Abstract
Multiprotein complexes play an important role in nearly all cell functions, therefore, the characterization of protein-protein interactions in living cells constitutes an important step in the analysis of a cellular signaling pathways. Using fluorescence resonance energy transfer (FRET) as a "molecular ruler" is a powerful approach for identifying biologically-relevant molecular interactions with high spatiotemporal resolution. Here, we describe two methods that use FRET to detect a physical interaction between the T cell antigen receptor (TCR) and the CXCR4 chemokine receptor in living T lymphocytes. These FRET approaches use two different sets of chromophores. We discuss the design strategies, control experiments, and pitfalls involved in using these FRET approaches. Although there is no perfect pair of chromophores for FRET, the two FRET methods described here provide complementary and reliable insight into the molecular interactions between these receptor molecules.
1. Introduction
There is compelling evidence that dynamic physical interactions among proteins play key roles in cellular signal transduction pathways. Visualizing the intracellular locations of signaling molecules in live cells is now possible because of the development of new fluorescent probes and advances in the design of fluorescence microscopy systems. Assays utilizing the technology of fluorescence resonance energy transfer (FRET) allow high spatial resolution of protein-protein interactions in living cells, and can be used to detect protein-protein interactions such as those that mediate signal transduction pathways. This is in contrast to immunofluorescence microscopy which lacks the resolution to distinguish whether two proteins are actually close in molecular terms or merely located in the same cell biological neighborhood. The use of FRET in cell biological experiments has accordingly exploded over the past few years.
Many articles describe the general theory and applications of FRET assays (Ciruela, 2008; Jares-Erijman and Jovrin, 2003; Shaner et al., 2007; Vamosi et al., 2008; Xia and Liu, 2001). Here, we describe in detail two different FRET assays that we used to investigate the formation of a physical complex between CXCR4 and the T cell antigen receptor (TCR) in living T lymphocytes in response to CXCR4 binding to its chemokine ligand, SDF-1 (CXCL12) (Kumar et al., 2006). We will focus particularly on our experimental use of a FRET assay that employs the less commonly-utilized phycobiliprotein fluorophores, phycoerythrin (PE) and allophycocyanin (APC). We will also describe our detailed protocol for using CFP / YFP FRET to examine CXCR4 - TCR interactions. Finally, will address the advantages in FRET assays of using the PE / APC fluorophore pair relative to the CFP / YFP FRET fluorophore pair, and the complementary benefits that can be realized by using both systems to investigate CXCR4-TCR proximity in T cells.
1.1 What is FRET?
In the late 1940s, Theodor Förster described the nonradiative transfer of energy from a chromophore in an exited state to another chromophore. A key feature of this energy transfer, termed FRET, is that it occurs only if the two chromophores are close together ; (Forster, 1948; Stryer, 1967; Stryer, 1978). In addition to chromophore proximity, FRET requires that the emission spectrum of one chromophore (the donor) overlaps the excitation spectrum of the other chromophore (the acceptor). FRET also requires an appropriate relative orientation of the two chromophores. FRET occurs when an excitated donor transfers energy to the acceptor, resulting in the acceptor's excitation and subsequent fluorescence. The distance parameter determining the efficiency of energy transfer depends on the inverse sixth power of intermolecular separation, therefore, the detection of FRET indicates that two molecules are within approximately 5–10 nm of each other (Forster, 1965; Lakowicz, 1999). These distances are on the order of the size of many individual proteins of biological significance, including enzymes and receptors. FRET has therefore been extensively employed to monitor the activity of cellular signaling cascades in live cells (He et al., 2005; Janetopoulos et al., 2001; Tertoolen et al., 2001; Vilardaga and Nikolaev, 2007). It should be noted that because FRET depends not only on chromophore proximity but also on the orientation of the chromophores and their relative abundance, the absence of a FRET signal cannot be taken as evidence for the absence of chromophore proximity.
1.2 Advantages of PE / APC mAb FRET
We describe below a phycobiliprotein FRET assay that we used to show that SDF-1 -dependent signaling increases the proximity of endogenous CXCR4 and TCR of T cells (Kumar et al., 2006). Although discussed at length in the historical FRET literature (Stryer, 1978), in recent years PE / APC FRET has been relatively unused for detecting molecular proximity in biologically-relevant contexts. Yet the expermental use of phycobiliprotein FRET to detect protein-protein interactions has several advantages as compared to CFP / YFP FRET.
First, the PE / APC FRET method presented below has the advantage of not requiring specialized reagents or equipment. The method utilizes commercially available PE- and APC-conjugated antibodies as chromophores, and a standard two laser flow cytometer such as that routinely available to many research laboratories for FRET detection.
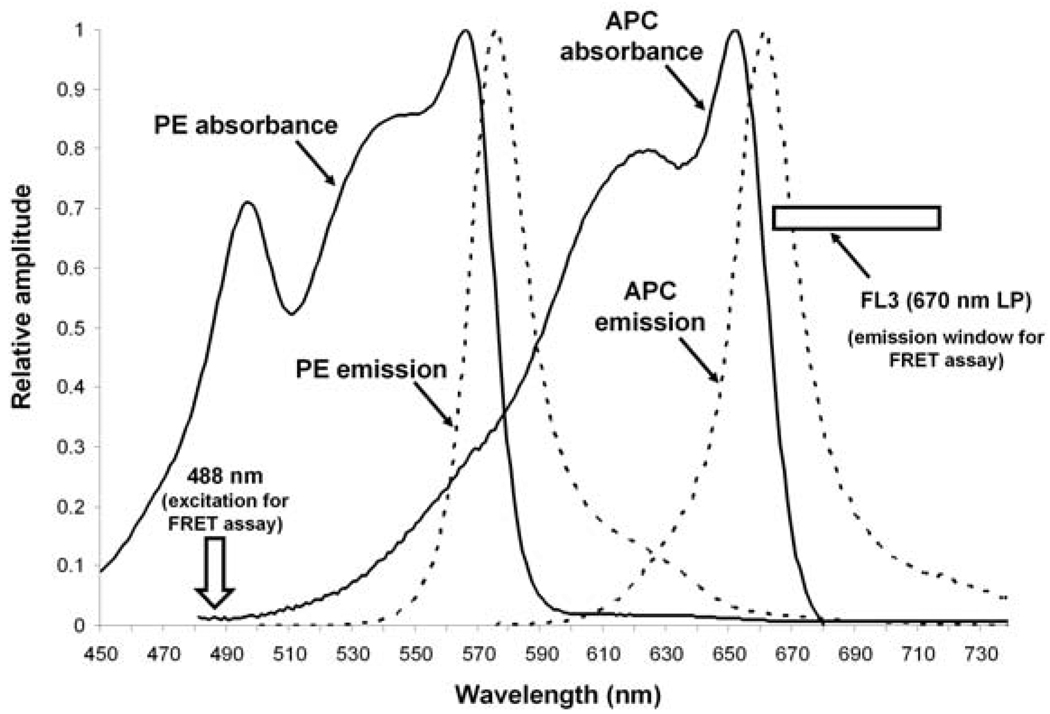
Second, the PE / APC fluorophore pair displays spectral properties that make it nearly ideal for use in FRET assays. A theoretically ideal pair of fluorophores for FRET studies should display the following characteristics: 1) The excitation spectra of the donor and acceptor fluorophores should be well separated, 2) the emission spectrum of the donor fluorophore should overlap the excitation spectrum of the acceptor fluorophore, and 3) the emission specta of the donor and the acceptor fluorophores should be well separated. Fig. 2 shows the excitation and emission spectra of PE and APC and illustrates how well the PE / APC fluorophore pair meets these three characteristics. First, the excitation spectra of PE and APC show little overlap. APC is maximally excited by 615 – 655 nm light, but APC is not excited by 488 nm light that maximally excites PE. Second, PE fluoresces at wavelengths that show good overlap with the excitation range of APC. Third, APC emission at long wavelengths does not overlap significantly with PE emission. For FRET assay, then, one may use a standard two laser flow cytometer to detect APC fluorescence > 670 nm via a long-pass filter (indicated by the rectangle in Fig. 2). APC fluorescence can be detected when APC is either directly excited by the 635 nm laser (as when using the cytometer's FL4 channel), or when APC is indirectly excited from PE via FRET when PE is stimulated by the 488 nm laser (as when using the cytometer's FL3 channel).
