Abstract
Neuronal differentiation is characterized by neuritogenesis and neurite outgrowth, processes that are dependent on membrane biosynthesis. Thus, the production of phosphatidylcholine (PtdCho), the major membrane phospholipid, should be stimulated during neuronal differentiation. We demonstrate that during retinoic acid (RA)-induced differentiation of Neuro-2a cells, PtdCho synthesis was promoted by an ordered and sequential activation of choline kinase α (CKα) and choline cytidylyltransferase α (CCTα). Early after RA stimulation, the increase in PtdCho synthesis is mainly governed by the biochemical activation of CCTα. Later, the transcription of CKα- and CCTα-encoding genes was induced. Both PtdCho biosynthesis and neuronal differentiation are dependent on ERK activation. A novel mechanism is proposed by which PtdCho biosynthesis is coordinated during neuronal differentiation. Enforced expression of either CKα or CCTα increased the rate of synthesis and the amount of PtdCho, and these cells initiated differentiation without RA stimulation, as evidenced by cell morphology and the expression of genes associated with neuritogenesis. The differentiation resulting from enforced expression of CCTα or CKα was dependent on persistent ERK activation. These results indicate that elevated PtdCho synthesis could mimic the RA signals and thus determine neuronal cell fate. Moreover, they could explain the key role that PtdCho plays during neuronal regeneration.
Keywords: Mitogen-activated Protein Kinases (MAPKs), Membrane Lipids, Neurodifferentiation, Neurotrophic Factor, Phosphatidylcholine
Introduction
The sprouting of neurites, the growth of an axon, and the extension of neurite trees are key morphological features characterizing neuronal differentiation. Neurite outgrowth is important for neuronal plasticity as well as for neuronal regeneration after injuries or neuropathological conditions. The in vitro differentiation to a neuron-like phenotype by the Neuro-2a mouse neuroblastoma cell line has often been used as a model system to investigate the mechanisms underlying neurite formation (1–3). These cells respond to trans-retinoic acid (RA)3 with a halt in proliferation and morphological changes that include the formation of an elaborate network of neurites (4). The development of neurites involves a major increase in the surface area of the cell, and the mechanisms that account for the growth of surface membrane structures are poorly understood. Inhibition of protein (5) or membrane lipid (6, 7) synthesis reduces neurite extension, thus indicating that these biochemical processes are critical to the morphological alteration. Phosphatidylcholine (PtdCho) is the major phospholipid building block of membranes, and the supply of PtdCho can be regulated by the biochemical activity of key enzymes (8), gene expression of biosynthetic or degradative enzymes (9–11), or intracellular trafficking (12). PtdCho can be synthesized de novo in mammalian cells by two pathways as follows: 1) the Kennedy pathway, also known as the CDP-choline pathway (Fig. 1A) (13), and 2) the phosphatidylethanolamine methylation pathway (14). Although the rate of PtdCho biosynthesis by the Kennedy pathway is governed in many instances by the rate of conversion of phosphocholine to CDP-choline in a reaction catalyzed by the CTP:phosphocholine cytidylyltransferase (CCT), ample evidence suggests a regulatory role for choline kinase (CK) as well (15).
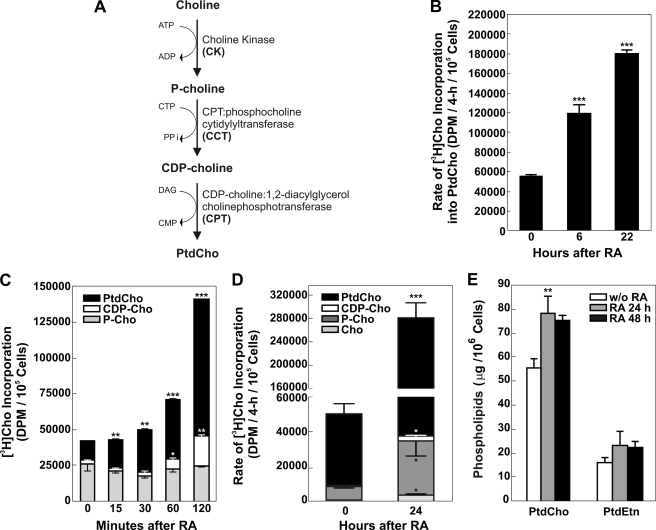
FIGURE 1.
A, schematic representation of CDP-choline Kennedy pathway. DAG, diacylglycerol. B, stimulation of the CDP-choline pathway after RA. Neuro-2a cells were incubated with 20 μm RA for 0–24 h. At the times indicated, cells were radiolabeled with [3H]choline for 4 h, and lipids were extracted, and the radiolabel associated with PtdCho was quantified by scintillation counting. C, cells were radiolabeled with [3H]choline continuously from 0 to 120 min after addition of RA. The distribution of significant amounts of radioactivity among the pathway components was quantified by scintillation counting following lipid extraction of the cells and thin layer chromatography as described under “Experimental Procedures.” Data represent the mean of two determinations ± S.D. D, at times indicated, cells were radiolabeled with [3H]choline for 4 h; lipids were extracted, and the radiolabel associated with choline (Cho), phosphocholine (P-Cho), cytidine diphosphocholine (CDP-Cho), and phosphatidylcholine (PtdCho) was quantified by scintillation counting following thin layer chromatography as described under “Experimental Procedures.” The data represent the mean of four determinations ± S.D. E, cells were incubated with RA for the times indicated, and the amount of PtdCho or PtdEtn was determined following lipid extraction and quantification using the Iatroscan as described under “Experimental Procedures.” The data represent the mean of four determinations ± S.D. Significance was determined comparing untreated cells and treated with RA with the Student's t test: *, p < 0.05; **, p < 0.01; ***, p < 0.001.
The following two genes encode CCT: Pcyt1a, located on murine chromosome 16, expresses alternative transcripts termed CCTα1 and CCTα2 that, when translated, produce the same protein called CCTα (16); Pcyt1b, located on the X chromosome, expresses the CCTβ2 and CCTβ3 proteins from alternative promoters and differentially spliced transcripts (17, 18). Although CCTα is expressed in all tissues, CCTβ2 and CCTβ3 are most highly expressed in brain (16, 19). CK catalyzes the phosphorylation of choline by ATP yielding phosphocholine and has some reactivity with ethanolamine (20). At least three protein isoforms of CK, termed CKα1, CKα2, and CKβ, have been identified. The first two isoforms are derived from the same gene, Chka, by alternative splicing, and the third isoform is the product of a distinct gene named Chkb (21).
PtdCho biosynthesis takes place in cell bodies and in distal axons of neurons (22, 23). However, limited information is available that describes the molecular mechanisms by which the supply of new membrane meets the demand for neuritogenesis (24). PtdCho is required for axonal elongation and growth, and inhibition of PtdCho biosynthesis by choline deficiency inhibits neurite elongation (25, 26), implicating the CDP-choline pathway as essential. PtdCho synthesis increases in PC12 cells when neurite outgrowth is induced by nerve growth factor (NGF). Carter et al. (27, 28) revisited the differentiation of PC12 cells and demonstrated that the expression of the CCTβ isoform and CCT activity were enhanced during neuronal differentiation, promoting neurite outgrowth and branching. CCTβ2 was thought to be selectively up-regulated, but independent quantitative analysis of transcripts showed that the expression of both isoforms CCTα and CCTβ2 increased following NGF induction (19). The expression of CCTα was either the same (27) or increased (19) following neurite formation in PC12 cells. Araki and Wurtman (29) concluded that the increase in PtdCho biosynthesis induced by NGF treatment was exclusively due to an activation of the final step enzyme in the CDP-choline pathway, CDP-choline:1,2-diacylglycerol cholinephosphotransferase (CPT), due to its saturation by rising levels of diacylglycerol. CK was not investigated in any of these later studies. In light of these varied results, we hypothesized that a coordinated gene expression mechanism involving more than one activity may exist to stimulate PtdCho biosynthesis during neuronal differentiation. Here, we report that an increase in PtdCho biosynthesis is mediated by enhanced gene expression of key enzymes in the CDP-choline pathway, namely CKα and CCTα. We also provide evidence demonstrating that the mechanism by which RA activates this genetic program involves ERK1/2 activation. To evaluate the role of PtdCho in neuritogenesis, we found that enforced expression of CCTα or CKα is sufficient to induce PtdCho biosynthesis, a persistent ERK activation, and trigger cell differentiation. These results provide new insight into the mode of action of RA and suggest that an aspect of PtdCho metabolism acts as a neurotrophin-like signal to help guide the development of a neuroblast into a mature neuron.
