Abstract
Salmonella enterica sv. typhimurium (S. enterica sv. Typhimurium) has two metal-transporting P1-type ATPases whose actions largely overlap with respect to growth in elevated copper. Mutants lacking both ATPases over-accumulate copper relative to wild-type or either single mutant. Such duplication of ATPases is unusual in bacterial copper tolerance. Both ATPases are under the control of MerR family metal-responsive transcriptional activators. Analyses of periplasmic copper complexes identified copper-CueP as one of the predominant metal pools. Expression of cueP was recently shown to be controlled by the same metal-responsive activator as one of the P1-type ATPase genes (copA), and copper-CueP is a further atypical feature of copper homeostasis in S. enterica sv. Typhimurium. Elevated copper is detected by a reporter construct driven by the promoter of copA in wild-type S. enterica sv. Typhimurium during infection of macrophages. Double mutants missing both ATPases also show reduced survival inside cultured macrophages. It is hypothesized that elevated copper within macrophages may have selected for specialized copper-resistance systems in pathogenic microorganism such as S. enterica sv. Typhimurium.
Keywords: ATPases, Bacteria, Copper, Macrophage, Metals, CopA, CueP, CueR, GolT, Infection
Introduction
Salmonella enterica serovars cause serious medical and veterinary problems world-wide and are responsible for substantial morbidity as well as mortality (1, 2). The disease manifestation varies depending upon the infectious serovar and the host susceptibility, with systemic enteric fever (typhoid) and intestinal/diarrheal disease representing the most common syndromes in humans. Salmonella enterica sv. typhimurium (S. enterica sv. Typhimurium)4 is broadly host adapted and an important serotype for animal to human transmitted salmonellosis, being a major contributor to the 93.8 million cases of Salmonella gastroenteritis disease occurring globally each year (2). The ability to survive within macrophage phagosomes is critical for S. enterica sv. Typhimurium virulence during systemic disease (3). Within this compartment the pathogen must sense and respond rapidly to a variety of fluctuating conditions, including reactive oxygen species resulting from the actions of the respiratory burst (NADPH) oxidase (4–6).
Copper-requiring proteins in S. enterica sv. Typhimurium include extracytoplasmic copper, zinc superoxide dismutases (SodC proteins), which catalyze the dismutation of superoxide into oxygen and hydrogen peroxide and are associated with S. enterica sv. Typhimurium phagosomal survival (7, 8). However, copper can also be toxic, even at low concentrations, due to binding to adventitious sites, for example displacing iron from iron-sulfur dehydratases (9), and by reacting with hydrogen peroxide to generate highly toxic hydroxyl radicals via Fenton chemistry (9, 10). Indeed, this toxicity has led to its widespread use to control microbial growth in agriculture and food processing (11, 12). Copper is also known to contribute to host immunity (13), although little is known about its direct mechanism of action. Respiratory burst oxidase activity and the ability of phagocytes to kill ingested S. enterica sv. Typhimurium has been shown to be diminished during copper deficiency (13, 14), with copper-deficient animals being highly vulnerable to pathogen (including S. enterica sv. Typhimurium) infection (15, 16).
In a recent study (17), increased bacterial (Escherichia coli) killing in activated macrophages was associated with increased copper uptake plus trafficking of the copper exporting P1B-type ATPase, ATP7A, from the Golgi apparatus to phagosome-associated vesicles (17). Furthermore, a copper-sensitive E. coli mutant, lacking the CopA copper-exporter, showed reduced viability in macrophages. Although these studies were performed with a non-pathogenic strain of E. coli, they are consistent with a model in which copper-toxicity contributes to pathogen killing within macrophage phagosomes. Genes encoding P1B-type ATPases associated with copper resistance are among the major genes expressed during infection of macrophages and/or lungs by the intracellular pathogens S. enterica sv. Typhimurium, Mycobacterium tuberculosis and Legionella pneumophila (18–23), while disruption of related genes in other bacterial pathogens causes reduced survival in mice (24, 25). In this study we have used a copper-responsive promoter to directly monitor copper-levels in macrophage phagosomes infected with pathogenic S. enterica sv. Typhimurium and confirm an increase in copper levels during infection. We also established a requirement for copper-resistance in S. enterica sv. Typhimurium within macrophage phagosomes.
S. enterica sv. Typhimurium and E. coli are co-linear for most genes with divergence largely associated with pathogenesis (26). However, the Cus system for copper export across the outer membrane (27, 28) is notably absent from S. enterica sv. Typhimurium. The S. enterica sv. Typhimurium Cue system consists of a copper-responsive MerR-family transcriptional regulator CueR, (alias SctR) that up-regulates expression of copA and cueO (alias cuiD), encoding a P1B-type ATPase copper transporter and a multi-copper oxidase, respectively, in response to copper (22, 29–31). Most recently, CueR was also shown to regulate a previously uncharacterized gene cueP, which encodes a periplasmic protein that can also contribute to copper-resistance, particularly under anaerobic conditions (32). In addition to the cue genes, S. enterica sv. Typhimurium also possesses a cluster of genes designated gol due to an association with gold resistance (33). This cluster encodes a second P1B-type ATPase metal transporter (GolT), a second CueR-type sensor (GolS) and a CopZ/Atx1 copper-chaperone like protein (GolB). The golT and golS genes are co-transcribed immediately upstream of golB, with all three genes under the control of GolS (33). A lack of copper sensitivity in a golT mutant and lack of gol induction by copper previously suggested no ancillary role in copper homeostasis (33).
Here we have re-investigated copper homeostasis in S. enterica sv. Typhimurium. We reveal a primary role for GolT, in addition to CopA, in S. enterica sv. Typhimurium copper export, with both proteins acting to reduce cellular copper loads. No difference in gold tolerance or gold accumulation was detectable for copA and golT single or double mutants compared with wild-type, using defined minimal medium indicating that neither ATPase can export gold. Furthermore, crude fractionation of copper complexes coupled, via principal component analysis, to denaturing protein separation and mass fingerprinting, identified CueP as an abundant periplasmic copper-binding protein in S. enterica sv. Typhimurium. Notably, copper-sensitive mutants of S. enterica sv. Typhimurium, lacking copA and golT, were more sensitive to macrophage-mediated killing than wild-type cells.
EXPERIMENTAL PROCEDURES
Bacterial Strains and DNA Manipulations
S. enterica sv. Typhimurium strain SL1344 was used as wild-type and strain LB5010a was used as a restriction-deficient modification-proficient host for DNA manipulations, both were obtained from the Salmonella Genetic Stock Centre. E. coli strains JM109 and DH5α were used for routine cloning. Bacteria were cultured with shaking at 37 °C in Luria-Bertani (LB) medium or M9 minimal medium (34) supplemented with l-histidine (20 μg ml−1), ampicillin (100 μg ml−1), kanamycin (50 μg ml−1), and/or chloramphenicol (34 μg ml−1), where appropriate. Cells were transformed to antibiotic resistance as described (34, 35). All generated plasmid constructs were checked by sequence analysis.
