Summary
Plasmacytoid dendritic cells (pDCs) are specialized dendritic cells (DCs) that produce large amounts of type I interferon (IFN) after Toll-like receptor (TLR) activation. Human pDCs preferentially express immunoglobulin-like transcript 7 (ILT7; LILRA4), which couples with a signaling adapter to activate a prominent immunoreceptor tyrosine-based activation motif (ITAM)–mediated signaling pathway. ILT7 protein directly binds to and can be activated by bone marrow stromal cell antigen 2 (BST2; CD317) protein, the expression of which is found on cells preexposed to IFN or on the surface of human cancer cells. The interaction between ILT7 and BST2 functions to assure an appropriate TLR response by pDCs during viral infection and likely participates in pDC-tumor crosstalk. Two opposing modes of receptor-mediated regulatory mechanisms work jointly to fine tune the innate immunity of pDCs.
Keywords: dendritic cells, plasmacytoid dendritic cells, ILT7, BST2, dendritic cell receptors, receptor signal transduction, regulation of IFN production, regulation of TLR response
Introduction
Cell surface receptors are proteins associated with the plasma membrane that participate in diverse functions of a cell. In the immune system, leukocytes are foremost defined by collective specific surface markers that enable their identification and isolation. Through interaction with biological ligands—molecules that bind to the receptor—surface receptors undergo a conformational change that triggers certain downstream signaling events and ordinarily leads to a cellular response. For example, a cytokine or chemokine receptor can acutely ‘sense’ its ligands present in the environment and subsequently initiate proliferation, differentiation, or migratory responses of the cell. Likewise, other immune receptors, such as pattern recognition receptors (PRRs), killer activated receptors, killer inhibitor receptors (KIRs), complement receptors, Fc receptors, B-cell receptors (BCRs), and T-cell receptors (TCRs), all play crucial roles in the physiological functions of immune cells.
Since their initial discovery by Steinman and Cohn in 1973 (1), dendritic cells (DCs) in mammals have been well recognized as an important immune cell population consisting of multiple subsets that display distinct surface markers and carry out important functions such as innate pathogen sensing, antigen presentation, maintenance of tolerance, and orchestration of immune activation (2–4). DC subsets can be distinguished by their unique surface markers, distinct repertories of PRRs, and tissue distribution, etc. In particular, plasmacytoid DCs (pDCs), a distinct DC subset, are a specialized immune cell population corresponding to natural interferon α/β (IFNα/β)–producing cells, which secrete high amounts of type I IFN (IFNα/β) in response to viruses (5–7). pDCs constitute only 0.2%–0.8% of peripheral blood in a human individual but induce 95% of the IFN produced by blood peripheral blood mononuclear cells (PBMCs) in response to herpes simplex virus (HSV) (8).
During the last 10 years since the identification of pDCs (9, 10), collective studies by researchers have uncovered a list of features of pDCs that are unique among immune cells and other DCs and support their important physiological functions. On the cell surface, human pDCs lack expression of common lineage markers for other leukocytes but can be stained positively for CD4, CD123, human leukocyte antigen DR (HLA-DR), and BDCA2, the latter of which is a C-type lectin receptor (CLR) (8, 11). Because the function of any autonomous cell is directly influenced by environmental factors that frequently act as ligands to engage surface receptors, it is important to understand the principal mechanisms that initiate from the cell surface and subsequently modulate the key functions of pDCs. We recently described and characterized immunoglobulin-like transcript 7 (ILT7), a specific surface receptor for human pDCs (12) and have since discovered a biological ligand for ILT7 that serves as an important extrinsic and also perhaps an intrinsic factor to regulate pDCs under several physiological scenarios (13). This review details the biology of ILT7 in relation with that of pDCs and explores the potential applications of such knowledge.
Molecular features supporting the innate immunity of pDCs
pDCs readily respond to variety of nucleic acid-bearing stimuli—live or inactivated viral particles, immune complexes containing DNA or RNA moiety, or synthetic agonists in the forms of single-stranded DNA (ssDNA) containing unmethylated CpG motifs or simple nucleic acid analogues (5, 7). Regardless of origin, mammalian pDCs (from humans, rodents, primates, or pigs) bear the same functionality that distinguishes them from other immune cells: the ability to rapidly produce a copious amount of type I IFN in response to nucleic acid–type ligands (5–7). Genome-wide expression profiling analysis revealed highly conserved gene expression shared between mouse and human pDCs (14). The extraordinary capability of pDCs to mount an antiviral IFN response is supported by their exclusive usage of certain innate immune receptors and selective expression of key molecules constituting a signaling network.
Multiple germ line–encoded sensor systems have evolved in mammals for detecting pathogen-associated pattern molecules during microbial infection (15–17). Among the PRRs, Toll-like receptors (TLRs) are a family of ancient innate immune receptors that can bind to pathogen-associated molecules via their leucine-rich repeats in the ectodomain (18, 19). Interestingly, pDCs primarily use an endosomal TLR system to sense nucleic acids and produce type I IFNs. In parallel, the RIG-I–like receptor (RLR) signaling pathway constitutes a sensor system that operates independently of TLRs to detect nucleic acid in the cytosol. The RLR pathway is activated widely in all nucleated cells after viral infection or exposure to double-stranded RNA or DNA in the cytosol (15, 20, 21). Despite this, type I IFN production by pDCs normally does not require the RLR machinery. Finally, absent in melanoma 2 (AIM2) was recently identified as a cytosolic DNA sensor that activates the inflammasome and caspase-1 (22). However, there is no report yet that AIM2 is involved in type I IFN induction or that inflammasome is activated in pDCs.
The innate immune capacity of human pDCs is underscored in three aspects at the molecular level. First, pDCs selectively express TLR7 and TLR9 but lack most other TLRs (23–25). Importantly, ligands for TLR7 and TLR9 are ssRNA/synthetic nucleic acid analogues (26–28) or ssDNA (29–31), respectively, consistent with the dual sensing of RNA and DNA by pDCs. In contrast, human myeloid DCs (mDCs) preferentially express TLR1, TLR2, and TLR3, whereas monocytes express TLR1, TLR2, TLR4, TLR5, and TLR8 (23, 24, 32). Such polarized TLR expression by DC subsets explains in part (i) pDCs’ unresponsiveness, in contrast to mDCs’ hypersensitivity, to poly(IC) or lipopolysaccharide (LPS), which activates TLR3 or TLR4, respectively, and (ii) pDCs’ predominant responsiveness to DNA- and RNA-containing agonists. Interestingly, both TLR7 and TLR9 are maintained in the intracellular endosomal-lysosomal compartment where they encounter their ligands (33–35). TLR7/9 activation leads to recruitment of myeloid differentiation primary response gene 88 (MyD88), an exclusive adapter molecule in TLR7/9 signaling (15, 16), and assembly of a multiprotein signal-transducing complex including MyD88, interleukin-1 receptor-associated kinases (IRAKs), tumor necrosis factor (TNF) receptor-associated factors (TRAFs), and IFN-regulatory factor-7 (IRF-7) in the cytoplasm (reviewed in 7, 15). Subsequently, activated IRF7 initiates type I IFN transcription, whereas nuclear factor–κB (NF-κB) and mitogen-activated protein kinases (MAPKs) promote the expression of proinflammatory cytokines, chemokines, and costimulatory molecules (7, 15).
pDCs constitutively express IRF7, the master mediator for type I IFN production (36, 37). The IRF family of transcription factors can bind to the IFN-stimulated response element (ISRE), a consensus DNA sequence found in the promoters of the genes that encode type I IFNs and many others, and initiate gene transcription (38). The high levels of endogenous IRF7 expression by pDCs is licensed by pDC-specific lineage factor E2-2, which drives IRF7 transcription (39), and the concomitant absence of 4E-binding proteins (4E-BPs), translational repressors for IRF7 (40). Furthermore, another IRF member, IRF8, amplifies the type I IFN response during the second phase of IFN transcription in pDCs (41, 42). Additional factors involved in IFN receptor–mediated feedback signaling may also play important roles in TLR-mediated IFN production by pDCs (43, 44).
pDCs display unique cell biology properties help assert their immune functions. These cells contain a prominent rough endoplasmic reticulum (ER) structure resembling that of immunoglobulin (Ig)-secreting plasma cells (45). Electron microscopic analysis has revealed that TLR9 primarily resides in the ER of pDCs (46). Full-length TLR7 and TLR9 are processed by protease cleavage in the endolysosomes of DCs (34, 35). The processed TLR9 then can bind to nucleic acid agonists and recruit MyD88 for prolonged activation in the early endosome, leading to prominent type I IFN production (46–48). The compartmentalization of intracellular TLR7/9 serves to minimize nonspecific responses to self-nucleic acids (49) but requires assistance by additional cellular factors. For example, UNC93B, an ER-resident transmembrane protein abundant in pDCs, interacts with TLR7/9 and is crucial for ligand-induced TLR7/9 trafficking to endolysosomes (50–52). Additionally, gp96, an ER chaperone, also associates with these TLRs thus facilitates their maturation (53).
ILT receptor family
The human ILT [also known leukocyte Ig-like receptor (LILR) or as monocyte Ig-like receptor (MIR)] gene family is composed of 13 loci that encode 11 expressed genes and 2 pseudogenes (54–56). ILT genes are clustered adjacent to the KIR family genes within the leukocyte receptor complex at human chromosome 19q13.4. Encoded in the genomes of humans and primates but not those of rodents, ILTs are a gene family that has recently evolved through intergenic recombination and gene duplication (57).