Fig. 2. Absorbance and emission spectra of PE and APC.
For the absorbance spectra, PE and APC (in the context of anti-CXCR4-PE and anti-TCR-ζ-APC) were excited by 488 nm and 580 nm light, respectively. Absorption spectra were recorded on a Cary 4000 UV/VIS spectrophotometer (Varian Inc. Palo Alto CA). Emission spectra were recorded on a SPEX Fluorolog-3 spectrofluorimeter using a 5 nm slit width (Horiba Jobin Yvon, Edison, NJ). FRET signals are detected in our FRET assay when PE is excited at 488 nm (indicated by the arrow) and the emission of APC > 670 nm is measured using a long pass filter (the detection window is indicated by the rectangle).
A third advantage of using the PE / APC fluorophore pair for FRET assays derives from the fact that these molecules have evolved to mediate FRET in biological systems, specifically within the phycobilisomes of Cyanobacteria and eukaryotic algae. Both PE and APC are therefore highly stable molecules and are also highly efficient at FRET. PE, APC, and other phycobiliproteins employ covalently-linked open chain tetrapyrrole groups to capture light energy. The phycobiliproteins used in our FRET assay (i.e. PE and APC conjugated to monoclonal antibodies (mAbs)) are in the form of six α/β monomers arranged as a disc. In phycobilisomes, these discs are stacked to form a highly-ordered array. PE, APC, and other phycobiliproteins in the phycobilisomes transfer light energy from one to another via FRET and ultimately to chlorophyll to achieve photosynthesis (Glazer et al., 1985;Viskari and Colyer, 2001). The FRET occurring during this process is characterized by high quantum yields (up to 98 %) and large extinction coefficients and Stoke's shifts. Although this very high FRET efficiency relies on the specific, ordered structure of the phycobilisome, experimental protocols utilizing less-ordered phycobiliproteins, such as the PE and APC forms conjugated to mAbs, can nevertheless achieve impressive FRET efficiencies. The relative efficiency of phycobiliprotein FRET aids in the detection of FRET signals and simplifies using the PE / APC FRET chromophore pair for experimental purposes.
Despite these significant advantages, some disadvantages are also associated with using the PE / APC fluorophore pair for FRET assays. Chief among these is the large size of these phycobiliprotein chromophores. The hexameric form of PE and APC is approximately 150 kD, which makes this FRET pair unsuitable for fine distance measurements, for example, within a single molecule. This problem is compounded in our protocol since we utilize mAbs to link the chromophores to the receptors. As discussed below, this disadvantage can be ameliorated by using multiple approaches to confirm molecular interactions.
1.3 CFP / YFP fusion protein FRET
To avoid potential ambiguity due to the use of receptor-binding antibodies and the bulky nature of the PE and APC fluorophores and their mAb-mediated attachment to the receptors, we also used a second FRET approach for assaying CXCR4-TCR interactions in living T cells (Kumar et al., 2006). In recent years the use of green fluorescent protein (GFP) and its color variants cyan (CFP) and yellow (YFP) fluorescent proteins has become a prevalent approach for measuring protein-protein interactions by FRET (Chan et al., 2001; He et al., 2003b; He et al., 2003a; Shaner et al., 2005; Tsien, 1998). In this FRET approach, the entire fluorescent sensor is encoded and expressed in cells as a fusion with the protein(s) of interest. Molecular genetic manipulation of expression plasmids makes this easy to achieve simply by transfecting cells with appropriate expression plasmids. In the CFP / YFP FRET assay, the excitation of CFP is achieved by 433 nm light, which leads to the emission of cyan fluorescence with a peak at 475 nm. If YFP is close by, energy from CFP can be transferred to YFP, and consequently emission from YFP will occur with a peak wavelength at 528 nm.
Unfortunately, the CFP / YFP FRET system displays several non-ideal spectral characteristics. In contrast to the PE / APC FRET pair discussed above, the CFP / YFP FRET pair suffers from weak overlap of excitation spectra, poor separation of excitation spectra, and also poor separation of emission spectra. It is therefore more difficult to avoid potential excitation and emission spectra overlap in CFP / YFP FRET systems than in PE / APC FRET systems. Problems arising from overlap of the excitation spectra can be avoided by using 433 nm light for excitation (as recommended in our protocol, below), whereas use of 458 nm light for excitation is not recommended. Problems arising from emission spectra overlap are more difficult to avoid. Very sensitive ratio imaging by a charge-coupled device (CCD) camera can be used (Jares-Erijman and Jovrin, 2003) to reliably establish FRET using this system. Alternatively, as we describe below, CFP / YFP FRET can be established by using a spectrofluorimeter that allows examination multiple regions of the entire emission spectra. This permits the confirmation of FRET by assuring that increases in YFP (acceptor) fluorescence occur concomitant with a decrease in CFP (donor) fluorescence.
1.4 Using both FRET approaches to study CXCR4 - TCR proximity
We used two different FRET approaches to detect CXCR4-TCR interactions (Kumar et al., 2006). Each FRET approach has its advantages and disadvantages, but by combining the approaches we were able to obtain complementary data sufficient to provide strong support for our conclusions.
In addition to the technical advantages discussed above, our PE / APC FRET approach has the advantage of examining interactions between native receptors expressed on the cell surface at endogenous levels. Because it does not require transfection, this approach can be used to examine CXCR4-TCR interactions of normal T lymphocytes and other cells that are difficult to transfect. On the other hand, this PE / APC FRET approach may not be generally applicable for studying all protein-protein interactions, primarily because it relies on using mAbs to link the chromophores to the proteins. The use of mAbs requires that the proteins to be assayed must be abundantly expressed on the cell-surface and that specific antibodies directed against a cell-surface-accessible epitopes are available. The mAbs might affect receptor function upon labeling to the proteins, or they might also bind to epitopes too distant to allow the chromophores to interact. Although we have solved these problems specifically for our CXCR4-TCR FRET assay by carefully selecting mAbs for use in the FRET assay, these issues must be considered when modifying this approach to analyze interactions between other cell-surface proteins. Another general disadvantage of the PE / APC FRET system is that both the mAbs and the PE and APC chromophores are quite large, and this necessarily reduces the molecular resolution that can be obtained from the FRET assay.