Generation of S. enterica sv. Typhimurium Deletion Mutants
Deletion derivatives of S. enterica sv. Typhimurium SL1344 were obtained using the λ Red method (35) using primers: 5′-GGAGTTTTTACTATGTCTCAAACTATCGACCTGACCCTGGACGGGTGTAGGCTGGAGCTGCTTC-3′ and 5′-ATCAGACTATGGCGCATCAGCGTAATCGCCGCGGTTTCAATCGCCACATCCATATGAATATCCTCCTTAG-3′ for copA; 5′-GAATAGTCAGGATGGGGAAGTCGTCATGAGTCAGTCAGAAAATCGTCACGGTGTAGGCTGGAGCTGCTTC-3′ and 5′-CCAGTGATCATGGCGACCTTAATGCCGAGCTGATGTAAAGCGTTAATTGCCATATGAATATCCTCCTTAG-3′ for golT; 5′-GACACATCCACGACATGAGGAGGAGCGTCATGAACATCGGTAAAGTGTAGGCTGGAGCTGCTT-3′ and 5′-GTAATCTCAGGACTTACAGACGCTTTGCCAGTCCGTGGCGACGAGGACCATATGAATATCCTCCTT-3′ for golS; 5′-GCAAGGTTAAGGTCAAGGGGGAAATATGAATATTAGCGATGTGGCGGTGTAGGCTGGAGCTGCTTC-3′ and 5′-GTGGCTTTTGCGCCTTGTGATGACAGCAGCCGGAAAGATTATCCATATGAATATCCTCCTTAG-3′ for cueR; and 5′-GGAACCCCTATAGTAGGCAGGGAGATTGTTCACAAGGAATTGAAGTTATGGTGTAGGCTGGAGCTGCTT-3′ and 5′-GATAACCCATTATGTTATCGGGCATTTTTTTAACGTAATGGTAATTCCGTCATATGAATATCCTCCTTA-3′ for cueP. Mutagenesis was performed using strain LB5010a and selection of mutants achieved using LB medium supplemented with 10 μg ml−1 chloramphenicol. Mutations were subsequently moved to SL1344 or derivatives using P22 phage transduction. Antibiotic-resistance cassettes were subsequently removed using the helper plasmid pCP20 carrying the FLP recombinase. For complementation of generated mutants, gene coding regions were amplified from S. enterica sv. Typhimurium SL1344 genomic DNA using primers 5′-GCGAGGATCCTTTAGGCTACGTAATGGCGG-3′ and 5′-GTCAAAGCTTCCTGCATAACTGACGGCG-3′ (for copA), 5′-CAACAGATCTAATAGCAAGCGTTCCTG-3′ and 5′-GCAAAGATCTAACATCAGCCTGGG-3′ (for golT), 5′-GCTGGATCCCGTAGAACGCAATGACC-3′ and 5′-GTTTAAGCTTGATGCCGCGTTAGTG-3′ (for cueR), 5′-GGCAGGATCCTGTCACCGGTATTC-3′, and 5′-CATCAAGCTTGAACTGCATAGTGAAC-3′ (for golS), digested with BamHI and HindIII or BglII (golT) and ligated into the BamHI/HindIII or BamHI (golT) site of the multi-copy vector pACYC184. The resulting constructs were introduced into strain LB5010a prior to strain SL1344.
Determination of Metal Tolerance and Metal Quotas
Overnight cultures were diluted 1:50 in M9 medium supplemented with various concentrations of copper or gold and grown until mid-logarithmic phase. Growth was monitored by measuring A595 nm, using a Multiskan Ascent Microplate Reader (Thermo Electron), and metal quotas of chelate-washed cells were determined as previously (36), but using inductively-coupled plasma mass spectroscopy (ICP-MS). Metal contents were determined as atoms mg−1 cellular protein (determined using the Bradford assay with bovine serum albumin as a calibration standard) and atoms cell−1 (from viable counts on LB agar). All assays were performed in triplicate on at least three separate occasions.
Generation of Promoter-lacZ Fusion Constructs and β-Galactosidase Assays
The operator-promoter regions of copA and golTS were amplified from S. enterica sv. Typhimurium SL1344 genomic DNA using primers 5′-GTCTAGACCCGGGTTTTTTTCGCCACATCGC-3′ and 5′-GGATCCGATATCCAGGGTCAGGTCGATAGTTTGAG-3′ (for PcopA) or 5′-GTCTAGACCCGGGTTCTACGCATGTCGTTTCCCTC-3′, and 5′-GGATCCGATATCCAGGGTCAGGTCGATAGTTTGAG-3′ (for PgolTS), ligated to pGEM-T prior to subcloning into the SmaI/BamHI site of pRS415 (37). The resulting constructs were introduced into S. enterica sv. Typhimurium strain LB5010a prior to strain SL1344. β-galactosidase assays were performed as described (36) in triplicate on at least three separate occasions. Overnight cultures were grown in LB medium or M9 minimal medium, diluted 1:100 or 1:50, respectively, in fresh medium supplemented with various concentrations of metals, hydrogen peroxide, and/or methyl viologen (described in individual experiments) and grown to mid-logarithmic phase prior to assays. The metal salts used were ZnSO4, CoCl2, NiSO4, CuSO4, AgNO3, AuHCl4, MnCl2, and C6H8O7·Fe·H3N. It is noted that inhibitory concentrations of manganese and iron were not achieved in LB medium, possibly relating to precipitation at higher concentrations. To examine the effect of pH and extracellular superoxide, cells grown to mid-logarithmic phase were washed with LB medium followed by incubation for 60 min at 37 °C in fresh medium adjusted for pH with 0.1 m phosphate buffer (34) or supplemented with 0.1 units of xanthine oxidase and 125 μm hypoxanthine, respectively. Superoxide generation was confirmed by monitoring nitroblue tetrazolium (25 μm) reduction by A560 nm measurements.
Copper Profiling Experiments
Overnight cultures of S. enterica sv. Typhimurium grown in LB medium were diluted 1:100 in fresh LB medium supplemented with 250 μm CuSO4 and 25 μm ZnSO4 (to induce expression of copper homeostatic genes and for expression of the periplasmic copper, zinc superoxide dismutases (38)), and incubated for 16 h at 37 °C with shaking to reach stationary phase. Cold osmotic shock was used to liberate periplasmic contents and the metalloprotein metal pools were identified and quantified as described (39), with the exception that anion exchange fractions (1 ml) were concentrated 4-fold using Vivaspin 2 centrifugal concentrators (with a 5-kDa nominal molecular mass cutoff) and 0.2 ml loaded onto a Superdex 75 10/300 column (GE Healthcare) for size exclusion chromatography.