EXPERIMENTAL PROCEDURES
Tissue Culture
The mouse neuroblastoma cell line Neuro-2a (ATCC CCL-131) was cultured in modified Eagle's medium (MEM), 10% fetal bovine serum (FBS) supplemented with penicillin G (100 units/ml), streptomycin (100 μg/ml) and maintained in a 5% CO2 humidified incubator at 37 °C. To induce neuritogenesis, the medium was changed to Dulbecco's modified Eagle's medium (DMEM) plus 2% FBS containing up to 10–20 μm trans-RA (Sigma) as indicated (4). Stable cell lines overexpressing CCTα or CKα were established following transfection with the plasmid vector pTandem-1 (Novagen) alone or vectors containing the cDNAs encoding Pcyt1a or Chka followed by clonal selection in medium containing 500 μg/ml Geneticin (Invitrogen). Individual clones were screened for overexpression by Western blotting or by increased enzyme-specific activity. CKα promoter-luciferase plasmid was constructed by cloning the 1753 bp corresponding to the proximal promoter region into pGL3-Basic (Promega). The promoter was amplified by PCR using the indicated primer (Table 1). Transient transfections with the CCTα (30) and CKα promoter-luciferase reporter plasmids (1 μg) were performed using a cationic liposome method (Lipofectamine 2000, Invitrogen). All dishes received 0.5 μg of pSV-β-galactosidase (Promega) as a control for transfection efficiency. Luciferase and β-galactosidase assays were performed using Promega luciferase assay systems, as recommended by the manufacturer, and luminometric measurements were made using Fluskan Ascent FL type 374 (Thermo Labsystems). Luciferase activity was normalized to β-galactosidase activity and expressed as fold of induction, which was calculated as a ratio between luciferase/β-galactosidase in undifferentiated cells and luciferase/β-galactosidase in RA-treated cells.
TABLE 1.
Primers and probes
Morphometric Analysis
The corresponding cell lines were plated at density of 2 × 104/35-mm dish for 12 h, after which the medium was changed to DMEM supplemented with 2% FBS and RA (10 μm). Control cells were maintained in MEM supplemented with 10% FBS. After 24 h, cells were observed by phase contrast microscopy (Olympus CK2), and 15–20 random fields of view were sampled. Cells bearing at least one neurite equal to or longer than the soma diameter were considered to be differentiated. To calculate the different parameters (percent of cells bearing neurites, total neurite length per differentiated cell, neurite length average, total neurite number per differentiated cell, and absolute frequency of cells bearing neurites equal to or longer than 1 or 2 soma diameter), the number and length of neurites per differentiated cell and total cell number were counted and/or measured in each field, using the “ImageJ” (National Institutes of Health) Software.
Western Blot Analysis and Immunofluorescence
For Western blot analysis, cell lines were plated at a density of 5 × 105/100-mm dish for 12 h, after which the medium was changed to DMEM supplemented with 2% FBS and RA (10 μm). The MEK1/2 U0126 inhibitor was preincubated during 15 min at a final concentration of 10–30 μm. Control cells were maintained in MEM supplemented with 10% FBS. After 48 h of treatment with RA, cells were collected, resuspended in 1× lysis buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 2 mm EDTA, 1 mm dithiothreitol, 0.1 mm phenylmethylsulfonyl fluoride, and 100 μg/ml leupeptin), and sonicated five times for 5 s at 5% (Sonics and Materials Inc-Vibra CellTM). Proteins concentrations were determined using bovine serum albumin as standard protein and “Sedmak and Grossberg” reagent (31). Briefly, an appropriate volume of samples or bovine serum albumin was mixed with Sedmak and Grossberg reagent and incubated during 5 min. Absorbance (A) at 620 and 465 nm was measured, and the ratio A620/465 nm was related to micrograms of bovine serum albumin. 40 μg of cell lysate were resolved on 12% SDS-PAGE and transferred to a nitrocellulose membrane (Amersham Biosciences). After blocking with 5% milk in 1× TBS (10 mm Tris-HCl, pH 7.5, 137.8 mm NaCl) and washing with 1× TBS, blots were incubated overnight with anti-βIII-tubulin primary antibody (1:5000, Covance). The membranes were then incubated with anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:7000, Amersham Biosciences) and developed using a chemiluminescence detection kit (Thermo Scientific). For p-ERK1/2 immunoblotting, cells were washed and raised with washing buffer (1× phosphate-buffered saline, 15 mm NaF, 1 mm Na3VO4, 1 mm EGTA, 100 μm phenylmethylsulfonyl fluoride, and Protease Inhibitor Mixture (Sigma)). Cells were collected, resuspended in 1× lysis buffer (20 mm Tris-HCl, pH 7.5, 150 mm NaCl, 15 mm NaF, 1 mm Na3VO4, 1 mm EGTA, 100 μm phenylmethylsulfonyl fluoride, and protease inhibitor mixture (Sigma)), and processed as described above. 10–20 μg of cell extracts were resolved on 12% SDS-PAGE and transferred to a nitrocellulose membrane (Amersham Biosciences). After blocking and washing, blots were incubated overnight with anti-p-ERK1/2 primary antibody (1:500, Santa Cruz Biotechnology). Secondary antibody was used as described previously. Loading protein control was demonstrated by measuring the levels of β-actin or α-tubulin using anti-β-actin (1:600, Santa Cruz Biotechnology) and anti-α-tubulin (1:5000), respectively. For immunofluorescence, cells were plated at low cell density in a coverslip and grown for 12 h, after which medium was changed to DMEM supplemented with 2% FBS and RA (10 μm) or maintained in MEM containing 10% FBS. The cells were then fixed using 4% formaldehyde and permeabilized with 0.05% saponin. The following antibodies were used: βIII-tubulin (1:1000, Covance); p-ERK1/2 (1:500, Santa Cruz Biotechnology); and anti-rabbit Alexa Fluor® 488-labeled (1:800, Invitrogen). Slides were mounted with Prolong 4′,6-diamidino-2-phenylindole (Invitrogen). Microscopy was carried out at room temperature using a confocal microscope (Nikon model Eclipse TE-2000-E2 C1 plus) equipped with Plan Apochromat 20.0×/0.75/1.00 dry objective; Nikon C1 standard detector, and Nikon EZ-C1 3.60 software. Images were adjusted for contrast and gamma (60/1.4 for βIII-tubulin staining, 65/1.3 for p-ERK staining) using Nikon EZ-C1 3.70 FreeViewer software.
Lipid Extraction
Cell pellets were resuspended in 1 ml of water or 1× phosphate-buffered saline. Lipids were extracted by the method of Bligh and Dyer (see Ref. 32) using 2.4 ml of acidified methanol (2% acetic acid, v/v) and 1 ml of chloroform in the first step, followed by 1.5 ml of chloroform and 1.2 ml of water in the second step to yield two phases, organic and aqueous. The organic phase was collected and dried.
Metabolic Labeling
Cells were incubated with 10 μm RA for the times indicated. For rate measurements, cells were harvested and resuspended in medium supplemented with 5 μCi/ml methyl[3H]choline (specific activity, 85 Ci/mmol) or 2 μCi/ml [2-14C]acetate (specific activity, 55 mCi/mmol), obtained from American Radiolabeled Chemicals. After labeling for 2 or 4 h, cells were harvested, counted, and subjected to extraction according to the method of Bligh and Dyer (32). For continuous labeling experiments, 5 μCi/ml methyl[3H]choline (specific activity, 85 Ci/mmol) was added together with the RA, and the cells were incubated for the times indicated. After labeling, cells were harvested, counted, and subjected to extraction according to the method of Bligh and Dyer (32). The amount of radiolabel incorporated into the organic and aqueous phases was quantified by scintillation counting. A 50-μl aliquot of each aqueous phase was spotted onto preadsorbent Silica Gel H layers (Analtech) that were developed in methanol, 0.1 m NaCl, ammonium hydroxide (50:50:5, v/v). Choline, phosphocholine, and CDP-choline were identified by co-migration with standards. The bands were excised, and the fractional distribution of choline-labeled intermediates was determined by scintillation counting of the excised bands. The [2-14C]acetate incorporation into fractionated lipid molecular species was determined following two-dimensional thin layer chromatography of organic phase on Silica Gel H layers developed in chloroform/methanol/water (75:25:2.5, v/v) and chloroform/methanol/acetic acid/water (80:9:12:2, v/v). Bands co-migrated with authentic lipid standards. Radioactivity on the plates was visualized using a Typhoon 9200 PhosphorImager screen and quantified using ImageQuant software (version 5.2). These layers were used to analyze the total phospholipids, and they were stained using CuSO4/H3PO4 method (33) and quantified using ImagePro3 software.
