Abstract
The phagocytic NADPH-oxidase [NOX] has been implicated in the generation of superoxides in the pancreatic β-cell. Herein, using normal rat islets and clonal INS 832/13 cells, we tested the hypothesis that activation of the small G-protein Rac1, which is a member of the NOX holoenzyme, is necessary for palmitate [PA]-induced generation of superoxides in pancreatic β-cells. Incubation of isolated β-cells with PA potently increased the NOX activity culminating in a significant increase in the generation of superoxides and lipid peroxides in these cells; such effects of PA were attenuated by diphenyleneiodonium [DPI], a known inhibitor of NOX. In addition, PA caused a transient, but significant activation [i.e., GTP-bound form] of Rac1 in these cells. NSC23766, a selective inhibitor of Rac1, but not Cdc42 or Rho activation, inhibited Rac1 activation and the generation of superoxides and lipid peroxides induced by PA. Fumonisin B-1 [FB-1], which inhibits de novo synthesis of ceramide [CER] from PA, also attenuated PA-induced superoxide and lipid peroxide generation and NOX activity implicating intracellularly generated CER in the metabolic effects of PA; such effects were also demonstrable in the presence of the cell-permeable C2-CER. Further, NSC23766 prevented C2-CER-induced Rac1 activation and production of superoxides and lipid peroxides. Lastly, C2-CER, but not its inactive analogue, significantly reduced the mitochondrial membrane potential, which was prevented to a large degree by NSC23766. Together, our findings suggest that Tiam1/Rac1 signaling pathway regulates PA-induced, CER-dependent superoxide generation and mitochondrial dysfunction in pancreatic β-cells.
Keywords: NADPH Oxidase, Rac1, Tiam1, palmitate, ceramide, oxidative stress, pancreatic β-cells
1. Introduction
Several lines of evidence from multiple laboratories suggests that chronic exposure of isolated β-cells to elevated saturated fatty acids [e.g., palmitic acid; PA] leads to a significant metabolic dysregulation and eventual demise of the β-cell [1–3]. Multiple mechanisms have been put forth to explain PA-induced metabolic defects; one of these include generation of intracellular oxidative stress [e.g., reactive oxygen species; ROS; 4–6], albeit recent studies by Moore et al [7] appear to argue against fatty acid-induced oxidative stress in the pancreatic β-cell. A signaling step involved in the increased generation of ROS and associated induction of intracellular oxidative stress in the pancreatic β-cell is the activation of the phagocytic NOX system, which is a highly regulated membrane-associated protein complex that catalyzes the one electron reduction of oxygen to superoxide anion involving oxidation of cytosolic NADPH. The phagocytic NOX is a multicomponent system comprised of membrane as well as cytosolic components. The membrane-associated catalytic core is a complex consisting of gp91phox, p22phox and the small G-protein Rap1. The cytosolic regulatory components include p47phox, p67phox and the small G-protein Rac1 [8–12]. Following stimulation, the cytosolic components of NADPH oxidase translocate to the membrane for association with the catalytic core for holoenzyme assembly. Available evidence also suggests that a protein kinase Cζ-sensitive phosphorylation of p47phox leads to its translocation to the membrane fraction [13]. It has also been shown that functional activation of Rac [i.e., GTP-Rac] is vital for the holoenzyme assembly and activation of NOX [14–16].
Several recent studies have demonstrated localization and functional activation of the NOX in clonal β-cells, normal rat islets and human islets under the duress of various stimuli including elevated levels of glucose, saturated fatty acids and proinflammatory cytokines [6, 17–19]. It has also been demonstrated that pharmacological inhibition of NOX by diphenyleneiodoinium chloride [DPI] or anti-sense oligonucleotides for p47phox markedly attenuated glucose-induced ROS production and oxidative stress, suggesting a critical involvement of NOX in the metabolic dysfunction induced by glucose [20]. These data implicate a significant contributory role for NOX in the metabolic dysfunction of the β-cell under conditions of oxidative stress [21–23].
Despite the aforementioned compelling lines of evidence, very little has been studied with regards to the potential contributory roles of small G-proteins [e.g., Rac1] in the cascade of events leading to PA-induced NOX-mediated superoxides generation in β-cells. Based on this reasoning, we undertook the current investigation to test our overall hypothesis that PA-induces generation of superoxides and lipid peroxides in INS 832/13 cells and rodent islets by increasing Rac1 activation, which represents one of the signaling events necessary for the functional regulation of the endogenous NOX holoenzyme assembly and its catalytic activity. Herein, we describe evidence to implicate NOX signaling pathway in the generation of superoxides and lipid peroxides in PA-mediated effects on isolated β-cells. We also present the first evidence to suggest a critical modulatory role for Tiam1, a guanine nucleotide exchange factor [GEF] for Rac1 [28], in this signaling pathway leading to the onset of mitochondrial dysfunction.
2. Materials and methods
2.1. Materials
C2-Ceramide, Dihydroceramide and NSC23766 were from Calbiochem [San Diego, CA]. Nitroblue tetrazolinium salt, malondialdehyde, thiobarbituric acid, diphenyleneiodonium chloride, butylated hydroxytoulene, oleic acid and palmitic acid were from Sigma [St. Louis, MO]. Antibodies directed against p47phox and actin were from Santa Cruz Biotechnology [Santa Cruz, CA]. Rac1 activation kit was purchased from Cytoskeleton Inc. [Denver, CO]. JC-1 assay kit was from Cell Technology Inc. [Mountain View, CA]. Palmitate stock solutions were prepared as we described in [24].
2.2. Insulin-secreting cells
INS 832/13 cells were provided by Dr. Chris Newgard [Duke University Medical Center, Durham, NC] and were cultured in RPMI 1640 medium containing 10 % heat-inactivated fetal bovine serum supplemented with 100 IU/ml penicillin and 100 IU/ml streptomycin, 1 mM sodium pyruvate, 50 µM 2-mercaptoethanol, and 10 mM HEPES [pH 7.4]. The medium was changed twice and cells were subcloned weekly. Islets were isolated from normal Sprague–Dawley rats using the collagenases digestion method described previously [24].
2.3. Quantitation of superoxide generation by nitroblue tetrazolium [NBT] assay
INS 832/13 cells were plated in six-well plates and grown to subconfluency and then treated with PA [100 µM], C2-CER [30 µM], FB-1 [10 µM], DPI [5 µM] or NSC23766 [20 µM] in different combinations as described in the text. The medium was then removed and the cells were washed once with PBS and further incubated with 0.25 % NBT for 30 min at 37 °C. Cells were then harvested and pelleted by low-speed centrifugation. The resulting pellet was resuspended in 50 % acetic acid. The reduced NBT formazan product was quantified by measuring the absorbance at 510 nm using Beckman DU640 spectrophotometer.
2.4. Quantitation of superoxide generation by malondialdehyde [MDA] assay
INS 832/13 cell lysates derived from control or treated cells [100 µg protein] were treated with 10 % trichloro acetic acid, 2 % butylated hydroxytoulene, and freshly prepared 0.67 % thiobarbituric acid. Following this, the samples were boiled for 15–20 min and then allowed to cool down at 4–8 °C for 15–20 min. The samples were then gently vortexed and centrifuged at 3500 rpm for 15 min. The resulting supernatant was used to measure the absorbance at 532 nm. A standard concentration curve was used to extrapolate MDA generated from various samples.
2.5. NOX assay
INS 832/13 cells were plated in six-well plates, grown to subconfluency and then treated with either diluent or PA [100 µM] or C2-CER [30 µM] for 6 hr. After treatment the medium was removed and the cells were washed once with PBS and further incubated with 5 µM of 2',7'-dichlorodihydrofluorescein diacetate [DCHFDA] for 30 min at 37 °C. Cells were then harvested and pelleted by low-speed centrifugation and the protein content of the pellet was determined using Bradford’s assay. Following to this, equal amount of proteins were taken and fluorescence in each condition was recorded [excitation − 485 nm and emission − 530 nm]. The amount of fluorescence recorded is directly correlated to the amount of superoxide radicals generated due to NOX activity.
2.6. Rac1 activation assay
INS 832/13 cells were treated with either diluent or NSC23766 [20 µM] or C2-CER or PA or oleate. Before treatment, cells were incubated overnight with NSC23766 in a low serum - low glucose containing medium. Cells were further incubated with PA or C2-CER as indicated in the text in the continuous presence of either NSC23766 or diluent. Lysates [~500 µg protein] were clarified by centrifugation for 5 min at 4800×g, and PAK-PBD [p21-activated kinase-binding domain] beads [20 µl] were added to the supernatant. The mixture was then rotated for 1 hr at 4 °C and pelleted by centrifugation at 4,000×g for 3 min. The resulting pellet was washed once with lysis buffer followed by a rinse [3x] in wash buffer [25 mM Tris, pH 7.5, 30 mM MgCl2, 40 mM NaCl, and 150 mM EDTA]. Proteins in the pellet were resolved by SDS-PAGE and transferred onto a nitrocellulose membrane, and Western blotting method determined the relative abundance of activated Rac1.