Cell Culture and Macrophage Infections
RAW 264.7 macrophages (European Collection of Cell Cultures) were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% (v/v) heat-inactivated fetal bovine serum at 37 °C, 5% CO2. Infections were performed in Dulbecco's PBS (D-PBS) with stationary phase bacteria grown overnight in M9 minimal medium at a multiplicity of infection (MOI) of 10:1 (bacteria:macrophage) as previously (40). For competition infection assays, wild-type and mutant strains of S. enterica sv. Typhimurium were individually grown overnight and mixed in a 1:1 ratio in D-PBS to give a combined MOI of 10:1, serial dilutions of the inoculum were also plated onto LB agar with and without selection to verify the dose and ratio of wild-type to mutant bacteria. At 1-h postinfection, D-PBS was replaced with serum-free DMEM and extracellular bacteria killed using gentamicin (100 μg ml−1) for 1 h. The medium was then replaced (i.e. 2 h postinfection) with fresh serum-free DMEM containing gentamicin (20 μg ml−1) for the duration of the infection. At various time points postinfection, cells were washed four times with D-PBS and intracellular S. enterica sv. Typhimurium released using D-PBS containing 0.5% Triton X-100 and incubation at 37 °C for 5 min. For β-galactosidase assays, 200-μl samples were immediately frozen in liquid nitrogen and stored at −80 °C until required. Aliquots of the inoculating bacteria or bacteria grown in M9 media supplemented with 25 μm CuSO4, and bacteria maintained in M9 media in parallel to the infections, were also diluted with D-PBS containing 0.5% Triton X-100 and treated similarly. β-Galactosidase assays were performed in triplicate using the fluorescent substrate 4-methylumbelliferone β-d-galactopyranoside, as described previously (40). The number of intracellular bacteria was assessed by viable counts on non-selective and selective LB agar to confirm plasmid maintenance, where appropriate, or discrimination of recovered wild-type and mutant bacteria in competition infection assays to determine the competitive index in proliferation, which is calculated using the formula (mutant output/wild-type output)/(mutant input/wild-type input), with output defined as the colony forming units recovered at the various time points postinfection and input represents the colony-forming units recovered at 1-h postinfection (intracellular bacteria after the initial treatment with gentamicin) (41). All infection assays were carried out using three separate wells for each condition and repeated on at least four separate occasions. The statistical significance of the results was determined using the unpaired Student's t test.
Animal Infections
C57BL/6 mice were purchased from Harlan Olac Ltd. (Blackthorn, Bicester, UK) and mice matched for sex and age, between 9 and 12 weeks old, were used for experiments. Experiments were covered by a Project License granted by the UK Home Office under the Animal (Scientific Procedures) Act 1986. This license was approved locally by the University of Cambridge Ethical Review Committee. Mice were infected by oral gavage with 0.2 ml (∼1 × 109 colony-forming units) of stationary phase bacteria in PBS; prepared from single colony cultures which were grown overnight in 100 ml of LB at 37 °C without shaking, pelleted by centrifugation (6,000 rpm, 10 min), and resuspended in 10 ml of PBS. Inocula were enumerated by plating onto LB agar. Mice were killed by cervical dislocation and livers and spleens aseptically removed. The organs were placed into a stomacher bag and homogenized in 10 ml of sterile water in a Colworth Stomacher 80. The resulting homogenate was 10-fold serially diluted in PBS and pour plated with LB agar to obtain viable counts.
RESULTS
CopA and GolT Contribute to Copper, but Not Gold, Resistance
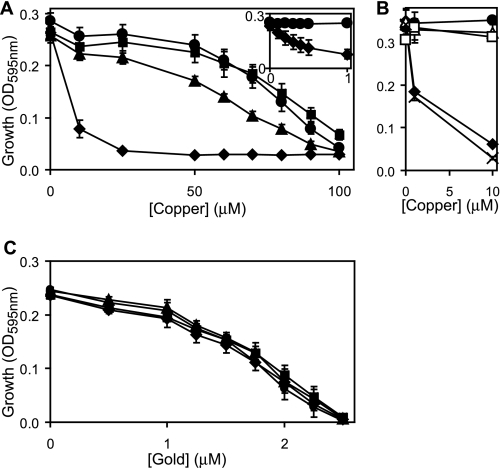
The two related P1B-type ATPases, CopA and GolT, have previously been associated with S. Typhimurium metal-resistance in studies that, most notably, used complex (LB) medium (22, 33). Whereas some reduction in copper tolerance was observed for a ΔcopA strain, no such reduction was observed for ΔgolT (22, 33). Decreased gold tolerance was detected for ΔgolT (as well as for ΔcopA) leading to a proposal that the Gol system has evolved primarily to adapt to toxic concentrations of gold (33). We have re-examined the tolerance of wild-type S. enterica sv. Typhimurium (SL1344) and mutants lacking copA and/or golT, to copper and gold using defined minimal medium (M9) as opposed to complex LB medium. Under these conditions, wild-type S. enterica sv. Typhimurium is sensitive to copper above 50 μm (Fig. 1A), which contrasts dramatically with the millimolar doses of copper required to inhibit S. enterica sv. Typhimurium growth on complex media (LB) agar plates (22, 33). In defined medium, mutants lacking golT display similar copper tolerance to wild-type cells and ΔcopA show merely a slight loss of copper tolerance corroborating previous findings (22, 33). Crucially, double mutants deficient in both golT and copA are extremely (>100-fold) sensitive to copper in minimal medium with growth inhibited below 1 μm copper (Fig. 1A, inset). Copper resistance is restored to the ΔcopA/ΔgolT strain by re-introducing either copA or golT on a plasmid (Fig. 1B). These findings therefore demonstrate that either CopA or GolT can confer S. enterica sv. Typhimurium copper tolerance with, at least some, functional redundancy.
FIGURE 1.
Both copA and golT have a role in copper export. A–C, final A595 nm measurements of wild-type S. enterica sv. Typhimurium (filled circles), ΔcopA (filled triangles), ΔgolT (filled squares), ΔcopA/ΔgolT (filled diamonds), ΔcopA/ΔgolT containing copA on plasmid pACYC184 (open triangles), ΔcopA/ΔgolT containing golT on plasmid pACYC184 (open squares) or ΔcopA/ΔgolT containing pACYC184 alone (crosses) following growth (4 h) in M9 minimal medium supplemented with increasing concentrations of copper (A and B) or gold (C). Inset, A595 nm (y axis) of wild-type S. enterica sv. Typhimurium (circles) and ΔcopA/ΔgolT (diamonds) following growth with up to 1 μm copper (x axis).
Gold is more toxic to S. enterica sv. Typhimurium than copper, with growth of wild-type inhibited above 1 μm gold in M9 minimal medium (Fig. 1C), and 40 μm required on LB-agar plates (33). Unexpectedly, and in contrast to previous findings where loss of copA or golT caused similar reductions in S. enterica sv. Typhimurium survival on LB-agar plates supplemented with 40 μm gold (33), the tolerance of the ΔcopA and ΔgolT single and double mutants to gold is indistinguishable from wild-type in M9 minimal medium (Fig. 1C). It is possible that this difference in the phenotype for the copA and golT mutants, with respect to gold tolerance, reflects differences in metal availability between the different media used in this and the previous (33) study. However, it is noteworthy that we also detected no difference in gold tolerance between wild-type and ΔcopA/ΔgolT in liquid LB cultures (data not shown). Alternatively, there may be subtle differences between the S. enterica sv. Typhimurium strains used (14028s versus SL1344 herein). Notably in E. coli deletion of copA also had no affect on gold-tolerance (42) or cytosolic gold levels (43) in LB medium, despite CueR activating copA expression in response to gold (42).