For these reasons we also examined CXCR4-TCR interactions using the CFP / YFP FRET system. This system has the advantages of employing smaller-sized fluorescent probes (YFP and CFP are each approximately only 25 kD in size) and of not requiring mAbs to attach the chromophores to the proteins. Another advantage is that simple genetic manipulations are all that is required in order to investigate interactions between any two proteins. However, this system has its own disadvantages. Possible alterations of proteins' structure and function may occur simply because they are fused to the CFP or YFP proteins, and overexpression of fusion proteins might disrupt normal cellular functions in unanticipated ways. Moreover, this system can only be used to examine protein-protein interactions in cells that are transfectable or otherwise permissive of genetic manipulation. Finally, use of the CFP / YFP FRET system is significantly complicated by its non-ideal spectral characteristics, as described above.
Both FRET assays as we used them might be criticized because they attach a fluorescent probe (and therefore assayed receptor proximity) to only one subunit of the TCR. However, this criticism is ameliorated by our using two FRET assays that probe behavior of different TCR subunits: the PE / APC FRET assay measures CXCR4 - TCR-ε interactions, while the CFP / YFP FRET assay measures CXCR4 - TCR-ζ interactions. Additional assurance that the two CXCR4-TCR FRET assays are measuring CXCR4 interactions with the whole TCR comes from the fact that the PE / APC FRET assay only labels receptors located on the cell surface. It has previously been shown that the majority of TCR on the cell-surface are holoreceptors consisting of all α, β, γ, δ, ε, and ζ TCR subunits (Alarcon et al., 1988).
2. Assaying CXCR4-TCR proximity via the PE / APC mAb FRET approach: flow cytometric detection of FRET between fluorescent monoclonal antibodies bound to endogenous cell-surface CXCR4 and TCR-CD3-ε
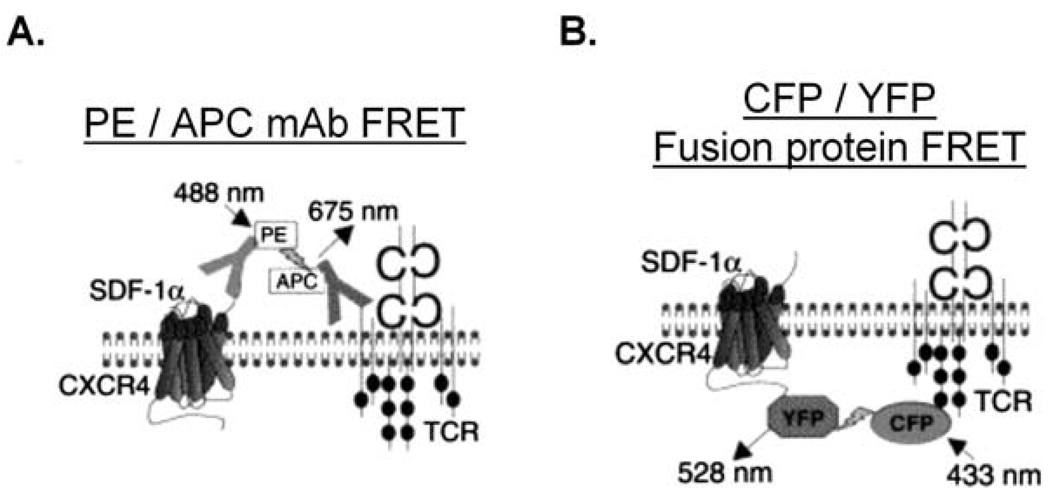
We successfully used this first FRET assay (depicted in Fig. 1A) to detect SDF-1 -induced increases in proximity between CXCR4 and the TCR in both normal, human peripheral blood T cells isolated from peripheral blood mononuclear cell preparations (PBMC) and the Jurkat T cell line (Kumar et al., 2006). Unless otherwise indicated, cells are stimulated at 37° C with 0.5 – 10−7 – 1.0 × 10−7 M SDF-1 (SDF-1α from R&D Systems, Minneapolis, MN). SDF-1 is resuspended at 105 M in PBS supplemented with 0.5 % BSA, aliquotted, and stored at −70 °C. Cells must be metabolically active in order to form complexes. The small quantities of azide used to preserve stocks of mAbs will not inhibit receptor complex formation, however, it is important to otherwise employ azide-free solutions. Solutions should alo be prepared using the highest quality and purest bovine serum albumin (BSA) available.
Fig. 1. Two experimental approaches for using FRET to assay CXCR4-TCR complex formation in response to SDF-1 treatment of T lymphocytes: PE / APC mAb FRET and CFP / YFP fusion protein FRET.
A) Cartoon depicting FRET between endogenous CXCR4 and TCR receptors, as assayed by using mAbs to link PE and APC fluorophores to the endogenous cell-surface receptors. B) Cartoon depicting FRET between fluorescent CFP and YFP fusion proteins of CXCR4 and the TCR. Used by permission from Kumar et al., 2006.
2.1 Important considerations for labeling cell-surface CXCR4 and TCR
Several aspects of the labeling step are critical to ensure success of the FRET assay. First, it is important to use the particular mAb clones specified. These are CXCR4 mAb conjugated to PE (Clone #44717; R&D Systems) and TCR-CD3-ε mAb conjugated to APC (Clone #UCHT1; Pharmingen, San Diego, CA). Using different mAbs may not provide the appropriate orientation and/or proximity of the conjugated fluorophores to permit the detection of FRET following CXCR4-TCR complex formation. Second, to increase the ability to detect FRET, it is important to label as high a fraction as possible of the two cell-surface receptors with the fluorescently-conjugated mAbs. It is particularly critical to achieve optimal labeling of the FRET donor, which in this case is CXCR4. Care must therefore be taken to bind the antibodies to the cell-surface receptors under conditions of considerable antibody excess and for a sufficient amount of time. For this reason, it is recommended that before attempting FRET assays, the anti-CXCR4-PE reagent be titrated for optimal labeling conditions as determined by traditional flow cytometric analysis. We have found that some lots of anti-CXCR4-PE reagent need to be used at double or more of the per-cell dose recommended by the manufacturer for traditional flow cytometry in order to achieve maximal labeling of CXCR4. Different lots of anti-CXCR4-PE reagent may also vary in their PE/IgG coupling ratio and thereby affect sensitivity of the FRET assay. When the cell-surface CXCR4 of Jurkat T cells is optimally-labeled for FRET assay, the cells are nearly 103 times brighter in the PE/FL2 flow cytometry channel than unstained cells. Finally, it is important to avoid any warming of the samples that may permit receptor internalization during the antibody labeling step and before SDF-1 treatment. Using an ice-water bath for sample incubation during these operations is therefore preferable to using ice alone.
