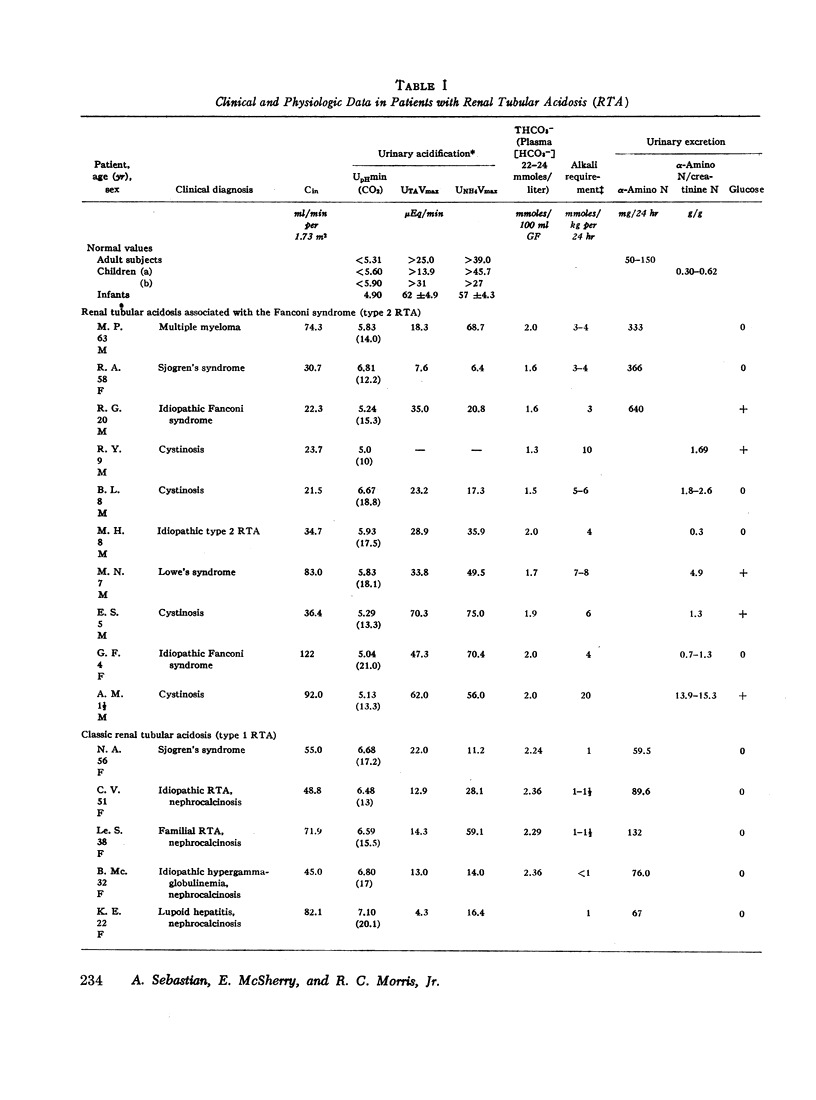
Abstract
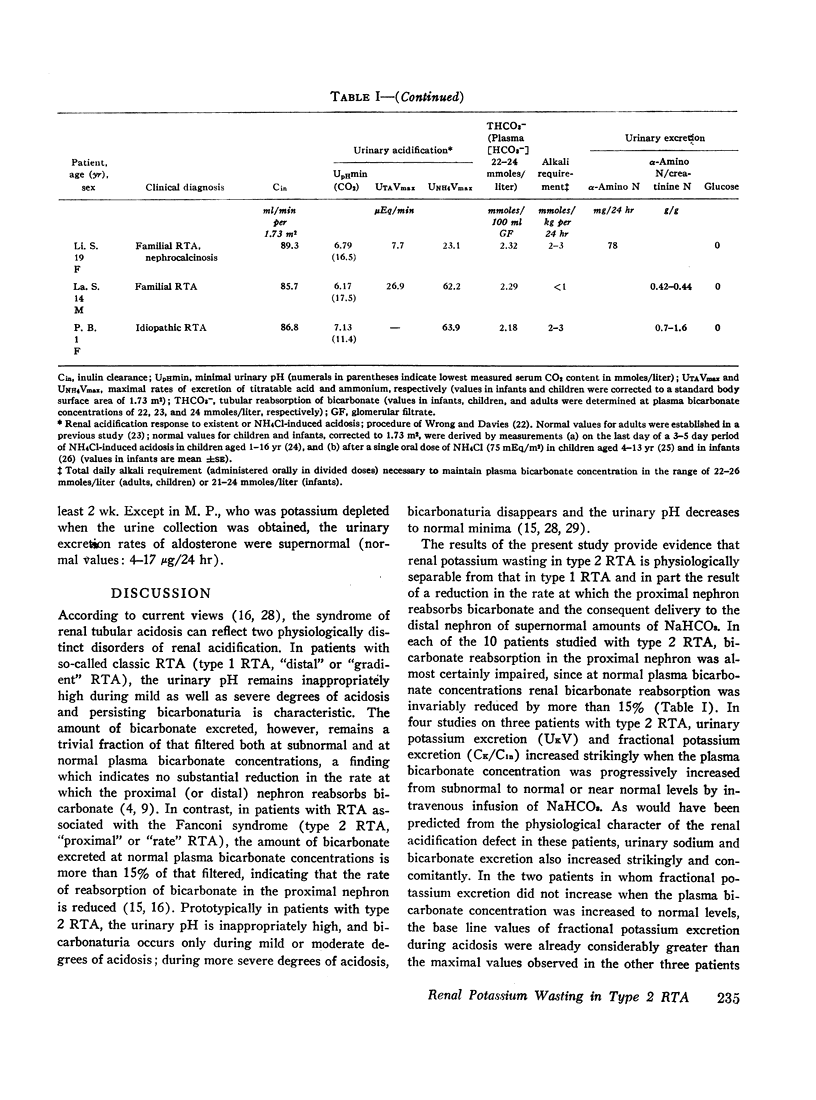
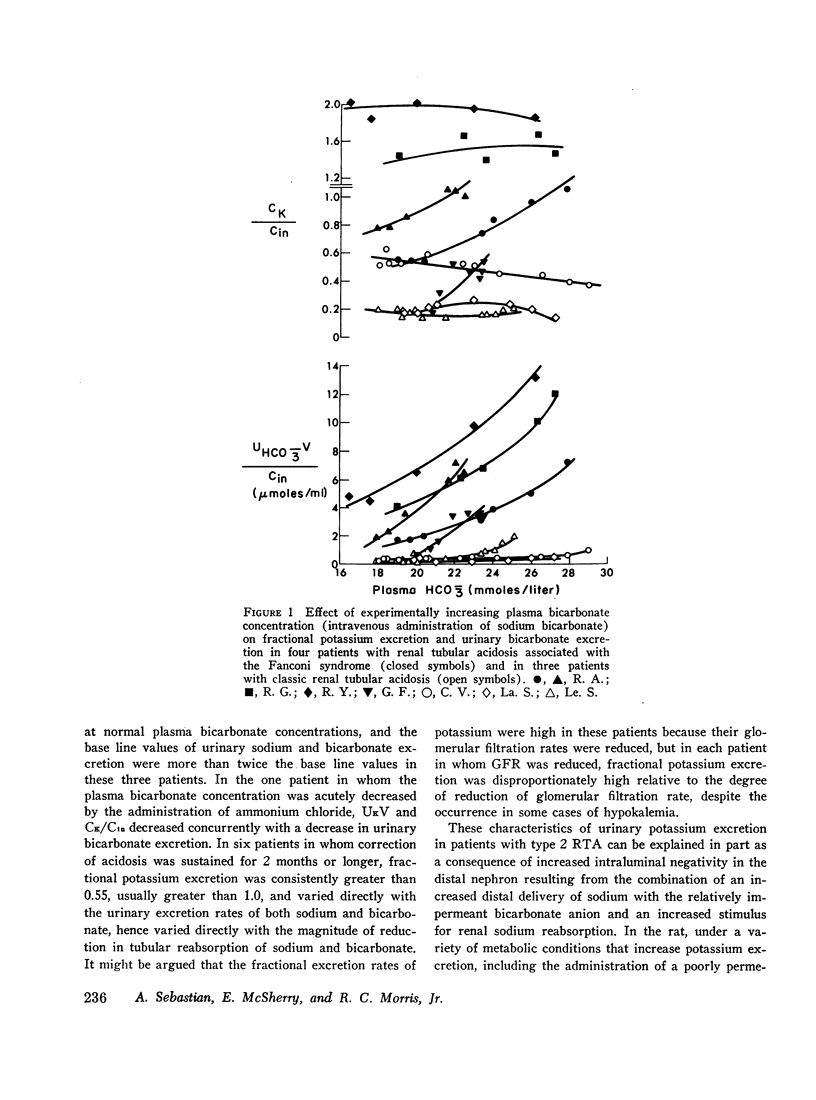
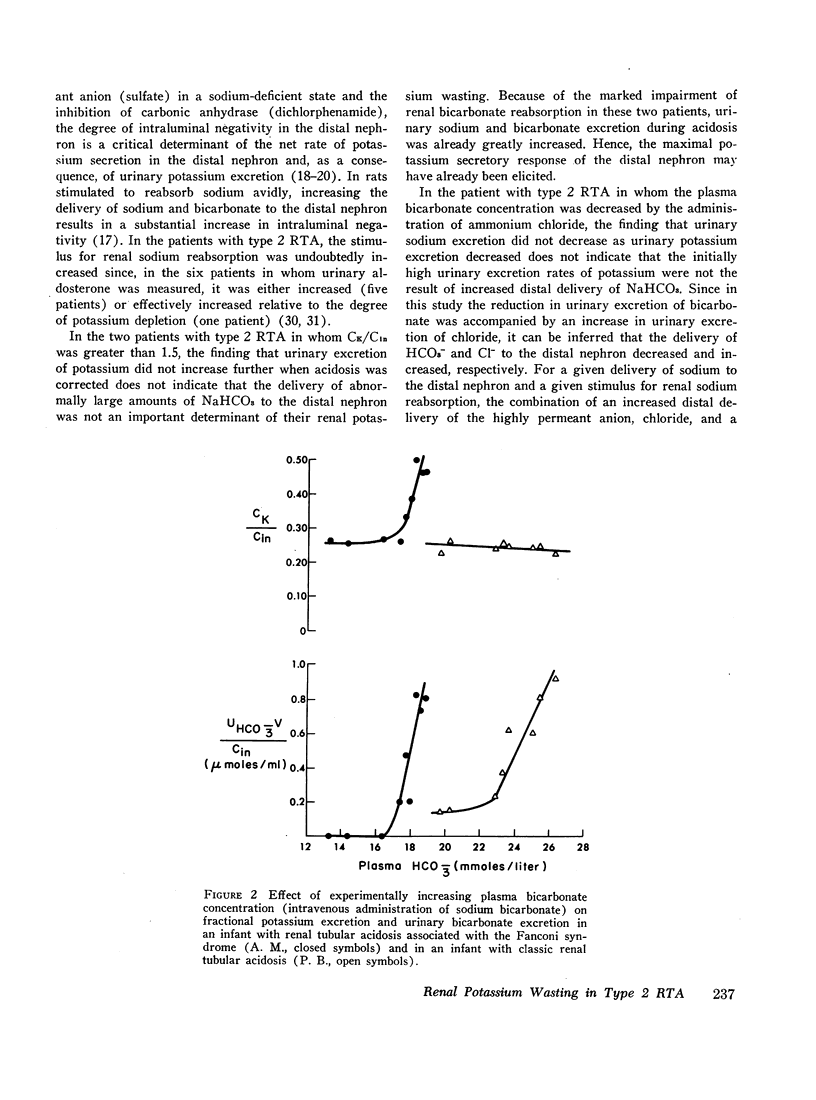
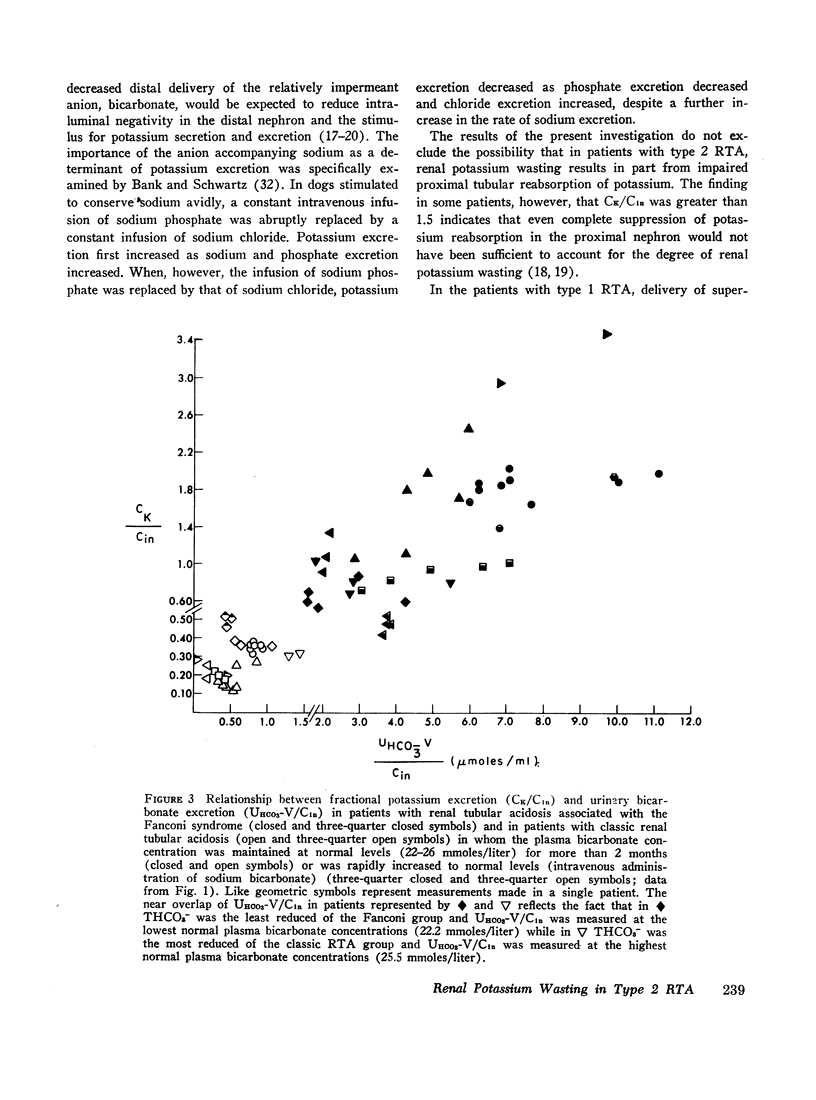
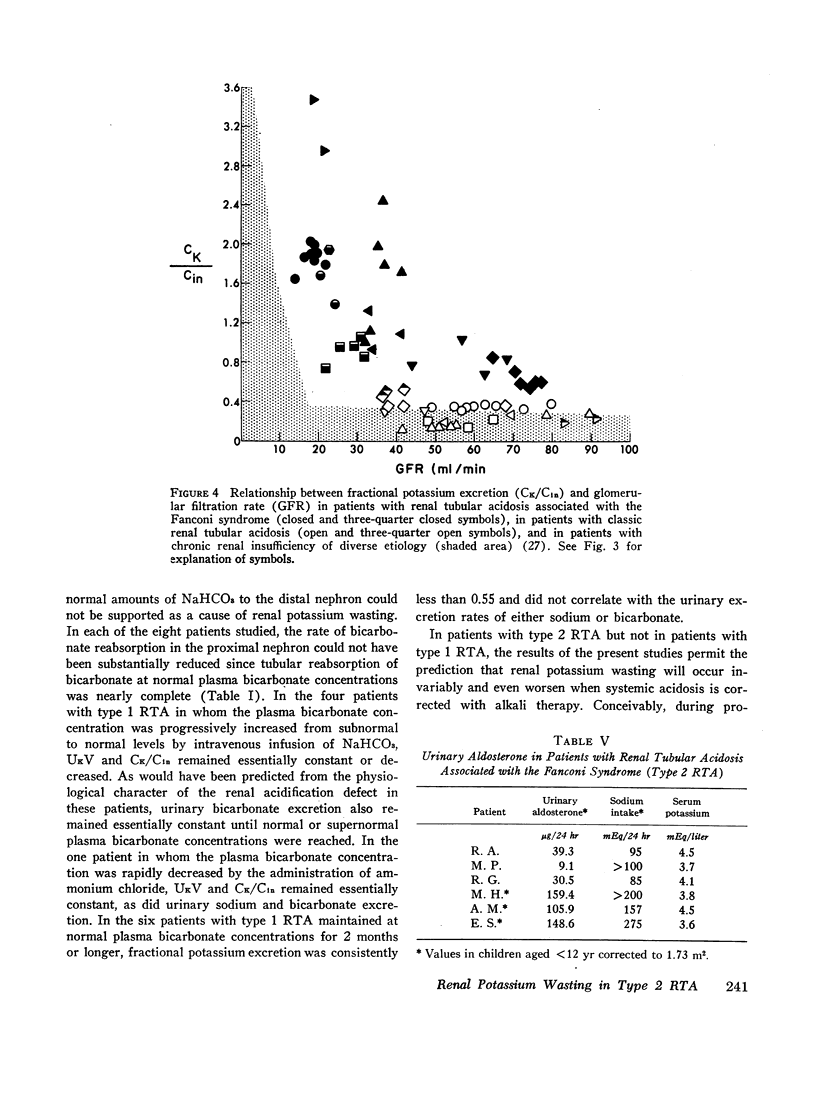
The mechanism of renal potassium wasting in renal tubular acidosis associated with the