GolT Does Not Reduce Gold Accumulation
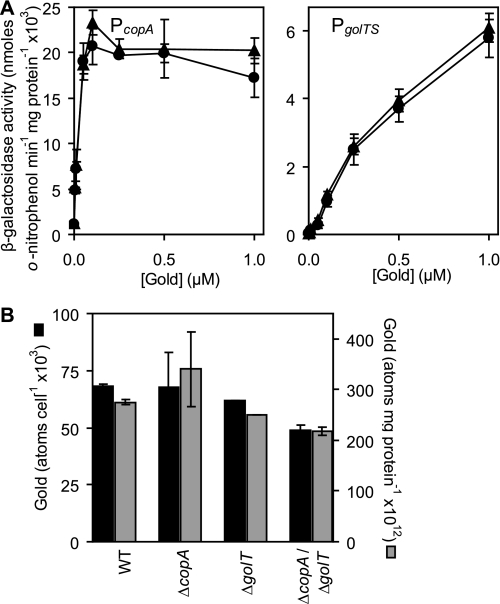
The lack of detectable gold sensitivity for ΔcopA, ΔgolT, or ΔcopA/ΔgolT in minimal medium implies that neither CopA nor GolT can export gold in S. enterica sv. Typhimurium SL1344. The previous suggestion (33) that the S. enterica sv. Typhimurium Gol system has primarily evolved to cope with toxic gold levels led us to investigate this further. Expression from the S. enterica sv. Typhimurium copA and golTS promoters is gold-responsive (33). Hence, in an attempt to assess cytosolic gold levels we examined the effects of a golT deletion on gold-responsive expression from the copA and golTS operator-promoter regions. Expression from both PcopA and PgolTS increased in response to gold in a concentration-dependent manner, consistent with the detection of elevated cytosolic gold levels (Fig. 2A). Importantly, there was no increase in the sensitivity or fold-induction from PcopA and PgolTS in cells lacking golT, compared with wild-type (Fig. 2A), revealing that GolT does not reduce cytosolic gold levels. Total cellular gold contents were also assessed using ICP-MS of chelate washed extracts, of wild-type S. enterica sv. Typhimurium and of the transporter mutants, ΔcopA, ΔgolT, or ΔcopA/ΔgolT, following growth in defined minimal medium in the presence of 1 μm gold (noninhibitory). Values were expressed as number of atoms per cell and relative to protein content (Fig. 2B). Wild-type cells and the various mutants showed similar gold contents. These data do not support a role for the GolT P1B-type ATPase in gold transport.
FIGURE 2.
Cytoplasmic gold levels are unaffected by GolT. A, β-galactosidase activity was measured in wild-type S. enterica sv. Typhimurium (circles) or ΔgolT (triangles) containing PcopA or PgolTS fused to lacZ following growth in M9 minimal medium supplemented with a range of permissive gold levels. B, gold contents of wild-type S. enterica sv. Typhimurium (WT), ΔcopA, ΔgolT, and ΔcopA/ΔgolT grown in M9 minimal medium in the presence of 1 μm added gold. Metal contents are shown as atoms cell−1 (black) or atoms mg−1 cellular protein (gray).
CueP Is a Dominant Periplasmic Copper Protein
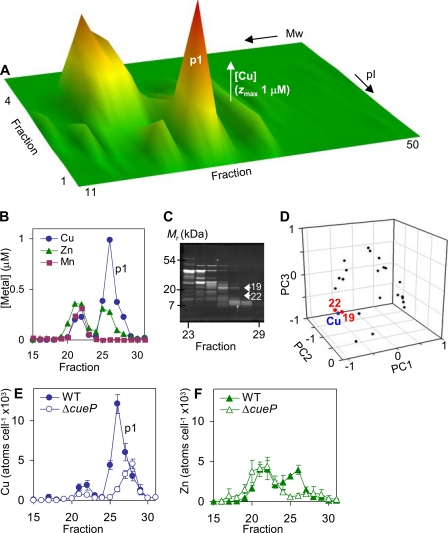
In contrast to E. coli, S. enterica sv. Typhimurium lacks facilitated export of copper across the outer membrane via Cus or any analogous known transporter (27, 28), yet has dual systems, Cue and Gol, facilitating copper transport to the periplasm. We therefore examined the protein-bound copper pool(s) in periplasmic fractions from S. enterica sv. Typhimurium cells exposed to copper stress to identify the protein targets for copper. Periplasm contents were liberated by cold osmotic shock, resolved by native two-dimensional liquid chromatography (anion exchange followed by size exclusion), and eluant fractions analyzed for metals by ICP-MS (Fig. 3A). A predominant copper complex (p1) contains ∼27 × 103 atoms of copper per cell and coincides with a zinc complex containing ∼11 × 103 atoms of zinc per cell (Fig. 3B). To characterize the protein(s) binding the copper in complex p1, the abundance of individual proteins in the p1 region was estimated by integrating peak areas from scanned SDS-PAGE gels (Fig. 3C) followed by principal component analysis to compare the rise and fall of p1 copper with the rise and fall of each protein by their proximity on scatter plots (Fig. 3D and supplemental Fig. S1) (6). Two proteins (designated 19 and 22, Fig. 3D) clustered with the p1 copper. These were excised from the gel and mass fingerprinting identified protein 19 as the product of STM3650 (8 peptides matched, 73% coverage, e-score 2 × 10−6), whereas protein 22 could not be identified because of low abundance. The product of STM3650 is a deduced periplasmic or exported protein of 19.5 kDa. During the preparation of this manuscript, this gene product was independently identified as a periplasmic protein under the control of the copper-responsive regulator CueR and designated CueP (32). CueP has a role in copper resistance, particularly in the absence of oxygen, leading to the suggestion that CueP may functionally substitute for a periplasmic copper export system in S. enterica sv. Typhimurium (32).
FIGURE 3.
The distribution of protein-bound periplasmic copper in S. enterica sv. Typhimurium. A, periplasmic extracts from wild-type S. enterica sv. Typhimurium were resolved by anion exchange (pI) into 1-ml fractions, then aliquots (0.2 ml) of 4-fold concentrated eluant resolved by size exclusion (Mr) chromatography into 0.5 ml fractions and analyzed for metals by inductively coupled plasma-mass spectrometry; the full profile for copper is shown and similar profiles were obtained with three independent extracts. B, [copper], [zinc], and [manganese] in fractions 15–31 (0.5 ml) following size exclusion of proteins eluted by 100 mm NaCl during anion exchange, which includes the major copper complex p1. C and D, the abundances of individual proteins in fractions corresponding to p1 were estimated by integrating peak areas from scanned SDS-PAGE gels of these fractions, visualized by Sypro Ruby (C), followed by principal component analysis, comparing the rise and fall of copper with the rise and fall of each protein (D). Two proteins (19 and 22) closely matching the p1 copper profile were excised from the gel and 19 was identified by mass fingerprinting as STM3650 (CueP), while 22 was not identified. E and F, the copper and zinc content of fractions 15–31 (0.5 ml) obtained following size exclusion of proteins eluted by 100 mm NaCl during anion exchange of periplasmic proteins from wild-type S. enterica sv. Typhimurium and ΔcueP shown as atoms cell−1, data points represent the mean (±S.E.) for three independent experiments.
To unequivocally confirm that CueP is a predominant p1 copper protein, mutants lacking cueP were generated. The p1 copper peak was confirmed to be absent from ΔcueP periplasmic fractions (Fig. 3E and supplemental Fig. S2), although it is noted that an overlapping copper peak was retained containing ∼11 × 103 atoms of copper cell−1. These data therefore demonstrate that CueP binds copper in vivo. It is also notable that protein-bound zinc (∼6 × 103 atoms of zinc cell−1), which coincides with p1 is also diminished in ΔcueP (Fig. 3F). It is possible that some zinc may also associate with CueP in wild-type cells or alternatively a separate zinc complex may have some dependence upon CueP (the remaining zinc profile for ΔcueP was similar to that obtained for wild-type cells, data not shown).