2.2 Detailed procedure
Jurkat cells are grown in Medium A (RPMI with phenol red supplemented with L-glutamine and 5 % fetal calf serum and 5 % calf serum). Cells are used for experiments when grown to a density between 0.6 × 106 cells/ml and 0.9 × 106 cells/ml. The protocol below is also appropriate for use with normal, human PBMC T cells. Purify PBMC the same day as blood draw via a standard ficoll gradient method from blood bank buffy coat or apheresis cone preparations. PBMC can then be used for FRET experiments following 24 hr of cell culture at 37 °C in Medium A. When using PBMC, flow cytometric FRET results should be gated to include only CD3+ cells in the analysis.
Prepare FACS buffer without phenol red or azide (Hanks Balanced Salt Solution supplemented with 10 mg/ml BSA and 10 mM HEPES pH 7.4). Prepare Fixing Solution (PBS with 2 % paraformaldehyde). Sterilize solutions by filtering through 0.2 µm tissue-culture filters. Solutions may be stored at 4 °C for several weeks.
Determine the number of cell samples required for the assay. Each sample should contain 0.5 × 106 cells. A minimum assay will require control samples for setting flow cytometry channel voltage and compensation in addition to experimental samples + and - SDF-1. For example, prepare the following cell samples: 1) unstained cells, 2) cells stained with anti-CXCR4-PE only, 3) cells stained with anti-TCR-ε-APC only, 4) cells stained only with any perCP-conjugated antibody that binds to the cells (to confirm FL3 channel setup on the cytometer), and 5&6) cells stained with both anti-CXCR4-PE anti-TCR-ε-APC that will be stimulated later with either vehicle or SDF-1.
Wash cell samples in ice-cold FACS buffer and aliquot into 1.5 ml eppendorf tubes on wet ice. Stain cells with the appropriate antibodies by resuspending cell pellets in the appropriate antibody or mixture of antibodies. For anti-CXCR4-PE anti-TCR-ε-APC use the optimal concentration and volumes determined by prior titration. Incubate cells on wet ice 20–40 min to obtain optimal labeling of cell-surface receptors. Wash cells twice with 0.2 ml ice-cold FACS buffer.
To assay SDF-1-dependent FRET, stimulate the appropriate cell samples with SDF-1 (or vehicle) as follows. Immediately after staining cells with mAbs and washing, resuspend each cell pellet in 0.2 ml FACS buffer. Add an appropriate amount of SDF-1 stock solution (or vehicle) and place test tubes in a 37 °C circulating water bath for exactly 20 min. Care must be taken to control the time of the stimulation +/− 10 sec since FRET signals increase with time (Kumar et al., 2006). We previously showed that 20 min of SDF-1 stimulation achieves nearly maximal FRET signals (Kumar et al., 2006). To stop the reactions, transfer each sample into a chilled test tube containing 0.2 ml ice-cold Fixing Solution. Place cells at 4 °C for at least 1 hr to fix them, then analyze by flow cytometry immediately or after further incubation at 4 °C. Fixation is not required for FRET detection if cells are kept on wet ice until flow cytometric analysis, however, fixation is recommended in order to prevent temperature- and time-dependent variations in FRET signals.
2.2.1 Flow cytometer specifications
For flow cytometry, we used a dual-laser (488 nm Ar and 635 nm He-Ne) FACS Caliber (Becton Dickinson, Franklin Lakes, NJ), as described (Batard et al., 2002; Kumar et al., 2006). Excitation / emission windows were 488 nm/585 ± 21 nm (FL2), 488 nm/>670 nm (FL3), and 635 nm/661 ± 8 nm (FL4). The optimal voltage settings for each channel are determined as in conventional flow cytometry, i.e. so that the unstained cell peak is completely on-scale. Compensation is similarly determined as in conventional flow cytometry, except that greater care should be taken to not either over- or under- compensate any channels. Saved instrument settings on the instrument specified above are usually approximately correct from day-to-day, although compensation may require fine daily adjustment.
2.3 Examples and results
Fig. 3–Fig. 5 shows examples of using the "mAb FRET" approach to analyze the SDF-1 -dependent formation of CXCR4-TCR complexes. CXCR4 and TCR-CD3-ε of Jurkat T cells were labeled as above with receptor-specific mAbs conjugated to PE or APC, respectively. After stimulation with SDF-1, cellular fluorescence changes associated with FRET were analyzed by flow cytometry.
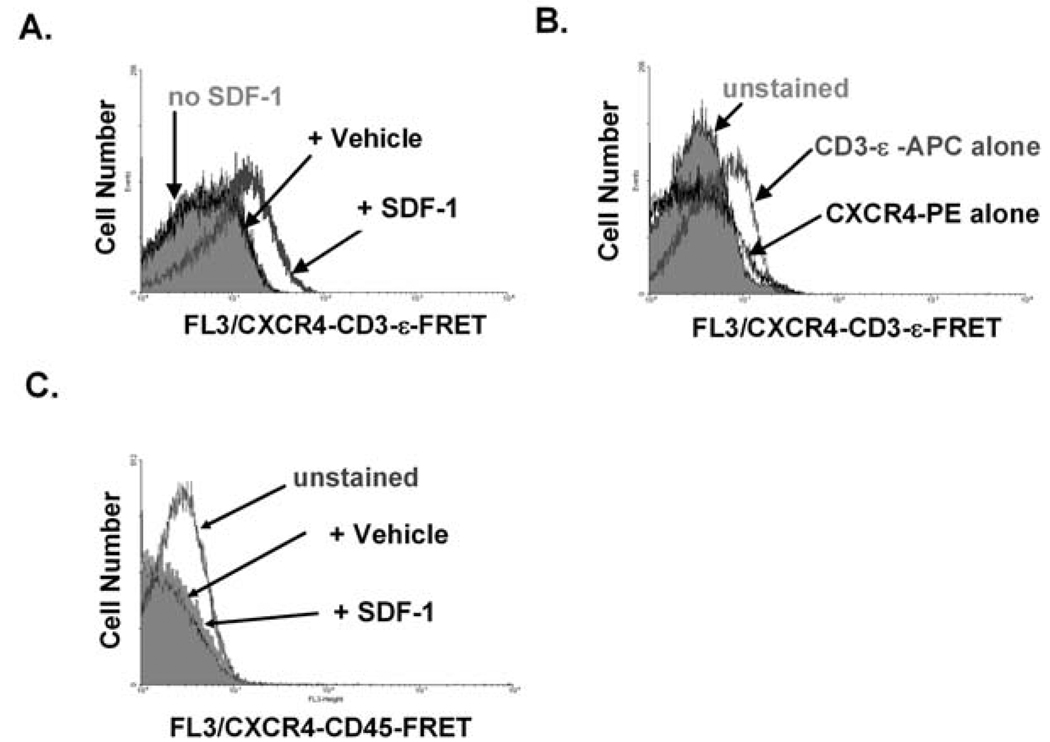
Fig. 3. Using mAb FRET and flow cytometry to assay SDF-1 - dependent CXCR4-TCR complex formation: typical responses of Jurkat T cells and control data.