ILT gene products are surface receptors with two or four C2-type Ig-like domains. They are expressed widely by various antigen-presenting cell (APC) populations, such as monocytes, macrophages, DCs, and B cells, and by other leukocytes such as T cells, natural killer (NK) cells, basophils, and mast cells (54, 55). By sequence alignment, human receptor ILTs are divided into separate groups: five inhibitory ILTs containing long cytoplasmic tails harboring intracellular immunoreceptor tyrosine-based inhibitory motifs (ITIMs), one ILT (LILRA3) without transmembrane domain, and 5 stimulatory ILTs that have short cytoplasmic tails that lack ITIMs but the presence of a charged residue in the transmembrane domain. The charged residue in the latter group would allow their association with signaling adapter molecules that possess ITAMs, a conserved sequence through which many receptors on hematopoietic cells signal. The signature sequence of an ITAM is Yxx[L/I]-x6–8-Yxx[L/I] (x denotes any amino acid). The ITAM-mediated signaling pathway performs the functions of BCRs, TCRs, NK cell receptors, Fc receptors, certain lectins, and ILTs (58, 59). Conversely, the consensus sequence for ITIM is S/I/V/LxYxxI/V/L. Ligand engagement by ITIM-containing receptors results in ITIM phosphorylation and recruitment of phosphotyrosine phosphatases, such as Src homology 2-containing protein tyrosine phosphatase-1 (SHP-1) and SHP-2, or the inositol polyphosphate-5-phosphatase (SHIP), which work opposite of protein kinases to dampen signal activation (60, 61).
Members of inhibitory ILT family receptors recognize three classes of ligands and play powerful inhibitory functions on APCs. Both ILT2 (also known as LILRB1 or CD85j) and ILT4 (also known as LILRB2 or CD85d) can bind to ‘self’ proteins encoded by multiple classic (HLA-A and -B) and non-classic major histocompatibility complex class I (MHC-I) alleles (HLA-F and –G1) with moderate affinity (62, 63). Besides these endogenous molecules, ILT2 binds with high affinity to UL18, a human cytomegalovirus (HCMV) gene product that resembles MHC-I protein (63), whereas ILT4 interacts with a human immunodeficiency virus (HIV) escape variant-HLA complex with enhanced affinity (64). Finally, ILT2 may further serve as a receptor for bacteria Staphylococcus aureus and Escherichia coli and thus directly participate and modulate TLR-mediated inflammatory responses (65). Ligand engagement with ILT2 significantly suppresses the ability of monocyte-derived DCs to produce cytokines, upregulate costimulatory molecules, and stimulate T-cell proliferation (66, 67). Elevated ILT3 and ILT4 expression on monocytes and DCs renders these APCs tolerogenic, with severely comprised ability to stimulate antigen-specific CD4+ T-helper cells (68). HLA-G ligation of ILT4 is sufficient to tolerize DCs and allow prolonged allograft survival (69).
In contrast, the biological ligands for the stimulatory group of ILTs are largely unknown (54). Only LILRA1 binding with HLA-B27 has been reported (70). However, structural comparison has revealed significant differences in MHC-I binding sites between inhibitory and stimulatory ILTs, suggesting that distinct ligand recognition may occur between the two groups of ILTs (71). ILT1 (also known as LILRA2 or CD85h), which is abundantly expressed by monocyte/macrophage lineage cells, associates with the transmembrane adapter molecule FcεRIγ (72). ILT1 activation by antibody cross-linking inhibits DC differentiation and abrogates antigen presentation to T cells (73). Ligation of ILT1 or ILT11 (also known as LILRA5 or CD85f) induces the secretion of proinflammatory cytokines by monocytes (73, 74). However, ILT1, expression of which is upregulated on CD14+CD68+ cells from lesions of lepromatous patients, tends to shift the balance of cytokine profile, favoring interleukin-10 (IL-10) over IL-12, and thus contributing to the Th2 cytokine profiles of the disease (73, 75). Since the natural ligand for ILT1 is unknown, yet to be defined is the precise physiological conditions that trigger ILT1 signaling and therefore regulate the functions of APCs.
ILT7 as a unique receptor for human pDCs
ILT7 (also known as LILRA4 and CD85g) encodes a preprotein of 499 amino acids that gives rise to a surface receptor with four extracellular immunoglobulin domains, a transmembrane domain, and a short intracellular tail (Fig. 1). A positively charged arginine residue at position 449 is located within the predicated transmembrane segment of ILT7 protein. An ILT7 ortholog from chimpanzee is closely related to human ILT7; however, both of them cluster away from other ILT proteins on the phylogenetic tree (57). Similar to many other activating ILTs, ILT7 has been thought of as an orphan receptor without a known ligand.
Fig. 1. Signaling activation of ILT7 downregulates TLR-mediated IFN and cytokine responses by pDCs.
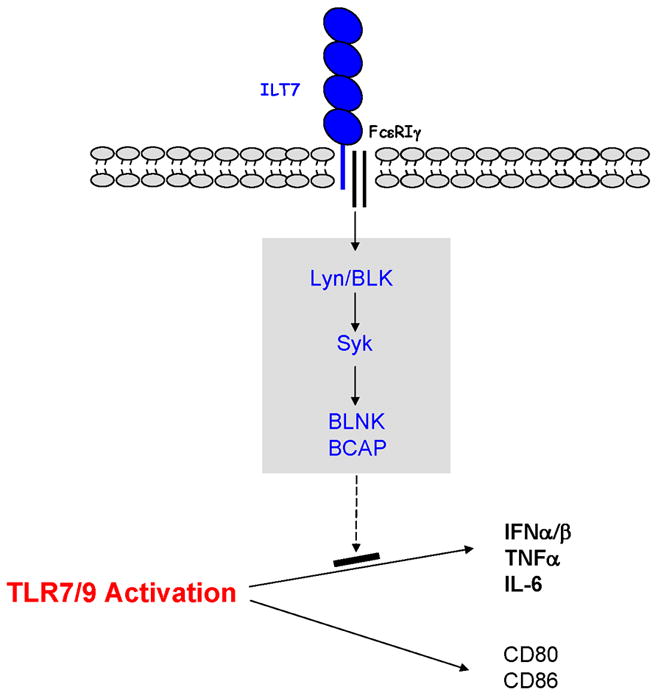
Mature ILT7 protein with an extracellular portion containing four immunoglobulin-like domain associates via its transmembrane region with the ITAM adapter FcεRIγ. The ILT7/FcεRIγ complex activates a receptor-proximal signal transduction cascade involving several protein kinases and cell type–specific signaling adapters, which potently suppresses TLR7/9-mediated IFN and cytokine production by pDCs while minimally affecting pDC maturation.
Using a polymerase chain reaction (PCR)–based subtraction technique, Rissoan et al. (76) found that ILT7 is one of a group of interesting genes differentially expressed by human pDCs compared with monocyte-derived DCs. A marker gene expression screening that we conducted with a human leukocyte expression database including all major immune cell types in peripheral blood further pinpointed ILT7 as a gene expressed abundantly and exclusively by human pDCs (12). Independently, ILT7 was isolated as a pDC-specific gene from an effort screening SAGE libraries constructed from human DCs (77). The ILT7 expression profile is drastically different from that of other ILTs. For example, transcripts of ILT2 and ILT3 are expressed by pDCs, monocytes, and mDCs, as well as monocyte-derived DCs (12).
To confirm the expression of the ILT7 protein, we generated a monoclonal antibody (mAb) that recognizes a mouse T-cell line transfected with human ILT7 (12). In total PBMCs, anti-ILT7 mAb stained a rare cell population of non-T, non-B, non-monocyte, and non-NK cells. When double-stained with mAbs against the human pDC marker BDCA2, almost all ILT7+ cells were BDCA2high, suggesting that circulating blood pDCs preferentially express ILT7 (12). When activated by TLR agonists, such as CpG or HSV, or treated with cytokine IL-3, pDCs downregulate ILT7 surface expression (12), as a result of reduced transcript expression by activated pDCs (76, 78). Flow cytometry analysis using independent mAbs against ILT7 confirmed the exclusive expression by resting pDCs and its downregulation in activated or differentiated pDCs (77). Of note, ILT7 is absent on several pDC-like cell lines derived from CD4+CD56+ leukemia cells (77, Cao et al., unpublished observation).
ILT7 transcripts are minimally detected in most human tissues but are moderately enriched in lymphoid organs, where pDCs reside (77). The pDC-restricted ILT7 expression is remarkable given the wide expression range of other ILTs among APCs and various leukocytes. Promoter analysis of ILT1, ILT2, and ILT4 implies a transcriptional mechanism likely involving PU.1, which acts as a common trans-acting factor along the myeloid lineage, yet each ILT is regulated in a distinct manner (79). E2-2, an E protein transcription factor that specifies pDC lineage in vivo, can directly bind to the regulatory regions of ILT7 promoter; additionally, pDCs from patients with Pitt-Hopkins syndrome bearing E2-2 haploinsufficiency consistently express lower levels of ILT7. Therefore, E2-2 is critical in controlling pDC-specific expression of ILT7 (39). Although enhanced ILT3 and ILT4 expression on monocytes can be induced by IL-10 exposure or contact-dependent interaction with T-suppressor cells (55, 68), a condition(s) to upregulate ILT7 expression by pDCs has yet to be established.
Signal transduction by ILT7
Based on the presence of arginine 449 in the transmembrane domain of ILT7, we hypothesized that ILT7 could pair with an adapter molecule via charged interaction within the plasma membrane. The candidates for such adapters include a list of transmembrane molecules containing ITAMs, a motif capable of triggering potent cell activation signaling. Although human pDCs lack the expression of ITAM components found in TCRs or BCRs, they do express FcεRIγ and DAP12, two ITAM-bearing adapters commonly found in KIRs, NK cell receptors, and CLRs (80, 81). DAP10, a non-ITAM adapter that signals via a YINM motif permitting activation of phosphoinositol 3-kinase (PI3K) (82), is also expressed by pDCs. An ‘adapter trap’ reporter cell system was used to determine unequivocally which adapter pairs with ILT7 (12). Specifically, mouse BaF/3 pro-B cells stably transfected with FcεRIγ, DAP12, or DAP10 were transduced with ILT7, which itself cannot be stably expressed on the cell surface (12). FcεRIγ enhanced the cell surface expression of ILT7 but not that of DAP12 or DAP10. Similarly, ILT7 stabilized the surface expression of FcεRIγ. Moreover, the ILT7-FcεRIγ complex could be coimmunoprecipitated from BaF/3 cells transfected with both genes (12). Therefore, similar to ILT1, ILT7 associates FcεRIγ to form a stable receptor complex with signaling potential.