2.7. Assessment of mitochondrial dysfunction by JC-1 assay
Loss of mitochondrial membrane potential in cells has been estimated using JC-1 assay kit. Briefly, INS 832/13 cells were grown at 80 % confluency on the cover slips and were incubated with and without NSC23766 [20 µM] overnight in low serum – low glucose media. Cells were then treated with C2-CER [30 µM] or DHC [30 µM] for 6 hr with or without NSC23766. At the end of incubation, cells were washed twice with assay buffer (provided with the kit) and were further incubated for 15 min with JC-1 dye [1X]. Cells were then washed twice with assay buffer and the cover slips were mounted onto a glass slide and images were taken at 40X magnification using Olympus IX71 microscope [Olympus America Inc., Center Valley, PA]
2.8. Other assays
Protein concentrations were determined by Bradford’s dye-binding method using bovine serum albumin as the standard. Statistical significance of differences between diluent and experimental groups was determined by Student’s t test and ANOVA analysis. p < 0.05 was considered significant.
3. Results
3.1. PA induces generation of lipid peroxides and ROS in insulin-secreting cells
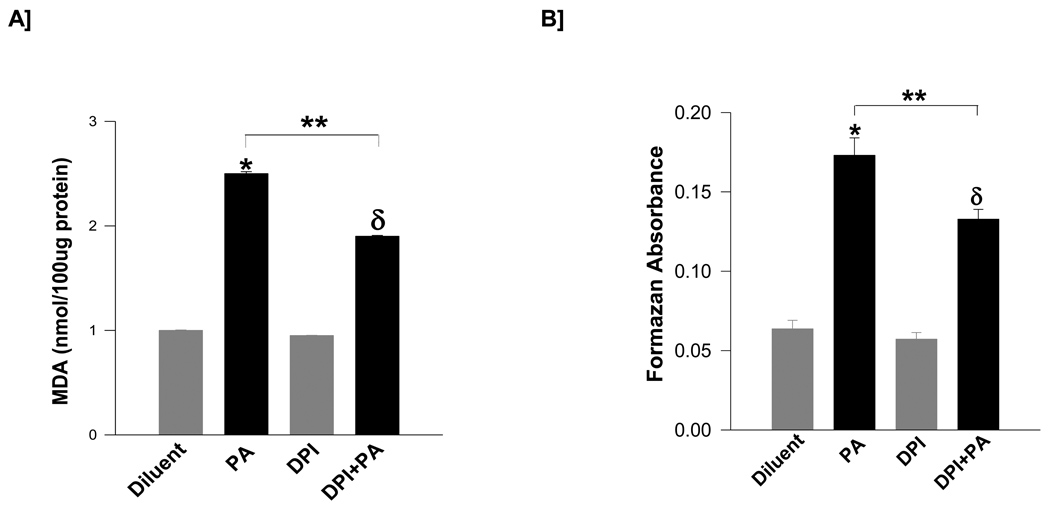
At the outset, we determined if exposure of isolated β-cells to PA results in the generation of superoxides and lipid peroxides. Data shown in Figure 1 suggests that incubation of INS 832/13 cells with PA [100 µM; 6 hr] significantly increased lipid peroxide levels [~2.5-fold; expressed as MDA equivalents; Panel A] and ROS levels [~2.7-fold; Panel B]. Furthermore, coprovision of DPI, a known inhibitor of NOX attenuated the PA-induced lipid peroxide levels [~37 %] and ROS generation [~31 %]. Together, these data suggest that PA-mediated generation of lipid peroxides and ROS in isolated β-cells may, in part, be due to activation of NOX.
Figure 1. PA induces generation of lipid peroxides and superoxides in INS832/13 cells: Protection by DPI.
INS 832/13 cells were incubated [6 hr] with either diluent or PA [100 µM] and/or DPI [5 µM] as indicated in the figure. Lipid hydroperoxide levels were measured as MDA equivalents [Panel A] and superoxide levels [Panel B] were quantitated as formazan equivalents. Data are mean ± SEM from three independent determinations. Values were considered significant at p < 0.05. * represents significant effect of PA to diluent; δ represents significance between DPI and DPI+PA; ** denotes significance between PA and DPI+PA.
3.2. PA induces activation of NOX in pancreatic β-cells
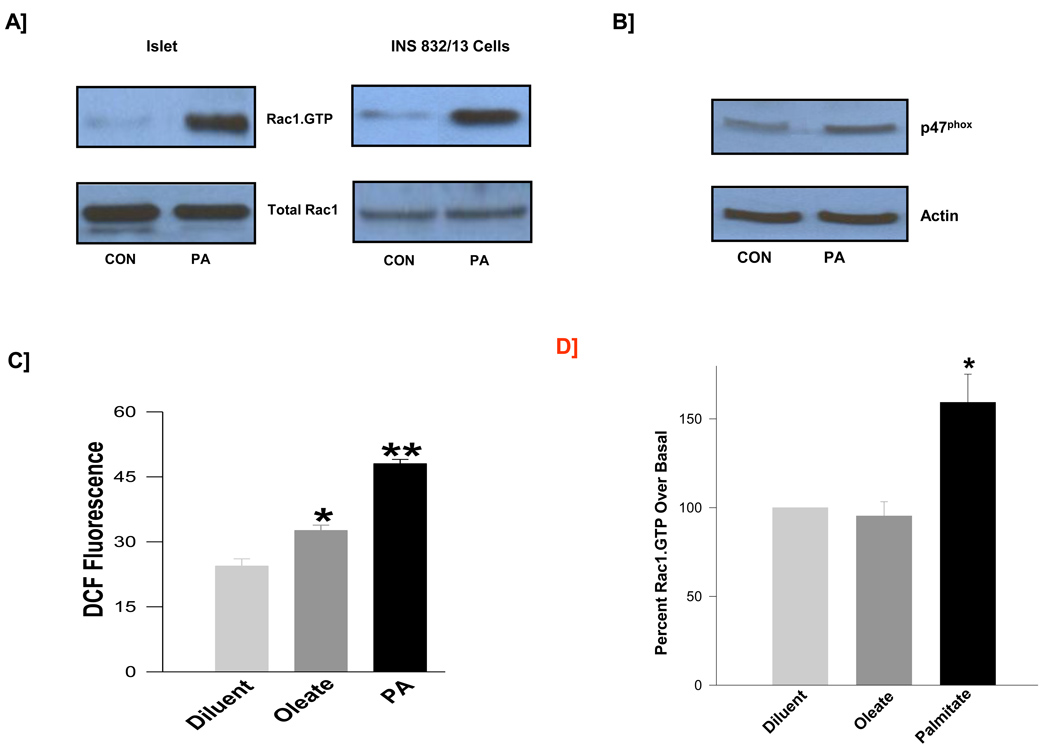
Data described above prompted us to further investigate potential regulation of NOX activity by PA in insulin-secreting cells. As stated above, p47phox represents one of the subunits of the NOX holoenzyme which is subjected to regulation in cells under the duress of oxidative stress. It has been shown that small G-protein Rac1, also a member of the NOX assembly, is also activated under conditions of oxidative stress leading to activation of NOX activity. Data described in Figure 2 suggested that incubation of normal rat islets [Panel A; left] or INS 832/13 cells [Panel A; right] with PA significantly increased the activation [i.e., GTP-bound form] of Rac1 as determined by the PAK-pulldown assay [see Methods for additional details]. In addition, we observed a marked increase in the expression of p47 phox in these cells following exposure to PA [Figure 2; Panel B]. Together, data in Panels A and B suggests upregulation of expression and function of key components of NOX holoenzyme in cells exposed to PA. We next quantitated the NOX activity to determine if PA-induced activation of Rac1 [Panel A] and p47phox expression [Panel B] culminates in the functional activation of the enzyme. Indeed, findings described in Figure 2; Panel C suggested a significant increase [~97 %] in the catalytic activation of NOX in cells treated with PA. It should be noted that under these conditions, oleate exerted a modest effect on the NOX activity [Figure 2; Panel C] without significantly affecting Rac1 activation in INS 832/13 cells [Figure 2; Panel D]. Together, our findings suggest that PA, but not oleate, elicits stimulatory effects on Rac1 activation and NOX activity.
Figure 2. PA, but not oleate, induces Rac1 activation and NOX activation in β-cells.
