Abstract
Objective
Following guidelines, women evaluated by colposcopy, but not found to have a precancerous lesion, could be tested again at 12 months for carcinogenic human papillomavirus (HPV). Compared with pooled-probe testing, measuring HPV genotype–specific persistence might better predict subsequent grade 3 cervical intraepithelial neoplasia (CIN3).
Methods
For women enrolled in the immediate colposcopy arm of the Atypical squamous cells of undetermined significance (ASCUS) and Low-grade squamous intraepithelial lesion (LSIL) Triage Study (ALTS), who underwent enrollment colposcopy but were without prevalently detected CIN2 or worse (CIN2+; n = 671), we compared 1-year HPV persistence, as measured by a pooled HPV genotype test (hybrid capture 2; hc2) versus a research PCR HPV genotyping test (line blot assay; LBA) as predictors of “missed prevalent” or possibly incident CIN3 diagnosed between 12 and 24 months.
Results
Thirty-two (4.8%) women were diagnosed with subsequent CIN3. Testing repeatedly hc2-positive (hc2+) was more common (49.0%) than genotype-specific persistence as detected by LBA (30.3%, P < 0.01). Although absolute risks of CIN3 following repeat hc2+ or genotype-specific persistence were similar (8.8% versus 8.4%, P = 0.86), repeat hc2+ was more sensitive for identifying CIN3 than genotype-specific persistence (90.6% versus 53.1%, P < 0.01). Among 329 women repeatedly hc2+, women with persistent HPV16 were at higher risk of CIN3 than non–HPV16-persistent women (23.1% versus 7.0%, P < 0.01).
Conclusions
For postcolposcopy management, 1-year HPV persistence as measured by hc2 would recall more women but was more sensitive and similarly predictive for CIN3 in the following year than detection of genotype-specific persistence by LBA.
Impact
Although find little utility for measuring type-specific persistence, testing for persistent HPV16 might be clinically useful.
Introduction
Cervical cytologic interpretations of atypical squamous cells of undetermined significance (ASCUS) and low-grade squamous intraepithelial lesion (LSIL) are the most commonly reported cytologic abnormalities. Testing for the presence of carcinogenic human papillomavirus (HPV) can effectively triage women with ASCUS (equivocal) cytology, as HPV-negative women are at very low risk of disease (1–4). Such testing is not useful among women with definite minor abnormalities of LSIL because these are usually HPV-positive (3, 4).
In the United States, more than 2 million women ages 30 or older with LSIL cytology or HPV-positive ASCUS cytology are referred to colposcopy each year. Most women were found to have cervical intraepithelial neoplasia (CIN) grade 1 or less. Guidelines in the United States recommend that these women be subsequently followed with either repeat cytology at 6 and 12 months or HPV DNA test at 12 months (3–5). The most commonly used Food and Drug Administration–approved test is hybrid capture 2 (hc2), which detects the presence of 1 or more of 13 carcinogenic HPV genotypes as well as some cross-reactive borderline carcinogenic genotypes (6). Although hc2 testing at 12 months after the colposcopy exam has high sensitivity (~90% for CIN2+; ref. 7), the referral rate for repeat colposcopy is ~50% (7) and the absolute risk of CIN2+ when hc2 is positive at that visit is only ~20% (8). Consequently, many thousands of women following this protocol would be referred for repeat colposcopy although only a minority would be found to have CIN2+ (7, 8).
Persistent cervical infection by ~15 carcinogenic or high-risk HPV genotypes causes virtually all cervical cancer and its immediate precursor lesions (9–11), although not all women with persistent carcinogenic HPV develop precancer or cancer. Detection of persistent carcinogenic HPV infection is associated with increased risk of cervical precancer/cancer (12). HPV persistence has been variably defined as two positive tests within an interval of 6, 12, or more months using different HPV DNA detection strategies. At one extreme, the definition has included repeat HPV positivity using a pooled test such as hc2; on the other, it has been restricted to the identification of HPV genotype–specific persistence (12). In theory, HPV genotype–specific testing should better identify women at risk for precancer when compared with a pooled test, as testing HPV positive by a pooled test could also be the result of one HPV genotype clearing and another being acquired. A few natural history studies, comparing risk of precancer among women with persistent infection defined as HPV genotype–specific persistence versus repeatedly detecting any carcinogenic HPV, have found genotype-specific persistence posing greater (13) or similar (12, 14) risk compared with repeatedly testing positive for any carcinogenic HPV type (as with a pooled test).