A hallmark of ITAM-mediated signaling is calcium mobilization and nuclear factor of activated T cell (NFAT) activation (80, 81, 83). To reveal the cellular signals transduced by ILT7, we introduced a human ILT7-FcεRIγ complex into a mouse 2B4 T-cell hybridoma line that contained an intracellular NFAT–green fluorescent protein (GFP) construct (12). Cross-linking ILT7 with immobilized anti-ILT7 mAb resulted in robust GFP expression in an FcεRIγ–dependent manner, indicating that NFAT activation is a result of ILT7 ligation.
Although pDCs represent a major DC subset, they do express a list of lymphoid-restricted genes that are absent in DCs of myeloid lineage, such as pre-Tα, Spi-B, and IgH D-J rearrangement (76, 84–86). In lymphocytes, several well-established signaling pathways exist downstream of BCRs and TCRs that linked through their ITAM-bearing subunits (87, 88). Specifically, after receptor activation, two core tyrosine residues within the ITAMs are phosphorylated by Src family protein tyrosine kinases (PTKs). The phosphorylated tyrosines within the ITAMs are associated with the src homology 2 domains of Syk-family PTKs, which in turn phosphorylate cell type–specific intracellular adapters to initiate a multitude of signaling events. Each type of lymphocyte expresses and uses a distinct set of proteins to carry out the receptor-proximal signal transduction (87, 88). Thus, it would be important to establish a detailed map of the ITAM-mediated signaling pathway for pDCs. From both transcript expression and protein analysis, we found that strikingly pDCs express many members of the BCR signaling cascade, such as B-lymphoid tyrosine kinase (BLK), Lyn kinase, Syk, as well as important cell-type specific adapter molecules, BLNK and BCAP, but none involved in TCR proximal signaling (89, 90).
As predicted, both Src family kinases and Syk are phosphorylated rapidly after ILT7 activation of human primary pDCs (12), indicating the onset of ITAM signaling (Fig. 1). Such signaling was absent when pDCs were cross-linked with isotype-matched control antibody or with mAb against BDCA4, a surface molecule expressed on pDCs (12). Furthermore, ILT7 cross-linking effectively triggered prominent intracellular calcium mobilization in pDCs (12). This activity is sensitive to a compound that interferes with the function of Src family kinases as well as an inhibitor specific to Syk kinase. Therefore, the ILT7-FcεRIγ complex is capable of activating the ITAM-mediated pathway in human pDCs.
Interaction between BST2 and ILT7
Identification of ILT7 ligands
To discover a ligand that would interact with ILT7 and trigger downstream signaling activation, we took advantage of the ILT7/FcεRIγ 2B4 NFAT-green fluorescence protein (GFP) reporter cell line, which reliably expresses GFP in response to ILT7 surface ligation. To search the presence of ILT7 ligands (ILT7-L), we used these cells to screen virus-infected cells and a large panel of human tumor cell lines (13). Common laboratory mammalian cell lines, such as HEK293, Vero, CHO, Cos7, and Jurkat, do not stimulate the ILT7 reporter cells. Therefore, we further tested a list of human cancer cell lines derived from breast, ovarian, colon, melanoma, glioma, and lung cancers. GFP expression was induced after coculturing ILT7 reporter cells with several human breast carcinoma cells (such as MDA-MB-468, MCF7, and T47D cells) and melanoma lines (such as WM35 and Mel938) (13).
The breast cancer line T47D was most potent in triggering ILT7 in our study. The ability of T47D cells to activate the reporter cells is strictly ILT7-dependent, given that (i) 2B4 NFAT-GFP reporter cells expressing solely FcεRIγ in the absence of ILT7 were not activated and (ii) the induction of GFP could be completely abolished by a neutralizing anti-ILT7 mAb. Interestingly, pretreatment with IFNα and TNF–α significantly boosted ILT7-L activity, suggesting that ILT7-L expression may be influenced by inflammatory conditions imposed on the cells (13).
ILT7 activation by cancer cells requires direct cell-cell contact, as demonstrated by the result that cells cultured in separate chambers of a transwell dish fail to induce GFP (13). However, in contrast to other ILTs recognizing MHC-I ligands, the putative ILT7-L appears to be unrelated to either MHC-I or MHC-II, given that antibodies against MHC-I or MHC-II cannot block the ILT7-L activity and both the MHC-I– and MHC-II–expressing cell lines fail to activate ILT7 reporter cells (13). Therefore, the ILT7-L likely represents an entirely novel entity.
To facilitate the identification of the putative ILT7-L, we immunized mice with cells positive with ILT7-L and obtained two clones of mAbs with activity toward blocking T47D-induced ILT7 reporter cell activation (13). In flow cytometry, both mAbs positively stained the breast tumor cell lines that activate ILT7 reporter cells but minimally reacted with breast tumor cell lines that failed to activate ILT7 reporters (13). Dose dependently, both mAbs completely neutralized the ability of the tumor cell line T47D to activate the ILT7 reporter cells (13). Therefore, the antigen(s) recognized by these mAbs are likely the ILT7-L. Subsequently, these anti-ILT7-L mAbs were used to screen HEK293 cells transfected with clones of an expression cDNA library enriched with genes encoding human transmembrane proteins. Although recognizing non-overlapping epitopes, both mAbs recognized a single protein encoded by human bone marrow stromal cell antigen 2 (BST2) (CD317; HM1.24) (13).
Biology of BST2
BST2 is a protein of 180 amino acids that was initially identified as a membrane protein expressed by bone marrow stromal cells and later shown to be expressed by plasma cells and multiple types of cancer cells (91–93). It is a membrane-associated glycoprotein traversing between the cell surface and Golgi apparatus (91). The fully glycosylated, glycosylphosphatidylinisotol (GPI)-anchored form of BST2 is found in lipid rafts of plasma membranes and endocytosed vesicles (91, 94). BST2 mRNA is expressed by human lung, ovary, adrenal gland, spleen, thymus, heart, liver, placenta, and various cancer cells (95). BST2 is involved in the growth and development of plasma cells in multiple myeloma and has been identified as a differentially expressed gene promoting tumor invasion (92, 93, 96). Recently, BST2 is also termed ‘tetherin’, a molecule involved in human immunodeficiency virus particle release from infected cells (97, 98). Moreover, BST2 protein is prominently expressed by resting mouse pDCs as PDCA1 antigen (99).
Within the promoter region of human BST2, there are multiple cis-regulatory elements for transcription factors such as signal transducer and activator of transcription (STAT), activating enhancer binding protein-2 (AP-2), GATA1, and IRFs (92, 100). In particular, STAT binding allows inducible BST2 expression on many different types of cells after exposure to both type I and II IFN (97–99). Using the mAbs we generated, we confirmed such wide range IFNα–inducible BST2 expression by human embryonic kidney (HEK293) cells, dermal fibroblast (NHDF) cells, umbilical vein endothelial (HUVEC) cells, and keratinocyte (HaCat) cells (13).
BST2 as ILT7 ligand
The notion that BST2 serves as the ligand for ILT7 is supported by three lines of evidence (13). First, recombinant ILT7 protein directly and specifically binds to a recombinant BST2-GST fusion protein in a dose-dependent manner. The specific interaction between recombinant BST2-Fc and ILT7-Fc occurs with an estimated affinity of 10−6 M as measured by surface plasma resonance, in line with the published dissociation constant of MHC-I binding to ILT2 and ILT4 (Kd between 2–100 μM) (63, 101). Second, rBST2 protein strongly activates the ILT7 reporter cells through direct ILT7 and BST2 engagement. Last and most importantly, BST2 expressed on the surface of HEK293 cells induces GFP expression in ILT7 NFAT-GFP reporter cells but not in ILT7-negative reporter cells, which can be blocked by neutralizing antibodies against either ILT7 or BST2. Therefore, BST2 can serve as a physiological ligand that specifically binds to and activates ILT7.
On primary human pDCs, the interaction between BST2 and ILT7 induces changes similar to those of cross-linking by anti-ILT7 mAb (12, 13). For example, both rBST2 protein and anti-ILT7 mAb induce prominent calcium mobilization, which depends on Syk activation downstream of ITAM phosphorylation. When cross-linked by either anti–ILT7 mAb or recombinant BST2 protein, pDCs stimulated by the TLR9 ligand CpG oligonucleotide and TLR7 ligand influenza virus produce less IFNα and TNFα (12, 13). ILT7 cross-linking effectively reduced the TLR response even after pDCs were preexposed to CpG for 1 h (12). ILT7 activation consistently reduces the transcription of type I IFN subtypes, including IFNα1, IFNα4, IFNα8, and IFNβ, plus IL-6 by pDCs when activated by TLR agonists (12, 13). In contrast, expression of costimulatory molecules, such as CD80 and CD86, was not affected (12, 13). Furthermore, in contact with HEK293 cells expressing surface BST2 but not control HEK293 cells, pDCs secrete reduced levels of IFNα when challenged by influenza virus (13). These data strongly suggest that the interaction between BST2 and ILT7 mainly function to modulate pDCs’ TLR-induced cytokine responses.
ILT7-BST2 cis-interaction
Resting mouse pDCs prominently express BST2 (99) but lack the expression of a direct ortholog of ILT7 (54). Given that both ILT7 and BST2 may be expressed on human pDCs, we investigated their potential cis-interaction on pDCs. Different from mouse pDCs, low amounts of surface BST2 are expressed by freshly isolated human pDCs (13). Although BST2 transcripts are elevated in TLR-activated pDCs, BST2 surface expression remains moderate on human pDCs stimulated with TLR ligands or cultured with cytokines (including both type I and type II IFN). This is unexpected, given that all other cell types analyzed readily upregulate BST2 in response to IFN.