Normal rat islets and INS 832/13 cells were treated with diluent or PA [100 µM; Panel A]. The relative amounts of activated Rac1 [i.e., Rac1-GTP] were determined from these lysates by PAK-PBD pull down assay. Data are representative of two independent experiments. Panel B: Lysates derived from INS 832/13 cells treated in the absence or presence of PA [100 µM] were separated by SDS-page, and probed for p47phox and actin expression. A representative blot from two independent experiments is shown here. Panel C: Lysates derived from INS 832/13 cells treated in the absence or presence of PA or oleate [100 µM each] were processed for NOX activity and was quantitated by the DCHFDA assay and are expressed as DCF fluorescence units. Data are mean ± SEM from two individual measurements for DCF fluorescence. *, ** p <0.05 vs. diluent. Panel D: INS 832/13 cells were treated with diluent and/or oleate [100 µM] or PA [100 µM] and the relative amounts of activated Rac1 were determined by PAK-PBD pull down assay. Data presented in here are densitometric analysis of the blots and are mean ± SEM from four independent experiments. * represents p < 0.05 vs. diluent.
3.3. Tiam1, a GEF for Rac1, is involved in PA-induced Rac1 activation and generation of superoxides and lipid peroxides in pancreatic β-cells
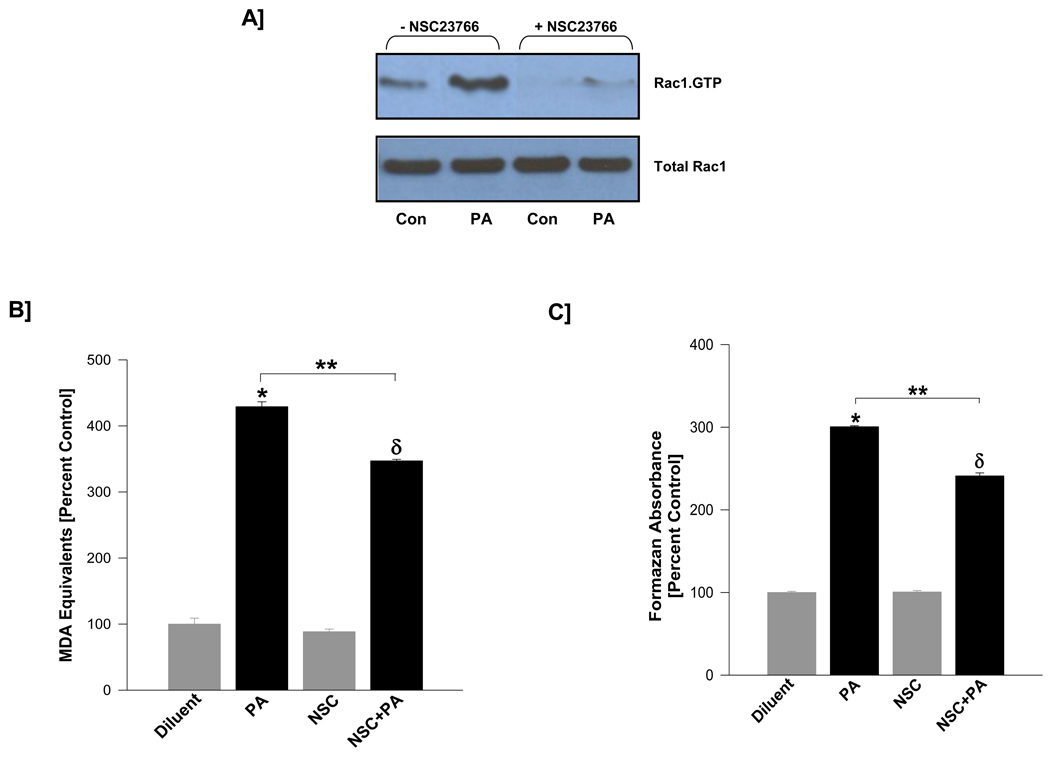
It has been demonstrated in many cells types, and more recently in pancreatic β-cells, that Rac1 activation is mediated by GEFs, such as Tiam1 [25, 26]. Recent studies from our laboratory have provided immunological evidence for Tiam1 in insulin-secreting cells, and further indicated that NSC23766, a specific inhibitor of Tiam1, specifically inhibits GTP loading onto Rac1, but not Cdc42 and Rho [25]. Therefore, we investigated if pre-treatment of isolated β-cells to NSC23766 prevents PA-induced Rac1 activation and associated increase in the generation of superoxides and lipid peroxides. Data shown in Figure 3 [Panel A] demonstrated a near complete inhibition of PA-induced Rac1 activation by NSC23766 suggesting potential requirement for Tiam1 in PA-induced Rac1 activation. Furthermore, we observed that PA-induced generation of lipid peroxides [Panel B] and reactive oxygen species [Panel C] in INS 832/13 cells were also reduced [~20 to 30 %] following inhibition of Tiam1-mediated activation of Rac1. Together, these data implicate a novel regulatory role(s) for Tiam1/Rac1 signaling step(s) in PA-mediated generation of superoxides and lipid peroxides in isolated β-cells.
Figure 3. NSC23766, a specific inhibitor of Tiam1-mediated activation of Rac1, markedly attenuates PA-induced Rac1 activation in INS 832/13 cells.
INS 832/13 cells were incubated overnight with either diluent or NSC23766 [20 µM]. The cells were further incubated [3 hr] in the presence of either low glucose [5 mM] or PA [100 µM] in the continuous presence of NSC23766 or diluent. The degree of Rac1 activation was determined by PAK-PBD pull down assay. Panel A: Data are representative of two independent experiments. Levels of lipid hydroperoxides [Panel B] or ROS [Panel C] generated in PA or diluent-treated INS 832/13 cells in the absence or presence were measured as MDA equivalents or formazan equivalents, respectively. Data are mean ± SEM from three determinations. Values were considered significant at p < 0.05. * represents significant effect of PA to diluent; δ represents significance between NSC and NSC+PA; ** denotes significance between PA and NSC+PA.
3.4. PA-induced generation of lipid peroxides and superoxides may, in part, be due to intracellular generation of CER via the de novo pathway
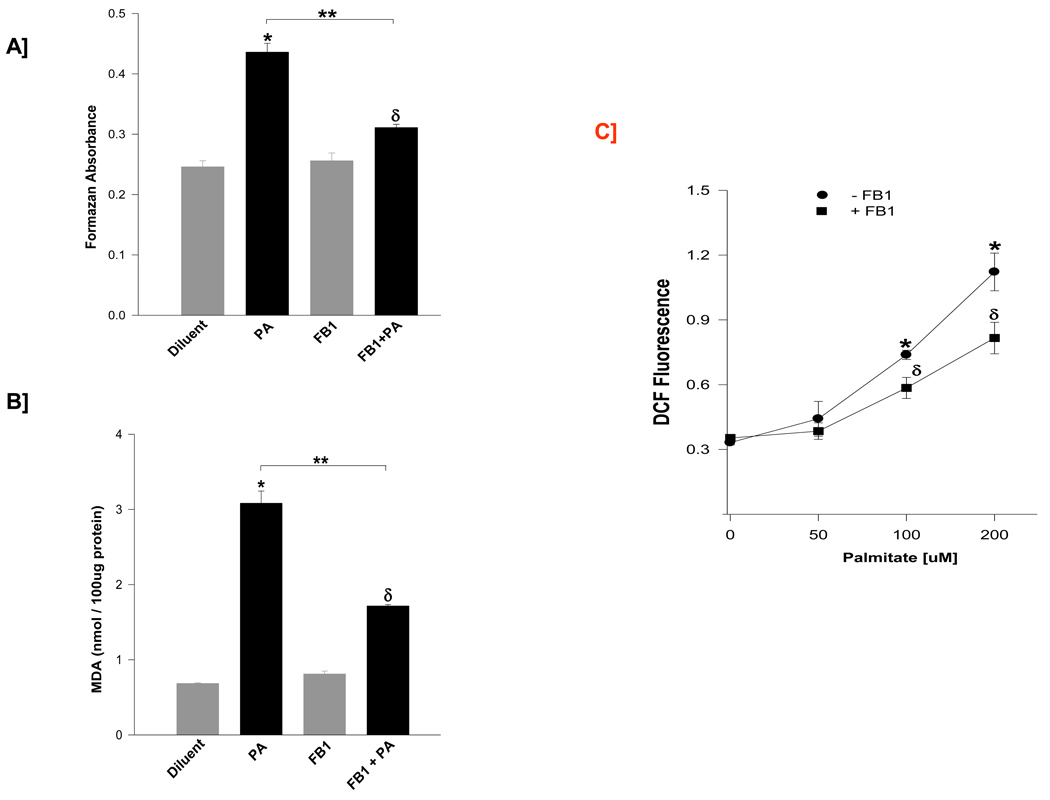
Since PA is the precursor for the de novo biosynthesis of CER, in the next series of studies we investigated potential roles of intracellularly generated CER in aforementioned PA-induced effects on isolated β-cells. To address this, we quantitated PA-induced generation of reactive oxygen species and lipid peroxides in cells pre-treated in the absence or presence of FB-1, a known inhibitor of de novo biosynthesis of CER from PA [28], incubation of isolated β-cells with 100 µM PA in presence of FB-1 significantly reduced PA-induced generation of ROS [~72 %; Panel A] and lipid peroxides [~62 %; Panel B] without significantly affecting these parameters in cells incubated with the diluent.
