection, Mueller and colleagues showed previously that de novo gB synthesis can be detected by gB-CD8 cells as early as 2 h postinfection (19).
HSV-1 latency differs from chronic infections in that viral DNA replication does not take place and viral protein expression is largely silenced. However, HSV-1 latency is no longer considered an entirely antigenically silent state. The recognition of latent virus by CD8+ T cells was demonstrated by the observations that HSV-specific CD8+ T cells surround neurons in latently infected murine TG, form immunological synapses with the neurons, and release lytic granules into the synapse in situ and that HSV-specific CD8+ T cells can block HSV-1 reactivation from latency in dispersed TG cultures (9,10). Moreover, non-HSV-specific CD8+ T cells that enter the TG during acute infection are lost during latency, suggesting that an antigenic encounter is required to maintain the CD8+ T-cell population in the TG (27). Presumably, these antigenic encounters occur during partial reactivation events that are terminated by CD8+ T-cell effector functions, including IFN-γ and lytic granule release (3, 10, 15). The fact that the HSV-1 latent genome copy number remains constant during latency and that CD8+ T cells can terminate reactivation prior to the expression of the true late gC gene in TG cultures (16) suggests that gB-specific CD8+ T cells recognize their epitope on neurons early in the viral life cycle prior to viral DNA synthesis. This raises the question of whether gB expression prior to HSV-1 DNA synthesis is required for the retention of gB-CD8 cells in latently infected TG and for their ability to block HSV-1 reactivation from latency.
One of the most striking observations of our study was that the frequency of gB-CD8 cells was diminished over time in TG harboring latent gCp-gB. These findings suggest a lack of antigenic exposure to gB-CD8 when gB is expressed as a true late gene in gCp-gB. We conclude that reduced antigenic exposure due to the altered kinetics of gB expression and not the reduced latent genome copy number in gCp-gB-infected TG is responsible for the loss of gB-CD8 based on four observations. First, reducing the infectious dose of the rescue virus 3-fold caused a corresponding approximately 3-fold reduction in the latent genome copy number in the TG but did not alter the frequency of gB-CD8 cells (data not shown). Second, although the gCp-gB, US3KO, and gB-EGFP viruses all induced comparable latent genome copy numbers in the TG, the gB-CD8 cell frequency was significantly lower in the TG harboring gCp-gB. Third, the fact that gB-specific CD8+ T cells are selectively lost from the TG argues against the possibility that delaying gB expression simply reduces the levels of inflammatory mediators such as chemokines and cytokines, which would not be expected to result in the selective loss of CD8+ T cells of a particular specificity. Fourth, the activation of gB-CD8 cells, as indicated by intracellular granzyme B levels, was dramatically reduced in TG harboring latent gCp-gB compared to those harboring rescuant virus, even when the load of latent virus was equalized. We are currently investigating whether the loss of gB-CD8 cells from the TG and the concomitant loss of granzyme B expression within gB-CD8 cells in TG harboring gCp-gB correspond to lower levels or an absence of gB transcripts within latently infected TG.
We have previously shown that gene expression kinetics are similar during reactivation and lytic replication, at least with respect to the γ1 (gB) and γ2 (gC) genes (24). In a wild-type virus, gB is expressed relatively early, so it seems obvious to surmise that gB-CD8 cells can recognize their viral antigen early and shut down reactivation before DNA replication. We hypothesized that once DNA replication takes place, it is too late in the viral life cycle for CD8+ T cells to block reactivation, as the virus has already committed to assembly and egress. However, our findings establish that gB-CD8 cells can indeed block reactivation even after viral DNA replication. This finding suggests that even if a gB-CD8 cell encounters a neuron late in the reactivation process, it will still be able to block full reactivation and virion formation. This is consistent with the ability of the CD8+ T-cell effector molecule IFN-γ to block reactivation even after late gene expression (3). CD8+ T cells can employ lytic granules and IFN-γ to block the reactivation of wild-type HSV-1 from latency without neuronal destruction (10). It remains to be determined if the effector mechanism(s) employed by gB-CD8 cells to block reactivation when gB is expressed only after viral DNA synthesis is compatible with neuronal preservation. The fact that the CD8+ T cells in infected TG that are not specific for the immunodominant gB498-505 epitope appear to be HSV specific implies that the reduced frequency of gB-CD8 cells in TG harboring gCp-gB is associated with an increased frequency of HSV-specific CD8+ T cells reactive to subdominant epitopes or to new epitopes that arise in these mice. Clarification of this issue will await the identification of the subdominant epitopes recognized by these cells.