Thus, to address assay and definition choice for clinical practice in follow-up of women with HPV-positive ASCUS and LSIL in whom no CIN2+ was found at initial colposcopy, we compared the predictive values of repeatedly testing positive using a pooled assay of carcinogenic HPV genotypes and HPV genotype–specific detection of viral persistence for subsequent CIN3 using data from the ASCUS-LSIL Triage Study (ALTS).
Materials and Methods
ALTS was a randomized trial directed by the National Cancer Institute (NIH) that compared three triage strategies for women with ASCUS or LSIL. The details of the design, methods, and primary results of ALTS have been published extensively elsewhere (15–17). The National Cancer Institute and local institutional review boards approved the study. Briefly, a total of 5,060 women with ASCUS4 or LSIL cytology were enrolled in the study at four clinical centers and referred to colposcopy depending on the study arm to which they were randomly assigned. In the immediate colposcopy arm, all women had standard colposcopy (17) at, or soon after, enrollment regardless of enrollment test results. In the HPV triage arm, women were referred to colposcopy if the enrollment HPV test was missing (3.9%) or positive (56.4% for ASCUS and LSIL combined; triage of LSIL was discontinued early), or if the enrollment cytology was high-grade squamous intraepithelial lesion. In the conservative management arm, women were referred to colposcopy if enrollment cytology was interpreted as high-grade squamous intraepithelial lesion (10%). During the 2-year follow-up, all women were evaluated every 6 months with cytology (HPV testing was done but not provided to the clinical teams until the exit visit of the study) and invited for colposcopy at the 24-month exit visit. Clinical management was based on the clinical center pathologists’ cytologic and histologic diagnoses. In addition, all cytology and histology slides were sent to quality control pathology for independent review. Pathology quality control histologic diagnoses were masked to all cytology results and were used in this data analysis to avoid center-specific variation.
After liquid-based (ThinPrep; Hologic) cytology slides were prepared, 4-mL aliquots of the residual PreservCyt samples were tested for HPV DNA by hc2 (Qiagen), a pooled-probe, signal amplification DNA test that targets a group of 13 HPV genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68). A positive cutoff point of 1.00 relative light units per positive control was used. hc2 does not distinguish which HPV genotype(s) are present. HPV genotyping was done by line blot assay (LBA; Roche Molecular Systems), a research-use-only L1-based PCR assay that uses a primer set designated PGMY09/11, done on the standard transport medium specimen (20, 21). Amplimers were subjected to reverse-line blot hybridization for 27 or 38 HPV genotypes. We considered women as LBA PCR–positive if at least 1 of 13 genotypes targeted by hc2 was detected. We also considered LBA results for HPV16 alone. Because cross-reactivity with borderline carcinogenic HPV types has been documented with hc2 (6), we also considered whether including these HPV genotypes (53, 66, 67, 70, 73, and 82) could explain differences in performance between hc2 and LBA.
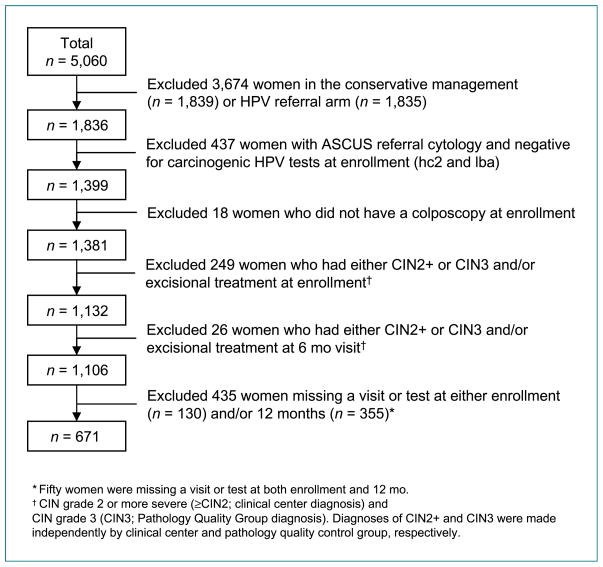
For this analysis, we excluded women enrolled in the conservative management arm (n = 1,839) because the diagnosis of CIN2+ during the year 1 period depended on finding high-grade squamous intraepithelial lesion and was insensitive in this arm (Fig. 1). Women in the HPV referral arm (n = 1,835) were also excluded because women were referred for colposcopy based on their hc2 results, biasing comparisons with other HPV testing strategies. We included only the subset of women from the immediate colposcopy arm who would have been referred to colposcopy based on current standards of practice (3, 4), i.e., women with LSIL or HPV-positive ASCUS. Therefore, women were excluded if they had an ASCUS referral cytology and were HPV-negative (by hc2 and LBA) because they would not have been referred for colposcopy according to current standard practices (n = 437). Women who did not have a colposcopic exam at enrollment despite referral were also excluded (n = 18).