We next quantitated PA-induced effects on NOX activity as a function of period of incubation and the concentration of PA. Data in Table 1 indicated that PA elicited significant stimulatory effects on NOX activity. Maximal effects were seen between 3–6 hr of incubation. Interestingly, PA effects were not seen beyond 6 hr time point as the NOX activity fell even below the control values. In addition, pre-incubation of these cells with FB-1 resulted in a significant inhibition in NOX activity at 6 hr time point suggesting potential regulation of NOX activity by intracellularly generated CER [Table 1]. We next quantitated NOX activity in these cells as a function of PA concentration [0–200 µM] in the absence or presence of FB-1. Data in Figure 4 [Panel C] suggested a concentration-dependent activation of NOX by PA. Further, we noticed a significant inhibition of PA-induced NOX activity by FB-1. Together, these data suggest that PA-induced effects on lipid and superoxide levels and NOX activity may, in part, be due to the intracellularly generated CER.
Table 1.
Time-dependent effects of PA on NOX activity in INS 832/13 cells: Potential roles for ceramide
| Time (hr) | 0 | 3 | 6 | 12 | 24 |
|---|---|---|---|---|---|
| Treatment | |||||
| PA | 100 ± 3.11δ | 157 ± 5.73 * δ | 145 ±2.99* | 72 ± 1.41 * δ | 62 ± 5.06 * δ |
| PA + FB1 | 109 ± 2.27δ | 156 ± 3.70δ | 113 ±1.84 † | 65 ± 4.64 δ | 60 ± 9.02 δ |
INS 832/13 cells were treated with PA [100 µM] in the absence or presence of FB-1 [10 µM] for different time intervals as indicated. NOX activity was quantitated by the DCHFDA assay. Data are expressed as DCF fluorescence units and are mean ± SEM from three determinations.
represents p < 0.05 between PA induced ROS vs. control (0 hr).
represents p < 0.05 between PA induced ROS in the presence of FB1 vs. PA alone.
represents no significant difference between the two treatment groups.
Figure 4. Fumonisin B-1, an inhibitor of de novo biosynthesis of CER from PA, markedly reduces PA-induced generation of lipid peroxides and superoxides in INS 832/13 cells.
INS 832/13 cells were pre-treated in the presence or absence of FB-1 [10 µM] prior to the addition of PA [100 µM] and lysates derived from these cells were assessed for generation of superoxides and lipid peroxides. Superoxide generation was quantitated by NBT method and expressed as formazan equivalents [Panel A]. Lipid peroxide levels were quantitated by the MDA assay, and expressed as nmoles of MDA formed /100 µg protein [Panel B]. Data are mean ± SEM from three determinations. Values were considered significant at p < 0.05. * represents significant effect of PA to diluent; δ represents significance between FB1 and FB1+PA; ** denotes significance between PA and FB1+PA. Furthermore, cells were pre-treated in the presence or absence of FB1 [10 µM] prior to the addition of PA at different concentrations [0 –200 µM]. Lysates derived were processed for NOX activity and was quantitated by the DCHFDA assay [Panel C] and are expressed as DCF fluorescence. Data are mean ± SEM from three determinations. Graph with different symbols are statistically significant at p < 0.001. * represents PA-induced ROS vs. diluent; δ represents PA+FB1 induced ROS vs. FB1.
3.5. A cell-permeable analog of CER mimics PA effects in isolated β-cells
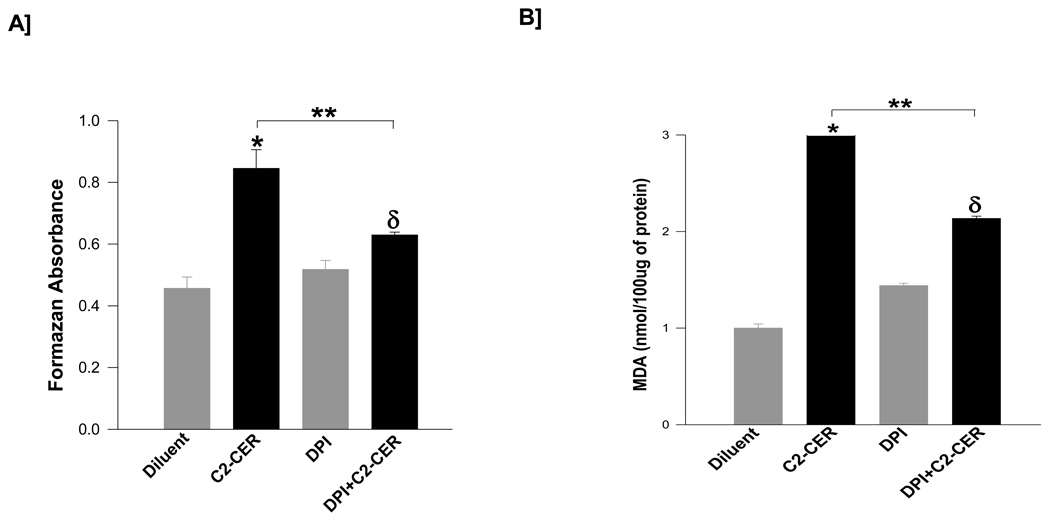
We next investigated if coprovision of a cell-permeable CER [e.g., C2-CER] mimics PA-induced oxidative stress in INS 832/13 cells, and if such an increase is mediated via activation of endogenous NOX. To address this, INS 832/13 cells were incubated with diluent or C2-CER, which has been effectively used to determine CER-induced metabolic dysfunction in isolated β-cells [27, 28] in the absence or presence of DPI to inhibit endogenous NOX. Data described in Figure 5 showed a marked reduction in C2-CER-induced ROS levels [~71 %; Panel A] or lipid peroxides [~69 %; Panel B] in cells exposed to DPI. It should be noted that DPI exerted a modest increase in the generation of lipid peroxides in the absence of C2-CER without significantly affecting the basal superoxide generation [Panels A and B; lanes 1 vs. 3]. Taken together, these findings implicate NOX activity in C2-CER-induced generation of ROS and lipid peroxides in pancreatic β-cells.
Figure 5. C2-CER promotes generation of lipid peroxides and ROS in INS 832/13 cells by activating endogenous NADPH oxidase activity.
INS 832/13 cells were treated with either diluent or C2-CER [30 µM] and/or DPI [5 µM] in various combinations as indicated in the figure. The degree of ROS generation was quantitated by the NBT method and is expressed as formazan equivalents [Panel A]. The amount of lipid hydroperoxide generation was quantitated by the MDA assay and is expressed as MDA equivalents [Panel B]. Data are mean ± SEM from three determinations in each case. Values were considered significant at p < 0.05. * represents significant effect of C2-CER vs. diluent; δ represents significance between DPI and DPI+C2-CER; ** denotes significance between C2-CER and DPI+C2-CER.