RNA Transcript Measurements
Total RNA from cells cultured with or without retinoic acid for the times indicated was isolated using TRIzol (Invitrogen). Contaminating genomic DNA was removed by digestion with DNase I, and aliquots were stored as ethanol precipitates at −20 °C. cDNA was prepared from RNA by reverse transcription using SuperScript II RNase H reverse transcriptase (Invitrogen) and random primers. Primers and probes for real time RT-qPCR were designed using Primer Express® software (version 2.0, Applied Biosystems) and are listed in Table 1. Real time RT-qPCR was carried out using the 7300 Real Time PCR System and 7300 System SDS software (version 1.2.3, Applied Biosystems). The TaqMan Rodent glyceraldehyde-3-phosphate dehydrogenase control reagent (Applied Biosystems) was the source of the primers and probes for quantifying the Gapdh mRNA. Primers and probes used to quantify control β-actin mRNA (Actb) are listed in Table 1. The collected data were analyzed using the CT method (41); the amount of target RNA was normalized to the endogenous Gapdh or Actb reference and related to the amount of target RNA in untreated cells. The specific number of experiments (n) and p values for statistical significance as evaluated by Student's t test (unpaired) are reported in each legend; the following convention was used for representing significance: *, 0.01 < p < 0.05; **, 0.001 < p < 0.01; and ***, p < 0.001.
Affymetrix Array Analysis
Total RNA was used to prepare cDNA for hybridizing, washing, and scanning of a GeneChip® Mouse Genome 430 2.0 array (Affymetrix, Inc., Santa Clara, CA) using a GeneChip® Fluidics Station 400 and a GeneArrayTM scanner. Data were collected using Microarray Suite software (formerly known as GeneChip® Suite software). Comparison and statistical analysis of all the Affymetrix data were achieved using Spotfire® DecisionSiteTM 8.11 (Spotfire, Inc.) software. The number of experiments and p values for statistical significance as evaluated by Student's t test (unpaired) are reported in the footnote to Table 1.
Inhibitor Treatment
A 10 mm stock solution of MAPK (MEK1/2) U0126 inhibitor (Promega) was prepared in DMSO, stored at −20 °C in the dark, and diluted with media before use. For the assay, cells either were pretreated with or without the inhibitor for 15 min and then stimulated with RA.
Cells were incubated with 10 μm RA, and 0.1 μm cycloheximide, 0.1 μg/ml actinomycin D, or 20 μm U0126 was added at the times indicated. The cells were radiolabeled for 4 h, at the corresponding times, using [2-14C]acetate. After radiolabeling, lipids were extracted by the Bligh and Dyer method and were analyzed by thin layer chromatography.
RESULTS
PtdCho Biosynthesis Is Increased during Neuritogenesis in Neuro-2a Cells
The rate of PtdCho biosynthesis was measured in Neuro-2a cells at different times (0, 6, and 22 h) during incubation with 20 μm RA. Cells were radiolabeled with [3H]choline for 4 h; lipids were extracted at the indicated time points, and the radiolabel associated with PtdCho was quantified by scintillation counting. The data in Fig. 1B show that the rate of PtdCho biosynthesis doubled after 6 h of RA treatment and increased further thereafter, as measured after 22 h of treatment. To determine the particular steps in the Kennedy pathway that were activated, cells were radiolabeled with [3H]choline continuously from 0 min up to 2 h after RA addition (Fig. 1C) and for another 2 h after 24 h of RA treatment (Fig. 1D). The distribution of radioactivity among the choline metabolites (choline, phosphocholine, and cytidine diphosphocholine (CDP-Cho)) was quantified. The levels of phosphocholine remained constant during the first 2 h, although the levels of CDP-Cho increased after 1 h, indicating an early stimulation of CCT activity, either by biochemical or gene expression mechanisms, which resulted in increased PtdCho synthesis. The increase in PtdCho synthesis within 30 min after RA addition could suggest a very early activation of the CPT step, prior to CDP-Cho accumulation. It is unlikely that changes in gene expression would occur within this short time, and so the data suggest biochemical activation of CPT, perhaps by an increase in the diacylglycerol levels, as was reported previously (29). Later, after 24 h of RA treatment, there was a significant increase in the amounts of choline and phosphocholine, in addition to the increase in CDP-Cho and PtdCho (Fig. 1D). These data indicated a stimulation of choline uptake and CK activity after CCT activation and during RA-induced PtdCho synthesis and suggested differential induction of CKα and CCTα activity during neuritogenesis, both contributing to increase the rate of PtdCho biosynthesis. The increased flux through the CDP-Cho pathway resulted in more PtdCho per cell after 24 or 48 h of RA treatment (Fig. 1E). Phosphatidylethanolamine (PtdEtn), the second most abundant phospholipid in mammalian membranes, increased proportionally, and a ratio of about 3.4 PtdCho-to-PtdEtn was maintained during RA-induced differentiation (Fig. 1E).
PtdCho Biosynthetic Gene Expression in Neuro-2a Cells
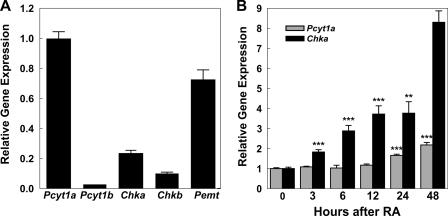
We first surveyed gene expression using an Affymetrix microarray to identify those genes encoding enzymes involved in the PtdCho biosynthetic pathways that were expressed in Neuro-2a cells and that changed in response to RA. Both CKα and CKβ were expressed, but only CKα increased substantially following RA treatment. The survey also revealed an increase in CCTα expression. We were unable to determine changes in CCTβ expression due to its low expression, and the gene encoding the CPT remained unaffected by RA treatment. The expression of the Pcyt2 gene, encoding the ethanolamine cytidylyltransferase, increased following RA as was confirmed by RT-PCR, and this was consistent with the concomitant increase in the PtdEtn content of the cells after RA treatment (Fig. 1E). Real time RT-qPCR was used to confirm and quantify the levels of transcripts in the PtdCho biosynthetic pathway. The transcripts encoding enzymes of the Kennedy pathway (Pcyt1a, Pcyt1b, Chka, and Chkb) and also the phosphatidylethanolamine methylation (Pemt) were expressed in unstimulated Neuro-2a cells at strikingly different levels (Fig. 2A), and we found that Pcyt1a and Chka transcripts significantly increased during differentiation (Fig. 2B). Pemt and Chkb expression did not exhibit any change, and the data obtained for Pcyt1b were not statistically reliable, probably due to its low expression levels (Fig. 2A). The data indicated an early accumulation of Chka mRNA levels, followed by a later increase of Pcyt1a mRNA levels. Thus, the early stimulation of PtdCho biosynthesis was driven by the CCTα activity due, at least in part, to its biochemical activation, probably through association of the protein with membrane lipids (34), whereas the later increase in Pcyt1a (CCTα) transcript levels extended and sustained the increased PtdCho biosynthesis. In contrast, CKα mRNA increased early during RA-induced neuritogenesis.
FIGURE 2.
Expression of genes involved in PtdCho biosynthesis. A, RNA was isolated from Neuro-2a cells without treatment, and transcript levels were quantified by real time RT-qPCR using gene-specific primers and probes. Data were normalized to glyceraldehyde dehydrogenase mRNA and expressed relative to Pcyt1a levels. Pcyt1a encodes choline cytidylyltransferase α; Pcyt1b encodes choline cytidylyltransferase β2; Chka encodes choline kinase α; Chkb encodes choline kinase β. Data represent the mean of three determinations ± S.D. B, Neuro-2a cells were incubated with 20 μm RA for the indicated times, and transcript levels were quantified by real time RT-qPCR using gene-specific primers and probes. Data were normalized to glyceraldehyde dehydrogenase mRNA and expressed relative to the levels in untreated cells. Data represent the mean of three determinations ± S.D. Significance was determined comparing untreated cells and treated with RA with the Student's t test: **, p < 0.01; ***, p < 0.001.