Figure 1.
Consort diagram.
We were interested in the clinical management by HPV testing at the 12-month return visit of women without immediate evidence of disease requiring treatment (who did not have CIN2+ diagnosed prevalently). Thus, we further excluded women who at enrollment (n = 249) or 6 months (n = 26) were diagnosed with CIN2+ by clinical center pathologists or CIN3 by the quality control pathology team and/or underwent excisional treatment within the first 6 months of follow-up. Finally, we further restricted our main analytic population to those women with hc2 and LBA PCR results at both enrollment and 12-month visits, yielding a final analytic sample of 671 women.
We calculated the cumulative absolute risk for CIN3 as diagnosed by the quality control group during the 12-, 18-, or 24-month visits because it is the preferred scientific surrogate for cancer risk. We also calculated the cumulative absolute risk of CIN2+ as diagnosed by clinical center pathologists during the 12-, 18-, or 24-month visits because it is the clinically relevant threshold for treatment. There was one case of cancer that was included as an endpoint by either definition.
We compared risks of CIN2+ or CIN3 given repeat positive hc2, type-specific persistence by LBA for 1 of 13 carcinogenic HPV genotypes, and persistence of HPV16 by LBA at enrollment and 12-month visits. We calculated the sensitivity of persistently positive hc2 and type-specific persistence by LBA to trigger referral at 12 months among women found in the subsequent year to have CIN2+ and CIN3. We took into account possible confounding or mediating variables such as age, referral cytology, clinic center, and characteristics of the enrollment colposcopy exam (colposcopic impression, number of biopsies, and enrollment histology result).
Among women who were repeatedly hc2 positive, we examined whether those with LBA genotype–specific persistent HPV infection had a greater absolute risk of CIN2+ and CIN3 than women who were not LBA genotype–specific persistent.
Comparisons of estimates of risk were tested using standard contingency table analysis with χ2 statistics; binomial exact 95% confidence intervals (95% CI) were calculated where indicated. Analyses were done using SAS 9.1.3 analytic software (SAS Institute, Inc.).
Results
A majority of the 671 women had a positive HPV test result at enrollment prior to the colposcopy visit, although women were more likely to have tested positive for carcinogenic HPV by hc2 than by LBA (87.0% versus 73.8%, respectively; P < 0.01); 15.8% tested positive for HPV16 by LBA (Table 1).
Table 1.
Enrollment characteristics among women without prevalently detected CIN grade 2 or worse (CIN2+) in the immediate colposcopy arm
| Total |
||
|---|---|---|
| N | Column (%) | |
| 671 | 100.0 | |
| Center | ||
| Center 1 | 230 | 34.3 |
| Center 2 | 132 | 19.7 |
| Center 3 | 106 | 15.8 |
| Center 4 | 203 | 30.3 |
| Referral cytology | ||
| ASCUS | 342 | 51.0 |
| LSIL | 329 | 49.0 |
| Patient characteristics at enrollment visit | ||
| Age (median = 23 y) | ||
| 18–29 y | 546 | 81.4 |
| 30+ y | 125 | 18.6 |
| LBA PCR for carcinogenic HPV types* | ||
| Negative | 176 | 26.2 |
| Positive for one or more genotypes | 495 | 73.8 |
| HPV16 positive | 106 | 15.8 |
| Carcinogenic HPV types other than 16† | 444 | 66.2 |
| hc2 | ||
| Negative | 87 | 13.0 |
| Positive | 584 | 87.0 |
| Characteristics of enrollment colposcopic exam | ||
| Colposcopic impression‡ | ||
| Normal/cervicitis/atrophy/polyp | 138 | 20.6 |
| Atypical metaplasia | 60 | 8.9 |
| Low grade or worse | 469 | 69.9 |
| Number of biopsies | ||
| 0 | 165 | 24.6 |
| 1 | 386 | 57.5 |
| 2+ | 120 | 17.9 |
| Enrollment histology result§ | ||
| Normal | 328 | 48.9 |
| CIN1 | 173 | 25.8 |
| CIN2|| | 22 | 3.3 |
Includes 13 HPV types: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68.