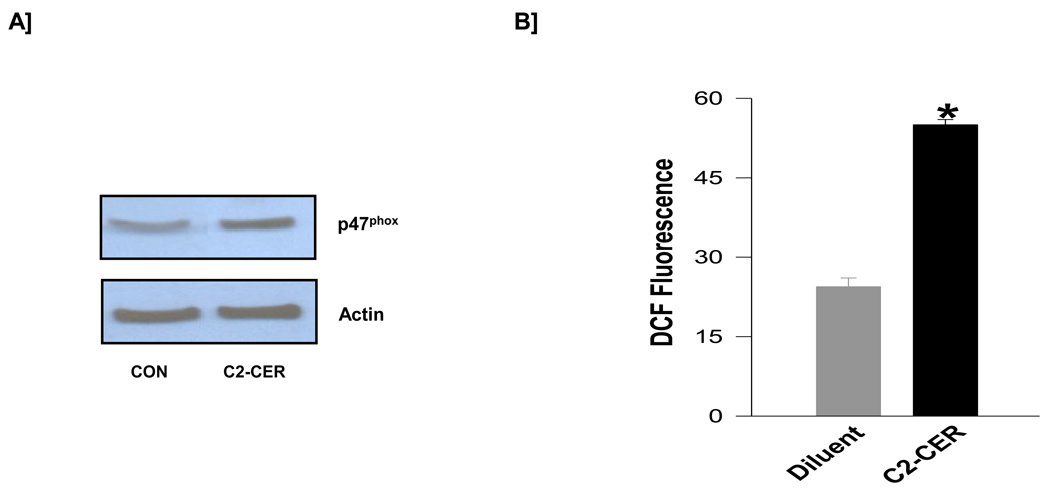
3.6. C2-CER mimics PA effects in inducing p47phox expression and NOX activity in isolated β-cells
As a logical extension to the studies described in Figure 5, we examined if C2-CER induces p47phoxexpression and NOX activity in pancreatic β-cells. Data in Figure 6 [Panel A] shows that incubation of INS 832/13 cells with C2-CER significantly increased p47phox expression. Moreover, in a manner akin to PA, C2-CER increased [more than 2-fold] the NOX activity in INS 832/13 cells [Figure 6; Panel B]. Together, these data in Figures 5 and 6 demonstrate that a cell-permeable analog of C2-CER mimics the effects of PA on isolated β-cells by increasing the NOX activity.
Figure 6. C2-CER increases the expression of p47phox and NOX activity in INS 832/13 cells.
INS 832/13 cells were treated with either diluent or C2-CER [30 µM] and examined for relative increases in p47phox expression and NADPH oxidase activity. Panel A: Lysate proteins derived from diluent or C2-CER-treated cells were separated by SDS-page and probed for p47phox and actin expression. A representative blot from two independent experiments is shown here. Panel B: NOX activity in diluent or C2-CER-treated cells was quantitated by the DCHFDA fluorescence assay and is expressed as DCF fluorescence. Data are mean ± SEM from two independent determinations. * represents p <0.05 vs. diluent.
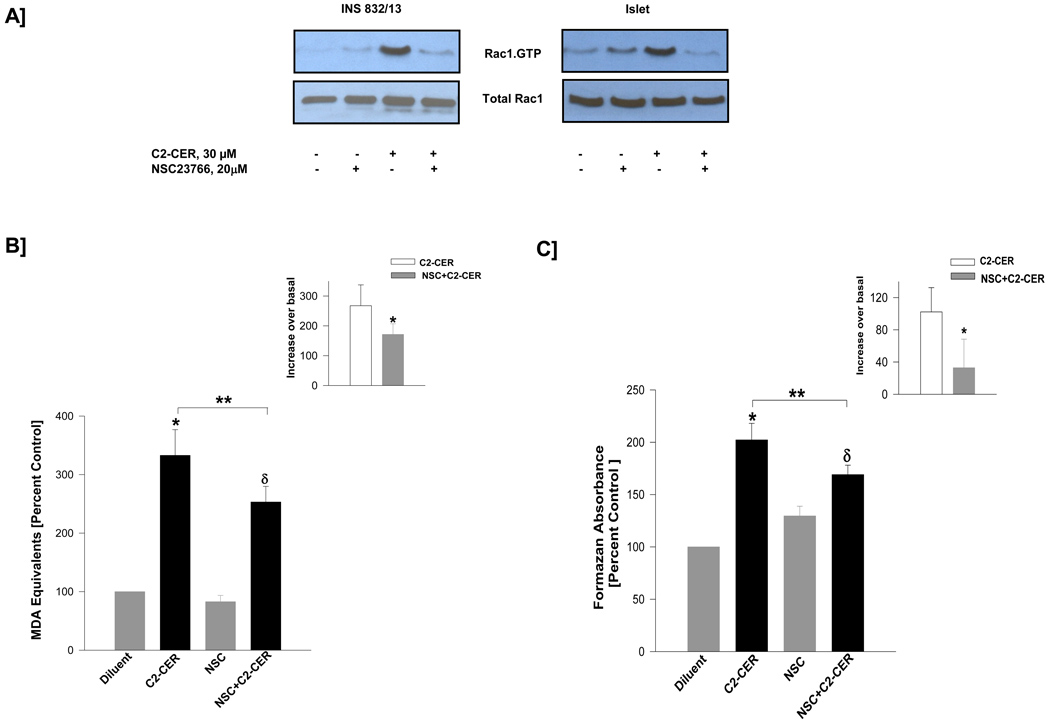
3.7. C2-CER-induced generation of superoxides and lipid peroxides are mediated by the Tiam1/Rac1 signaling pathway
Herein, we examined the possible involvement of Tiam1/Rac1 signaling cascade in C2-CER-induced oxidative stress in β-cells. Data shown in Figure 7 suggested a significant activation of Rac1 by C2-CER in INS 832/13 cells [Panel A; left] and normal rat islets [Panel A; right]. Moreover, coprovision of NSC23766 substantially inhibited C2-CER-induced Rac1 activation in both cell types. These data clearly suggest that C2-CER-induced effects on isolated β-cells may, in part, be due to activation of a Rac1-dependent signaling mechanism. Furthermore, we noticed that C2-CER-induced generation of lipid peroxides [Figure 7; Panel B] or superoxides [Figure 7; Panel C] were reduced [~27–60 %] by NSC23766, thus suggesting novel regulation of CER-mediated effects by a Tiam1/Rac1-dependent signaling mechanism [see below].
Figure 7. NSC23766 inhibits C2-CER-induced Rac1 activation and generation of lipid peroxides and superoxides in pancreatic β-cells.
INS 832/13 cells and rat islet were treated with either diluent or NSC23766 [20 µM] and cultured overnight in low glucose / low serum media. Cells were further incubated in the presence of C2-CER [30 µM] for 30 min in INS 832/13 cells and 3 hr in Islet in the continuous presence of NSC23766 or diluent. The relative amounts of activated Rac1 [i.e., Rac1-GTP] were determined by PAK-PBD pull down assay. Data are representative of two independent experiments [Panel A]. Panel B: INS 832/13 cells were incubated [6 hr] with either diluent or with C2-CER [30 µM] or NSC23766 [20 µM; alone or in combination]. Lipid hydroperoxides were measured as MDA equivalents and plotted as increase over basal. Panel C: INS 832/13 cells were incubated [6 hr] with either diluent or with C2-CER [30 µM] or NSC23766 [20 µM; alone or in combination as indicated in the figure]. Superoxide generation was measured as formazan equivalents and plotted as increase over basal. Data in the insets represents incremental response to C2-CER in the absence or presence of NSC23766. Data are mean ± SEM from three determinations in each case. Values were considered significant at p < 0.05. * represents significant effect of C2-CER vs. diluent; δ represents significance between NSC and NSC+C2-CER; ** denotes significance between C2-CER and NSC+C2-CER.
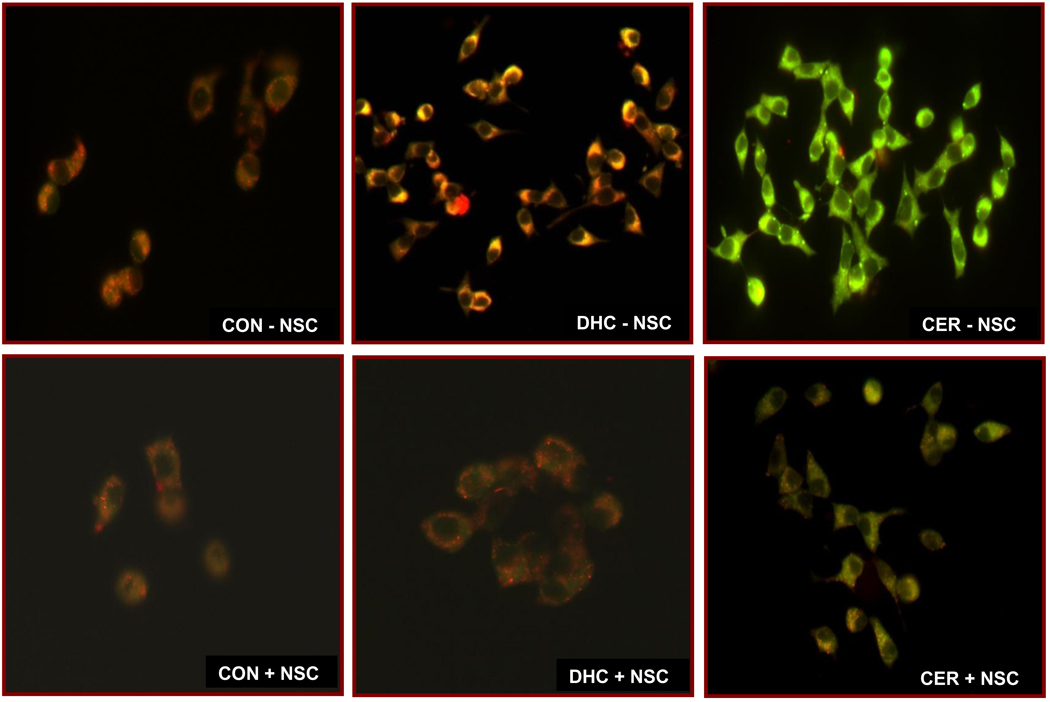
3.8. C2-CER, but not its inactive analogue, promotes mitochondrial dysfunction in INS 832/13 cells in a Tiam1/Rac1 signaling pathway
We have recently reported that exposure of isolated β-cells to C2-CER results in significant abnormalities in mitochondrial function including loss in membrane potential and leakage of cytochrome-C into the cytosolic compartment [28]. Therefore, in the last set of experiments we verified if Tiam1/Rac1 signaling step might underlie the CER-induced mitochondrial dysfunction in INS 832/13 cells. To address this, mitochondrial membrane potential [MMP] was quantitated by the JC-1 staining method in cells exposed to diluent or C2-CER in the absence or presence of NSC23766. To determine the specificity of CER effects, we also utilized Dihydroceramide [DHC], an inactive analogue of CER, on MMP in INS 832/13 cells. Data in Figure 8 indicated that exposure of these cells to C2-CER [lower left panel], but not DHC [middle left panel] significantly lowered the MMP as evidenced by staining of the majority of cells in green due to reduced MMP. Furthermore, NSC23766 prevented C2-CER-induced loss in membrane potential [as evidenced by a strong J-aggregation; red color] in these cells, further supporting the hypothesis that Tiam1/Rac1 signaling pathway contributes to CER-induced metabolic dysfunction in the pancreatic β-cell.