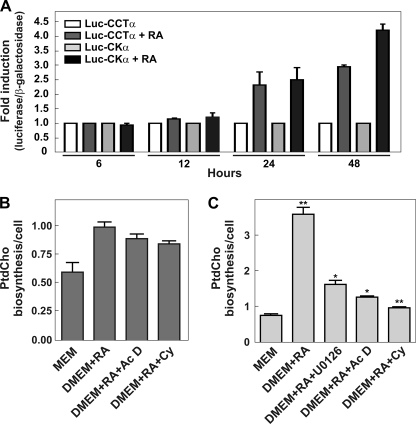
We used promoter-reporter assays to investigate transcriptional activation of the genes encoding CKα and CCTα in response to RA. The activities were measured in Neuro-2a cells that were transfected with plasmid constructs containing the previously identified and characterized proximal promoters for CCTα1 (−2100/+38) or CKα (−1606/+146) (30, 35, 36) upstream of luciferase cDNA, plus a plasmid carrying CMV-β-galactosidase as a control for transfection efficiency. RT-PCR revealed that the CCTα1, but not the CCTα2 transcript, was expressed in this cell type and thus guided our selection of the promoter upstream of CCTα1. RA was added to the cells 24 h after transfection, and relative luciferase activities were measured after 6, 12, 24, and 48 h of RA treatment (Fig. 3A). No differences were observed at the shorter time points; however, after 24 h of RA treatment, we detected a 2-fold induction of both CCTα and CKα transcriptional activities. RA treatment for 48 h resulted in a 3-fold increase in CCTα and a 4.5-fold increase in CKα promoter activities. No effect was observed when the assay was performed in differentiation medium (DMEM plus 2% FBS) but in the absence of RA, confirming that the induction of the transcription of these genes was specifically due to RA and not due to serum deprivation (supplemental Fig. S1). These data revealed an RA-dependent activation of CCTα and CKα transcription by 24 h, and indicated that CKα message stabilization at the earlier times after RA addition was responsible for raising its transcript levels (Fig. 2B). These results were confirmed by measuring PtdCho biosynthesis in cells treated with actinomycin D, which inhibits RNA synthesis, during the first 4 h and after 24 h of RA stimulus (Fig. 3, B and C). The increase in PtdCho biosynthesis was not affected by actinomycin D early after RA stimulation, whereas a clear attenuation of the rate of PtdCho biosynthesis was observed after 24 h. These data supported the view that transcriptional stimulation of both CKα and CCTα expressions maintained the later phase of membrane expansion. Treatment of RA-stimulated cells with cycloheximide, an inhibitor of protein synthesis, did not have an effect on PtdCho synthesis after 4 h but reduced the rate of PtdCho formation when added 24 h after RA (Fig. 3C). These data indicated that the transcriptional activation of both CKα and CCTα expressions was dependent on new protein synthesis and thus the genes encoding these enzymes could not be classified as immediate early genes. These data also support the conclusion that the early phase of RA-stimulated PtdCho synthesis was mediated by biochemical mechanisms that were not reliant on new protein synthesis.
FIGURE 3.
Transcriptional analysis of Pcyt1a and Chka genes during Neuro-2a differentiation. A, Neuro-2a cells were transfected with Luc-CCTα or Luc-CKα proximal promoter reporter constructs together with pSV-β-galactosidase as a transfection control. 24 h after transfection, cells were grown in proliferating media (MEM, 10% FBS) or in differentiating media (DMEM supplemented with 2% FBS) supplemented with 20 μm RA. Luciferase (Luc) and β-galactosidase activities were measured at 6, 12, 24, and 48 h after differentiation was initiated. Graphs represent the ratio between luciferase/β-galactosidase obtained in differentiating versus no-differentiating conditions from three independent experiments. The values are means ± S.E. B and C, Neuro-2a cells were treated with U0126 (10 μm), actinomycin D (Ac D) (0.1 μg/ml), and/or cycloheximide (Cy) (0.1 μm) in presence of RA (20 μm) for 30 min (early stimulation (B)) or 20 h (later stimulation (C)). After that, cell were labeled with 2 μCi/ml [2-14C]acetate during 4 h; samples were collected and lipids analyzed as described previously. Results are expressed as PtdCho biosynthesis/cells, and the graphs are representative of two independent experiments ± S.E. Significance was determined by comparing untreated cells and cell treated with RA with or without inhibitors with the Student's t test: *, p < 0.05; **, p < 0.01.
Neuritogenesis Is Stimulated by Increased PtdCho Biosynthesis
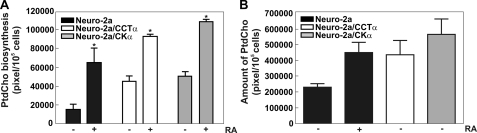
Differentiation of Neuro-2a cells was accompanied by increased PtdCho biosynthesis (Fig. 1) and by the enhanced expression of phospholipid biosynthetic genes (Figs. 2 and 3). Our results highlight Chka as an inducible gene during neuritogenesis along with Pcyt1a, a well characterized gene encoding the enzyme that is recognized to have a regulatory role in the PtdCho biosynthetic pathway (34, 37). Published biochemical experiments indicated that CCTα overexpression promoted PtdCho biosynthesis in other cells (11), and so we determined whether enforced expression of CCTα or CKα was capable of promoting PtdCho biosynthesis and driving the process of neurite outgrowth. Stable Neuro-2a cell lines were derived that expressed CCTα (Neuro-2a/CCTα) or CKα (Neuro-2a/CKα) constitutively, and enhanced expression of each gene in the absence of RA was confirmed by CCT in vitro activity assays or immunoblotting with anti-CKα antibody, respectively. The clonal cell lines that exhibited increased levels of expression were selected for further investigation. In the absence of RA, the clonal cell lines with constitutive expression of CCTα or CKα exhibited increased basal rates of PtdCho biosynthesis that were comparable with those observed upon RA treatment of the control Neuro-2a cells (Fig. 4A). The rate of PtdCho formation before stimulation was 2.9-fold higher in the Neuro-2a/CCTα cells compared with Neuro-2a cells and increased up to 2-fold after 24 h of RA stimulation. Similarly, the rate of PtdCho production was 3.2-fold higher in the unstimulated Neuro-2a/CKα cells and increased 2.1-fold after RA treatment. These data demonstrated that increased expression of either CCTα or CKα was sufficient to drive PtdCho synthesis without requiring RA stimulation. The increased rate of PtdCho synthesis was reflected in the total amount of PtdCho per cell, which was greater in the Neuro-2a/CCTα and Neuro-2a/CKα cell lines prior to RA stimulation and comparable with that observed upon RA stimulation of the control Neuro-2a cells (Fig. 4B).
FIGURE 4.
Increased PtdCho synthesis after RA treatment in cells that overexpress CDP-choline pathway proteins. A, cell lines were incubated with 20 μm RA, and at the indicated times were pulsed with [2-14C]acetate for 4 h, and lipids were extracted, and the radiolabel associated with PtdCho was analyzed by thin layer chromatography and quantified by ImageQuant software as described under “Experimental Procedures.” B, graph shows the amount of PtdCho/cells obtained in Neuro-2a ± RA and in the indicated cell lines analyzed by thin layer chromatography and quantified by ImageQuant software. Graphs are representative of three independent assays ± S.E. Significance was determined comparing untreated Neuro-2a cells and each stable cell line with the Student's t test: *, p < 0.05.
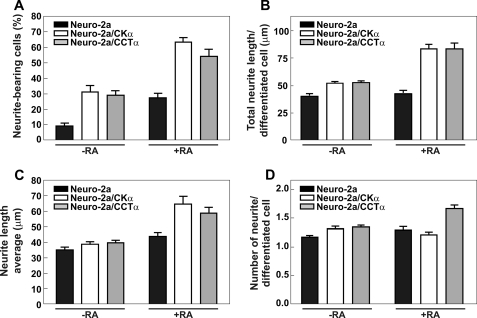
We next determined whether the enhancement of PtdCho biosynthesis in the cells that expressed CCTα or CKα in a constitutive manner corresponded to an increased number of neurite-bearing cells. In contrast to the Neuro-2a control progenitor cells that were transfected with empty vector, both the Neuro-2a/CCTα and Neuro-2a/CKα cell lines exhibited an increased number of cells with budding neurites even prior to the addition of RA, being 3.2- and 3.4-fold greater for Neuro-2a/CCTα and Neuro-2a/CKα, respectively (Fig. 5A). In addition, the fractions of cells that were differentiating exceeded the maximal fraction of differentiating control cells after 24 h of RA treatment (Fig. 5A). Comparison of the data among the three cell lines indicated that both CKα and CCTα expression promoted neurite outgrowth. The total neurite length(s) per cell (Fig. 5B), the average length of the neurites (Fig. 5C), and the number of neurites per cell (Fig. 5D) were measured, and both engineered cell lines showed augmented total neurite length(s) per cell in the presence of RA (Fig. 5B). This parameter indicates either that cells increased the number of neurites or generated longer neurites. In addition, Neuro2-a/CCTα cells showed an increased number of neurites per cell (Fig. 5D).