Fifty-five women were positive for both HPV16 and another carcinogenic HPV type.
Colposcopic impression missing for four (0.6%) women.
Pathology Quality Control Group diagnosis of histology results from colposcopically-guided biopsies, LEEPs and other excisional procedures. No biopsies were taken for 143 (21.3%) examinations, suggesting no lesions as judged by the colposcopists. Results were inadequate or missing for five (0.8%) women.
Clinical center diagnosis was CIN1 (n = 8), normal (n = 13), or unsatisfactory (n = 1).
We found more women were persistently hc2-positive than had HPV genotype–specific persistent infection detected by LBA (49.0% versus 30.3%, P < 0.01; Table 2). Overall, 32 of 671 women (4.8%) had a CIN3 diagnosis during the 12 to 24 months of follow-up. Twenty-nine of 329 women (8.8%) who tested repeated hc2-positive and 17 of 203 women (8.4%) of women with an HPV genotype–specific persistent infection had a CIN3 diagnosis during the 12 to 24 months of follow-up (P = 0.86).
Table 2.
Absolute risk of and sensitivity to detect subsequent CIN2+/CIN3 given definition of carcinogenic HPV persistence among women without prevalently detected CIN2+ at enrollment colposcopy
| Total |
Clinical center CIN2+ at 12–24 mo* | Absolute risk |
Sensitivity |
Quality control CIN3 at 12–24 mo* | Absolute risk |
Sensitivity |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Col % | Row % | 95% CI | Col % | 95% CI | Row % | 95% CI | Col % | 95% CI | |||
| Total | 671 | 100.0 | 64 | 9.5 | (7.3–11.8) | 100.0 | 32 | 4.8 | (3.2–6.4) | 100.0 | ||
| HPV persistent at 12 mo visit by pooled hc2+ | 329 | 49.0 | 55 | 16.7 | (12.7–20.8) | 85.9 | (77.2–94.7) | 29 | 8.8 | (5.7–11.9) | 90.6 | (79.9–100.0) |
| HPV persistent for specific genotype at 12 mo visit by LBA PCR for 13 HPV genotypes† | 203 | 30.3 | 34 | 16.8 | (11.6–21.9) | 53.1 | (40.6–65.7) | 17 | 8.4 | (4.5–12.2) | 53.1 | (34.8–71.4) |
| HPV16 persistent at 12 mo visit by LBA PCR‡ | 42 | 6.3 | 13 | 31.0 | (16.4–45.5) | 20.3 | (10.2–30.4) | 9 | 21.4 | (8.5–34.4) | 28.1 | (11.7–44.6) |
NOTE: Women were enrolled into the immediate colposcopy arm after ASCUS/HPV+ or LSIL referral cytology.
Endpoint is CIN grade 2 or more severe (≥CIN2; clinical center diagnosis) and CIN grade 3 (CIN3; Pathology Quality Control Group diagnosis). Diagnoses of CIN2+ and CIN3 were made independently by clinical center and pathology quality control group, respectively.
HPV types: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68. Twenty-five women had type-specific persistent HPV but were not repeatedly hc2+ and none of them had CIN2+ or CIN3 detected at 12 to 24 mo.
Thirty-nine of these women were persistently hc2+.