CopA and GolT Reduce Total Cell Copper Accumulation
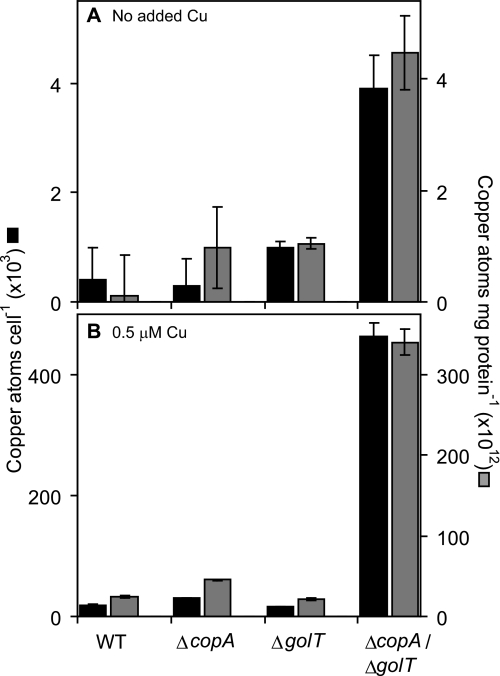
The lack of a functional Cus system and possession of CueP in S. enterica sv. Typhimurium might suggest that copper transport to the periplasm by CopA and GolT does not contribute to a reduction in total cellular copper load but rather serves to relocate surplus copper to the periplasm. We determined total cellular copper contents, using ICP-MS of chelate washed extracts, of wild-type S. enterica sv. Typhimurium and of copA and/or golT copper transporter mutants following growth in defined minimal medium in the presence and absence of 0.5 μm copper (noninhibitory to ΔcopA/ΔgolT). Values were expressed as number of atoms per cell and relative to protein content (Fig. 4). As anticipated, addition of 0.5 μm copper to the media caused an increase in the copper contents of wild-type cells and the various mutants (compare Fig. 4, panels A and B). However, ΔcopA accumulated slightly more copper than wild-type cells (∼1.7-fold and ∼1.8-fold increase in copper atoms cell−1 or atoms mg protein−1, respectively) (Fig. 4B). No increase in copper content was detected for ΔgolT compared with wild-type cells. Crucially, the ΔcopA/ΔgolT double mutant showed substantially elevated copper accumulation in media supplemented with 0.5 μm copper (∼25-fold and ∼14-fold increase in copper atoms cell−1 or atoms mg protein−1, respectively) (Fig. 4B). Copper contents were also elevated for ΔcopA/ΔgolT in minimal medium with no metal supplement (∼9-fold, Fig. 4A). The overaccumulation of copper in ΔcopA/ΔgolT confirms that both CopA and GolT contribute to cellular copper export with substantial functional redundancy. It is not known whether copper efflux from the periplasm is facilitated by an unidentified exporter and/or results from passive diffusion.
FIGURE 4.
Mutants lacking CopA and GolT accumulate copper. Copper contents of wild-type S. enterica sv. Typhimurium (WT), ΔcopA, ΔgolT, and ΔcopA/ΔgolT grown in M9 minimal medium in the absence (A) or presence (B) of 0.5 μm added copper. Metal contents are shown as atoms cell−1 (black) or atoms mg−1 cellular protein (gray). Note the different axes scales for A and B.
The total cellular copper contents of the cueP mutant and wild-type S. enterica sv. Typhimurium were also compared following growth in M9 minimal medium supplemented with a non-inhibitory concentration of copper (25 μm). Copper contents were slightly elevated for ΔcueP (∼3-fold and ∼2-fold increase in copper atoms cell−1 or atoms mg protein−1, respectively, supplemental Fig. S3), consistent with CueP also contributing to copper homeostasis in these cells.
Expression from PcopA Is Responsive to Copper and Gold but Unaffected by Other Environmental Conditions Encountered within Macrophage Phagosomes
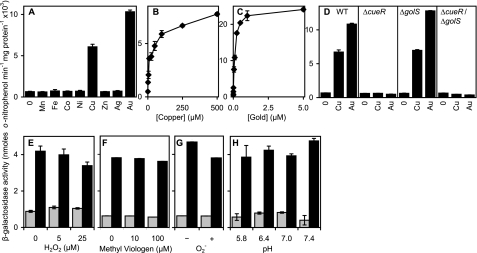
In a recent study (17) using RAW264.7 macrophages, it was demonstrated that trafficking by the ATP7A copper transporter to phagosome associated vesicles contributes to the bactericidal activity of these cells, presumably by supplying copper into the phagosomal compartment. Within macrophages the copper status of the Salmonella containing vesicle is not known. To use PcopA to monitor phagosomal copper levels, it was first necessary to establish that PcopA expression is unaffected by other environmental stresses that S. enterica sv. Typhimurium might face inside macrophages including exposure to other metal ions, oxidative stress, and changes in pH. Hence, to examine the metal-responsiveness of PcopA further, expression from PcopA was monitored following growth of S. enterica sv. Typhimurium in medium (LB) supplemented with biologically significant concentrations of a range of different metal ions. Consistent with previous findings (22, 33), expression from PcopA was highly elevated in cells grown in medium supplemented with maximum permissive concentrations of copper or gold (Fig. 5A), but was unaffected by any of the other metal ions tested. Expression from PcopA is highly specific for copper and gold, in a concentration-dependent manner, with gold being the more potent inducer at permissive concentrations (Fig. 5, B and C). Copper-responsive expression from PcopA in S. enterica sv. Typhimurium is under the control of the MerR-family transcriptional regulator CueR (22). However, S. enterica sv. Typhimurium possesses a closely related regulator, GolS, which has been shown to confer gold-responsive expression of PgolTS and PgolB (33). Both CueR and GolS bind to similar inverted repeat sequences between the unusually spaced (19 bp) −35 and −10 elements of their target promoters, indicating possible overlap in the genes regulated by the two sensors (12). Hence, we examined the contributions of CueR and GolS to the regulation of PcopA. Copper and gold responsiveness was completely abolished in ΔcueR and ΔcueR/ΔgolS mutants, but unaffected in ΔgolS (Fig. 5D), revealing that CueR is responsible for both copper and gold-responsiveness of PcopA. Thus CueR is highly selective for gold and copper, with gold being the most potent inducer at permissive concentrations (Fig. 5, A–D), despite the negligible gold-related growth phenotypes (Fig. 1).
FIGURE 5.