Figure 8. NSC23766 inhibits C2-CER-induced mitochondrial dysfunction in pancreatic β-cells.
INS 832/13 cells were treated with either diluent or NSC23766 [20 µM] and cultured overnight in low glucose and low serum media. Cells were further incubated in the presence of C2-CER [30 µM] and / or DHC [30 µM] for 6 hr in the continuous presence of NSC23766 or diluent. Mitochondrial dysfunction was determined by JC-1 assay. Data are representative of two independent experiments.
4. Discussion
One of the main objectives of this study was to test the hypothesis that activation of the Tiam1/ Rac1 signaling pathway is necessary for PA-mediated generation of superoxides and lipid peroxides in isolated β-cells. The salient findings of our study are: [i] exposure of isolated β-cells to PA leads to the generation of reactive oxygen species and lipid peroxides, which may, in part, be due to increased NOX activity; [ii] PA-induced effects on NOX activity are largely due to intracellularly generated CER from the de novo biosynthetic pathway; [iii] PA-induced, CER-mediated activation of NOX and the resultant increase in intracellular oxidative stress require activation of Rac1; [iv] PA-induced, CER-sensitive activation of Rac1 requires the intermediacy of Tiam1, a GEF for Rac1; and [v] inhibition of Tiam1/Rac1 signaling pathway leads to restoration of mitochondrial membrane potential. Together, our data provide the first evidence for Tiam1/Rac1 signaling pathway in PA-induced, CER-mediated increase in the oxidative environment in INS 832/13 cells and normal rodent islets.
As stated above, our current findings implicate the involvement of Tiam1 in PA- or C2-CER-induced activation of Rac1. In the context of potential regulation of Rac1, multiple GEFs have been identified in other cell types. These constitute the diffuse B cell lymphoma [Dbl] family of GEFs, including Trio and Tiam1. Recently, Zheng et al have developed NSC23766, which is a soluble first generation small molecule inhibitor of Tiam1-mediated activation of Rac1 [29]. These investigators have reported significant inhibition of Rac1-GTP-loading by NSC23766 without significantly affecting the GTP-loading onto other small G-proteins including Cdc42 and Rho A. Under these conditions, NSC23766 also attenuated cell proliferation induced by Tiam1, which is a Rac1-specific GEF. Based on these data, Zheng et al concluded that NSC23766 represents a specific inhibitor of Tiam1-mediated activation of Rac1. Several other laboratories have utilized NSC23766 since then to decipher the potential contributory roles for Tiam1/Rac1 signaling pathway in cellular functions [25, 30 and references therein]. Recently, we have confirmed the selectivity of NSC23766-mediated inhibition of Rac1 activation in insulin-secreting cells [25]. In the present study, we demonstrated that NSC23766 not only attenuated PA or C2-CER-induced Rac1 activation, but also markedly reduced PA or C2-CER-induced generation of superoxides and lipid peroxides, implicating novel regulatory roles for Tiam1/Rac1 signaling pathway in the activation of phagocytic-like NOX in β-cells. Using molecular biological approaches Yi F and Chen et al [31] have recently demonstrated roles of Vav2, another GEF for Rac1, in homocysteine-induced Rac1/NOX activation in mesangial cells.
Several recent studies have demonstrated regulatory roles of Rac1 in high glucose-induced metabolic dysregulation and cell death. For example, Shen et al [30] have recently reported a significant increase in cardiomyocyte apoptosis under hyperglycemic conditions. Using cultured myocytes, these investigators demonstrated a significant upregulation of Rac1 and NOX activity which was attenuated in cells overexpressing a dominant negative mutant of Rac1. Moreover, treatment of diabetic animals with NSC23766 significantly reduced NOX activity and cell demise followed by restoration of myocardial function [30]. These findings further support the involvement of Tiam1/Rac1 signaling pathway in hyperglycemia-induced metabolic dysfunction and demise of myocytes. It may be germane to point out that unpublished observations from our laboratory have suggested similar regulatory roles of Rac1 in high glucose-induced activation of NOX activation and the associated increase in oxidative stress in INS 832/13 cells and normal rat islets [Syed and Kowluru, unpublished].
Along these lines, studies by Cacicedo and coworkers in cultured retinal pericytes have demonstrated a role for NOX in PA-induced apoptosis [32]. A significant increase in NOX activity, oxidative stress and caspase-3 activity was demonstrable in cells exposed to PA. Overexpression of dominant negative mutants of p67phox and Rac1 [N17Rac1] markedly inhibited the increase in caspase-3 activation. Furthermore, overexpression of an active mutant of Rac1 [V12Rac1] increased caspase-3 activity suggesting that constitutive activation of Rac1 results in NOX activation culminating in the generation of oxidative stress and metabolic dysfunction in these cells.
Using FB-1, a specific inhibitor of de novo synthesis of CER from PA, we have demonstrated that PA-induced effects may, in part, be due to intracellularly generated CER. Data accrued in studies using C2-CER further support this postulation. Published evidence along these lines suggests that CER-mediated effects are indeed mediated via activation of Rac1 in many cell types. For example, using C2-CER, Kim and Kim have reported activation of c-fos serum response element via the Rac1 signaling pathway in Rat-2 fibroblasts [33]. Interestingly, using NIH 3T3 cells, Embade and coworkers have demonstrated novel relationships between FasL generation and CER production in Rac1-induced apoptosis [34]. In another study, Deshpande and coworkers [35] have demonstrated intermediacy of intracellularly generated CER in Rac1-induced mitochondrial oxidative stress and premature senescence in human umbellical vein endothelial cells. Together, these data appear to implicate CER/Rac1 signaling pathways in oxidative stress and metabolic dysfunction in multiple cells types. Therefore, based on these and other supporting evidence we presented in this study, we believe that PA effects on lipid peroxides, superoxides and NOX activity are specific and that they require the intermediacy of Tiam1/Rac1 signaling pathway. It should be noted that we also observed modest effects of oleate on NOX activity without significantly affecting the Rac1 activation [Figure 2] suggesting a clear distinction between the modes of action of these two fatty acids.