FIGURE 5.
CCTα and CKα overexpression promotes neuritogenesis. Neuro-2a, Neuro-2a/CCTα, or Neuro-2a/CKα cells were grown in the presence or absence of RA (20 μm) for 24 h. A, graph represents the percentage (%) of neurite-bearing cells for each cell line grown in the presence and in the absence of RA. B, graph represents total neurite length (μm) per differentiated cell for each cell line growth in the presence and absence of RA. C, graph represents the average of neurite length (μm) per differentiated cell for each cell line growth in presence and absence of RA. D, graph represents the number of neurites per differentiated cell for each cell line growth in the presence and absence of RA. Graphs are representative of five independent experiments ± S.E.
βIII-Tubulin Expression as a Differentiation Marker
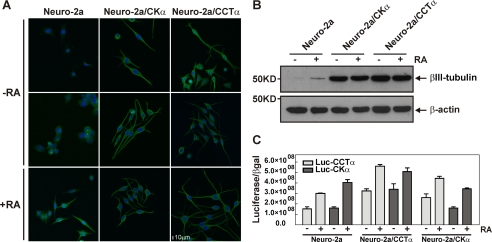
The βIII-tubulin is one of the earliest cytoskeletal proteins specifically associated with neuronal development (38). Thus, it is considered to be a marker protein for neuronal differentiation (39). We investigated whether Neuro-2a cells that constitutively expressed either CCTα or CKα had increased expression of βIII-tubulin in addition to an increased rate of PtdCho biosynthesis (Fig. 4) and greater neurite formation (Fig. 5). In the absence of RA, Neuro-2a progenitor cells did not express detectable levels of βIII-tubulin, as measured either by Western blot or immunocytochemistry (Fig. 6, A and B). However, this protein was readily detectable after RA treatment. Interestingly, Neuro-2a/CKα and Neuro-2a/CCTα cell lines initiated neurite formation and expressed high levels of βIII-tubulin even in the absence of RA. These results suggested that increased expression of either CCTα or CKα promoted the expression of other genes associated with neuronal differentiation. An Affymetrix microarray analysis comparing the expression of specific neuronal genes in unstimulated Neuro-2a/CCTα cells with those expressed in the parental Neuro-2a line revealed a large group of genes whose expression was enhanced more than twice when CCTα was overexpressed (Table 2). Among the annotated genes, we highlight the induction of syntaxin-3, a plasma membrane protein present in the neuronal growth cone and essential for neurite outgrowth (40). Furthermore, the engineered cell lines without RA showed increased levels of CCTα and CKα transcription, reaching similar levels to those of the control cells after RA treatment. The exception is the transcription of CKα in Neuro-2a/CKα cells that showed levels similar to control cells. In all the cases, the levels of transcription were enhanced after RA treatment (Fig. 6C).
FIGURE 6.
βIII-tubulin expression in cells that trigger differentiation when PtdCho biosynthesis is stimulated. A, detection of βIII-tubulin (green) by immunocytochemistry in Neuro-2a, Neuro-2a/CKα, and Neuro-2a/CCTα cells treated with (+RA) or without (−RA) RA. Images were adjusted for contrast and gamma (60/1.4) using Nikon EZ-C1 3.70 Free Viewer software. Bar, 10 μm. B, Western blot analysis shows the levels of βIII-tubulin in each condition for each cell lines. Samples were collected after RA treatment, and equal amounts of cell lysates (40 μg) were subjected to SDS-PAGE and immunoblotting with anti-βIII-tubulin or anti-β-actin antibodies. Immunoblot is representative of three independent experiments. C, Neuro-2a/CCT α, Neuro-2a/CCTα, and Neuro-2a cell lines were transfected with Luc-CCTα or Luc-CK α proximal promoter reporter constructs together with pSV-β-galactosidase as a transfection control. 24 h after transfection, cells were grown in proliferating media (MEM supplemented with 10% FBS) or in differentiating media (DMEM supplemented with 2% FBS) supplemented with 20 μm RA. Luciferase and β-galactosidase activities were measured at 24 h after differentiation was initiated. Graph represents the ratio between luciferase/β-galactosidase and is representative of two independent experiments each one performed by duplicate.
TABLE 2.
Neuronal gene expression stimulated by increased PtdCho
The statistical analyses were from six data sets (three untreated Neuro-2a control cells and three untreated Neuro-2A/CCTα cells) that were independently processed and hybridized. The significance of enforced CCTα expression was determined using two-tailed, unpaired t tests with the confidence intervals set at 95%. Data with p values ≤0.05 were included in the table.
| Gene | Protein | Fold inductiona |
|---|---|---|
| Axl | AXL receptor tyrosine kinase | 7 |
| Ccl2 | Chemokine (C-C motif) ligand 2 | 6 |
| Pctk3 | PCTAIRE-motif protein kinase 3 | 5.4 |
| Runx3 | Runt-related transcription factor | 5.3 |
| Fst | Follistatin | 4.6 |
| Cxcl1 | Chemokine (CXC) ligand 1 | 3.6 |
| Frk | Fyn-related kinase | 3.6 |
| 03-Sep | Septin 3 | 3.3 |
| Epha7 | Eph receptor A7 | 3.2 |
| Gfra1 | Glial cell line-derived neurotrophic factor family receptor | 3.1 |
| Nfasc | Neurofascin | 2.7 |
| Stx3 | Syntaxin 3 | 2.6 |
| Prrxl1 | Paired related homeobox protein-like 1 | 2.5 |
| Fzd2/5 | Frizzled homolog 2/5 | 2.4 |
| Insl6 | Insulin-like 6 | 2.3 |
| Klf4 | Kruppel-like factor 4 | 2.3 |
| Nrbp2 | Nuclear receptor-binding protein 2 | 2.1 |
| FgFr1 | Fibroblast growth factor receptor 1 | 2 |
| Gsn | Gelsolin | 2 |
| Mapkapk2 | MAPK-activated protein kinase 2 | 2 |
| Six4 | Sine oculis-related homeobox 4 homolog | 2 |
a Fold induction was calculated by comparing the expression of each gene in unstimulated Neuro-2a/CCTa respect to Neuro-2a control cell line.