Metal-responsive expression from PcopA is unaffected by pH or reactive oxygen species. A–C, β-galactosidase activity measured in S. enterica sv. Typhimurium containing PcopA fused to lacZ following growth (150 min) in LB medium with no metal supplement or 1 mm Mn(II), 1 mm Fe(III), or maximum permissive concentrations of Co(II) (0.1 mm), Ni(II) (0.5 mm), Cu(II) (0.5 mm), Zn(II) (1 mm), Ag(I) (2 μm), or Au(III) (5 μm) (A), or up to inhibitory concentrations of Cu(II) (B) or Au(III) (C). D, expression from PcopA in S. enterica sv. Typhimurium (WT), ΔcueR, ΔgolS or ΔgolS/ΔcueR following growth in LB medium with no metal supplement or maximum permissive concentrations of Cu(II) or Au(III). E–H, expression from PcopA in S. enterica sv. Typhimurium following growth (150 min) in LB medium with no metal supplement (gray) or with added copper (25 μm) to achieve ∼half-maximal copper-induced expression (black), with or without hydrogen peroxide (E) or the intracellular superoxide generator methyl viologen (F), or prior to washing and exposure (1 h) to fresh medium with or without 0.1 units ml−1 of the extracellular superoxide generator xanthine oxidase (G) or at different pH (H). Expression from a control promoter, PpolA (40), was unaffected by any of the conditions tested (data not shown).
Other environmental stresses that S. enterica sv. Typhimurium might face inside the micro-environment of the macrophage phagosome include acidic pH and oxidative stress. No increase in expression was detected in the presence of hydrogen peroxide, superoxide, or low pH and importantly, copper-responsiveness from PcopA was retained in all cases (Fig. 5, E–H). In some cases, a slight reduction in expression was detected in the presence of the additional stress, however this was coincident with reduced growth, and hence the inhibitory effects on expression most likely relate to toxicity. These data establish the feasibility of using PcopA to exclusively report upon phagosomal copper levels (with an assumption that gold is not encountered here).
S. enterica sv. Typhimurium Is Exposed to Copper in Macrophage Phagosomes
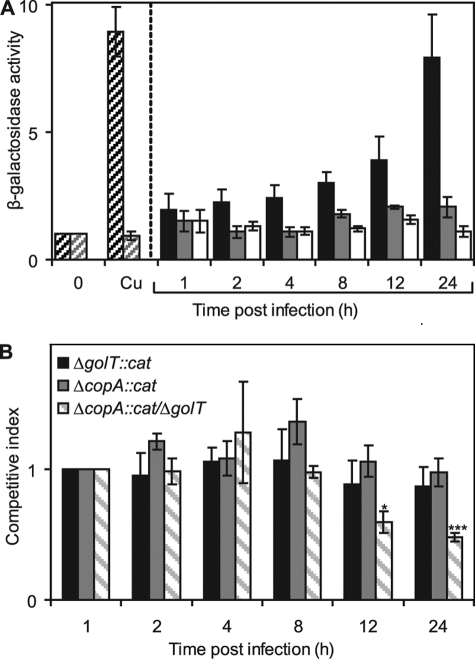
S. enterica sv. Typhimurium SL1344 possessing the PcopA-lacZ fusion construct was used to infect RAW264.7 macrophages. Intracellular S. enterica sv. Typhimurium were isolated at various time points postinfection, the number of bacteria assessed by viable counts and β-galactosidase activity determined. Maintenance of the reporter constructs during this time course and copper-responsive β-galactosidase activity in recovered bacteria was also confirmed (data not shown). Importantly, expression from PcopA increased steadily over the 24-h infection period with ∼4-fold and ∼8-fold increase being observed at 12 and 24 h, respectively, postinfection (Fig. 6A). The level of expression observed at 24-h postinfection was comparable to that of extracellular bacteria grown in the presence of 25 μm copper (Fig. 6A). To confirm that the increase in PcopA expression was directly due to copper induction of PcopA, expression was also monitored in a ΔcueR/ΔgolS background during infection. In the double mutant, PcopA is expressed at basal levels but is not copper-inducible (Fig. 5D). The survival of ΔcueR/ΔgolS is indistinguishable from wild-type S. enterica sv. Typhimurium during the infection period (data not shown). No significant increase in PcopA expression was observed during the 24-h infection period in the absence of CueR and GolS (Fig. 6A), consistent with loss of copper-sensing. No change in expression from PcopA was observed in bacteria maintained in minimal medium in parallel experiments (Fig. 6A). These data imply that S. enterica sv. Typhimurium CueR detects elevated copper within the macrophage phagosome.
FIGURE 6.
Copper export is important for intracellular survival of S. enterica sv. Typhimurium. A, macrophages (RAW264.7) were infected with wild-type S. enterica sv. Typhimurium (black) or the ΔgolS/ΔcueR derivative (gray) containing PcopA fused to lacZ following growth in M9 minimal medium and β-galactosidase activity was measured in bacteria isolated at indicated time points postinfection. β-Galactosidase activity was also measured in wild-type S. enterica sv. Typhimurium containing PcopA-lacZ maintained in M9 minimal medium (extracellular) for the duration of the infection (non-filled). Expression levels in bacteria used for the infection, grown with no added Cu(II) or grown in parallel with 25 μm Cu(II) are also shown (wild-type, dark diagonal shading; ΔgolS/ΔcueR, light diagonal shading). B, competitive infections were performed with wild-type S. enterica sv. Typhimurium and either ΔcopA::cat (gray), ΔgolT::cat (black), or ΔcopA::cat/ΔgolT (diagonal shading), with the competitive index defined as the colony-forming unit ratio of mutant and wild-type strains recovered at the indicated time points postinfection, divided by their ratio in the input (intracellular bacteria after the initial treatment with gentamicin, 1-h postinfection). In each case, bacterial strains were mixed at a ratio of 1:1 and used at a final MOI of 10:1 (bacteria:macrophage). Data points represent the mean (±S.E.) for at least four independent experiments, each performed in triplicate (***, p < 0.000005; *, p < 0.005 by Student's t test).
Copper Export Is Important for S. enterica sv. Typhimurium Survival in Macrophages
Enhanced expression from PcopA during macrophage infection is consistent with an elevated bacterial copper load and a requirement for copper export. Hence, to test whether or not there is a requirement for CopA and GolT during macrophage infection, the survival of wild-type S. enterica sv. Typhimurium and mutants lacking copA and/or golT were examined within RAW267.4 macrophages. This was achieved by a competitive infection experiment in which macrophages were co-infected with a 1:1 ratio of wild-type S. enterica sv. Typhimurium and a strain lacking one or both copper exporters, and measuring the contribution of each strain to the total number of bacteria recovered at various time points postinfection. Mutant strains harbored an antibiotic resistance marker (cat, conferring chloramphenicol resistance) to allow selection from wild-type cells and were generated by P22 transduction of either copA::cat or golT::cat elements from LB5010a to an SL1344 background or copA::cat to a ΔgolT SL1344 background.
Competition infection assays involving wild-type and mutants lacking a single transporter, ΔcopA::cat or ΔgolT::cat, revealed that loss of either transporter conferred no significant reduction in the level of uptake (data not shown) or ability to replicate within RAW264.7 macrophages up to 24-h postinfection (Fig. 6B). However, significantly more wild-type bacteria than ΔcopA::cat/ΔgolT were recovered at time points beyond 8-h postinfection (Fig. 6B), with a competitive index of 0.48 obtained at 24 h, thus revealing that the ability to export copper confers a selective advantage within the macrophage phagosome. There was no difference in the number of wild-type and ΔcopA::cat/ΔgolT cells recovered following competitive growth in M9 minimal media (data not shown), consistent with neither strain having a competitive advantage in the extracellular environment lacking elevated copper. These data therefore establish a requirement for S. enterica sv. Typhimurium copper-resistance during intracellular survival in cultured macrophages.