To investigate a potential cis-interaction between ILT7 and BST2, ILT7 and FcεRIγ were transduced into a human Burkitt lymphoma cell line expressing endogenous BST2 (13). Interestingly, expression of the ILT7/FcεRIγ complex reduces the levels of surface BST2. This downregulation did not occur when another human pDC-specific receptor complex BDCA2/FcεRIγ was expressed. A reciprocal downregulation was clearly observed in 2B4 cells when both ILT7/FcεRIγ and BST2 were expressed – ILT7 and BST2 limit the surface expression of each other when expressed on the same cell (Cao et al., unpublished results). Thus, the ILT7-BST2 cis-interaction likely results in BST2 internalization, which might have novel functional consequences in human pDCs.
Functional implications of the interaction between BST2 and ILT7
In the immune system, pDCs play a critical role in antiviral innate immune responses by secreting large quantities of IFNα/β, which is essential to provide protection against systemic viral dissemination. Following a viral infection, type 1 IFN responses are normally transient and self-limiting. Deregulated and prolonged IFN exposure will not only interfere regular hematopoiesis and lead to lymphopenia (102–104) but also increase the risk of autoimmunity (105). Therefore, mechanisms that ensure a specific and brief IFN response to viruses is important for the host to minimize pathological malady. Given that BST2 is robustly induced on the surface of a variety of cells in an environment following exposure to IFN and other proinflammatory cytokines (92, 97–99), the interaction between BST2 and ILT7 may well serve as an important negative feedback mechanism to prevent prolonged IFN production after viral infection (Fig. 2).
Fig. 2. Effects of the interaction between BST2 and ILT7 on pDCs.

(Top) By sensing viral infection, pDCs can rapidly and rigorously produce large amounts of type I IFN via TLR7 or TLR9 activation. IFN then may induce the neighboring cells to express BST2, which in turn engages with ILT7 on pDCs to downregulate the magnitude of IFN and cytokine responses in a negative-feedback manner. (Bottom left) In a tumor environment where BST2 is endogenously expressed, infiltrating pDCs may be functionally suppressed to elicit normal IFN response to TLR ligands as a result of the interaction between BST2 and ILT7. (Bottom right) When coexpressed, ILT7 sequesters BST2 by inducing BST2 internalization as a result of cis-recognition.
Cells of the innate immune system are frequently under tight regulatory control by ‘paired receptors’. Such receptors and their ligands are well documented for several receptor families, such as KIRs, Ly49 family receptors, and now ILTs. Many of the inhibitory receptors participate in self-tolerance by recognizing ubiquitously expressed endogenous molecules, whereas stimulatory receptors may recognize ‘alert’ molecules upregulated following immune activation or viral infection (80, 106). In Ly49 family in mouse, Ly49A, a prototype inhibitory Ly49 receptors found on NK cells, recognizes endogenous MHC-I molecule H-2Dd (107), whereas the stimulatory NK receptor Ly49H binds to glycoprotein M157 of MCMV (108, 109), Ly49P recognizes the H-2Dk-restricted MCMV–infected cells (110), and NKG2D interacts with MHC-I-related ligands RAE-1α–ε, H60, and MULT1 (111–114), the expression of which is controlled by TLR activation, viral infections, and stress (115–117). In humans, NKG2D engages with stress-induced MHC-I–like molecules MICA and MICB (118), as well as ULBP1–4 (119, 120), the expression of which is influenced by viral infections or TLR stimulation (121). The current finding that BST2 is a gene product whose expression is under the control of inflammatory signals supports the notion that stimulatory ILT receptors may also regulate immune functions by interacting with inducible or pathogen-associated ligands.
As other leukocytes, pDCs have been found in multiple cancers and may potentially interact with tumor cells. Immature pDCs are detected in the local tumor environment of breast cancer (122), melanoma (123, 124), ovarian cancer (125, 126), Kaposi sarcoma (127), leukemia (128, 129), and head and neck squamous-cell carcinoma (130). Even though pDC infiltration is found roughly in one of every eight primary breast tumors, their presence strongly correlates with an adverse outcome of the disease (122). The breast cancer infiltrating pDCs display lower capacity to produce IFNα in response to TLR ligands (131). Because IFNα is required for the cancer immunoediting process to deter the growth of primary tumors (132, 133), interactions between BST2 and ILT7 that suppress the IFN responses of pDCs may contribute to tumor tolerance (Fig. 2). On the other hand, BST2 expressed by multiple cancer types has been identified as a potent inducer of NF-κB (134) and one of the candidate genes expressed in malignant cells that promote tumor invasion (91–93). Thus, the direct interaction between BST2 and ILT7 can potentially be bidirectional in a cancer environment, and the consequences of ILT7 engagement with BST2 expressed on tumor cells need to be investigated.
Cis-binding between receptor and ligand constitutes an important functional aspect of some Ly49 family receptors and ILT-related molecules. In mast cells, the constitutive cis-interaction between human MHC-I and ILT4 or mouse MHC-I and paired immunoglobulin-like receptor–B, a close relative of ILTs, delivers inhibitory signaling that plays an essential role in regulating allergic responses (135). In NK cells, inhibitory Ly49A not only binds to its ligand H-2Dd in trans but also constitutively associates with H-2Dd in cis. The H-2Dd cis-interaction sequesters Ly49A and thus restricts the number of Ly49A receptors available for binding to H-2Dd on target cells. This mode of interaction proves to be important for both NK education and optimal NK cell activation (136, 137). We have observed the interaction between ILT7 and BST2 both in trans and in cis. The cis-interaction is at least partly responsible for low levels of surface BST2 detectable on human pDCs, which contrasts with BST2 dominant expression on mouse pDCs (Fig. 2). The functional consequence of this interaction has yet to be established.
Receptor-mediated regulation of pDC functions
Nucleic acids as a product of viral infection are most potent in triggering IFN production; however, nucleic acid–sensing TLRs have a limited ability to discriminate nucleic acids originating from the host versus those derived from foreign pathogens (31). In the autoimmune conditions such as systemic lupus erythematosus (SLE) and psoriasis, the constitutive activation of pDCs and consequent IFN production contributes significantly to the disease pathogenesis (138–140). Several host factors, including anti-DNA antibodies, anti-microbial peptide LL37, and the nuclear DNA-binding protein HMGB1, alone or in combination, facilitate entry of self-DNA into pDCs, where they trigger TLR9 to induce type I IFN (138, 140, 141). Similarly, autoantibody-self small nuclear ribonucleoprotein complexes can activate TLR7 through FcγRII to induce IFNα/β (142, 143). Therefore, tight regulatory mechanisms are essential to prevent the spontaneous activation of pDCs by endogenous nucleic acids and subsequent production of type I IFN. First of all, the intracellular compartmentalized action of TLR7 and TLR9 reduces the likelihood of nonspecific activation of pDCs by free nucleic acids (49, 144). TLR activation is under control by an array of surface signaling receptors that exert regulatory functions when engaging with their ligands in the environment (7, 145).
In addition to ILT7, BDCA2 is a human pDC receptor that couples with FcεRIγ (89, 90). BDCA2, a gene product of the C-type lectin CLEC4C, is specifically expressed by pDCs in human peripheral blood and tissues (11). Similar to ILT7 activation, BDCA2 cross-linking by a specific mAb induces tyrosine phosphorylation of Syk and Src kinases, triggers calcium influx, and potently suppresses TLR7/9-mediated cytokine responses by pDCs (11, 89). Two virus-associated ligands for BDCA2 have been reported—envelope protein gp120 of HIV (146) and surface antigens of hepatitis B virus (147)—both of which selectively interfere with the function of TLR9 but not TLR7, in contrast to the effect of anti-BDCA2 mAb (11). Several other receptors expressed by human pDCs—such as the high-affinity IgE receptor FcεRIα in association with FcεRIγ, NKp44, a receptor that signals through the ITAM-bearing adapter DAP12, and Fc receptor CD32a, which has an ITAM with its intracellular domain—also inhibit IFNα production by pDCs when activated by ligand and/or antibody (148–151). All of these receptors presumably signal through a common preexisting ITAM-mediated BCR-like regulatory pathway in pDCs to exert their regulatory function (Fig. 3).
Fig. 3. Innate immunity of pDCs is under control by two classes of surface receptors.
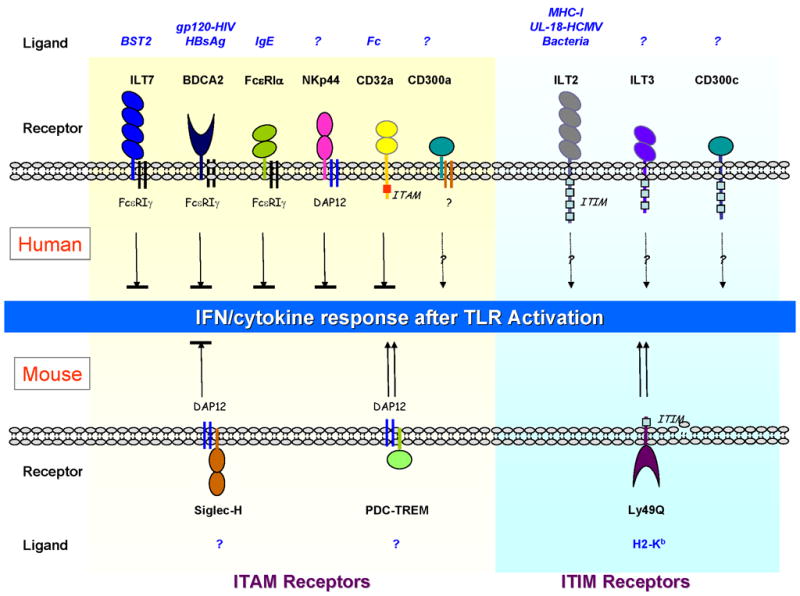
Receptors signal through a shared ITAM-mediated pathway that mostly negatively regulates TLR7/9-mediated IFN and cytokine responses, whereas receptors containing ITIMs may have opposing effects on pDCs. Shown are the pDC receptors expressed by human (top) and mouse (bottom) pDCs.