A) Results from an individual experiment performed as described in Fig. 1A and the text. Both CXCR4-PE and TCR-ε-APC mAb were bound to the CXCR4 and TCR molecules of Jurkat T cells, then cells were stimulated with either SDF-1 or Vehicle (water) at 37 °C for 10 min. The data show that SDF-1, but not Vehicle, treatment induced an increase in per-cell CXCR4-PE - TCR-ε-APC FRET fluorescence (detected using the FL3 channel of the flow cytometer). B) Control samples in which cells were analyzed as in A) after being bound to either CD3-ε-APC or CXCR4-PE alone. These control cells do not display FL3 fluorescence at the level of that induced by SDF-1 on the dually-labeled cells shown in Fig. 3A. C) Control sample in which cells were analyzed as in A) after being bound to anti-CD45-APC instead of anti-TCR-ε-APC. Cells were also stained with anti-CXCR4-PE. No increase in FL3/FRET fluorescence was detected in response to SDF-1. Used by permission from Kumar et al., 2006.
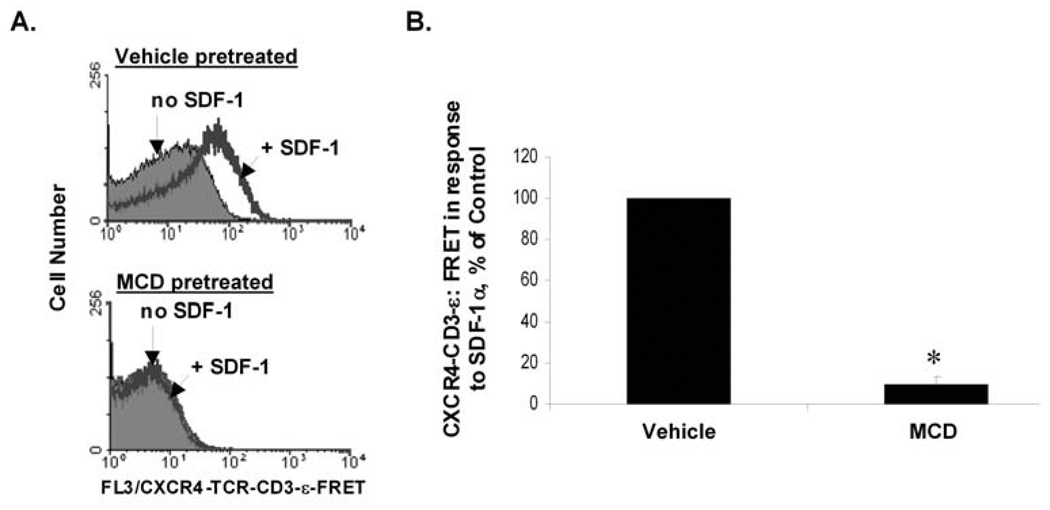
Fig. 5. Methyl-β-cyclodextrin (MCD) inhibits the ability of SDF-1 to induce CXCR4-TCR FRET, suggesting that this process requires cholesterol and/or membrane fluidity.
Wild-type Jurkat T cells were pretreated for 20 min with 25 mM of the cholesterol chelator, methyl-β-cyclodextrin, or an equivalent amount of vehicle (RPMI). SDF-1 - dependent CXCR4-TCR complex formation was then assayed by the "mAb FRET" approach described in Fig. 1A, Fig. 3, and the text. A) Histograms showing examples of individual experiments. B) Bar graph showing a summary of multiple experiments as in A). Each bar denotes the mean SDF-1 -dependent FRET response ± S.E.M. for 3 independent experiments; results are shown as a % of the responses of control cells pre-treated with vehicle alone and analyzed the same day; *, significantly different from control responses (p < 0.05).
2.3.1 Jurkat results and controls
Fig. 3A shows typical results for Jurkat T cells and recommended controls. SDF-1 treatment increased per-cell FRET fluorescence, which is reflected by the increase in FL3 channel fluorescence. The CXCR4-TCR FL3/FRET response was specific for SDF-1, since no CXCR4-TCR FRET signals were induced when cells were stimulated with Vehicle (water) alone (Fig. 3A). Cells stimulated at 4 °C fail to induce SDF-1 -dependent FRET signals, suggesting that CXCR4-TCR complex formation requires a metabolic process (Kumar et al., 2006). The levels of FL3 fluorescence associated with FRET were not seen using cells labeled with either PE or APC alone (Fig. 3B). The controls shown in Fig. 3A & B should be performed in every experiment to provide assurance of proper cytometer setup.
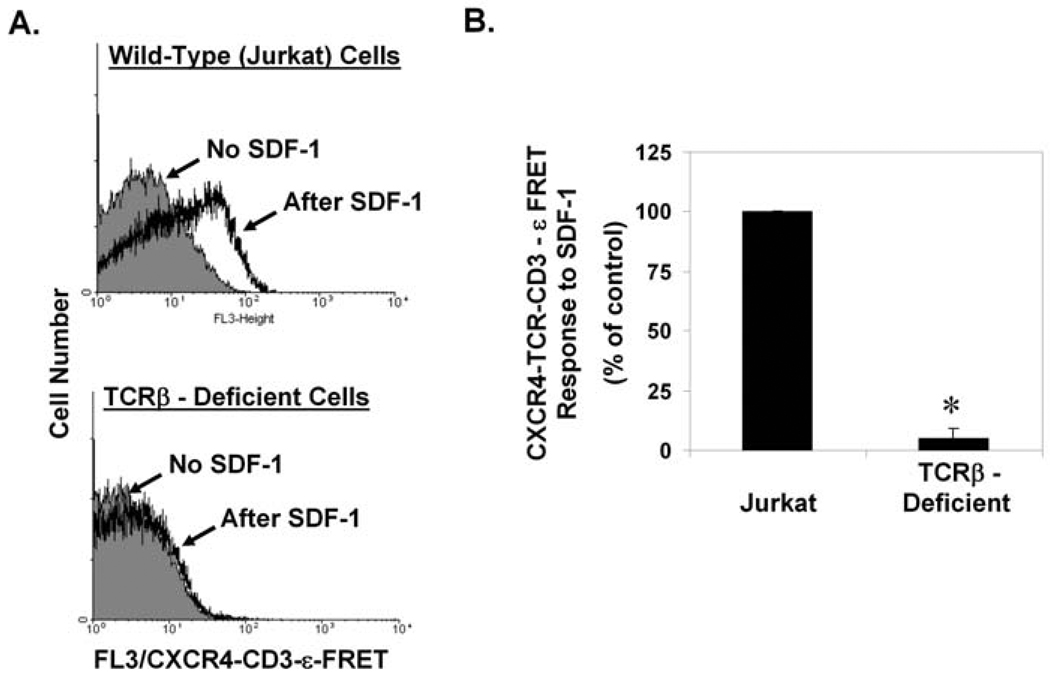
An additional control is shown in Fig. 3C. No FL3/FRET signals were detected when a similar approach was used to assay the proximity of CXCR4 to another abundantly expressed cell-surface receptor, CD45. The results of this control experiment indicate that the flexibility and length of the mAbs used to link the fluorophore to the receptors are not, in themselves, sufficient to permit promiscuous FRET between any pair of cell-surface receptors. Fig. 4 shows an additional control. No FL3/FRET signals were detected using a somatic mutant of the Jurkat T cell line that is deficient in TCRβ expression and consequently also deficient in cell-surface TCR expression (Kumar et al., 2006), providing assurance that a positive readout in this assay requires expression of the TCR.