The C57/BL6 murine model is routinely used for in vivo infection studies with Salmonella. In contrast to humans, S. enterica sv. Typhimurium infections of mice cause a systemic typhoid-like disease, usually resulting in death of the animal within a few days (44). Following oral administration, the bacteria pass through the stomach to the small intestine and invade through specialized M-cells in the Peyer's patches where they are engulfed by macrophages and disseminated throughout the body (45). Surprisingly, we detected no difference in the number of wild-type S. enterica sv. Typhimurium or the ΔcopA/ΔgolT mutant isolated from the liver and spleen of C57/BL6 mice orally infected with 1 × 109 bacteria 5 days postinfection; with 6.31 (±1.12) × 106 wild-type or 6.39 (±1.08) × 106 ΔcopA/ΔgolT isolated per liver, and 6.23 (±1.59) × 106 wild-type or 6.76 (±1.00) × 106 ΔcopA/ΔgolT isolated per spleen (n = 20). This implies that additional factors circumvent the requirement for S. enterica sv. Typhimurium copper export, at least within our mouse model. However, it remains possible that an attenuation phenotype may be detected in vivo using a different model of infection, such as an alternative animal host (e.g. different mouse strains) or monitoring different tissues, disease progression, and/or mortality.
DISCUSSION
We have demonstrated that two copper exporting P1B-type ATPases, CopA and GolT, are involved in copper homeostasis in S. enterica sv. Typhimurium. Mutants lacking both of these transporters are extremely copper sensitive in M9 minimal medium (Fig. 1A) and hyperaccumulate copper (Fig. 4) relative to wild-type, or crucially relative to either single mutant. Contrary to expectations, no difference in the tolerance of golT and copA single or double mutants to gold was detectable in defined minimal medium, compared with wild-type S. enterica sv. Typhimurium (Fig. 1C), and gold accumulated to similar levels in all four strains (Fig. 2). Activation of copA (Fig. 5) and golT (33) expression by gold is gratuitous. We show that CueP is a copper-binding protein (Fig. 3E), and furthermore a predominant copper complex in periplasmic extracts of S. enterica sv. Typhimurium (Fig. 3A). Replication within macrophage phagosomes is a feature of S. enterica sv. Typhimurium virulence. We used copper-responsive expression from PcopA to monitor copper-availability to Salmonella in macrophage-phagosomes and reveal that elevated copper is a feature of this bactericidal compartment (Figs. 5 and 6A). Copper resistance aids survival in this compartment (Fig. 6B).
Both CopA and GolT are proposed to contribute to S. enterica sv. Typhimurium copper resistance by pumping copper from the cytosol to the periplasm. CueP also contributes to S. enterica sv. Typhimurium copper resistance (32) and confirmation that CueP binds copper within the periplasm (Fig. 3) might suggest that CueP prevents copper toxicity in the periplasm. However, copper binding by CueP may alternatively or additionally limit the return of copper to the cytosol, which would be consistent with CueP reducing the cellular copper load. Genes encoding CueP-like proteins are present in the genomes of other Gram-negative and Gram-positive bacteria, many of which are notably pathogens including Yersinia sp., Citrobacter sp., Erwinia caratovora, Corynebacterium sp., and Shewanella sp. It is therefore plausible that this represents a widespread mechanism of copper resistance in some manner adapted to virulence, although the role of CueP in the periplasm remains to be established.
Induction of copA expression is specific to copper and gold (Fig. 5) and increased expression from PcopA in S. enterica sv. Typhimurium within macrophage phagosomes is consistent with an increased copper load within the intracellular environment. Accordingly, increased expression of a gene (iviX) corresponding to copA has been identified previously by in vivo expression technology (IVET) as being up-regulated in S. enterica sv. Typhimurium during infection of murine macrophages (19). Our results show that copper-sensitive mutants of S. enterica sv. Typhimurium (ΔcopA/ΔgolT) have considerably reduced (∼50%) survival in macrophages compared with wild-type cells, suggesting that copper export is a S. enterica sv. Typhimurium defense mechanism against macrophage-mediated killing. However, in a murine model of infection, we detected no difference in the bacterial loads in the liver and spleen for wild-type S. enterica sv. Typhimurium and the copper export mutant, implying that other determinants may circumvent the requirement for the ATPases in this model system and most likely relates to the involvement of additional host immune factors in bacterial killing. A converse set of experimental observations were recently documented in a study (29) of the S. enterica sv. Typhimurium multi-copper oxidase CueO. This copper-resistance gene was shown to be important for virulence in the murine infection model but not in cultured macrophages. These data were taken to imply that additional unidentified host factors are involved in mouse clearance of the CueO mutant (29). Nonetheless, taken together, all of these studies do reveal the importance of copper homeostasis in S. enterica sv. Typhimurium for virulence, with the different components argued to make contributions at different stages of infection.
In addition to a role in copper resistance, copper-transporting P1B-type ATPases in eukaryotes and cyanobacteria also traffick copper to intracellular compartments for copper-requiring proteins (46, 47). It is therefore possible that the transport of copper by CopA and/or GolT to the periplasm is important for supplying this cofactor to proteins in this compartment, such as periplasmic copper, zinc superoxide dismutases, SodCI and SodCII, which protect against extracellular superoxide stress and phagosomal killing (7, 8, 38).
S. enterica sv. Typhimurium and E. coli have distinct defenses against copper toxicity which likely correlate with the different copper challenges in their two lifestyles. Salmonella infections represent a considerable burden in both developed and developing countries. Atypical copper homeostasis in this organism therefore offers opportunities to develop novel antimicrobial agents targeted to these systems, or indeed to use elevated copper, or potentially an analogue such as silver, to control infections and reduce Salmonella transmission by food or other routes.
Supplementary Material
This work was supported by a grant from the Biotechnology and Biological Science Research Council (BBSRC) Agri-food Committee (BB/G010765/1) and BBSRC studentship (to D. O.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- S. enterica sv. Typhimurium
- Salmonella enterica sv. typhimurium
- LB
- Luria-Bertani
- DMEM
- Dulbecco's modified Eagle's medium
- MOI
- multiplicity of infection
- ICP-MS
- inductively-coupled plasma mass spectroscopy
- PBS
- phosphate-buffered saline.