In mouse, pDCs uniquely express a sialic acid–binding Ig-like lectin family receptor Siglec-H, which associates with the ITAM adapter protein DAP12 (152, 153). The natural ligand for Siglec-H is not known; however, stimulation of pDCs with a Siglec-H–specific antibody significantly diminishes the secretion of type I IFN in response to CpG in vitro and in vivo (152). In accordance with this result, pDCs from DAP12-deficient mice produce increased levels of IFNα in response to TLR9 activation (154). Therefore, the regulatory function of the ITAM-mediated pathway on TLR activation is seemingly preserved between human and mouse pDCs. As a member of the triggering receptor expressed on myeloid cells (TREM) family, PDC-TREM is preferentially expressed on TLR-stimulated mouse pDCs, where it complexes with Plexin-A1 and DAP12, and required for IFNα production (44). The mechanism for the distinct functions of two DAP12-associated pDC receptors (i.e. Siglec-H and PDC-TREM) is not clear at this time.
Like NK cells and other APCs, pDCs simultaneously express several classical inhibitory surface receptors containing ITIMs, which presumably functions opposing the ITAM-containing receptors. In mice, pDCs express high levels of Ly49Q, a CLR that specifically binds to the H-2Kb allele of MHC-I (155, 156). Despite having an ITIM, Ly49Q enhances pDC innate immune function in vitro, and Ly49Q-deficient pDCs are defective to produce cytokines in response to TLR7/9 stimulation in vitro and in vivo (157). This finding strongly suggests, paradoxically in the context of the pDC signaling network, that ITIM-mediated signals can synergize with and promote TLR activation. Interestingly when Siglec-H and Ly49Q are cross-linked simultaneously, Ly49Q-mediated enhancement of IFNα production is diminished (157). Therefore, ITAM-mediated inhibitory signals appear to dominate over the ITIM-mediated signals in pDCs.
Human pDCs express both CD300a and CD300c, two CD300 family molecules that share 80% sequence in the extracellular Ig domain (158). Dual CD300a/c triggering by cross-linking antibody reduced TNFα and increased IFNα secretion by pDCs. However, the signaling events responsible for such effect remain to be determined, given that CD300a contains 3 ITIMs where as CD300c has a short intracellular domain with a charged amino acid in the transmembrane domain, which may be associated with signaling adapters. As mentioned before, human pDCs also positively express two other ILT inhibitory family receptors, ILT2 and ILT3, the functional impact of which awaits formal investigation. It is possible that, similar to ILT3+ILT4+ DCs, these receptors may also participate in peripheral tolerance in addition to modulating innate immunity, especially because pDCs are required for maintenance of mucosal, oral, and transplantation tolerance (159–161).
Future studies and clinical perspectives
The human pDC receptor ILT7 in complex with FcεRIγ recognizes its physiological ligand BST2, which is expressed on the surface of various cells in an inflamatory millieu or on the surface of cancer cells and, as a result of signaling activation along the ITAM-mediated pathway, significantly regulates the innate immune responses of pDCs. BST2 represents the first non–MHC-I–type ligand for a member of the ILT receptor family (54). The NFAT-GFP reporter cell system, which sensitively detects ILT7 surface ligation in the presence of an ILT7-L was critical for the successful identification of BST2, a glycoprotein unrelated to MHC molecules. This system should be useful also for the identification of novel ligands with moderate binding affinity for other orphan surface receptors. Further structural analysis will be necessary to define the binding interface between BST2 and ILT7 in trans and in cis, which may also shed light on the structural basis of ligand recognition by ILT7 and other stimulatory ILTs.
In autoimmune diseases such as SLE and psoriasis, in which type I IFN is constitutively present and pathologically implicated (138–140), BST2 expression is expected to be upregulated on the surface of multiple cell types. It will be of great interest to investigate whether the BST2-ILT7–mediated controlling mechanism is breached in these patients. Given the ability of both recombinant BST2 protein and anti-ILT7 mAb to bind and stimulate ILT7 in vitro, strategies to activate ILT7 signaling may present a therapeutic option to downmodulate pDC activation during autoimmune processes.
On a separate note, ligation of antigen uptake receptors can significantly increase the efficiency with which antigens are delivered to the immune system in vivo. One strategy to improve current DC vaccines is to deliver the vaccines via specific DC receptors with the goal to improve the efficacy of T-cell mobilization (2, 3, 162). Compared with mDCs, pDCs are generally less proficient to spontaneously endocytose or phagocytose antigens (5, 6). Because many antigen uptake receptors on DCs are lectins, pDCs express limited number of CLRs including BDCA2 (on resting pDCs) (11) and LLT1 (CLEC2D; on activated pDCs) (163), whereas human mDCs and monocyte-derived DCs broadly express on the surface DEC-205 (CD205), mannose receptor MRC1 (CD206), Dectin-1, DC-SIGN (CD209), ASGPR, OLR1 (LOX-1), and CLEC4A/DCIR (2). Nonetheless, similar to BDCA2, anti-ILT7 mAb can be rapidly internalized from the surface of human pDCs, a process that likely relies on the function of Syk (77, Cao et al., unpublished observations). Therefore, ILT7 can be further explored as a receptor to enhance antigen delivery selectively to pDCs as a part of a DC vaccination regimen.
Acknowledgments
The bulk of the work summarized here was conducted in the laboratory of Dr. Yong-Jun Liu, who greatly supported the research efforts. Grant funding for W.C. was from the National Institutes of Health (AI074809) and from UT MD Anderson Cancer Center.
References
- 1.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM. Dendritic cells: versatile controllers of the immune system. Nat Med. 2007;13:1155–1159. doi: 10.1038/nm1643. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 5.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y-J. IPC: professional Type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 7.Gilliet M, Cao W, Liu Y-J. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 8.Siegal FP, et al. The nature of the principal Type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 9.Rissoan M-C, et al. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–1186. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 10.Cella M, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 11.Dzionek A, et al. BDCA-2, a novel plasmacytoid dendritic cell-specific Type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon α/β induction. J Exp Med. 2001;194:1823–1834. doi: 10.1084/jem.194.12.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao W, et al. Plasmacytoid dendritic cell-specific receptor ILT7-FcεRIγ inhibits Toll-like receptor-induced interferon production. J Exp Med. 2006;203:1399–1405. doi: 10.1084/jem.20052454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao W, et al. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J Exp Med. 2009;206:1603–1614. doi: 10.1084/jem.20090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robbins S, et al. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol. 2008;9:R17. doi: 10.1186/gb-2008-9-1-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar H, Kawai T, Akira S. Pathogen recognition in the innate immune response. Biochem J. 2009;420:1–16. doi: 10.1042/BJ20090272. [DOI] [PubMed] [Google Scholar]
- 16.Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009;21:317–337. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medzhitov R. Approaching the asymptote: 20 years later. Immunity. 2009;30:766–775. doi: 10.1016/j.immuni.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 19.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Microbes Infect. 2004;6:1382–1387. doi: 10.1016/j.micinf.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Yoneyama M, Fujita T. Function of RIG-I-like Receptors in Antiviral Innate Immunity. J Biol Chem. 2007;282:15315–15318. doi: 10.1074/jbc.R700007200. [DOI] [PubMed] [Google Scholar]