Fanconi syndrome (type 2 RTA) was investigated in 10 patients, each of whom had impaired proximal renal tubular reabsorption of bicarbonate as judged from a greater than 15-20% reduction of renal tubular bicarbonate reabsorption (THCO3-) at normal plasma bicarbonate concentrations. When the plasma bicarbonate concentration ([HCO3-]p) was experimentally increased to normal levels in three patients with a fractional potassium excretion (CK/Cin) of less than 1.0 during acidosis, CK/Cin and urinary potassium excretion (UKV/Cin) increased strikingly and concurrently with a striking increase in urinary sodium (UNaV/Cin) and bicarbonate (UHCO3-V/Cin) excretion. When [HCO3-]p was increased to normal levels in two patients with a CK/Cin of greater than 1.0 during acidosis and in whom UNaV/Cin and UHCO3-V/Cin were already markedly increased, CK/Cin did not increase further. When [HCO3-]p was decreased to subnormal levels in a patient given ammonium chloride, UKV/Cin, CK/Cin, and UHCO3-V/Cin decreased concurrently. In the six patients in whom [HCO3-]p was maintained at normal levels (oral alkali therapy) for 2 months or longer, CK/Cin was directly related to the urinary excretion rates of sodium and bicarbonate, hence was directly related to the magnitude of reduction of THCO3- at normal [HCO3-]p; CK/Cin was greater than 0.55 in all six patients and greater than 1.0 in four.