Fig. 4. SDF-1 - dependent CXCR4-TCR complex formation requires expression of the TCR.
Wild-type Jurkat T cells, or a somatic mutant of Jurkat deficient in expression of TCRβ and consequently deficient in cell-surface TCR expression (Batard et al., 2002), were assayed for SDF-1 - dependent CXCR4-TCR complex formation by the "mAb FRET" approach described in Fig. 1A, Fig. 3, and the text. A) Histograms showing examples of individual experiments. B) Bar graph showing a summary of multiple experiments as in A). Each bar denotes the mean SDF-1 - dependent FRET response ± S.E.M. for 3 independent experiments; results are shown as a % of the responses of control (ie wild-type Jurkat) cells analyzed the same day; *, significantly different from control responses (p < 0.05).
Several additional controls useful for confirming the authenticity of results obtained from using this "mAb FRET" assay are described in our published paper (Kumar et al., 2006). First, we showed that in addition to cells from the Jurkat cell line, normal, human PBMC T cells display SDF-1 - dependent CXCR4-TCR FRET responses. Second, we showed that the SDF-1 -induced CXCR4-TCR FRET signals gradually increase with time after SDF-1 addition and plateau after approximately 20 min. Third, we showed that SDF-1 - induced CXCR4-TCR FRET signals are appropriately inhibited by increasing doses of a CXCR4 antagonist. Finally, we showed that SDF-1 - induced CXCR4-TCR FRET signals display a dose-response relationship consistent with SDF-1 acting by binding to CXCR4 (Kumar et al., 2006). Together, the results in Fig. 3&Fig. 4 and additional controls described here indicate that SDF-1 stimulation causes CXCR4 and the TCR to move into close, physical proximity in both the Jurkat T cell line and normal human T cells.
2.3.2 Effects of methyl-β-cyclodextrin
Here, we use this PE / APC mAb FRET assay to further characterize SDF-1 - dependent CXCR4-TCR complex formation of T cells. Jurkat T cells were pretreated with either the cholesterol chelation agent, methyl- β -cyclodextrin (MCD), or vehicle. Cell-surface CXCR4 and TCR-CD3-ε molecules were then stained with CXCR4-PE and TCR-CD3-ε-APC mAbs, and SDF-1 - dependent CXCR4-TCR complex formation was detected via FRET assay as in Fig. 2. Fig. 5 shows that MCD pretreatment abrogated the SDF-1 - dependent FRET signals of the cells, suggesting that SDF-1 - dependent CXCR4-TCR complex formation occurs via a process that requires cholesterol and/or membrane fluidity.
3. Assaying CXCR4-TCR proximity via the CFP / YFP fusion protein approach: fluorescence spectroscopic detection of FRET between CXCR4 and TCR fluorescent fusion proteins expressed in live cells
We also used a second FRET method (Fig. 1B) to assay SDF-1 - induced increases in the proximity between CXCR4-YFP and TCR-ζ-CFP in the Jurkat T cell line (Kumar et al., 2006).
3.1 Transient transfection of fusion proteins into Jurkat T cells
3.1.1 Plasmids and controls
To express CXCR4 and TCR-CD3-ζ fluorescent fusion proteins in the Jurkat T cell line, we employed transient transfection of expression plasmids encoding these proteins as fluorescent fusion proteins with YFP and CFP. For example, we amplified cDNA encoding human CXCR4 and TCR-CD3-ζ via PCR and subcloned them into pEYFP-N1 and pECFP-N1, respectively (Clontech, Mountain View, CA) (the construction of these plasmids is described in Kumar et al. (Kumar et al., 2006)). It is important to initially prepare not only cell samples dually-transiently-transfected with both YFP and CFP fusion protein-encoding plasmids, but also control cell samples transiently-transfected with either the YFP or CFP fusion protein-encoding plasmids individually. These first types of control samples are important in order to delineate the shape and intensity of the individual YFP or CFP fluorescence emission profiles under the detection conditions used and with the instrument employed. A second type of control is essential to include in each experiment: a cell sample tranfected only with pcDNA3 (Invitrogen, Carlsbad, CA) or a similar "empty" vector. This control is required for background subtraction of fluorescence produced by the cells and media. To account for effects of transfection-related cell death on the fluorescence of the sample, it is important that the total amount of DNA used for all transfections in a given experiment be kept constant for all samples and controls. This can be achieved by adding to each transfection an amount of an "empty" vector such as pcDNA3 so as to make the total amount of plasmid DNA in each transfection equivalent.
3.1.2 Preliminary experiments to determine optimal conditions
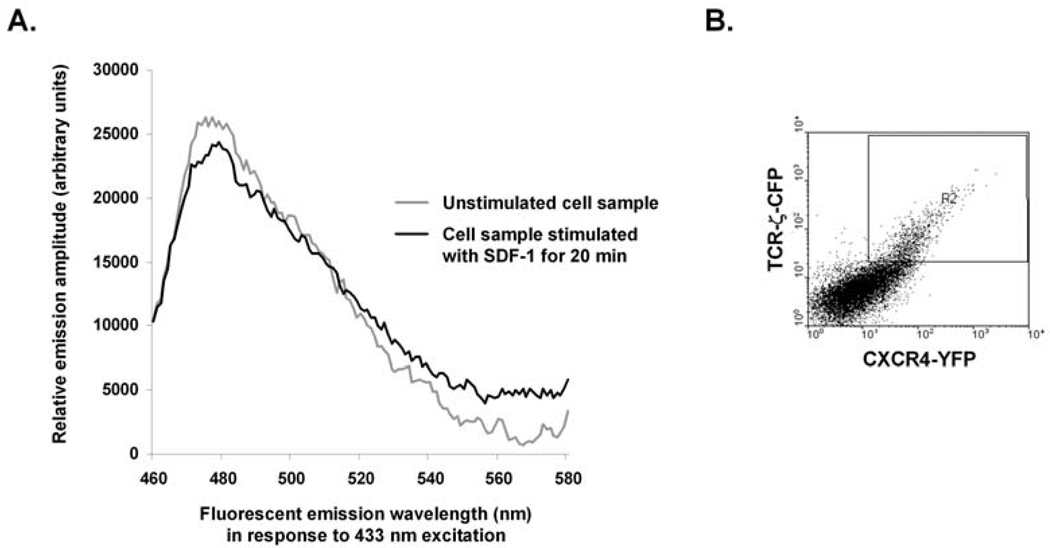
Optimal FRET between interacting fluorescent fusion proteins would ideally be achieved under conditions of high, but molar equivalent, expression of two interacting fluorescent fusion proteins. If either the donor or acceptor fusion protein is grossly overexpressed relative to the other, the opportunity for the two molecules to interact will be greatly decreased and/or the signal may be too weak to detect. On the other hand, depending on the cell type used and the proteins being expressed, too high a level of expression of one or both fusion proteins may be toxic to the cells. Thus, before beginning FRET experiments, it is recommended that test transfections be performed in which the amounts of the fusion protein-expressing plasmids are titrated. Such test transfections are also useful for optimizing transfection efficiency. Flow cytometry of the test transfections, and also of later, experimental transfections, is useful for confirming appropriate levels of protein expression, transfection effiency, and cell viability. Fig. 6B shows an example. For this purpose, we use a Becton-Dickinson (Franklin Lakes, NJ) LSRII special order flow cytometer with five lasers. For CFP detection, excitation is achieved via the 407 nm laser and 505–520 nm emitted light is detected via a combination of a 505 nm long-pass filter followed by a 500/40 nm band-pass filter. For YFP detection, the cells are excited with the 488 nm laser and 515–545 nm emitted light is detected via a combination of a 505 nm long-pass filter followed by a 530/30 nm band-pass filter. In our hands, transiently-transfecting 107 Jurkat T cells with 10 µg each of CXCR4-YFP and TCR-ζ-CFP - expressing plasmids yields the optimal detection of SDF-1 - induced FRET signals. FRET signals were detected with dual plasmid transient transfection efficiencies of 20–40 % (Fig. 6B). These conditions lead to approximately equal expression levels of CXCR4-YFP and TCR-ζ-CFP in the transfected cells, as illustrated in Fig. 6B.