Forty-two of 671 women (6.3%) had persistent HPV16 and virtually all (n = 39) were repeatedly hc2 positive. Of the three women who were persistently HPV16-positive but hc2-negative at enrollment or at 12-month visit, none had CIN2+ or CIN3 detected at 12 to 24 months. Notably, women with persistent HPV16 were at three times the risk of CIN3 (21.4%) than women with genotype-specific persistence for a type besides HPV16 (7.1% of 169—not in table; P = 0.01).
Although absolute risks of CIN3 were similar for hc2-positive versus genotype-specific persistence (with the exception of HPV16 persistent infection), the sensitivity for identifying women who were subsequently diagnosed with CIN3 was greater for repeat hc2-positive (90.6%) compared with detecting HPV genotype–specific persistent infection by LBA (53.1%, P < 0.01; Table 2). Twelve of the 32 women with CIN3 were repeatedly hc2-positive but did not test genotype-specific persistent by LBA. However, 5 of these 12 women did test genotype-specific persistent for a borderline carcinogenic HPV genotype (53, 67, 73, 82, and/or 66; data not shown).
Our stratified analyses showed that sensitivity to identify women who were subsequently diagnosed with CIN3 differed slightly by referral cytology. The sensitivity for repeat hc2 positivity was nonsignificantly higher among women with ASCUS compared with women with LSIL referral (100% versus 82.4%, respectively; P = 0.23; data not shown). Such differences were not observed among women with HPV genotype–specific persistent infection by LBA.
Among the 329 repeatedly hc2-positive women, approximately one-half (n = 178) had at least one HPV genotype–specific persistent infection and 11.9% had a persistent HPV16 infection (Table 3). Many of the women who did not have a HPV genotype–specific persistent infection but were repeatedly hc2-positive (151 of 329) had acquired a new HPV infection. These women had an 8.0% absolute risk of CIN3, not significantly lower than the 9.6% risk observed among women repeatedly hc2-positive with HPV genotype–specific persistence (P = 0.61). As an ancillary analysis to improve statistical power, we included women randomized to the HPV arm and the findings were similar.
Table 3.
For women who were without prevalently detected CIN2+ at enrollment colposcopy but repeatedly hc2 positive at enrollment and 12 mo visits, absolute risk of CIN2+/CIN3 given HPV genotype–specific persistence
| Total |
Clinical center CIN2+ at 12–24 mo* | Absolute risk |
Quality control CIN3 at 12–24 mo* | Absolute risk |
||||
|---|---|---|---|---|---|---|---|---|
| N | Col % | Row % | 95% CI | Row % | 95% CI | |||
| Women repeatedly hc2+ at enrollment and 12 mo visits | 329 | 100.0 | 55 | 16.7 | (12.7–20.8) | 29 | 8.8 | (5.7–11.9) |
| Genotype-specific persistent HPV by LBA PCR† | 178 | 54.1 | 34 | 19.1 | (13.3–24.9) | 17 | 9.6 | (5.2–13.9) |
| HPV16 persistent | 39 | 11.9 | 13 | 33.3 | (17.9–48.8) | 9 | 23.1 | (9.2–36.9) |
| Not HPV16 persistent | 47 | 15.2 | 7 | 14.0 | (4.0–24.0) | 3 | 6.0 | (0.0–12.8) |
| HPV16–/HPV16– | 240 | 73.0 | 35 | 14.6 | (10.1–19.1) | 17 | 7.1 | (3.8–10.4) |
| Not genotype-specific persistent HPV by LBA PCR† | 151 | 45.9 | 21 | 13.9 | (8.3–19.5) | 12 | 8.0 | (3.6–12.3) |
NOTE: Women were enrolled into the immediate colposcopy arm after ASCUS/HPV+ or LSIL referral cytology.
Endpoint is CIN grade 2 or more severe (≥CIN2; clinical center diagnosis) and CIN grade 3 (CIN3; Pathology Quality Control Group diagnosis. Diagnoses of CIN2+ and CIN3 were made independently by clinical center and pathology quality control group, respectively.
Includes 13 HPV types: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68.
We stratified our analysis by women ages 18 to 29 versus women ages 30 to 62, to conform to common age thresholds for HPV and cytology cotesting. We observed comparable risk of CIN3 by age group for repeated hc2 positivity and HPV genotype–specific persistent infection (4.9% and 4.4%, P = 0.77, respectively; data not shown). In addition, our analysis included 153 women ages 18 to 20 who would not have been referred to colposcopy based on current standards of practice in the United States (3, 4). Excluding these women from our analysis did not change our findings.