In eight patients with classic RTA (type 1 RTA), proximal renal tubular reabsorption of bicarbonate was largely intact as judged from a trivial reduction of THCO3- at normal [HCO3-]p. When [HCO3-]p was either increased from subnormal to normal levels, or decreased from normal to subnormal levels, UHCO3-V/Cin remained essentially constant, and UKV/Cin did not change significantly. When correction of acidosis was sustained, UHCO3-V/Cin remained a trivial fraction of that filtered, and CK/Cin was consistently less than 0.55.
These results provide evidence that renal potassium wasting in type 2 RTA is physiologically separable from that in type 1 RTA and in part the result of a reduction in the rate at which the proximal tubule reabsorbs bicarbonate and the distal delivery of supernormal amounts of sodium bicarbonate. With an increased stimulus to distal sodium reabsorption, indicated by the finding of hyperaldosteronism, delivery to the distal nephron of supernormal amounts of sodium with the relatively impermeant bicarbonate anion would be expected to increase intraluminal negativity in the distal nephron, and as a consequence, increase potassium secretion and promote renal potassium wasting.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANK N., SCHWARTZ W. B. The influence of anion penetrating ability on urinary acidification and the excretion of titratable acid. J Clin Invest. 1960 Oct;39:1516–1525. doi: 10.1172/JCI104171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAPP J. R., RECTOR F. C., Jr, SELDIN D. W. Effect of unreabsorbed anions on proximal and distal transtubular potentials in rats. Am J Physiol. 1962 Apr;202:781–786. doi: 10.1152/ajplegacy.1962.202.4.781. [DOI] [PubMed] [Google Scholar]
- Cannon P. J., Ames R. P., Laragh J. H. Relation between potassium balance and aldosterone secretion in normal subjects and in patients with hypertensive or renal tubular disease. J Clin Invest. 1966 Jun;45(6):865–879. doi: 10.1172/JCI105402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann C. M., Jr, Boichis H., Soriano J. R., Stark H. The renal response of children to acute ammonium chloride acidosis. Pediatr Res. 1967 Nov;1(6):452–460. doi: 10.1203/00006450-196711000-00004. [DOI] [PubMed] [Google Scholar]
- Edelmann C. M., Soriano J. R., Boichis H., Gruskin A. B., Acosta M. I. Renal bicarbonate reabsorption and hydrogen ion excretion in normal infants. J Clin Invest. 1967 Aug;46(8):1309–1317. doi: 10.1172/JCI105623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOURMAN P., McCANCE R. A. Tetany complicating the treatment of potassium deficiency in renal acidosis. Lancet. 1955 Feb 12;268(6859):329–331. doi: 10.1016/s0140-6736(55)90063-5. [DOI] [PubMed] [Google Scholar]
- GANN D. S., DELEA C. S., GILL J. R., Jr, THOMAS J. P., BARTTER F. C. CONTROL OF ALDOSTERONE SECRETION BY CHANGE OF BODY POTASSIUM IN NORMAL MAN. Am J Physiol. 1964 Jul;207:104–108. doi: 10.1152/ajplegacy.1964.207.1.104. [DOI] [PubMed] [Google Scholar]
- Gill J. R., Jr, Bell N. H., Bartter F. C. Impaired conservation of sodium and potassium in renal tubular acidosis and its correction by buffer anions. Clin Sci. 1967 Dec;33(3):577–592. [PubMed] [Google Scholar]
- Kleeman C. R., Okun R., Heller R. J. The renal regulation of sodium and potassium in patients with chronic renal failure (CRF) and the effect of diuretics on the excretion of these ions. Ann N Y Acad Sci. 1966 Nov 22;139(2):520–539. doi: 10.1111/j.1749-6632.1966.tb41226.x. [DOI] [PubMed] [Google Scholar]