Fig. 6. Using CFP / YFP fusion protein FRET and spectrophotometry to assay the formation of complexes between CXCR4-YFP and TCR-ζ-CFP in response to SDF-1 treatment of Jurkat T cells.
A) Results from using a fluorescence spectrometer to assay CXCR4-TCR FRET in Jurkat T cells using the CFP / YFP fusion protein FRET approach described in Fig. 1B and the text. Jurkat T cells were transiently-transfected with plasmid vectors expressing CXCR4-YFP and TCR-ζ-CFP, then analyzed for FRET. The background-subtracted fluorescence emission spectra of the same cell samples in response to 433 nm light stimulation before (grey line) and after (black line) 20 min of SDF-1 treatment are shown. B) Results from a control flow cytometric analysis of the cell sample used in A), indicating that approximately 20 % of live cells (boxed) express both CFP and YFP fusion proteins.
3.1.3 Detailed protocol for transient transfection
For analysis of complex formation between CXCR4-YFP and TCR-ζ-CFP, Jurkat cells are grown in Medium A (RPMI without phenol red but supplemented with L-glutamine and 10 % fetal calf serum) and are used for transient transfection when grown to a density between 0.6 × 106 cells/ml and 0.9 × 106 cells/ml. Jurkat cells grown to a density of greater than 106 cells/ml are only poorly transfectable by electroporation. Four cell samples are prepared for each experiment. For each sample, 107 cells in a volume of 350 µl of Medium A are mixed with plasmid DNA as follows: Sample 1, pcDNA3 ("empty" vector; 20 µg); Sample 2, CXCR4-YFP (10 µg) and pcDNA3 (10 µg); Sample 3, TCR-ζ-CFP (10 µg) and pcDNA3 (10 µg); and Sample 4, CXCR4-YFP (10 µg) and TCR-ζ-CFP (10 µg). Each cell sample is then transferred to a 4 mm gap BTX electroporation cuvette and subjected to one 315 V pulse for 10 msec using a BTX T820 square-wave electroporator (BTX, Holliston, MA). The cells from each transfection are then diluted in 5 ml Medium A and cultured for 24–28 hr.
3.2 Assaying CXCR4-YFP and TCR-ζ-CFP proximity by FRET
3.2.1 Preparation of transfected cell samples
The length of the time between cell transfection and FRET analysis critically affects the levels of fusion protein expression and therefore the experimental outcome. In our hands, and using the conditions and amounts of the fusion protein-encoding plasmids described here, CXCR4-YFP - TCR-ζ-CFP FRET is best detected following 24–28 hr of culture. It is recommended that other laboratories determine the optimal post-transfection culture time (which may vary between 18 and 36 hrs) for their experiments. After the transfected cells are given the appropriate time in culture to express the fusion proteins, the cells are resuspended to a density of 1 × 106 − 2 × 106 cells/ml in Medium B (Hanks Balanced Salt Solution supplemented with 5 % fetal calf serum and 5 mM HEPES, pH 7.4) and cultured at 37 °C with 5 % CO2 until FRET analysis. RPMI-based medium cannot be used at this point due to it's high background fluorescence under the conditions used.
3.2.2 Collection of fluorescent spectra
To detect CXCR4-YFP - TCR-ζ-CFP FRET, we used a Horiba Jobin Yvon (Edison, NJ) SPEX Fluorolog-3 spectrofluorimeter with 433 nm light for excitation. The emission spectra were collected at 1 nm/sec from 460 nm to 580 nm. The sample was maintained at 37 °C and stirred constantly using a heated cuvette and a magnetic stir bar, respectively, during all spectral assays and also throughout the entire time of the SDF-1 stimulation. SDF-1 treatment was performed while cells remained in the cuvette and the same samples were assayed to obtain spectra both before and after SDF-1 treatment. Spectral data was collected both before and after SDF-1 treatment for all four experimental cell samples (including all control cell samples). We previously determined that CXCR4-TCR complex formation detectable by the antibody FRET method increases during 20 min of SDF-1 treatment at 37 °C and plateaus after 20 min (Kumar et al., 2006), therefore, we assayed for CXCR4-YFP - TCR-ζ-CFP FRET by the spectrofluorimetric method after 20 min of SDF-1 treatment.
3.2.3 Analysis and interpretation of fluorescent emission spectra
Once the emission spectra have been gathered, it is then necessary to subtract from the sample spectrum the background fluorescence emission produced by the media and cells. This subtraction also accounts for any changes in cell shape or size that might effect the spectra. To do this, cells transfected only with a plasmid vector such as pcDNA3 were analyzed for fluorescence emission before and after SDF-1 treatment. The unstimulated pcDNA3 control spectrum was then subtracted from the unstimulated experimental sample spectrum. Likewise, the SDF-1-stimulated pcDNA3 control spectrum was subtracted from the SDF-1-stimulated experimental sample spectrum. The spectra of the background-subtracted experimental samples before and after SDF-1 stimulation were then overlayed and offset in order to detect FRET. To unambiguously identify FRET signals using CFP and YFP, the enhanced emission signal of YFP acceptor should occur together with a concomitant decrease in the CFP donor's emission signal. As discussed in the introduction, comparing the spectra at these two wavelengths and looking for reciprocal changes in emission intensity is particularly important when using the CFP/YFP fluorophor pair to investigate FRET.
3.3 Example
Fig. 6A shows data from a typical experiment. The grey line denotes the background-subtracted fluorescence emission spectrum of Jurkat T cells expressing both CXCR4-YFP and TCR-ζ-CFP before these cells have been treated with SDF-1. The background-subtracted spectrum shows a CFP emission peak centered at 475 nm and a smaller YFP emission peak centered at 528 nm. The black line denotes the background-subtracted fluorescence emission spectrum of the same cell sample following it's stimulation with 5 × 10−8 M SDF-1 for 20 min and analyzed again. Comparison of the two spectra indicates relative changes consistent with CXCR4-YFP - TCR-ζ-CFP FRET signals increasing following SDF-1 treatment: a decrease in the CFP emission peak because it is donating energy to YFP, and a consequent increase in the YFP emission peak.