ERK1/2 Activation Mediates the Extension of Neurites Associated with PtdCho Synthesis in Neuro-2a Cells
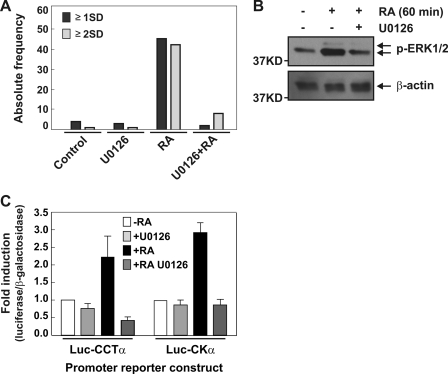
The effect of RA can be mediated by its binding to nuclear receptors, which act as ligand-inducible transcriptional factors that bind as heterodimers together with the retinoid X receptors to RA-response elements located in the promoter regions of target genes (41). Increasing evidence suggests that RA receptors can also mediate rapid extragenomic effects to stimulate signaling pathways by a yet ill-defined mechanism leading to activation of the ERK1/2 cascade (42). In PC12 cells, epidermal growth factor stimulates transient ERK activation and cell proliferation, whereas nerve growth factor induces sustained ERK activation and cell differentiation (43). The activation of ERK1/2 requires phosphorylation of threonine and tyrosine residues by the upstream activator kinase, MEK1/2 (MAPKK). Activated ERK phosphorylates various target molecules including cytoskeletal proteins and transcription factors (44), thus regulating proliferation, differentiation, and cell survival (45, 46). The promoter regions of the murine Pcyt1a and Chka genes lack RA-response elements, so we investigated whether ERK signaling mediates the effect of RA on PtdCho synthesis and neurite formation. Neuro-2a cells were plated at low density in proliferating or differentiating media in the presence or absence of U0126 (10 μm), the ERK1/2-specific MEK inhibitor, and neurite lengths were measured after 24 h. As Fig. 7A shows, treatment of the cells with the inhibitor blocked RA-induced neurite formation in Neuro-2a cells. Moreover, phospho-ERK (p-ERK) levels measured by Western blot analysis revealed that the agonist RA induced ERK phosphorylation and its concomitant activation in cells within 1 h, an effect that was prevented in the presence of U0126 (Fig. 7B). ERK phosphorylation was detected for up to 2 h after RA induction (supplemental Fig. S2). Considering that RA-induced differentiation promotes CCTα and CKα transcription (Fig. 3), we investigated if the transcriptional activity of one or both genes was affected by U0126 treatment. We assayed the activity of the promoter-reporter constructs described previously but in the presence or absence of U0126 (10 μm). The inhibitor prevented the RA-dependent induction of gene expression, maintaining the transcription of CCTα and CKα close to the basal levels (Fig. 7C) and also preventing the increase in PtdCho biosynthesis (Fig. 3C). Taken together, these data indicated that RA-dependent differentiation in Neuro-2a cells and the coordinated increase in CCTα and CKα gene transcription were mediated by ERK1/2 kinase activation.
FIGURE 7.
RA activates MAPK pathway in Neuro-2a cells. A, Neuro-2a cells were cultured in growing or differentiating media (containing RA 20 μm) in presence or absence of U0126 (10 μm) during 24 h and analyzed morphometrically. Graph depicts the absolute frequency of cells containing neurites equal to or longer than one soma diameter (≥1SD) or two soma diameters (≥2SD). B, Western blot analysis was used to investigate the phosphorylation state of ERK1/2 in Neuro-2a stimulated with RA (20 μm) during 1 h or without treatment. U0126 was used at 20 μm and preincubated during 15 min. C, Neuro-2a cells were transfected with Luc-CCTα and Luc-CKα promoter reporter construct. After 24 h, media were changed, and RA and U0126 (10 μm) were added to the indicated media and incubated 24 h. Luciferase and β-galactosidase activities were measured and data expressed as fold induction. The values are means ± S.E.
Differentiation Associated with Enhanced PtdCho Biosynthesis Involves ERK1/2 Activation
We next investigated whether ERK1/2 activation was associated with the enhanced PtdCho biosynthesis and neuritogenesis observed in unstimulated cells when the expression of CCTα or CKα was enforced. The levels of p-ERK in populations of Neuro-2a, Neuro-2a/CCTα, and Neuro-2a/CKα cells in the absence of RA were determined by both immunocytochemistry and Western blotting. Fig. 8 demonstrates that both of the engineered cell lines had persistent levels of p-ERK that were similar to the levels reached by the progenitor Neuro-2a cells after RA treatment (Fig. 8, A and B). This result indicated that ERK was activated by elevated PtdCho biosynthesis or increased cellular PtdCho content. We then measured the levels of p-ERK in Neuro-2a/CCTα and Neuro-2a/CKα cells treated with U0126 (10 and 30 μm) for 1 h. The inhibitor treatment resulted in decreased levels of p-ERK in these cell lines, indicating that MEK activity or unknown upstream effectors were positively influenced by PtdCho synthesis in this cellular context (Fig. 8C).
FIGURE 8.
Persistent activation of ERK1/2 accompanies differentiation of CCTα and CKα overexpressing cells. A, levels of p-ERK1/2 (green) were detected by immunocytochemistry in Neuro-2a, Neuro-2a/CCTα, and Neuro-2a/CKα cells with (+RA) or without (−RA) RA treatment. Images were adjusted for contrast and gamma (65/1.3) using Nikon EZ-C1 3.70 Free Viewer software. Bar, 10 μm. Pictures are representative of three independent experiments. B, Western blot analysis was used to investigate the amount of p-ERK1/2 in the indicated cell lines grown in undifferentiated media. Graph is representative of three independent experiments. βIII-actin was used as a control for loading. C, Western blot analysis was used to evaluate the inhibition of ERK phosphorylation by U0126 inhibitor. Each cell line was cultured in undifferentiated media and treated without or with U0126 (10 or 30 μm) during 1 h. Graphs are representative of three independent experiments.
DISCUSSION
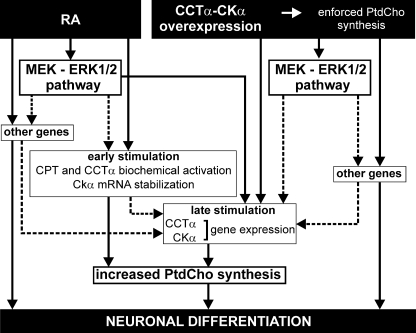
Neuroblastoma cells (Neuro-2a) readily emit neurites when treated with RA, presenting a convenient model of cell membrane expansion. Phosphatidylcholine as a major component of mammalian membranes plays an essential role during neurite outgrowth (6, 7); however, the multiple factors that coordinate PtdCho biosynthesis are poorly understood. PtdCho biosynthesis increases in Neuro-2a cells (Fig. 1B) due to the activation of CKα and CCTα (Figs. 1 and 2). After RA stimulation, cells respond to PtdCho demand by adapting CKα and CCTα activities by two sequential mechanisms (Fig. 9). The early stimulation involves a biochemical activation of CPT (29), CCTα (8), and CKα mRNA stabilization. The rate of PtdCho synthesis increases 3-fold as soon as 6 h after RA addition due to an increase in the pool of CDP-Cho, indicating increased CCTα biochemical activity (Fig. 1) in the absence of increased CCTα mRNA (Fig. 2B). CCTα activity is known to be dependent on its association with membrane phospholipids (37). CKα mRNA accumulates as soon as 3 h after RA (Fig. 2B) in the absence of increased transcription (Fig. 3), indicating stabilization of its mRNA. However, as early PtdCho stimulation was not affected by actinomycin D or by cycloheximide (Fig. 3B), we propose that CCTα activity drives this process, being the CKα mRNA stabilization a mechanism to support later biosynthesis. The increased rate of PtdCho synthesis is prolonged by enhanced transcription of the genes encoding CCTα and CKα within 24 h after RA stimulation and continuing at least until 48 h (Fig. 3). Both the early and late mechanisms of stimulation act coordinately to ensure enough PtdCho for the growing neurites.
FIGURE 9.
Schematic model representing the mechanisms involved in the induction of PtdCho biosynthesis during neuronal differentiation and the cross-talk with RA-induced signaling. Solid lines represent confirmed association between data, and the dotted lines indicate hypothetical relationships without data.
RA is one of the most potent differentiation inducers for human neuroblastoma (47, and it is utilized for the treatment of many types of tumors (48). Our experiments provide insight into the relationship between the RA signaling cascade and PtdCho biosynthesis that occurs during neuronal differentiation. ERKs play an important role in regulating typical neuronal functions such as synaptic plasticity, memory learning, and cell survival (49), so we investigated whether the ERK cascade is involved in the stimulation of PtdCho biosynthesis. ERK1/2 activation was found to be essential for RA-stimulated neuronal differentiation and for RA-stimulated PtdCho biosynthesis, because inhibition of MEK with U0126 abolished both of these processes (Figs. 3 and 7A). Phosphorylation of ERK1/2 is necessary to induce CCTα and CKα transcription during the later phase following RA stimulation, as U0126 also blocked the RA induction of the promoters that regulate the expression of the genes encoding these enzymes (Fig. 7C). Further studies are required to determine whether ERK regulates the activity (by phosphorylation) or the expression (42) of the transcriptional factor(s) that bind to the promoters of the genes encoding CCTα and CKα. We were unable to define the role of ERK1/2 on PtdCho synthesis early after stimulation (Fig. 9). Phosphorylation of CCT by ERK is known to reduce activity, rather than increase activity (50), so it is unlikely that CCT is a substrate for ERK in the context of differentiating neuronal cells.