We noted similar relative patterns when we used CIN2+ as diagnosed by the clinical center pathologists as our endpoints, although the absolute risks were, of course, higher with this broader disease outcome (Tables 2 and 3).
Discussion
Among this subcohort of women referred to ALTS for HPV+/ASCUS or LSIL cytology and without prevalently detected CIN2+ at enrollment colposcopy, 12-month HPV persistence as measured by a pooled test (hc2) would have recalled more women to colposcopy than detection of HPV genotype–specific persistence by a prototype PCR test (LBA). This finding was anticipated because measuring persistence using a pooled test such as hc2 counts sequential new infections as persistent.
Counter to our a priori intuition, HPV persistence as measured by the pooled test (hc2) would also have identified substantially more women who were subsequently diagnosed with CIN2+/CIN3 (greater sensitivity) compared with HPV genotype–specific persistence by LBA. Also noteworthy, repeat hc2 positivity and type-specific persistence yielded comparable absolute risks (positive predictive values) of subsequent CIN2+/CIN3. Only genotype-specific testing for HPV16 had a possibly important higher positive predictive value.
We explored several explanations for these unanticipated findings. First, the failure of HPV genotype–specific persistence to provide better identification of women at risk of CIN2+/CIN3 might be due in part to misclassification error by the assay to accurately identify 13 carcinogenic HPV genotypes individually at two visits. The LBA PCR research assay used in ALTS was observed to be slightly less clinically sensitive than hc2 at a single sampling (22). Because of this concern, we compared the enrollment LBA results with Linear Array (Roche Molecular Systems) and Amplicor (Roche Diagnostics) PCR results from previous analyses (23, 24) for women with ASCUS referral. Our conclusions were not changed. Second, hc2 cross-reacts with some borderline carcinogenic HPV genotypes such as HPV66, HPV67, and HPV73 (25), which could cause some CIN3 (26) that presumably have very low invasive potential. We identified five such cases.
Of note, our restriction of women with “prevalently detected” disease in the 1st year of follow-up excluded 24 women diagnosed with CIN2+ by the clinical center and 12 women diagnosed with CIN3 by quality control at 6 months. If these women tested hc2+ and HPV carcinogenic positive at enrollment and 12 months (missing the 6 month visit), the absolute risk and sensitivity would have been higher, although we did not expect a differential difference between the two tests. An ancillary analysis including these 24 women and using 6-month HPV results as a proxy for 12-month results found that the relative increases in absolute risk compared with the original analysis were 7% to 8% higher for all definitions of carcinogenic HPV persistence. Similarly, the relative increases in sensitivity compared with the original analysis were 9% to 10% higher for all definitions of carcinogenic HPV persistence.
It is possible that the newly acquired HPV infection called persistent in this study by a pooled HPV test such as hc2 and not by genotype-specific persistent infection conferred a rapid risk of CIN2+/CIN3 in ALTS. Indeed, among women persistently hc2-positive, those genotype-specific persistent were at similar risk to women not genotype-specific persistent. Furthermore, in an ancillary analysis, we found that the few women who acquired a new HPV infection as identified by a newly positive LBA or hc2 test result (HPV negative at enrollment and HPV positive at 12 months) were at higher risk of CIN3 (10.6% and 6.9%, respectively) than women who cleared their infection (HPV positive at enrollment and HPV negative at 12 months; 2.2% for LBA, 0.4% for hc2; data not shown).
Our findings parallel those of the National Cancer Institute cohort in Guanacaste, Costa Rica (14). Both studies showed similar absolute risks for measures of HPV persistence (pooled genotypes versus genotype specific). Both found higher positive predictive values for women with persistent HPV16. Notably, older women (30 years and older) in Guanacaste who tested repeatedly positive for a pool of carcinogenic HPV infections were more likely to have HPV genotype–specific persistence than younger women (<30 years). We had an insufficient sample size to examine the likelihood of type-specific HPV persistence among older women in ALTS who tested repeatedly positive by hc2 because of the generally young age of this ALTS population (median age, 23 years).