It is important to note that several recent studies have implicated physiological roles for a tonic increase in NOX activation and subsequent generation of reactive oxygen species in the stimulus-secretion coupling of glucose-stimulated insulin secretion [36]. Besides this, existing evidence in the literature clearly demonstrates increase in the insulin secretion by fatty acids under acute incubation conditions [17]. Therefore, one might ask the question if increase in Tiam1/Rac1 activation and NOX activation could contribute towards the physiological insulin secretion rather than inducing metabolic abnormalities in the isolated β-cell. While it appears likely, under specific experimental conditions, chronic activation of NOX by specific stimuli [e.g., high levels of glucose, fatty acids, CER or cytokines] leads to metabolic dysfunction and demise of the β-cell. For example, our recent observations [28] suggested significant abnormalities in mitochondrial function [i.e., loss in MMP] in cells exposed to C2-CER under acute conditions. Further, we have reported significant leakage of mitochondrial cytochrome C into the cytosolic compartment in C2-CER-treated cells [28]. Furthermore, it should be noted that our current observations [Figure 8] indicate that mitochondrial dysfunction, which is demonstrable in cells incubated with C2-CER, but not DHC, is prevented, to a large degree, by inhibition of Tiam1/Rac1 signaling pathway, further implicating the Tiam1-Rac1-NOX signaling pathway in the onset of metabolic dysfunction in the presence of C2-CER. Therefore, we speculate that early biochemical and cellular changes that we reported herein might be paving way to metabolic dysfunction and demise of the islet β-cell. It may be germane to point out that recent studies by Moore et al [7] have provided compelling evidence to argue against potential involvement of oxidative stress in fatty acid-induced metabolic dysfunction of the islet β-cell. It is, therefore, likely that additional regulatory mechanisms might underlie β-cell demise seen under the duress of lipotoxic conditions including those involving progressive alterations in the mitochondrial membrane permeability transition pore as suggested by recent studies of Koshkin et al [37] in MIN6 and INS-1 cells. Furthermore, PA-induced CER-mediated effects might also include regulation of key target proteins such as the CER-activated protein phosphatase 2A that we have characterized in isolated β-cells [27, 38], leading to the inactivation of key cellular events including inhibition of extracellular-regulated kinase and inhibition of proinsulin gene expression [39].
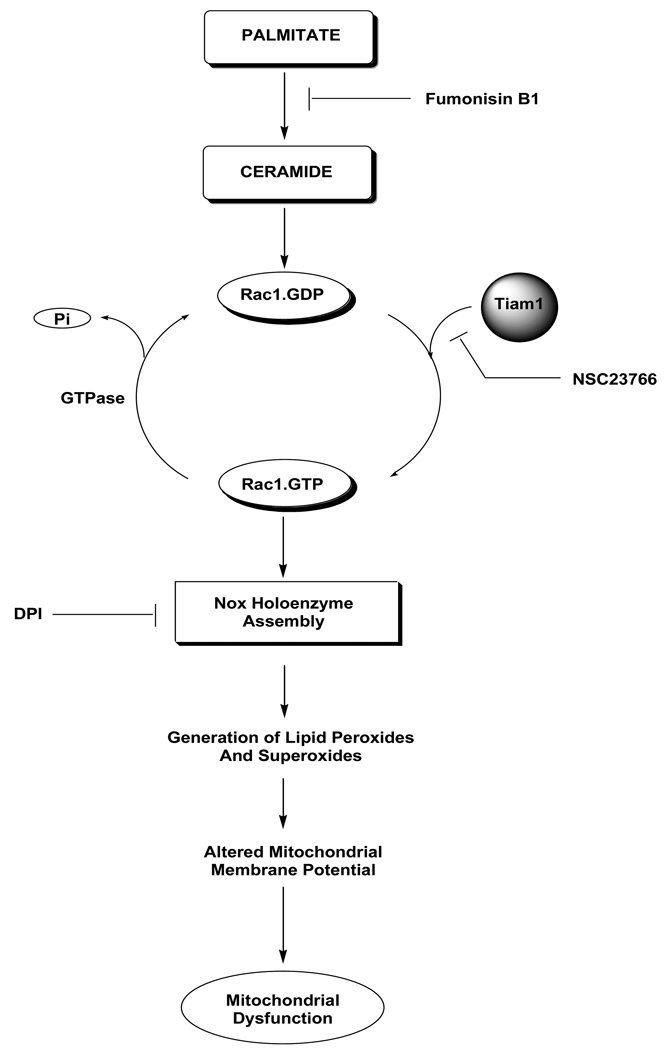
In conclusion, we present the first evidence for a novel role of Tiam1/Rac1 signaling pathway in PA- induced, CER-sensitive metabolic activation of NOX and associated production of superoxides and lipid peroxides in pancreatic β-cells. It is likely that Tiam1 could serve as a novel drug target for inhibition of generation of superoxides and lipid peroxides in isolated β-cells under lipotoxic conditions. Based on these data we propose a working model [Figure 9] to suggest that PA/CER increase the Rac1 activation [GTP-bound active form] and deactivation [GDP-bound inactive form] to generate signals that may be necessary for triggering cellular events leading to NOX activation, increased oxidative milieu, mitochondrial dysregulation in the pancreatic β-cell. It should be noted that while the proposed model principally addressed the roles of Tiam1-Rac1-NOX connection in PA/CER-mediated effects, relative contributory roles of other sources of reactive oxygen species, including the glutathione peroxidase, manganese-sensitive superoxide dismutase, catalase signaling cascades must also be recognized as key contributors to the mitochondrial dysfunction in isolated β-cells under the duress of lipotoxic conditions [1–3, 40]. However, additional studies are needed to further understand these signaling steps in the islet β-cell.
Figure 9. A model to implicate Tiam1/Rac1 signaling pathway in PA-induced, CER-mediated effects on isolated β-cells.
Based on the data accrued in the current studies we propose that incubation of isolated β-cells to either PA [but not oleate] or C2-CER [but not DHC] leads to Tiam1-mediated activation of Rac1, which is a key member of the NOX assembly. In addition, PA or C2-CER promotes the expression of p47phox in these cells. Together, these signaling steps promote the assembly and activation of NOX holoenzyme leading to the production of superoxides and lipid peroxides. These, in turn, exert damaging effects on the mitochondria including reduction in MMP leading to the onset of mitochondrial dysfunction. Appropriate sites of inhibition of these signaling steps by FB-1, NSC23766 and DPI are also indicated in the figure.
Acknowledgements
This research was supported by a Merit Review Award from the Department of Veterans Affairs, the National Institutes of Health [DK 74921], and the Grodman Cure Foundation. AK is also the recipient of the Senior Research Career Scientist Award from the Department of Veterans Affairs. The authors acknowledge the excellent technical assistance of Mr. Brandon Koch in these studies.
ABBREVIATIONS
- C2-CER
C2-Ceramide
- DCHFDA
2`, 7`- dichlorodihydrofluorescein diacetate
- DHC
Dihydroceramide
- DPI
Diphenyleneiodonium
- FB-1
Fumonisin B-1
- GEF
Guanine nucleotide exchange factor
- MDA
Malondialdehyde
- MMP
Mitochondrial membrane potential
- NBT
Nitroblue tetrazolium
- PA
Palmitic acid
- Rac1
Ras-related C3 botulinum toxin substrate 1
- ROS
Reactive oxygen species
- Tiam1
T-lymphoma invasion and metastasis 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of this work were presented in the 69th Annual Meetings of the American Diabetes Association Meetings held in New Orleans, USA in 2009 and the 2nd International Brussels Pancreatic islet Symposium held in Brussels, Belgium in 2009.