We hypothesized that enforcing PtdCho synthesis in Neuro-2a cells would result in a strong stimulatory effect on RA-induced differentiation. Derivative cell lines were created to overexpress either CCTα (Neuro-2a/CCTα) or CKα (Neuro-2a/CKα), and remarkably, these cell lines produce as much PtdCho as the control progenitor cells after RA treatment (Fig. 4). These data indicate that enforced expression of either CKα or CCTα is sufficient to promote phospholipid biosynthesis. Surprisingly, the engineered cell lines begin to differentiate even in the absence of RA as the number of budding cells increases significantly. Also, after RA treatment, the fraction of engineered cells that differentiate exceeds the maximal extent of differentiation in the absence of stimuli, suggesting the existence of a synergistic mechanism. In support of the hypothesis, the engineered cell lines have a substantially greater response to RA, with either longer neurites or more neurites per cell compared with the parental cells (Fig. 5). These data suggest that accelerating expression of the genes encoding either CK or CCT, thereby accelerating PtdCho synthesis, promotes neuritogenesis acting as a neurotrophin-like factor and also cooperates with early events after RA stimulation to further promote neurite formation. Consistent with this concept, we found in the engineered cell lines a persistent and RA-independent expression of early neuritogenesis markers, for example βIII-tubulin (Fig. 6) and syntaxin-3 (Table 2), which are essential for neurite outgrowth (40). Moreover, even Pcyt1a and Chkα transcription is induced in those cell lines to a similar extent as the control cells after RA addition (Fig. 6C). These results suggest that either CKα or CCTα overexpression, their metabolic products, or the PtdCho final product could act as a neurotrophin-like substance. Although it is clear that the RA signaling pathway and the signaling turned on by the PtdCho-derived substance overlap at some point, RA is still required for full differentiation of the engineered cell lines probably activating genes by its binding to the RA-response elements. In particular, those cellular components that halt proliferation appear to be dependent on RA; however, as proliferation and differentiation are opposite process in cell physiology, we expect changes in the rate of proliferation caused by increased PtdCho metabolism. Future work will be aimed at investigating the common mediators that enable cross-talk between RA signaling and PtdCho-dependent signals.
Activated ERK is required to drive RA-induced differentiation in the Neuro-2a cells (Fig. 7), and so we evaluated the levels of p-ERK that were expressed in the Neuro-2a/CKα and Neuro-2a/CCTα cells. There is a sustained activation of ERK in the stable cell lines even in the absence of RA, the condition where the parental Neuro-2a cells have undetectable levels (Fig. 8). ERK1/2 activation in the overexpressing cell lines is also dependent on MEK, as U0126 blocked ERK1/2 phosphorylation (Fig. 8C). Taken together, these results suggest that the MEKK pathway is positively influenced by PtdCho synthesis under these cellular conditions. These data provide a possible explanation for the ability of phospholipid precursors to increase the levels of phospholipid and neuronal proteins and to increase the number of dendrites in the brains of treated animals (51). However, we were unable to demonstrate if ERK activation is essential for the RA-independent differentiation.
Two potential mechanisms were considered to explain how neuritogenesis is turned on under these cellular conditions. The first one proposes that the increased levels of PtdCho attained by the engineered cell lines could have a structural function, providing a platform for cytoskeletal or signaling proteins to promote differentiation. Favoring this mechanism, and considering that polyunsaturated fatty acids represent 50% of the fatty acids in the plasma membrane phospholipids of neurons, previous work has demonstrated a significant increase in neurite length when neuronal cells are differentiated in media supplemented with the polyunsaturated fatty acids, arachidonic acid, or docosahexaenoic acid (52–55). Similarly, overexpression of acyl-CoA synthetase 2, which ligates free fatty acids to a coenzyme A carrier, stimulates fatty acid uptake, enhances lipid synthesis, and promotes neurite lengthening (56). Alternatively, polyunsaturated fatty acids released by phospholipase A2 may interact with syntaxin-3 to enhance NGF-induced neurite outgrowth in PC12 neuronal cells (40). Overall, in all these examples neuritogenesis occurs in the presence of NGF. The second possible explanation is to consider that PtdCho or its metabolites serve as signaling molecules (neurotrophin-like factors) driving neuritogenesis. In this sense, considerable evidence now exists demonstrating the potent effect of lysophospholipids on the biology of cells by eliciting responses to alter proliferation, survival, migration, and differentiation (57, 58). Furthermore, it was demonstrated that the steady-state concentration of phosphatidylcholine and phosphatidic acid regulates signal transduction through the MAPK and phosphatidylinositol 3-kinase/AKT survival pathway (59).
The cross-talk between membrane phospholipid synthesis/metabolism and signaling appears to be cell-type selective. In fact, in this study elevated PtdCho synthesis results in an accumulation of plasma membrane in neuronal cells and neuritogenesis, but in B-lymphocytes and fibroblasts, increased PtdCho synthesis promotes endoplasmic reticulum formation but is not enough to induce endoplasmic reticulum expansion fully, and the expression of endoplasmic reticulum-associated proteins is selective (9–11). On the other hand, rapidly proliferating HeLa cells respond to CCT overexpression and increased PtdCho synthesis by an equivalent stimulation of PtdCho degradation by a phospholipase A activity (60), resulting in no net increase in membrane phospholipid. Mechanistic details await future experimentation, but nevertheless, our results reinforce the role of PtdCho as an important molecule for nervous system reconstitution (61, 62).
Supplementary Material
Acknowledgments
We thank Dr. S. Peiru and C. Elena for critical reading of the manuscript, Jina Wang for technical expertise, Matthew Frank for Affymetrix analysis, and S. Scarpeci and J. Pellegrino for technical assistance in confocal microscopy analysis.
This work was supported by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina, Agencia de Promoción Cientifica y Tecnológica (ANPCyT), Argentina, Grant PICT 01-38027 (to C. B.), and by the American Lebanese and Syrian Associated Charity (to S. J.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- RA
- retinoic acid
- PtdCho
- phosphatidylcholine
- CK
- choline kinase
- CCT
- choline cytidylyltransferase
- ERK
- extracellular signal-regulated kinase
- DMEM
- Dulbecco's modified Eagle's medium
- FBS
- fetal bovine serum
- MAPK
- mitogen-activated protein kinase
- RT-qPCR
- reverse transcription-quantitative PCR
- NGF
- nerve growth factor
- CPT
- CDP-choline:1,2 diacylglycerol cholinephosphotransferase
- MEM
- modified Eagle's medium
- PtdEtn
- phosphatidylethanolamine
- CDP-Cho
- cytidine diphosphocholine
- MEK
- MAPK/ERK kinase.