REFERENCES
- 1.Coburn B., Grassl G. A., Finlay B. B. (2007) Immunol. Cell Biol. 85, 112–118 [DOI] [PubMed] [Google Scholar]
- 2.Majowicz S. E., Musto J., Scallan E., Angulo F. J., Kirk M., O'Brien S. J., Jones T. F., Fazil A., Hoekstra R. M. (2010) Clin. Infect. Dis. 50, 882–889 [DOI] [PubMed] [Google Scholar]
- 3.Fields P. I., Swanson R. V., Haidaris C. G., Heffron F. (1986) Proc. Natl. Acad. Sci. U.S.A. 83, 5189–5193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mastroeni P., Vazquez-Torres A., Fang F. C., Xu Y., Khan S., Hormaeche C. E., Dougan G. (2000) J. Exp. Med. 192, 237–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vazquez-Torres A., Jones-Carson J., Mastroeni P., Ischiropoulos H., Fang F. C. (2000) J. Exp. Med. 192, 227–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Segal A. W., Shatwell K. P. (1997) Ann. N.Y. Acad. Sci. 832, 215–222 [DOI] [PubMed] [Google Scholar]
- 7.Battistoni A. (2003) Biochem. Soc. Trans. 31, 1326–1329 [DOI] [PubMed] [Google Scholar]
- 8.De Groote M. A., Ochsner U. A., Shiloh M. U., Nathan C., McCord J. M., Dinauer M. C., Libby S. J., Vazquez-Torres A., Xu Y., Fang F. C. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 13997–14001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macomber L., Imlay J. A. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 8344–8349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macomber L., Rensing C., Imlay J. A. (2007) J. Bacteriol. 189, 1616–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aarestrup F. M., Hasman H. (2004) Vet. Microbiol. 100, 83–89 [DOI] [PubMed] [Google Scholar]
- 12.Osman D., Cavet J. S. (2008) Adv. Appl. Microbiol. 65, 217–247 [DOI] [PubMed] [Google Scholar]
- 13.Percival S. S. (1998) Am. J. Clin. Nutr. 67, 1064S–1068S [DOI] [PubMed] [Google Scholar]
- 14.Huang Z. L., Failla M. L. (2000) J. Nutr. 130, 1536–1542 [DOI] [PubMed] [Google Scholar]
- 15.Suttle N. F., Jones D. G. (1989) J. Nutr. 119, 1055–1061 [DOI] [PubMed] [Google Scholar]
- 16.Newberne P. M., Hunt C. E., Young V. R. (1968) Br. J. Exp. Pathol. 49, 448–457 [PMC free article] [PubMed] [Google Scholar]
- 17.White C., Lee J., Kambe T., Fritsche K., Petris M. J. (2009) J. Biol. Chem. 284, 33949–33956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham J. E., Clark-Curtiss J. E. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 11554–11559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heithoff D. M., Conner C. P., Hanna P. C., Julio S. M., Hentschel U., Mahan M. J. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 934–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talaat A. M., Lyons R., Howard S. T., Johnston S. A. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 4602–4607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu T., Ramesh A., Ma Z., Ward S. K., Zhang L., George G. N., Talaat A. M., Sacchettini J. C., Giedroc D. P. (2007) Nat. Chem. Biol. 3, 60–68 [DOI] [PubMed] [Google Scholar]
- 22.Espariz M., Checa S. K., Audero M. E., Pontel L. B., Soncini F. C. (2007) Microbiology 153, 2989–2997 [DOI] [PubMed] [Google Scholar]
- 23.Rankin S., Li Z., Isberg R. R. (2002) Infect. Immun. 70, 3637–3648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Francis M. S., Thomas C. J. (1997) Microb. Pathog. 22, 67–78 [DOI] [PubMed] [Google Scholar]
- 25.Schwan W. R., Warrener P., Keunz E., Stover C. K., Folger K. R. (2005) Int. J. Med. Microbiol. 295, 237–242 [DOI] [PubMed] [Google Scholar]
- 26.McClelland M., Sanderson K. E., Spieth J., Clifton S. W., Latreille P., Courtney L., Porwollik S., Ali J., Dante M., Du F., Hou S., Layman D., Leonard S., Nguyen C., Scott K., Holmes A., Grewal N., Mulvaney E., Ryan E., Sun H., Florea L., Miller W., Stoneking T., Nhan M., Waterston R., Wilson R. K. (2001) Nature 413, 852–856 [DOI] [PubMed] [Google Scholar]
- 27.Franke S., Grass G., Rensing C., Nies D. H. (2003) J. Bacteriol. 185, 3804–3812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Outten F. W., Huffman D. L., Hale J. A., O'Halloran T. V. (2001) J. Biol. Chem. 276, 30670–30677 [DOI] [PubMed] [Google Scholar]
- 29.Achard M. E., Tree J. J., Holden J. A., Simpfendorfer K. R., Wijburg O. L., Strugnell R. A., Schembri M. A., Sweet M. J., Jennings M. P., McEwan A. G. (2010) Infect. Immun. 78, 2312–2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim J. S., Kim M. H., Joe M. H., Song S. S., Lee I. S., Choi S. Y. (2002) FEMS Microbiol. Lett. 210, 99–103 [DOI] [PubMed] [Google Scholar]
- 31.Lim S. Y., Joe M. H., Song S. S., Lee M. H., Foster J. W., Park Y. K., Choi S. Y., Lee I. S. (2002) Mol. Cells 14, 177–184 [PubMed] [Google Scholar]
- 32.Pontel L. B., Soncini F. C. (2009) Mol. Microbiol. 73, 212–225 [DOI] [PubMed] [Google Scholar]
- 33.Checa S. K., Espariz M., Audero M. E., Botta P. E., Spinelli S. V., Soncini F. C. (2007) Mol. Microbiol. 63, 1307–1318 [DOI] [PubMed] [Google Scholar]
- 34.Sambrook J., Russell D. W. (2001) Molecular Cloning: A Laboratory Manual (Third Edition), Cold Spring Harbor Press, NY [Google Scholar]
- 35.Datsenko K. A., Wanner B. L. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cavet J. S., Meng W., Pennella M. A., Appelhoff R. J., Giedroc D. P., Robinson N. J. (2002) J. Biol. Chem. 277, 38441–38448 [DOI] [PubMed] [Google Scholar]
- 37.Simons R. W., Houman F., Kleckner N. (1987) Gene 53, 85–96 [DOI] [PubMed] [Google Scholar]
- 38.Ammendola S., Pasquali P., Pacello F., Rotilio G., Castor M., Libby S. J., Figueroa-Bossi N., Bossi L., Fang F. C., Battistoni A. (2008) J. Biol. Chem. 283, 13688–13699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tottey S., Waldron K. J., Firbank S. J., Reale B., Bessant C., Sato K., Cheek T. R., Gray J., Banfield M. J., Dennison C., Robinson N. J. (2008) Nature 455, 1138–1142 [DOI] [PubMed] [Google Scholar]
- 40.Taylor C. M., Osman D., Cavet J. S. (2009) Microb. Pathog. 46, 114–118 [DOI] [PubMed] [Google Scholar]
- 41.Segura I., Casadesús J., Ramos-Morales F. (2004) J. Microbiol. Methods 56, 83–91 [DOI] [PubMed] [Google Scholar]
- 42.Stoyanov J. V., Brown N. L. (2003) J. Biol. Chem. 278, 1407–1410 [DOI] [PubMed] [Google Scholar]
- 43.Stoyanov J. V., Magnani D., Solioz M. (2003) FEBS Lett. 546, 391–394 [DOI] [PubMed] [Google Scholar]
- 44.Carter P. B., Collins F. M. (1974) J. Exp. Med. 139, 1189–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jensen V. B., Harty J. T., Jones B. D. (1998) Infect. Immun. 66, 3758–3766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tottey S., Harvie D. R., Robinson N. J. (2005) Acc. Chem. Res. 38, 775–783 [DOI] [PubMed] [Google Scholar]
- 47.Field L. S., Luk E., Culotta V. C. (2002) J. Bioenerg. Biomembr. 34, 373–379 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.