4. Concluding Remarks
FRET experiments provide us with valuable insights into molecular dynamics, and can frequently be designed to examine this within living cellular systems. Combining different types of approaches, including FRET assays that employ different chromophores and that tag proteins of interest in different ways is the most reliable way to obtain conclusions about molecular interactions in the living cell. The addition of biochemical approaches, such as co-purification of proteins, can also be useful for confirming the presence of protein-protein complexes detected by FRET, and for examining the physiological significance of the interactions.
Reference List
- Alarcon B, Berkhout B, Breitmeyer J, Terhorst C. Assembly of the human T cell receptor-CD3 complex takes place in the endoplasmic reticulum and involves intermediary complexes between the CD3-γ, δ, ε core and single T cell receptor α or β chains. J. Biol. Chem. 1988;263:2953–2961. [PubMed] [Google Scholar]
- Batard P, Szollosi J, Luescher I, Cerottini J-C, MacDonald R, Romero P. The use of phycoerythrin and allophycocyanin for fluorescence resonance energy transfer analyzed by flow cytometry: advantages and limitations. Cytometry. 2002;48:97–105. doi: 10.1002/cyto.10106. [DOI] [PubMed] [Google Scholar]
- Chan FK-M, Sigel RM, Zacharias D, Swofford R, Holmes KL, Tsien RY. Fluorescence resonance energy transfer analysis of cell surface receptor interactions and signaling using spectral variants of the green fluorescent protein. Cytometry. 2001;44:361–368. doi: 10.1002/1097-0320(20010801)44:4<361::aid-cyto1128>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Ciruela F. Fluorescence-based methods in the study of protein-protein interactions in living cells. Curr. Opin. Biotechnol. 2008;19:1–6. doi: 10.1016/j.copbio.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Forster T. Zwischenmolekulare energiewanderung and fluoreszenz. Ann. Physik. 1948;2:55–75. [Google Scholar]
- Forster T. Delocalized excitation and excitation transfer. In: Sinanoglu O, editor. Modern Quantum Chemistry Part III: Action of Light and Organic Crystals. New York: Academic Press; 1965. pp. 92–137. [Google Scholar]
- Glazer AN, Chan C, Williams RC, Yeh SW, Clark JH. Kinetics of energy flow in the phycobilisome core. Science. 1985;230:1051–1053. doi: 10.1126/science.230.4729.1051. [DOI] [PubMed] [Google Scholar]
- He L, Bradrick TD, Karpova TS, Wu X, Fox MH, Fischer R, McNally JG, Knutson JR, Grammer AC, Lipsky PE. Flow cytometric measurement of fluorescence (Forster) resonance energy transfer from cyan fluorescent protein to yellow fluorescent protein using single-laser excitation at 458 nm. Cytometry. 2003a;53A:39–54. doi: 10.1002/cyto.a.10037. [DOI] [PubMed] [Google Scholar]
- He L, Olson DP, Wu X, Karpova TS, McNally JG, Lipsky PE. A flow cytometric method to detect protein-protein interaction in living cells by directly visualizing donor fluorophore quenching during CFP-YFP fluorescence resonance energy transfer (FRET) Cytometry. 2003b;55A:71–85. doi: 10.1002/cyto.a.10073. [DOI] [PubMed] [Google Scholar]
- He L, Wu X, Simone J, Hewgill D, Lipsky PE. Determination of tumor necrosis factor receptor-associated factor trimerization in living cells by CFP - YFP -mRFP FRET detected by flow cytometry. Nuc. Acids Res. 2005;33:e61. doi: 10.1093/nar/gni057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janetopoulos C, Jin T, Devreotes P. Receptor-mediated activation of heterotrimeric G proteins in living cells. Science. 2001;291:2408–2411. doi: 10.1126/science.1055835. [DOI] [PubMed] [Google Scholar]
- Jares-Erijman E, Jovrin TM. FRET imaging. Nature Biotechnol. 2003;21:1387–1395. doi: 10.1038/nbt896. [DOI] [PubMed] [Google Scholar]
- Kumar A, Humphreys TD, Kremer KN, Bramati PS, Bradfield L, Edgar CE, Hedin KE. CXCR4 physically associates with the T cell receptor to signal in T cells. Immunity. 2006;25:213–224. doi: 10.1016/j.immuni.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Lakowicz JR. Principles of fluorescence spectroscopic ruler. Annu. Rev. Biochem. 1999;47:819–846. doi: 10.1146/annurev.bi.47.070178.004131. [DOI] [PubMed] [Google Scholar]
- Shaner NC, Patterson GH, Davidson MW. Advances in fluorescent protein technology. J. Cell Sci. 2007;120:4247–4260. doi: 10.1242/jcs.005801. [DOI] [PubMed] [Google Scholar]
- Shaner NC, Steinbach PA, Tsien RY. A guide to choosing fluorescent proteins. Nat. Methods. 2005;2:905–909. doi: 10.1038/nmeth819. [DOI] [PubMed] [Google Scholar]
- Stryer L. Energy transfer: A spectroscopic ruler. Proc. Natl. Acad. Sci. U. S. A. 1967;58:719–726. doi: 10.1073/pnas.58.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L. Fluorescence energy transfer as a spectroscopic ruler. Annu. Rev. Biochem. 1978;47:819–846. doi: 10.1146/annurev.bi.47.070178.004131. [DOI] [PubMed] [Google Scholar]
- Tertoolen LGJ, Blanchetot C, Jiang G, Overvoorde J, Gadella TWJ, Jr, Hunter T, den Hertog J. Dimerization of receptor protein-tyrosine phosphatase alpha in living cells. BMC Cell Biol. 2001;2:8. doi: 10.1186/1471-2121-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien RY. The green fluorescent protein. Annu. Rev. Biochem. 1998;67:509–544. doi: 10.1146/annurev.biochem.67.1.509. [DOI] [PubMed] [Google Scholar]
- Vamosi G, Samjanovich S, Szollosi J. Dissecting interacting molecular populations by FRET. Cytometry. 2008;73A:681–684. doi: 10.1002/cyto.a.20601. [DOI] [PubMed] [Google Scholar]
- Vilardaga JP, Nikolaev O. Monitoring receptor signaling by intramolecular FRET. Curr. Opin. Pharmacol. 2007;7:547–553. doi: 10.1016/j.coph.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Viskari PJ, Colyer CL. Separation and quantitation of phycobiliproteins using phytic acid in capillary electrophoresis with laser-induced fluorescence detection. J. Chromatography. 2001;972:269–276. doi: 10.1016/s0021-9673(02)01085-3. [DOI] [PubMed] [Google Scholar]
- Xia Z, Liu Y. Reliable and global measurement of fluorescence resonance energy transfer using fluorescence microscopes. Biophys. J. 2001;81:2395–2402. doi: 10.1016/S0006-3495(01)75886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]