- 21.Chiu Y-H, MacMillan JB, Chen ZJ. RNA polymerase III detects cytosolic DNA and induces Type I interferons through the RIG-I pathway. Cell. 2009;138:576–591. doi: 10.1016/j.cell.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schroder K, Muruve DA, Tschopp J. Innate immunity: cytoplasmic DNA sensing by the AIM2 inflammasome. Curr Biol. 2009;19:R262–R265. doi: 10.1016/j.cub.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Kadowaki N, et al. Subsets of human dendritic cell precursors express different Toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–870. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hornung V, et al. Quantitative expression of Toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 25.Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol. 2001;31:3388–3393. doi: 10.1002/1521-4141(200111)31:11<3388::aid-immu3388>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 26.Lund JM, et al. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci USA. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heil F, et al. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 28.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 29.Bauer S, et al. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci USA. 2001;98:9237–9242. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hemmi H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740– 745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 31.Haas T, et al. The DNA sugar backbone 2′ deoxyribose determines Toll-like receptor 9 activation. Immunity. 2008;28:315–323. doi: 10.1016/j.immuni.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 32.Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol. 2001;31:3388–3393. doi: 10.1002/1521-4141(200111)31:11<3388::aid-immu3388>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 33.Wagner H. The immunobiology of the TLR9 subfamily. Trends Immunol. 2004;25:381– 386. doi: 10.1016/j.it.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 34.Park B, Brinkmann MM, Spooner E, Lee CC, Kim Y-M, Ploegh HL. Proteolytic cleavage in an endolysosomal compartment is required for activation of Toll-like receptor 9. Nat Immunol. 2008;9:1407–1414. doi: 10.1038/ni.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ewald SE, et al. The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature. 2008;456:658–662. doi: 10.1038/nature07405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawai T, et al. Interferon-α induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat Immunol. 2004;5:1061–1068. doi: 10.1038/ni1118. [DOI] [PubMed] [Google Scholar]
- 37.Honda K, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 38.Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006;6:644–658. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- 39.Cisse B, et al. Transcription ractor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell. 2008;135:37–48. doi: 10.1016/j.cell.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colina R, et al. Translational control of the innate immune response through IRF-7. Nature. 2008;452:323–328. doi: 10.1038/nature06730. [DOI] [PubMed] [Google Scholar]
- 41.Tailor P, et al. The feedback phase of Type I interferon induction in dendritic cells requires interferon regulatory factor 8. Immunity. 2007;27:228–239. doi: 10.1016/j.immuni.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsujimura H, Tamura T, Ozato K. Cutting edge: IFN consensus sequence binding protein/IFN regulatory factor 8 drives the development of Type I IFN-producing plasmacytoid dendritic cells. J Immunol. 2003;170:1131–1135. doi: 10.4049/jimmunol.170.3.1131. [DOI] [PubMed] [Google Scholar]
- 43.Kumagai Y, Kumar H, Koyama S, Kawai T, Takeuchi O, Akira S. Cutting edge: TLR- dependent viral recognition along with Type I IFN positive feedback signaling masks the requirement of viral replication for IFN-α production in plasmacytoid dendritic cells. J Immunol. 2009;182:3960–3964. doi: 10.4049/jimmunol.0804315. [DOI] [PubMed] [Google Scholar]
- 44.Watarai H, Sekine E, Inoue S, Nakagawa R, Kaisho T, Taniguchi M. PDC-TREM, a plasmacytoid dendritic cell-specific receptor, is responsible for augmented production of type I interferon. Proc Nat Acad Sci USA. 2008;105:2993–2998. doi: 10.1073/pnas.0710351105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grouard G, Rissoan M-C, Filgueira L, Durand I, Banchereau J, Liu Y-J. The Enigmatic Plasmacytoid T Cells Develop into Dendritic Cells with Interleukin (IL)-3 and CD40-Ligand. J Exp Med. 1997;185:1101–1112. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Latz E, et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. 2004;5:190–198. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 47.Honda K, et al. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature. 2005;434:1035–1040. doi: 10.1038/nature03547. [DOI] [PubMed] [Google Scholar]
- 48.Guiducci C, et al. Properties regulating the nature of the plasmacytoid dendritic cell response to Toll-like receptor 9 activation. J Exp Med. 2006;203:1999–2008. doi: 10.1084/jem.20060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barton GM, Kagan JC, Medzhitov R. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat Immunol. 2006;7:49–56. doi: 10.1038/ni1280. [DOI] [PubMed] [Google Scholar]
- 50.Casrouge A, et al. Herpes Simplex Virus Encephalitis in Human UNC-93B Deficiency. Science. 2006;314:308–312. doi: 10.1126/science.1128346. [DOI] [PubMed] [Google Scholar]
- 51.Tabeta K, et al. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat Immunol. 2006;7:156–164. doi: 10.1038/ni1297. [DOI] [PubMed] [Google Scholar]
- 52.Brinkmann MM, Spooner E, Hoebe K, Beutler B, Ploegh HL, Kim Y-M. The interaction between the ER membrane protein UNC93B and TLR3, 7, and 9 is crucial for TLR signaling. J Cell Biol. 2007;177:265–275. doi: 10.1083/jcb.200612056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Y, et al. Heat shock protein gp96 is a master chaperone for Toll-like receptors and is important in the innate function of macrophages. Immunity. 2007;26:215–226. doi: 10.1016/j.immuni.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown D, Trowsdale J, Allen R. The LILR family: modulators of innate and adaptive immune pathways in health and disease. Tissue Antigens. 2004;64:215–225. doi: 10.1111/j.0001-2815.2004.00290.x. [DOI] [PubMed] [Google Scholar]
- 55.Anderson KJ, Allen RL. Regulation of T-cell immunity by leucocyte immunoglobulin-like receptors: innate immune receptors for self on antigen-presenting cells. Immunology. 2009;127:8–17. doi: 10.1111/j.1365-2567.2009.03097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Katz HR, Frederick WA. Advances in Immunolology. Academic Press; 2006. Inhibition of inflammatory responses by leukocyte Ig-like receptors; pp. 251–272. [DOI] [PubMed] [Google Scholar]
- 57.Canavez F, et al. Comparison of Chimpanzee and Human Leukocyte Ig-Like Receptor Genes Reveals Framework and Rapidly Evolving Genes. J Immunol. 2001;167:5786–5794. doi: 10.4049/jimmunol.167.10.5786. [DOI] [PubMed] [Google Scholar]
- 58.Humphrey M, Lanier L, Nakamura M. Role of ITAM-containing adapter proteins and their receptors in the immune system and bone. Immunol Rev. 2005;208:50–65. doi: 10.1111/j.0105-2896.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 59.Reth M. Antigen receptor tail clue. Nature. 1989;338:383–384. [PubMed] [Google Scholar]
- 60.Barrow Alexander D, Trowsdale J. You say ITAM and I say ITIM, let’s call the whole thing off: the ambiguity of immunoreceptor signalling. Eur J Immnol. 2006;36:1646–1653. doi: 10.1002/eji.200636195. [DOI] [PubMed] [Google Scholar]
- 61.Ravetch JV, Lanier LL. Immune Inhibitory Receptors. Science. 2000;290:84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- 62.Cosman D, et al. A Novel Immunoglobulin Superfamily Receptor for Cellular and Viral MHC Class I Molecules. Immunity. 1997;7:273–282. doi: 10.1016/s1074-7613(00)80529-4. [DOI] [PubMed] [Google Scholar]
- 63.Chapman TL, Heikema AP, Bjorkman PJ. The Inhibitory Receptor LIR-1 Uses a Common Binding Interaction to Recognize Class I MHC Molecules and the Viral Homolog UL18. Immunity. 1999;11:603–613. doi: 10.1016/s1074-7613(00)80135-1. [DOI] [PubMed] [Google Scholar]
- 64.Lichterfeld M, et al. A viral CTL escape mutation leading to immunoglobulin-like transcript 4 mediated functional inhibition of myelomonocytic cells. J Exp Med. 2007;204:2813–2824. doi: 10.1084/jem.20061865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakayama M, et al. Paired Ig-Like Receptors Bind to Bacteria and Shape TLR-Mediated Cytokine Production. J Immunol. 2007;178:4250–4259. doi: 10.4049/jimmunol.178.7.4250. [DOI] [PubMed] [Google Scholar]
- 66.Young NT, Waller ECP, Patel R, Roghanian A, Austyn JM, Trowsdale J. The inhibitory receptor LILRB1 modulates the differentiation and regulatory potential of human dendritic cells. Blood. 2008;111:3090–3096. doi: 10.1182/blood-2007-05-089771. [DOI] [PubMed] [Google Scholar]
- 67.Wagner CS, Walther-Jallow L, Buentke E, Ljunggren H-G, Achour A, Chambers BJ. Human cytomegalovirus-derived protein UL18 alters the phenotype and function of monocyte-derived dendritic cells. J Leukoc Biol. 2008;83:56–63. doi: 10.1189/jlb.0307181. [DOI] [PubMed] [Google Scholar]
- 68.Chang CC, et al. Tolerization of dendritic cells by TS cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol. 2002;3:237–243. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- 69.Vladimir R, Siyuan L, Wei Z, Juan W, Anatolij H. Tolerization of dendritic cells by HLA-G. Eur J Imm. 2005;35:1133–1142. doi: 10.1002/eji.200425741. [DOI] [PubMed] [Google Scholar]
- 70.Allen RL, Raine T, Haude A, Trowsdale J, Wilson MJ. Cutting Edge: Leukocyte Receptor Complex-Encoded Immunomodulatory Receptors Show Differing Specificity for Alternative HLA-B27 Structures. J Immunol. 2001;167:5543–5547. doi: 10.4049/jimmunol.167.10.5543. [DOI] [PubMed] [Google Scholar]
- 71.Shiroishi M, Kajikawa M, Kuroki K, Ose T, Kohda D, Maenaka K. Crystal Structure of the Human Monocyte-activating Receptor, “Group 2” Leukocyte Ig-like Receptor A5 (LILRA5/LIR9/ILT11) J Biol Chem. 2006;281:19536–19544. doi: 10.1074/jbc.M603076200. [DOI] [PubMed] [Google Scholar]
- 72.Nakajima H, Samaridis J, Angman L, Colonna M. Cutting Edge: Human Myeloid Cells Express an Activating ILT Receptor (ILT1) That Associates with Fc Receptor γ-Chain. J Immunol. 1999;162:5–8. [PubMed] [Google Scholar]
- 73.Lee DJ, et al. LILRA2 Activation Inhibits Dendritic Cell Differentiation and Antigen Presentation to T Cells. J Immunol. 2007;179:8128–8136. doi: 10.4049/jimmunol.179.12.8128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Borges L, Kubin M, Kuhlman T. LIR9, an immunoglobulin-superfamily-activating receptor, is expressed as a transmembrane and as a secreted molecule. Blood. 2003;101:1484–1486. doi: 10.1182/blood-2002-05-1432. [DOI] [PubMed] [Google Scholar]