The data from this study can be used to design better therapeutic vaccines for HSV-1. While CD8+ T cells responding to late antigens are still able to block reactivation, they are not retained in high numbers in the TG during latency. This makes late viral antigens a poor choice as the sole immunogen. There is an obvious utility to targeting viral proteins expressed before DNA replication. A block in reactivation after viral DNA synthesis would permit an accumulation of viral genomes in neurons, which is associated with a greater likelihood of reactivation (8). A study reported previously by Hoshino and colleagues demonstrated that the rate of HSV-1 reactivation is proportional to the number of latent genomes and inversely proportional to the number of CD8+ T cells retained in the TG. Those researchers therefore suggested that vaccines should be evaluated for their ability to induce and maintain a virus-specific CD8+ T-cell response as well as a low genome load in the ganglia (8). Our findings suggest that immunization against viral α, β, and γ1 proteins that are expressed before DNA replication would optimize the capacity of the immune system to maintain HSV-1 in a latent state and prevent recurrent disease.
Acknowledgments
This work was supported by Public Health Service grants EY015291 (P.R.K.) and EY05945 (R.L.H.), a core grant from the NEI (EY08098), and unrestricted funds from Research To Prevent Blindness, Inc., and the Eye & Ear Foundation of Pittsburgh.
Footnotes
Published ahead of print on 23 June 2010.
REFERENCES
- 1.Ahn, K., T. H. Meyer, S. Uebel, P. Sempe, H. Djaballah, Y. Yang, P. A. Peterson, K. Fruh, and R. Tampe. 1996. Molecular mechanism and species specificity of TAP inhibition by herpes simplex virus ICP47. EMBO J. 15:3247-3255. [PMC free article] [PubMed] [Google Scholar]
- 2.Bedoui, S., P. G. Whitney, J. Waithman, L. Eidsmo, L. Wakim, I. Caminschi, R. S. Allan, M. Wojtasiak, K. Shortman, F. R. Carbone, A. G. Brooks, and W. R. Heath. 2009. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat. Immunol. 10:488-495. [DOI] [PubMed] [Google Scholar]
- 3.Decman, V., P. R. Kinchington, S. A. K. Harvey, and R. L. Hendricks. 2005. Gamma interferon can block herpes simplex virus type 1 reactivation from latency, even in the presence of late gene expression. J. Virol. 79:10339-10347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldman, L. T., A. R. Ellison, C. C. Voytek, L. Yang, P. Krause, and T. P. Margolis. 2002. Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proc. Natl. Acad. Sci. U. S. A. 99:978-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman, M. L., B. S. Sheridan, R. H. Bonneau, and R. L. Hendricks. 2007. Psychological stress compromises CD8+ T cell control of latent herpes simplex virus type 1 infections. J. Immunol. 179:322-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herold, B. C., R. J. Visalli, N. Susmarski, C. R. Brandt, and P. G. Spear. 1994. Glycoprotein C-independent binding of herpes simplex virus to cells requires cell surface heparan sulphate and glycoprotein B. J. Gen. Virol. 75:1211-1222. [DOI] [PubMed] [Google Scholar]
- 7.Honess, R. W., and B. Roizman. 1973. Proteins specified by herpes simplex virus. XI. Identification and relative molar rates of synthesis of structural and nonstructural herpes virus polypeptides in the infected cell. J. Virol. 12:1347-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoshino, Y., L. Pesnicak, J. I. Cohen, and S. E. Straus. 2007. Rates of reactivation of latent herpes simplex virus from mouse trigeminal ganglia ex vivo correlate directly with viral load and inversely with number of infiltrating CD8+ T cells. J. Virol. 81:8157-8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khanna, K. M., R. H. Bonneau, P. R. Kinchington, and R. L. Hendricks. 2003. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity 18:593-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knickelbein, J. E., K. M. Khanna, M. B. Yee, C. J. Baty, P. R. Kinchington, and R. L. Hendricks. 2008. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science 322:268-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kramer, M. F., S. H. Chen, D. M. Knipe, and D. M. Coen. 1998. Accumulation of viral transcripts and DNA during establishment of latency by herpes simplex virus. J. Virol. 72:1177-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kramer, M. F., and D. M. Coen. 1995. Quantification of transcripts from the ICP4 and thymidine kinase genes in mouse ganglia latently infected with herpes simplex virus. J. Virol. 69:1389-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krause, P. R., K. D. Croen, S. E. Straus, and J. M. Ostrove. 1988. Detection and preliminary characterization of herpes simplex virus type 1 transcripts in latently infected human trigeminal ganglia. J. Virol. 62:4819-4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laycock, K., S. Lee, R. Brady, and J. Pepose. 1991. Characterization of a murine model of recurrent herpes simplex viral keratitis induced by ultraviolet B radiation. Invest. Ophthalmol. Vis. Sci. 32:2741-2746. [PubMed] [Google Scholar]