References
- 1.Newsholme P, Haber EP, Hirabara SM, et al. Diabetes associated cell stress and dysfunction: role of mitochondrial and non mitochondrial ROS production and activity. J Physiol. 2007;583:9–24. doi: 10.1113/jphysiol.2007.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes. 2003;52:1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Morgan D, Oliver-Emilio HR, Keane D, et al. Glucose, palmitic and pro-inflammatory cytokines modulate production and activity of a phagocyte-like NADPH oxidase in rat pancreatic islets and a clonal cell line. Diabetologia. 2007;50:359–369. doi: 10.1007/s00125-006-0462-6. [DOI] [PubMed] [Google Scholar]
- 5.Inoguchi T, Li P, Umeda F, et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–1945. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- 6.Piro S, Anello M, Di Pietro C, et al. Chronic exposure to free fatty acids or high glucose induces apoptosis in rat pancreatic islets: possible role of oxidative stress. Metabolism. 2002;51:1340–1347. doi: 10.1053/meta.2002.35200. [DOI] [PubMed] [Google Scholar]
- 7.Moore PC, Ugas MC, Hagman DK, et al. Evidence against the involvement of oxidative stress in fatty acid inhibition of insulin secretion. Diabetes. 2004;53(10):2610–2616. doi: 10.2337/diabetes.53.10.2610. [DOI] [PubMed] [Google Scholar]
- 8.Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 9.Newsholme P, Morgan D, Rebelato E, et al. Insights into the critical role of NADPH oxidase(s) in the normal and dysregulated pancreatic beta cell. Diabetologia. 2009;52:2489–2498. doi: 10.1007/s00125-009-1536-z. [DOI] [PubMed] [Google Scholar]
- 10.Geiszt M. NADPH oxidases: new kids on the block. Cardiovasc Res. 2006;71:289–299. doi: 10.1016/j.cardiores.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Borregaard N, Tauber AI. Subcellular localization of the human neutrophil NADPH oxidase b-cytochrome and associated flavoprotein. J Biol Chem. 1984;259:47–52. [PubMed] [Google Scholar]
- 12.Kowluru A. Small G proteins in islet β-cell function. Endocr Rev. 2010;31:52–78. doi: 10.1210/er.2009-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dang PM, Fontayne A, Hakim J, El Benna J, Perianin A. Protein kinase zeta phosphorylates a subset of selective sites of the NADPH oxidase component p47 phox and participates in formyl peptide-mediated neutrophil respiratory burst. J Immunol. 2001;166:1206–1213. doi: 10.4049/jimmunol.166.2.1206. [DOI] [PubMed] [Google Scholar]
- 14.Abo A, Pick E, Hall N, Totty N, Teahan CG, Segal AW. Activation of the NADPH oxidase involves the small-GTP binding protein p21rac1. Nature. 1991;353:668–670. doi: 10.1038/353668a0. [DOI] [PubMed] [Google Scholar]
- 15.Knaus UG, Heyworth PG, Evans T, Curnutte JT, Bokoch GM. Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac 2. Science. 1991;254:1512–1515. doi: 10.1126/science.1660188. [DOI] [PubMed] [Google Scholar]
- 16.Hordijk PL. Regulation of NADPH oxidases: the role of Rac proteins. Circ Res. 2006;98:453–462. doi: 10.1161/01.RES.0000204727.46710.5e. [DOI] [PubMed] [Google Scholar]
- 17.Newsholme P, Keane D, Welters HJ, Morgan NG. Life and death decisions of the pancreatic β-cell: the role of fatty acids. Clin Sci (Lond) 2007;112:27–42. doi: 10.1042/CS20060115. [DOI] [PubMed] [Google Scholar]
- 18.Oliver HR, Verlengia R, Carvalho CR, Britto LR, Curi R, Carpinelli AR. Pancreatic beta-cells express phagocyte-like NAD(P)H oxidase. Diabetes. 2003;52:1457–1463. doi: 10.2337/diabetes.52.6.1457. [DOI] [PubMed] [Google Scholar]
- 19.Uchizono Y, Takeva R, Iwase M, Sasaki N, Oku M, Imoto H, Iida M, Sumimoto H. Expression of isoforms of NADPH oxidase components in rat pancreatic islets. Life Sciences. 2006;80:133–139. doi: 10.1016/j.lfs.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 20.Morgan D, Rebelato E, Abdulkader F, et al. Association of NAD(P)H oxidase with glucose-induced insulin secretion by pancreatic beta-cells. Endocrinology. 2009;150:2197–2201. doi: 10.1210/en.2008-1149. [DOI] [PubMed] [Google Scholar]
- 21.Inoguchi T, Nawata H. NAD(P)H oxidase activation: a potential target mechanism for diabetes vascular complications, progressive beta-cell dysfunction and metabolic syndrome. Cur Drug Targets. 2005;6:495–501. doi: 10.2174/1389450054021927. [DOI] [PubMed] [Google Scholar]
- 22.Sawada F, Inoguchi T, Tsubouchi H, et al. Differential effect of sulfonylureas on production of reactive oxygen species and apoptosis in cultured pancreatic beta-cell line, MIN6. Metabolism. 2008;57:1038–1045. doi: 10.1016/j.metabol.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 23.Guichard C, Moreau R, Pessayre D, Epperson TK, Krause KH. NOX family NADPH oxidase in liver and in pancreatic islets: a role in the metabolic syndrome and diabetes? Biochem Soc Trans. 2008;36:920–929. doi: 10.1042/BST0360920. [DOI] [PubMed] [Google Scholar]
- 24.Veluthakal R, Suresh MV, Kowluru A. Down-regulation of expression and function of nucleoside diphosphate kinase in insulin-secreting beta-cells under in vitro conditions of glucolipotoxicity. Mol Cell Biochem. 2009;329:121–129. doi: 10.1007/s11010-009-0113-6. [DOI] [PubMed] [Google Scholar]
- 25.Veluthakal R, Madathilparambil SV, McDonald P, Olson LK, Kowluru A. Regulatory roles for Tiam1, a guanine nucleotide exchange factor for Rac1, in glucose-stimulated insulin secretion in pancreatic beta-cells. Biochem Pharmacol. 2009;77:101–113. doi: 10.1016/j.bcp.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cruz-Monserrate Z, O'Connor KL. Integrin alpha 6 beta 4 promotes migration, invasion through Tiam1 upregulation, and subsequent Rac activation. Neoplasia. 2008;10:408–417. doi: 10.1593/neo.07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kowluru A, Metz SA. Ceramide-activated protein phosphatase-2A activity in insulin-secreting cells. FEBS Lett. 1997;418:179–182. doi: 10.1016/s0014-5793(97)01379-3. [DOI] [PubMed] [Google Scholar]
- 28.Veluthakal R, Palanivel R, Zhao Y, McDonald P, Gruber S, Kowluru A. Ceramide induces mitochondrial abnormalities in insulin-secreting INS-1 cells: potential mechanisms underlying ceramide-mediated metabolic dysfunction of the beta cell. Apoptosis. 2005;10:841–850. doi: 10.1007/s10495-005-0431-4. [DOI] [PubMed] [Google Scholar]
- 29.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen E, Li Y, Li Y, et al. Rac1 is required for cardiomyocyte apoptosis during hyperglycemia. Diabetes. 2009;58:2386–2395. doi: 10.2337/db08-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yi F, Chen QZ, Jin S, Li PL. Mechanism of homocysteine-induced Rac1/NADPH oxidase activation in mesangial cells: role of guanine nucleotide exchange factor Vav2. Cell Physiol Biochem. 2007;20:909–918. doi: 10.1159/000110451. [DOI] [PubMed] [Google Scholar]
- 32.Cacicedo JM, Benjachareowong S, Chou E, Ruderman NB, Ido Y. Palmitate-induced apoptosis in cultured bovine retinal pericytes: roles of NAD(P)H oxidase, oxidant stress, and ceramide. Diabetes. 2005;54:1838–1845. doi: 10.2337/diabetes.54.6.1838. [DOI] [PubMed] [Google Scholar]
- 33.Kim BC, Kim JH. Exogenous C2-ceramide activates c-fos serum response element via Rac-dependent signalling pathway. Biochem J. 1998;330:1009–1014. doi: 10.1042/bj3301009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Embade N, Valerón PF, Aznar S, López-Collazo E, Lacal JC. Apoptosis induced by Rac GTPase correlates with induction of FasL and ceramides production. Mol Biol Cell. 2000;11:4347–4358. doi: 10.1091/mbc.11.12.4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deshpande SS, Qi B, Park YC, Irani K. Constitutive activation of rac1 results in mitochondrial oxidative stress and induces premature endothelial cell senescence. Arterioscler Thromb Vasc Biol. 2003;23:e1–e6. doi: 10.1161/01.atv.0000047869.13737.53. [DOI] [PubMed] [Google Scholar]
- 36.Pi J, Bai Y, Zhang Q, Wong V, Floering FL, Daniel K, et al. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes. 2007;56:1783–1791. doi: 10.2337/db06-1601. [DOI] [PubMed] [Google Scholar]
- 37.Koshkin V, Dai FF, Robson-Doucette AC, et al. Limited mitochondrial permeabilization is an early manifestation of palmitate-induced lipotoxicity in pancreatic β-cells. J Biol Chem. 2008;283(12):7936–7948. doi: 10.1074/jbc.M705652200. [DOI] [PubMed] [Google Scholar]
- 38.Jangati GR, Veluthakal R, Kowluru A. siRNA-mediated depletion of endogenous protein phosphatase 2Acalpha markedly attenuates ceramide-activated protein phosphatase activity in insulin-secreting INS-832/13 cells. Biochem Biophys Res Commun. 2006;348(2):649–652. doi: 10.1016/j.bbrc.2006.07.100. [DOI] [PubMed] [Google Scholar]
- 39.Guo J, Qian YY, Xi XX, Hu XH, Zhu JX, Han X. Blockage of ceramide metabolism exacerbates palmitate inhibition of pro-insulin gene expresión in pancreatic β-cells. Mol Cell Biochem. 2010;338:283–290. doi: 10.1007/s11010-009-0362-4. [DOI] [PubMed] [Google Scholar]
- 40.D`Aleo V, Del Guerra S, Martano M, et al. The non-peptidyl low molecular weight radical scavenger IAC protects human pancreatic islets from lipotoxicity. Mol Cell Endo. 2009;309(1–2):63–66. doi: 10.1016/j.mce.2009.05.010. [DOI] [PubMed] [Google Scholar]