- 75.Bleharski JR, et al. Use of Genetic Profiling in Leprosy to Discriminate Clinical Forms of the Disease. Science. 2003;301:1527–1530. doi: 10.1126/science.1087785. [DOI] [PubMed] [Google Scholar]
- 76.Rissoan MC, et al. Subtractive hybridization reveals the expression of immunoglobulin-like transcript 7, Eph-B1, granzyme B, and 3 novel transcripts in human plasmacytoid dendritic cells. Blood. 2002;100:3295–3303. doi: 10.1182/blood-2002-02-0638. [DOI] [PubMed] [Google Scholar]
- 77.Cho M, et al. SAGE library screening reveals ILT7 as a specific plasmacytoid dendritic cell marker that regulates type I IFN production. Int Immunol. 2008;20:155–164. doi: 10.1093/intimm/dxm127. [DOI] [PubMed] [Google Scholar]
- 78.Ju X-S, et al. Immunoglobulin-like transcripts ILT2, ILT3 and ILT7 are expressed by human dendritic cells and down-regulated following activation. Gene. 2004;331:159–164. doi: 10.1016/j.gene.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 79.Nakajima H, et al. Transcriptional Regulation of ILT Family Receptors. J Immunol. 2003;171:6611–6620. doi: 10.4049/jimmunol.171.12.6611. [DOI] [PubMed] [Google Scholar]
- 80.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ivashkiv LB. Cross-regulation of signaling by ITAM-associated receptors. Nat Immunol. 2009;10:340–347. doi: 10.1038/ni.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lewis LL. DAP10- and DAP12-associated receptors in innate immunity. Immunol Rev. 2009;227:150–160. doi: 10.1111/j.1600-065X.2008.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mary Beth H, Lewis LL, Mary CN. Role of ITAM-containing adapter proteins and their receptors in the immune system and bone. Immunol Rev. 2005;208:50–65. doi: 10.1111/j.0105-2896.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 84.Shigematsu H, et al. Plasmacytoid Dendritic Cells Activate Lymphoid-Specific Genetic Programs Irrespective of Their Cellular Origin. Immunity. 2004;21:43–53. doi: 10.1016/j.immuni.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 85.Pelayo R, et al. Derivation of 2 categories of plasmacytoid dendritic cells in murine bone marrow. Blood. 2005;105:4407–4415. doi: 10.1182/blood-2004-07-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Corcoran L, et al. The Lymphoid Past of Mouse Plasmacytoid Cells and Thymic Dendritic Cells. J Immunol. 2003;170:4926–4932. doi: 10.4049/jimmunol.170.10.4926. [DOI] [PubMed] [Google Scholar]
- 87.Jun JE, Goodnow CC. Scaffolding of antigen receptors for immunogenic versus tolerogenic signaling. Nat Immunol. 2003;4:1057–1064. doi: 10.1038/ni1001. [DOI] [PubMed] [Google Scholar]
- 88.Koretzky GA, Abtahian F, Silverman MA. SLP76 and SLP65: complex regulation of signalling in lymphocytes and beyond. Nat Rev Immunol. 2006;6:67–78. doi: 10.1038/nri1750. [DOI] [PubMed] [Google Scholar]
- 89.Cao W, et al. BDCA2/FcεRIγ Complex Signals through a Novel BCR-Like Pathway in Human Plasmacytoid Dendritic Cells. PLoS Biol. 2007;5:e248. doi: 10.1371/journal.pbio.0050248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Röck J, et al. CD303 (BDCA-2) signals in plasmacytoid dendritic cells via a BCR-like signalosome involving Syk, Slp65 and PLCγ2. Eur J Immunol. 2007;37:3564–3575. doi: 10.1002/eji.200737711. [DOI] [PubMed] [Google Scholar]
- 91.Kupzig S. Bst-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic. 2003;4:694–709. doi: 10.1034/j.1600-0854.2003.00129.x. [DOI] [PubMed] [Google Scholar]
- 92.Ohtomo T, et al. Molecular Cloning and Characterization of a Surface Antigen Preferentially Overexpressed on Multiple Myeloma Cells. Biochem Biophys Res Comm. 1999;258:583–591. doi: 10.1006/bbrc.1999.0683. [DOI] [PubMed] [Google Scholar]
- 93.Walter-Yohrling J, et al. Identification of Genes Expressed in Malignant Cells That Promote Invasion. Cancer Res. 2003;63:8939–8947. [PubMed] [Google Scholar]
- 94.Masuyama N, et al. HM1.24 Is Internalized from Lipid Rafts by Clathrin-mediated Endocytosis through Interaction with α-Adaptin. J Biol Chem. 2009;284:15927–15941. doi: 10.1074/jbc.M109.005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ishikawa J, et al. Molecular cloning and chromosomal mapping of a bone marrow stromal cell surface gene, BST2, that may be involved in pre-B-cell growth. Genomics. 1995;26:527–534. doi: 10.1016/0888-7543(95)80171-h. [DOI] [PubMed] [Google Scholar]
- 96.Cai D, et al. Up-regulation of bone marrow stromal protein 2 (BST2) in breast cancer with bone metastasis. BMC Cancer. 2009;9:102. doi: 10.1186/1471-2407-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Neil SJD, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 98.Van Damme N, et al. The Interferon-Induced Protein BST-2 Restricts HIV-1 Release and Is Downregulated from the Cell Surface by the Viral Vpu Protein. Cell Host Microbe. 2008;3:245–252. doi: 10.1016/j.chom.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Blasius AL, Giurisato E, Cella M, Schreiber RD, Shaw AS, Colonna M. Bone Marrow Stromal Cell Antigen 2 Is a Specific Marker of Type I IFN-Producing Cells in the Naive Mouse, but a Promiscuous Cell Surface Antigen following IFN Stimulation. J Immunol. 2006;177:3260–3265. doi: 10.4049/jimmunol.177.5.3260. [DOI] [PubMed] [Google Scholar]
- 100.Ge Y, et al. Differential gene expression, GATA1 target genes, and the chemotherapy sensitivity of Down syndrome megakaryocytic leukemia. Blood. 2006;107:1570–1581. doi: 10.1182/blood-2005-06-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shiroishi M, et al. Human inhibitory receptors Ig-like transcript 2 (ILT2) and ILT4 compete with CD8 for MHC class I binding and bind preferentially to HLA-G. Proc Natl Acad Sci USA. 2003;100:8856–8861. doi: 10.1073/pnas.1431057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kamphuis E, Junt T, Waibler Z, Forster R, Kalinke U. Type I interferons directly regulate lymphocyte recirculation and cause transient blood lymphopenia. Blood. 2006;108:3253–3261. doi: 10.1182/blood-2006-06-027599. [DOI] [PubMed] [Google Scholar]
- 103.Lin Q, Dong C, Cooper MD. Impairment of T and B Cell Development by Treatment with a Type I Interferon. J Exp Med. 1998;187:79–87. doi: 10.1084/jem.187.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Passegue E, Ernst P. IFN-α wakes up sleeping hematopoietic stem cells. Nat Med. 2009;15:612–613. doi: 10.1038/nm0609-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gota C, Calabrese L. Induction of clinical autoimmune disease by therapeutic interferon- alpha. Autoimmunity. 2003;36:511–518. doi: 10.1080/08916930310001605873. [DOI] [PubMed] [Google Scholar]
- 106.Yamada E, McVicar D. Paired receptor systems of the innate immune system. Curr Protoc Immunol. 2008 doi: 10.1002/0471142735.ima01xs81. Appendix 1X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Karlhofer FM, Ribaudo RK, Yokoyama WM. MHC class I alloantigen specificity of Ly- 49+ IL-2-activated natural killer cells. Nature. 1992;358:66–70. doi: 10.1038/358066a0. [DOI] [PubMed] [Google Scholar]
- 108.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct Recognition of Cytomegalovirus by Activating and Inhibitory NK Cell Receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 109.Smith HRC, et al. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc Nat Acad Sci USA. 2002;99:8826–8831. doi: 10.1073/pnas.092258599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Desrosiers M-P, et al. Epistasis between mouse Klra and major histocompatibility complex class I loci is associated with a new mechanism of natural killer cell-mediated innate resistance to cytomegalovirus infection. Nat Genet. 2005;37:593–599. doi: 10.1038/ng1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413:165–171. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cerwenka A, et al. Retinoic Acid Early Inducible Genes Define a Ligand Family for the Activating NKG2D Receptor in Mice. Immunity. 2000;12:721–727. doi: 10.1016/s1074-7613(00)80222-8. [DOI] [PubMed] [Google Scholar]
- 113.Carayannopoulos LN, Naidenko OV, Fremont DH, Yokoyama WM. Cutting Edge: Murine UL16-Binding Protein-Like Transcript 1: A Newly Described Transcript Encoding a High-Affinity Ligand for Murine NKG2D. J Immunol. 2002;169:4079–4083. doi: 10.4049/jimmunol.169.8.4079. [DOI] [PubMed] [Google Scholar]
- 114.Diefenbach A, Hsia Jennifer K, Hsiung M-Yu B, Raulet David H. A novel ligand for the NKG2D receptor activates NK cells and macrophages and induces tumor immunity. Eur J Immunol. 2003;33:381–391. doi: 10.1002/immu.200310012. [DOI] [PubMed] [Google Scholar]
- 115.Hamerman JA, Ogasawara K, Lanier LL. Cutting Edge: Toll-Like Receptor Signaling in Macrophages Induces Ligands for the NKG2D Receptor. J Immunol. 2004;172:2001–2005. doi: 10.4049/jimmunol.172.4.2001. [DOI] [PubMed] [Google Scholar]
- 116.Lodoen M, et al. NKG2D-mediated Natural Killer Cell Protection Against Cytomegalovirus Is Impaired by Viral gp40 Modulation of Retinoic Acid Early Inducible 1 Gene Molecules. J Exp Med. 2003;197:1245–1253. doi: 10.1084/jem.20021973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nice TJ, Coscoy L, Raulet DH. Posttranslational regulation of the NKG2D ligand Mult1 in response to cell stress. J Exp Med. 2009;206:287–298. doi: 10.1084/jem.20081335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bauer S, et al. Activation of NK Cells and T Cells by NKG2D, a Receptor for Stress- Inducible MICA. Science. 1999;285:727–729. [PubMed] [Google Scholar]