In conclusion, in this study of women from ALTS who would be referred to colposcopy by current standards of practice, and without immediate evidence of disease requiring treatment at initial colposcopy, measures of genotype-specific persistent HPV infection were unable to provide greater risk stratification as compared with a pooled HPV assay recommended by current management guidelines (4, 5). With the possible exception of detecting HPV16 and specifically persistent HPV16, detection of HPV genotype–specific infections rather than a pool of carcinogenic HPV types was less sensitive and offered the only clinical difference of fewer referrals over testing for a pool of carcinogenic HPV genotypes. Other combinations of strategies for risk stratification such as HPV testing at 6-month intervals, colposcopic impression, and referral cytology failed to improve the negative or positive predictive values for CIN3 (data not shown). Although we did not observe a difference, it is possible that using a more analytically sensitive test would increase the type-specific sensitivity at the cost of lower specificity and positive predictive value. For women referred to colposcopy but without prevalently detected CIN2, new biomarkers are needed to better identify which women are at risk for subsequent detection of CIN3 (either missed prevalent or incident disease).
Acknowledgments
Some of the equipment and supplies used in this study were donated or provided at reduced cost by Qiagen (formerly Digene) Corporation (Gaithersburg, MD), Cytyc Corporation (Marlborough, MA), National Testing Laboratories (Fenton, MD), Denvu (Tucson, AZ), TriPath Imaging, Inc. (Burlington, NC), and Roche Molecular Systems, Inc. (Alameda, CA). We thank the ALTS Group Investigators for their help in planning and conducting the trial.
Grant Support
Intramural program of the National Cancer Institute, NIH, Department of Health and Human Services, and contracts CN-55153, CN-55154, CN-55155, CN-55156, CN-55157, CN-55158, CN-55159, and CN-55105.
Footnotes
ASCUS under the 1991 Bethesda system (18) was slightly more inclusive, particularly of probable reactive changes and ASC-H (atypical squamous cells, cannot rule out high-grade intraepithelial lesion), than the ASCUS category of the 2001 Bethesda system (19).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.ACOG Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin No. 109: Cervical cytology screening. Obstet Gynecol. 2009;114:1409–20. doi: 10.1097/AOG.0b013e3181c6f8a4. [DOI] [PubMed] [Google Scholar]
- 2.Arbyn M, Buntinx F, Van Ranst M, Paraskevaidis E, Martin-Hirsch P, Dillner J. Virologic versus cytologic triage of women with equivocal Pap smears: a meta-analysis of the accuracy to detect high-grade intraepithelial neoplasia. J Natl Cancer Inst. 2004;96:280–93. doi: 10.1093/jnci/djh037. [DOI] [PubMed] [Google Scholar]
- 3.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with abnormal cervical screening tests. J Low Genit Tract Dis. 2007;11:201–22. doi: 10.1097/LGT.0b013e3181585870. [DOI] [PubMed] [Google Scholar]
- 4.American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 99: management of abnormal cervical cytology and histology. Obstet Gynecol. 2008;112:1419–44. doi: 10.1097/AOG.0b013e318192497c. [DOI] [PubMed] [Google Scholar]
- 5.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. J Low Genit Tract Dis. 2007;11:223–39. doi: 10.1097/LGT.0b013e318159408b. [DOI] [PubMed] [Google Scholar]
- 6.Castle PE, Solomon D, Wheeler CM, Gravitt PE, Wacholder S, Schiffman M. Human papillomavirus genotype specificity of hybrid capture 2. J Clin Microbiol. 2008;46:2595–604. doi: 10.1128/JCM.00824-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guido R, Schiffman M, Solomon D, Burke L. Postcolposcopy management strategies for women referred with low-grade squamous intraepithelial lesions or human papillomavirus DNA-positive atypical squamous cells of undetermined significance: a two-year prospective study. Am J Obstet Gynecol. 2003;188:1401–5. doi: 10.1067/mob.2003.456. [DOI] [PubMed] [Google Scholar]