- MAHLER R. F., STANBURY S. W. Potassium-losing renal disease; renal and metabolic observations on a patient sustaining renal wastage of potassium. Q J Med. 1956 Jan;25(97):21–52. [PubMed] [Google Scholar]
- MALNIC G., KLOSE R. M., GIEBISCH G. MICROPUNCTURE STUDY OF RENAL POTASSIUM EXCRETION IN THE RAT. Am J Physiol. 1964 Apr;206:674–686. doi: 10.1152/ajplegacy.1964.206.4.674. [DOI] [PubMed] [Google Scholar]
- MILNE M. D., STANBURY S. W., THOMSON A. E. Observations on the Fanconi syndrome and renal hyperchloraemic acidosis in the adult. Q J Med. 1952 Jan;21(81):61–82. [PubMed] [Google Scholar]
- Malnic G., Klose R. M., Giebisch G. Microperfusion study of distal tubular potassium and sodium transfer in rat kidney. Am J Physiol. 1966 Sep;211(3):548–559. doi: 10.1152/ajplegacy.1966.211.3.548. [DOI] [PubMed] [Google Scholar]
- Malnic G., Klose R. M., Giebisch G. Micropuncture study of distal tubular potassium and sodium transport in rat nephron. Am J Physiol. 1966 Sep;211(3):529–547. doi: 10.1152/ajplegacy.1966.211.3.529. [DOI] [PubMed] [Google Scholar]
- Morris R. C., Jr An experimental renal acidification defect in patients with hereditary fructose intolerance. I. Its resemblance to renal tubular acidosis. J Clin Invest. 1968 Jun;47(6):1389–1398. doi: 10.1172/JCI105830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. C., Jr An experimental renal acidification defect in patients with hereditary fructose intolerance. II. Its distinction from classic renal tubular acidosis; its resemblance to the renal acidification defect associated with the Fanconi syndrome of children with cystinosis. J Clin Invest. 1968 Jul;47(7):1648–1663. doi: 10.1172/JCI105856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. C., Jr, Fudenberg H. H. Impaired renal acidification in patients with hypergammaglobulinemia. Medicine (Baltimore) 1967 Jan;46(1):57–69. doi: 10.1097/00005792-196701000-00003. [DOI] [PubMed] [Google Scholar]
- Morris R. C., Jr Renal tubular acidosis. Mechanisms, classification and implications. N Engl J Med. 1969 Dec 18;281(25):1405–1413. doi: 10.1056/NEJM196912182812508. [DOI] [PubMed] [Google Scholar]
- PEONIDES A., LEVIN B., YOUNG W. F. THE RENAL EXCRETION OF HYDROGEN IONS IN INFANTS AND CHILDREN. Arch Dis Child. 1965 Feb;40:33–39. doi: 10.1136/adc.40.209.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINES K. L., MUDGE G. H. Renal tubular acidosis with osteomalacia; report of 3 cases. Am J Med. 1951 Sep;11(3):302–311. doi: 10.1016/0002-9343(51)90167-2. [DOI] [PubMed] [Google Scholar]
- RELMAN A. S. RENAL ACIDOSIS AND RENAL EXCRETION OF ACID IN HEALTH AND DISEASE. Adv Intern Med. 1964;12:295–347. [PubMed] [Google Scholar]
- REYNOLDS T. B. Observations on the pathogenesis of renal tubular acidosis. Am J Med. 1958 Oct;25(4):503–515. doi: 10.1016/0002-9343(58)90040-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Soriano J., Edelmann C. M., Jr Renal tubular acidosis. Annu Rev Med. 1969;20:363–382. doi: 10.1146/annurev.me.20.020169.002051. [DOI] [PubMed] [Google Scholar]
- SIROTA J. H., HAMERMAN D. Renal function studies in a adult subject with the Fanconi syndrome. Am J Med. 1954 Jan;16(1):138–152. doi: 10.1016/0002-9343(54)90329-0. [DOI] [PubMed] [Google Scholar]
- WORTHEN H. G., GOOD R. A. The de Toni-Fanconi syndrome with cystinosis; clinical and metabolic study of two cases in a family and a critical review on the nature of the syndrome. AMA J Dis Child. 1958 Jun;95(6):653–688. [PubMed] [Google Scholar]
- WRONG O., DAVIES H. E. The excretion of acid in renal disease. Q J Med. 1959 Apr;28(110):259–313. [PubMed] [Google Scholar]