- 119.Cosman D, et al. ULBPs, Novel MHC Class I Related Molecules, Bind to CMV Glycoprotein UL16 and Stimulate NK Cytotoxicity through the NKG2D Receptor. Immunity. 2001;14:123–133. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 120.Chalupny J, Sutherland CL, Lawrence WA, Rein-Weston A, Cosman D. ULBP4 is a novel ligand for human NKG2D. Biochem Biophy Res Comm. 2003;305:129–135. doi: 10.1016/s0006-291x(03)00714-9. [DOI] [PubMed] [Google Scholar]
- 121.Ebihara T, et al. Induction of NKG2D ligands on human dendritic cells by TLR ligand stimulation and RNA virus infection. Int Immunol. 2007;19:1145–1155. doi: 10.1093/intimm/dxm073. [DOI] [PubMed] [Google Scholar]
- 122.Treilleux I, et al. Dendritic Cell Infiltration and Prognosis of Early Stage Breast Cancer. Clin Cancer Res. 2004;10:7466–7474. doi: 10.1158/1078-0432.CCR-04-0684. [DOI] [PubMed] [Google Scholar]
- 123.Benitez-Ribas D, et al. Plasmacytoid dendritic cells of melanoma patients present exogenous proteins to CD4+ T cells after FcγRII-mediated uptake. J Exp Med. 2006;203:1629–1635. doi: 10.1084/jem.20052364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Salio M, et al. Plasmacytoid dendritic cells prime IFN-γ-secreting melanoma-specific CD8 lymphocytes and are found in primary melanoma lesions. Eur J Immunol. 2003;33:1052–1062. doi: 10.1002/eji.200323676. [DOI] [PubMed] [Google Scholar]
- 125.Wei S, et al. Plasmacytoid Dendritic Cells Induce CD8+ Regulatory T Cells In Human Ovarian Carcinoma. Cancer Res. 2005;65:5020–5026. doi: 10.1158/0008-5472.CAN-04-4043. [DOI] [PubMed] [Google Scholar]
- 126.Zou W, et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med. 2001;7:1339–1346. doi: 10.1038/nm1201-1339. [DOI] [PubMed] [Google Scholar]
- 127.Della Bella S, et al. Quantitative and functional defects of dendritic cells in classic Kaposi’s sarcoma. Clin Immunol. 2006;119:317–329. doi: 10.1016/j.clim.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 128.Mohty M, et al. Circulating blood dendritic cells from myeloid leukemia patients display quantitative and cytogenetic abnormalities as well as functional impairment. Blood. 2001;98:3750–3756. doi: 10.1182/blood.v98.13.3750. [DOI] [PubMed] [Google Scholar]
- 129.Mohty M, Olive D, Gaugler B. Plasmacytoid DCs and cancer: a new role for an enigmatic cell. Trends Immunol. 2004;25:397–398. doi: 10.1016/j.it.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 130.Hartmann E, et al. Identification and Functional Analysis of Tumor-Infiltrating Plasmacytoid Dendritic Cells in Head and Neck Cancer. Cancer Res. 2003;63:6478–6487. [PubMed] [Google Scholar]
- 131.Sisirak V, et al., editors. The 10th International Symposium on Dendritic Cells. 2008. Breast tumor environment inhibits human plasmacytoid dendritic cells functions; p. P-4-03. [Google Scholar]
- 132.Wenzel J, Bekisch B, Uerlich M, Haller O, Bieber T, Tuting T. Type I interferon-associated recruitment of cytotoxic lymphocytes: a common mechanism in regressive melanocytic lesions. Am J Clin Pathol. 2005;124:37–48. doi: 10.1309/4EJ9KL7CGDENVVLE. [DOI] [PubMed] [Google Scholar]
- 133.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 134.Matsuda A, et al. Large-scale identification and characterization of human genes that activate NF-kappaB and MAPK signaling pathways. Oncogene. 2003;22:3307–3318. doi: 10.1038/sj.onc.1206406. [DOI] [PubMed] [Google Scholar]
- 135.Masuda A, Nakamura A, Maeda T, Sakamoto Y, Takai T. Cis binding between inhibitory receptors and MHC class I can regulate mast cell activation. J Exp Med. 2007;204:907–920. doi: 10.1084/jem.20060631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dahl ME, Dabbagh K, Liggitt D, Kim S, Lewis DB. Viral-induced T helper type 1 responses enhance allergic disease by effects on lung dendritic cells. Nat Immunol. 2004;5:337–343. doi: 10.1038/ni1041. [DOI] [PubMed] [Google Scholar]
- 137.Chalifour A, et al. A Role for cis Interaction between the Inhibitory Ly49A Receptor and MHC Class I for Natural Killer Cell Education. Immunity. 2009;30:337–347. doi: 10.1016/j.immuni.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 138.Lande R, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 139.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of Dendritic Cell Differentiation by IFN-alpha in Systemic Lupus Erythematosus. Science. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 140.Marshak-Rothstein A, Rifkin IR. Immunologically Active Autoantigens: The Role of Toll-Like Receptors in the Development of Chronic Inflammatory Disease. Annu Rev Immunol. 2007;25:419–441. doi: 10.1146/annurev.immunol.22.012703.104514. [DOI] [PubMed] [Google Scholar]
- 141.Tian J, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 142.Vollmer J, et al. Immune stimulation mediated by autoantigen binding sites within small nuclear RNAs involves Toll-like receptors 7 and 8. J Exp Med. 2005;202:1575–1585. doi: 10.1084/jem.20051696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Savarese E, et al. U1 small nuclear ribonucleoprotein immune complexes induce type I interferon in plasmacytoid dendritic cells through TLR7. Blood. 2006;107:3229–3234. doi: 10.1182/blood-2005-07-2650. [DOI] [PubMed] [Google Scholar]
- 144.Yasuda K, et al. Endosomal Translocation of Vertebrate DNA Activates Dendritic Cells via TLR9-Dependent and -Independent Pathways. J Immunol. 2005;174:6129–6136. doi: 10.4049/jimmunol.174.10.6129. [DOI] [PubMed] [Google Scholar]
- 145.Cao W. Molecular Characterization of Human Plasmacytoid Dendritic Cells. J Clin Immunol. 2009;29:257–264. doi: 10.1007/s10875-009-9284-x. [DOI] [PubMed] [Google Scholar]
- 146.Martinelli E, et al. HIV-1 gp120 inhibits TLR9-mediated activation and IFN-α secretion in plasmacytoid dendritic cells. Proc Natl Acad Sci USA. 2007;104:3396–3401. doi: 10.1073/pnas.0611353104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xu Y, et al. HBsAg inhibits TLR9-mediated activation and IFN-α production in plasmacytoid dendritic cells. Mol Immunol. 2009;46:2640–2646. doi: 10.1016/j.molimm.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 148.Novak N, et al. Characterization of FcεRI-bearing CD123+ blood dendritic cell antigen- 2+ plasmacytoid dendritic cells in atopic dermatitis. J Allergy Clin Immunol. 2004;114:364–370. doi: 10.1016/j.jaci.2004.05.038. [DOI] [PubMed] [Google Scholar]
- 149.Fuchs A, Cella M, Kondo T, Colonna M. Paradoxic inhibition of human natural interferon-producing cells by the activating receptor NKp44. Blood. 2005;106:2076–2082. doi: 10.1182/blood-2004-12-4802. [DOI] [PubMed] [Google Scholar]
- 150.Schroeder JT, et al. TLR9- and FcεRI-Mediated Responses Oppose One Another in Plasmacytoid Dendritic Cells by Down-Regulating Receptor Expression. J Immunol. 2005;175:5724–5731. doi: 10.4049/jimmunol.175.9.5724. [DOI] [PubMed] [Google Scholar]
- 151.Green DS, Lum T, Green JA. IgG-derived Fc down-regulates virus-induced plasmacytoid dendritic cell (pDC) IFNα production. Cytokine. 2004;26:209–216. doi: 10.1016/j.cyto.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 152.Blasius AL, Cella M, Maldonado J, Takai T, Colonna M. Siglec-H is an IPC-specific receptor that modulates type I IFN secretion through DAP12. Blood. 2006;107:2474–2476. doi: 10.1182/blood-2005-09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Blasius A, Vermi W, Krug A, Facchetti F, Cella M, Colonna M. A cell-surface molecule selectively expressed on murine natural interferon-producing cells that blocks secretion of interferon-alpha. Blood. 2004;103:4201–4206. doi: 10.1182/blood-2003-09-3108. [DOI] [PubMed] [Google Scholar]
- 154.Sjolin H, et al. DAP12 Signaling Regulates Plasmacytoid Dendritic Cell Homeostasis and Down-Modulates Their Function during Viral Infection. J Immunol. 2006;177:2908–2916. doi: 10.4049/jimmunol.177.5.2908. [DOI] [PubMed] [Google Scholar]
- 155.Kamogawa-Schifter Y, Ohkawa J, Namiki S, Arai N, Arai K-i, Liu Y. Ly49Q defines 2 pDC subsets in mice. Blood. 2005;105:2787–2792. doi: 10.1182/blood-2004-09-3388. [DOI] [PubMed] [Google Scholar]
- 156.Tai L-H, et al. Recognition of H-2Kb by Ly49Q suggests a role for class Ia MHC regulation of plasmacytoid dendritic cell function. Mol Immunol. 2007;44:2638–2646. doi: 10.1016/j.molimm.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 157.Tai L-H, et al. Positive regulation of plasmacytoid dendritic cell function via Ly49Q recognition of class I MHC. J Exp Med. 2008;205:3187–3199. doi: 10.1084/jem.20080718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ju X, Zenke M, Hart DNJ, Clark GJ. CD300a/c regulate type I interferon and TNF-{alpha} secretion by human plasmacytoid dendritic cells stimulated with TLR7 and TLR9 ligands. Blood. 2008;112:1184–1194. doi: 10.1182/blood-2007-12-127951. [DOI] [PubMed] [Google Scholar]
- 159.Ochando JC, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 160.de Heer HJ, et al. Essential Role of Lung Plasmacytoid Dendritic Cells in Preventing Asthmatic Reactions to Harmless Inhaled Antigen. J Exp Med. 2004;200:89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Goubier A, et al. Plasmacytoid Dendritic Cells Mediate Oral Tolerance. Immunity. 2008;29:464–475. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Palucka AK, Hideki U, Joseph WF, Jacques B. Taming cancer by inducing immunity via dendritic cells. Immunol Rev. 2007;220:129–150. doi: 10.1111/j.1600-065X.2007.00575.x. [DOI] [PubMed] [Google Scholar]
- 163.Rosen DB, et al. Functional Consequences of Interactions between Human NKR-P1A and Its Ligand LLT1 Expressed on Activated Dendritic Cells and B Cells. J Immunol. 2008;180:6508–6517. doi: 10.4049/jimmunol.180.10.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]