- 8.Walker JL, Wang SS, Schiffman M, Solomon D. Predicting absolute risk of CIN3 during post-colposcopic follow-up: results from the ASCUS-LSIL Triage Study (ALTS) Am J Obstet Gynecol. 2006;195:341–8. doi: 10.1016/j.ajog.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 9.Schiffman MH, Bauer HM, Hoover RN, et al. Epidemiologic evidence showing that human papillomavirus infection causes most cervical intraepithelial neoplasia. J Natl Cancer Inst. 1993;85:958–64. doi: 10.1093/jnci/85.12.958. [DOI] [PubMed] [Google Scholar]
- 10.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 11.Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 12.Koshiol J, Lindsay L, Pimenta JM, Poole C, Jenkins D, Smith JS. Persistent human papillomavirus infection and cervical neoplasia: a systematic review and meta-analysis. Am J Epidemiol. 2008;168:123–37. doi: 10.1093/aje/kwn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kjaer SK, van den Brule AJ, Paull G, et al. Type specific persistence of high risk human papillomavirus (HPV) as indicator of high grade cervical squamous intraepithelial lesions in young women: population based prospective follow up study. BMJ. 2002;325:572. doi: 10.1136/bmj.325.7364.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castle PE, Rodriguez AC, Burk RD, et al. Short term persistence of human papillomavirus and risk of cervical precancer and cancer: population based cohort study. BMJ. 2009;339:b2569. doi: 10.1136/bmj.b2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ASCUS-LSIL Traige Study (ALTS) Group. Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance. Am J Obstet Gynecol. 2003;188:1383–92. doi: 10.1067/mob.2003.457. [DOI] [PubMed] [Google Scholar]
- 16.ASCUS-LSIL Traige Study (ALTS) Group. A randomized trial on the management of low-grade squamous intraepithelial lesion cytology interpretations. Am J Obstet Gynecol. 2003;188:1393–400. doi: 10.1067/mob.2003.462. [DOI] [PubMed] [Google Scholar]
- 17.Schiffman M, Adrianza ME. ASCUS-LSIL triage study. Design, methods and characteristics of trial participants. Acta Cytol. 2000;44:726–42. doi: 10.1159/000328554. [DOI] [PubMed] [Google Scholar]
- 18.Broder S. From the National Institutes of Health. JAMA. 1992;267:1892. doi: 10.1001/jama.267.14.1892. [DOI] [PubMed] [Google Scholar]
- 19.Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114–9. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 20.Gravitt PE, Peyton CL, Alessi TQ, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38:357–61. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peyton CL, Gravitt PE, Hunt WC, et al. Determinants of genital human papillomavirus detection in a US population. J Infect Dis. 2001;183:1554–64. doi: 10.1086/320696. [DOI] [PubMed] [Google Scholar]
- 22.Schiffman M, Wheeler CM, Dasgupta A, Solomon D, Castle PE. A comparison of a prototype PCR assay and hybrid capture 2 for detection of carcinogenic human papillomavirus DNA in women with equivocal or mildly abnormal Papanicolaou smears. Am J Clin Pathol. 2005;124:722–32. doi: 10.1309/E067-X0L1-U3CY-37NW. [DOI] [PubMed] [Google Scholar]
- 23.Castle PE, Gravitt PE, Solomon D, Wheeler CM, Schiffman M. Comparison of linear array and line blot assay for detection of human papillomavirus and diagnosis of cervical precancer and cancer in the atypical squamous cell of undetermined significance and low-grade squamous intraepithelial lesion triage study. J Clin Microbiol. 2008;46:109–17. doi: 10.1128/JCM.01667-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wentzensen N, Gravitt PE, Solomon D, Wheeler CM, Castle PE. A study of Amplicor human papillomavirus DNA detection in the atypical squamous cells of undetermined significance-low-grade squamous intraepithelial lesion triage study. Cancer Epidemiol Biomarkers Prev. 2009;18:1341–9. doi: 10.1158/1055-9965.EPI-08-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouvard V, Baan R, Straif K, et al. A review of human carcinogens-Part B: biological agents. Lancet Oncol. 2009;10:321–2. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 26.Castle PE, Cox JT, Jeronimo J, et al. An analysis of high-risk human papillomavirus DNA-negative cervical precancers in the ASCUS-LSIL Triage Study (ALTS) Obstet Gynecol. 2008;111:847–56. doi: 10.1097/AOG.0b013e318168460b. [DOI] [PubMed] [Google Scholar]