- 15.Liu, T., K. M. Khanna, B. N. Carriere, and R. L. Hendricks. 2001. Gamma interferon can prevent herpes simplex virus type 1 reactivation from latency in sensory neurons. J. Virol. 75:11178-11184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu, T., K. M. Khanna, X. Chen, D. J. Fink, and R. L. Hendricks. 2000. CD8+ T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J. Exp. Med. 191:1459-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGeoch, D. J., M. A. Dalrymple, A. J. Davison, A. Dolan, M. C. Frame, D. McNab, L. J. Perry, J. E. Scott, and P. Taylor. 1988. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J. Gen. Virol. 69:1531-1574. [DOI] [PubMed] [Google Scholar]
- 18.Metcalf, J. F., and B. A. Michaelis. 1984. Herpetic keratitis in inbred mice. Invest. Ophthalmol. Vis. Sci. 25:1222-1225. [PubMed] [Google Scholar]
- 19.Mueller, S. N., C. M. Jones, W. Chen, Y. Kawaoka, M. R. Castrucci, W. R. Heath, and F. R. Carbone. 2003. The early expression of glycoprotein B from herpes simplex virus can be detected by antigen-specific CD8+ T cells. J. Virol. 77:2445-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulvey, M., C. Arias, and I. Mohr. 2007. Maintenance of endoplasmic reticulum (ER) homeostasis in herpes simplex virus type 1-infected cells through the association of a viral glycoprotein with PERK, a cellular ER stress sensor. J. Virol. 81:3377-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orr, M. T., M. A. Mathis, M. Lagunoff, J. A. Sacks, and C. B. Wilson. 2007. CD8 T cell control of HSV reactivation from latency is abrogated by viral inhibition of MHC class I. Cell Host Microbe 2:172-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peake, M. L., P. Nystrom, and L. I. Pizer. 1982. Herpesvirus glycoprotein synthesis and insertion into plasma membranes. J. Virol. 42:678-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rafield, L. F., and D. M. Knipe. 1984. Characterization of the major mRNAs transcribed from the genes for glycoprotein B and DNA-binding protein ICP8 of herpes simplex virus type 1. J. Virol. 49:960-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramachandran, S., J. E. Knickelbein, C. Ferko, R. L. Hendricks, and P. R. Kinchington. 2008. Development and pathogenic evaluation of recombinant herpes simplex virus type 1 expressing two fluorescent reporter genes from different lytic promoters. Virology 378:254-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rooney, J. F., M. L. Mannix, C. R. Wohlenberg, C. J. Wallington, A. L. Notkins, S. Banks, S. E. Straus, Y. Bryson, and M. Dillon. 1991. Prevention of ultraviolet-light-induced herpes labialis by sunscreen. Lancet 338:1419-1422. [DOI] [PubMed] [Google Scholar]
- 26.Sawtell, N. M., and R. L. Thompson. 1992. Rapid in vivo reactivation of herpes simplex virus in latently infected murine ganglionic neurons after transient hyperthermia. J. Virol. 66:2150-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheridan, B. S., T. L. Cherpes, J. Urban, P. Kalinski, and R. L. Hendricks. 2009. Reevaluating the CD8 T-cell response to herpes simplex virus type 1: involvement of CD8 T cells reactive to subdominant epitopes. J. Virol. 83:2237-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shirtcliff, E. A., C. L. Coe, and S. D. Pollak. 2009. Early childhood stress is associated with elevated antibody levels to herpes simplex virus type 1. Proc. Natl. Acad. Sci. U. S. A. 106:2963-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spear, P. G. 1975. Glycoproteins specified by herpes simplex virus type 1: their synthesis, processing and antigenic relatedness to HSV-2 glycoproteins. IARC Sci. Publ. 1975:49-61. [PubMed] [Google Scholar]
- 30.Sykulev, Y., M. Joo, I. Vturina, T. J. Tsomides, and H. N. Eisen. 1996. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity 4:565-571. [DOI] [PubMed] [Google Scholar]
- 31.Tal-Singer, R., T. M. Lasner, W. Podrzucki, A. Skokotas, J. J. Leary, S. L. Berger, and N. W. Fraser. 1997. Gene expression during reactivation of herpes simplex virus type 1 from latency in the peripheral nervous system is different from that during lytic infection of tissue cultures. J. Virol. 71:5268-5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Theil, D., T. Derfuss, I. Paripovic, S. Herberger, E. Meinl, O. Schueler, M. Strupp, V. Arbusow, and T. Brandt. 2003. Latent herpesvirus infection in human trigeminal ganglia causes chronic immune response. Am. J. Pathol. 163:2179-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner, A., B. Bruun, T. Minson, and H. Browne. 1998. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J. Virol. 72:873-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verjans, G. M., R. Q. Hintzen, J. M. van Dun, A. Poot, J. C. Milikan, J. D. Laman, A. W. Langerak, P. R. Kinchington, and A. D. Osterhaus. 2007. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc. Natl. Acad. Sci. U. S. A. 104:3496-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallace, M. E., R. Keating, W. R. Heath, and F. R. Carbone. 1999. The cytotoxic T-cell response to herpes simplex virus type 1 infection of C57BL/6 mice is almost entirely directed against a single immunodominant determinant. J. Virol. 73:7619-7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wisner, T. W., C. C. Wright, A. Kato, Y. Kawaguchi, F. Mou, J. D. Baines, R. J. Roller, and D. C. Johnson. 2009. Herpesvirus gB-induced fusion between the virion envelope and outer nuclear membrane during virus egress is regulated by the viral US3 kinase. J. Virol. 83:3115-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]