REFERENCES
- 1.Yanaka N., Nogusa Y., Fujioka Y., Yamashita Y., Kato N. (2007) FEBS Lett. 581, 712–718 [DOI] [PubMed] [Google Scholar]
- 2.Sato C., Matsuda T., Kitajima K. (2002) J. Biol. Chem. 277, 45299–45305 [DOI] [PubMed] [Google Scholar]
- 3.Crowder R. J., Enomoto H., Yang M., Johnson E. M., Jr., Milbrandt J. (2004) J. Biol. Chem. 279, 42072–42081 [DOI] [PubMed] [Google Scholar]
- 4.Shea T. B., Fischer I., Sapirstein V. S. (1985) Brain Res. 353, 307–314 [DOI] [PubMed] [Google Scholar]
- 5.Lein P. J., Higgins D. (1991) Brain Res. Dev. Brain Res. 60, 187–196 [DOI] [PubMed] [Google Scholar]
- 6.Harel R., Futerman A. H. (1993) J. Biol. Chem. 268, 14476–14481 [PubMed] [Google Scholar]
- 7.Vance J. E., De Chaves E. P., Campenot R. B., Vance D. E. (1995) Neurobiol. Aging 16, 493–499 [DOI] [PubMed] [Google Scholar]
- 8.Infante J. P. (1977) Biochem. J. 167, 847–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sriburi R., Bommiasamy H., Buldak G. L., Robbins G. R., Frank M., Jackowski S., Brewer J. W. (2007) J. Biol. Chem. 282, 7024–7034 [DOI] [PubMed] [Google Scholar]
- 10.Fagone P., Sriburi R., Ward-Chapman C., Frank M., Wang J., Gunter C., Brewer J. W., Jackowski S. (2007) J. Biol. Chem. 282, 7591–7605 [DOI] [PubMed] [Google Scholar]
- 11.Sriburi R., Jackowski S., Mori K., Brewer J. W. (2004) J. Cell Biol. 167, 35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jansen S. M., Groener J. E., Bax W., Suter A., Saftig P., Somerharju P., Poorthuis B. J. (2001) J. Biol. Chem. 276, 18722–18727 [DOI] [PubMed] [Google Scholar]
- 13.Kent C. (1997) Biochim. Biophys. Acta 1348, 79–90 [DOI] [PubMed] [Google Scholar]
- 14.Vance D. E., Walkey C. J. (1998) Curr. Opin. Lipidol. 9, 125–130 [DOI] [PubMed] [Google Scholar]
- 15.Infante J. P., Kinsella J. E. (1978) Biochem. J. 176, 631–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karim M., Jackson P., Jackowski S. (2003) Biochim. Biophys. Acta 1633, 1–12 [DOI] [PubMed] [Google Scholar]
- 17.Marcucci H., Elena C., Gilardoni P., Banchio C. (2008) Biochim. Biophys. Acta 1781, 254–262 [DOI] [PubMed] [Google Scholar]
- 18.Lykidis A., Murti K. G., Jackowski S. (1998) J. Biol. Chem. 273, 14022–14029 [DOI] [PubMed] [Google Scholar]
- 19.Jackowski S., Rehg J. E., Zhang Y. M., Wang J., Miller K., Jackson P., Karim M. A. (2004) Mol. Cell. Biol. 24, 4720–4733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aoyama C., Ohtani A., Ishidate K. (2002) Biochem. J. 363, 777–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aoyama C., Liao H., Ishidate K. (2004) Prog. Lipid Res. 43, 266–281 [DOI] [PubMed] [Google Scholar]
- 22.Vance J. E., Pan D., Campenot R. B., Bussière M., Vance D. E. (1994) J. Neurochem. 62, 329–337 [DOI] [PubMed] [Google Scholar]
- 23.Vance J. E., Pan D., Vance D. E., Campenot R. B. (1991) J. Cell Biol. 115, 1061–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Futerman A. H., Banker G. A. (1996) Trends Neurosci. 19, 144–149 [DOI] [PubMed] [Google Scholar]
- 25.Yen C. L., Mar M. H., Meeker R. B., Fernandes A., Zeisel S. H. (2001) FASEB J. 15, 1704–1710 [DOI] [PubMed] [Google Scholar]
- 26.Posse de Chaves E., Vance D. E., Campenot R. B., Vance J. E. (1995) J. Cell Biol. 128, 913–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carter J. M., Waite K. A., Campenot R. B., Vance J. E., Vance D. E. (2003) J. Biol. Chem. 278, 44988–44994 [DOI] [PubMed] [Google Scholar]
- 28.Carter J. M., Demizieux L., Campenot R. B., Vance D. E., Vance J. E. (2008) J. Biol. Chem. 283, 202–212 [DOI] [PubMed] [Google Scholar]
- 29.Araki W., Wurtman R. J. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 11946–11950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bakovic M., Waite K., Tang W., Tabas I., Vance D. E. (1999) Biochim. Biophys. Acta 1438, 147–165 [DOI] [PubMed] [Google Scholar]
- 31.Sedmak J. J., Grossberg S. E. (1977) Anal. Biochem. 79, 544–552 [DOI] [PubMed] [Google Scholar]
- 32.Iverson S. J., Lang S. L., Cooper M. H. (2001) Lipids 36, 1283–1287 [DOI] [PubMed] [Google Scholar]
- 33.Christine William W. C. (2003) Lipids Analysis, 3rd Ed., MRS Lipids Analysis Unit, Scottish Crop Research Institute, Invergowrie, Dungee, Scotland [Google Scholar]
- 34.Jackowski S., Fagone P. (2005) J. Biol. Chem. 280, 853–856 [DOI] [PubMed] [Google Scholar]
- 35.Aoyama C., Ishidate K., Sugimoto H., Vance D. E. (2007) Biochim. Biophys. Acta 1771, 1148–1155 [DOI] [PubMed] [Google Scholar]
- 36.Banchio C., Schang L. M., Vance D. E. (2003) J. Biol. Chem. 278, 32457–32464 [DOI] [PubMed] [Google Scholar]
- 37.Vance J. E., Vance D. E. (2004) Biochem. Cell Biol. 82, 113–128 [DOI] [PubMed] [Google Scholar]
- 38.Easter S. S., Jr., Ross L. S., Frankfurter A. (1993) J. Neurosci. 13, 285–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katsetos C. D., Herman M. M., Balin B. J., Vinores S. A., Hessler R. B., Arking E. J., Karkavelas G., Frankfurter A. (1998) Anat. Rec. 250, 351–365 [DOI] [PubMed] [Google Scholar]
- 40.Darios F., Davletov B. (2006) Nature 440, 813–817 [DOI] [PubMed] [Google Scholar]
- 41.Chambon P. (1996) FASEB J. 10, 940–954 [PubMed] [Google Scholar]
- 42.Cañón E., Cosgaya J. M., Scsucova S., Aranda A. (2004) Mol. Biol. Cell 15, 5583–5592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marshall C. J. (1995) Cell 80, 179–185 [DOI] [PubMed] [Google Scholar]
- 44.Leevers S. J., Paterson H. F., Marshall C. J. (1994) Nature 369, 411–414 [DOI] [PubMed] [Google Scholar]
- 45.Vaudry D., Stork P. J., Lazarovici P., Eiden L. E. (2002) Science 296, 1648–1649 [DOI] [PubMed] [Google Scholar]
- 46.Cobb M. H. (1999) Prog. Biophys. Mol. Biol. 71, 479–500 [DOI] [PubMed] [Google Scholar]
- 47.Sidell N., Altman A., Haussler M. R., Seeger R. C. (1983) Exp. Cell Res. 148, 21–30 [DOI] [PubMed] [Google Scholar]
- 48.Reynolds C. P., Matthay K. K., Villablanca J. G., Maurer B. J. (2003) Cancer Lett. 197, 185–192 [DOI] [PubMed] [Google Scholar]
- 49.Sweatt J. D. (2001) J. Neurochem. 76, 1–10 [DOI] [PubMed] [Google Scholar]
- 50.Agassandian M., Zhou J., Tephly L. A., Ryan A. J., Carter A. B., Mallampalli R. K. (2005) J. Biol. Chem. 280, 21577–21587 [DOI] [PubMed] [Google Scholar]
- 51.Cansev M., Wurtman R. J., Sakamoto T., Ulus I. H. (2008) Alzheimers Dement. 4, S153–S168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dehaut F., Bertrand I., Miltaud T., Pouplard-Barthelaix A., Maingault M. (1993) Neurosci. Lett. 161, 133–136 [DOI] [PubMed] [Google Scholar]
- 53.Ikemoto A., Kobayashi T., Emoto K., Umeda M., Watanabe S., Okuyama H. (1999) Arch. Biochem. Biophys. 364, 67–74 [DOI] [PubMed] [Google Scholar]
- 54.Okuda S., Saito H., Katsuki H. (1994) Neuroscience 63, 691–699 [DOI] [PubMed] [Google Scholar]
- 55.Williams E. J., Walsh F. S., Doherty P. (1994) J. Neurochem. 62, 1231–1234 [DOI] [PubMed] [Google Scholar]
- 56.Marszalek J. R., Kitidis C., Dararutana A., Lodish H. F. (2004) J. Biol. Chem. 279, 23882–23891 [DOI] [PubMed] [Google Scholar]
- 57.Pitson S. M., Pébay A. (2009) Neurosignals 17, 242–254 [DOI] [PubMed] [Google Scholar]
- 58.Anderson D. H. (2006) Prog. Lipid Res. 45, 102–119 [DOI] [PubMed] [Google Scholar]
- 59.Yalcin A., Clem B., Makoni S., Clem A., Nelson K., Thornburg J., Siow D., Lane A. N., Brock S. E., Goswami U., Eaton J. W., Telang S., Chesney J. (2010) Oncogene 29, 139–149 [DOI] [PubMed] [Google Scholar]
- 60.Baburina I., Jackowski S. (1999) J. Biol. Chem. 274, 9400–9408 [DOI] [PubMed] [Google Scholar]
- 61.Adibhatla R. M., Hatcher J. F., Larsen E. C., Chen X., Sun D., Tsao F. H. (2006) J. Biol. Chem. 281, 6718–6725 [DOI] [PubMed] [Google Scholar]
- 62.Adibhatla R. M., Hatcher J. F., Dempsey R. J. (2006) AAPS J. 8, E314–E321 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.