Abstract
Development of rod photoreceptors in the mammalian retina is critically dependent on the basic motif-leucine zipper transcription factor NRL (neural retina leucine zipper). In the absence of NRL, photoreceptor precursors in mouse retina produce only cones that primarily express S-opsin. Conversely, ectopic expression of NRL in post-mitotic precursors leads to a rod-only retina. To explore the role of signaling molecules in modulating NRL function, we identified putative sites of post-translational modification in the NRL protein by in silico analysis. Here, we demonstrate the sumoylation of NRL in vivo and in vitro, with two small ubiquitin-like modifier (SUMO) molecules attached to the Lys-20 residue. NRL-K20R and NRL-K20R/K24R sumoylation mutants show reduced transcriptional activation of Nr2e3 and rhodopsin promoters (two direct targets of NRL) in reporter assays when compared with wild-type NRL. Consistent with this, in vivo electroporation of the NRL-K20R/K24R mutant into newborn Nrl−/− mouse retina leads to reduced Nr2e3 activation and only a partial rescue of the Nrl−/− phenotype in contrast to the wild-type NRL that is able to convert cones to rod photoreceptors. Although PIAS3 (protein inhibitor of activated STAT3), an E3-SUMO ligase implicated in photoreceptor differentiation, can be immunoprecipitated with NRL, there appears to be redundancy in E3 ligases, and PIAS3 does not seem to be essential for NRL sumoylation. Our studies suggest an important role of sumoylation in fine-tuning the activity of NRL and thereby incorporating yet another layer of control in gene regulatory networks involved in photoreceptor development and homeostasis.
Keywords: Development, Gene Expression, Gene Regulation, Photoreceptors, Post-translational Modification, Retina, Sumoylation, Tissue-specific Transcription Factors, Transcription Factors, Transcription Regulation
Introduction
Spatiotemporal control of gene expression is critical for development and homeostasis (1). Cell type-specific expression patterns are established and maintained by transient or stable interactions between cis-regulatory elements in the target genes and trans-regulatory factors that together constitute gene regulatory networks (2). Signaling molecules, another key component of gene regulatory networks, can modify the activity of transcription factors by post-translational modifications (PTMs)3 such as phosphorylation, acetylation, ubiquitination, and sumoylation (3–6). Rapid and reversible modulation of the activity of transcription factors by PTMs is essential for adaptation to continuously changing cellular microenvironment(s) and is accomplished by altering protein stability, subcellular localization, and protein-DNA and/or protein-protein interaction (3, 6–8). Consequently, PTMs provide a higher level of control and complexity to gene regulation in a particular biological context.
The vertebrate retina exhibits a highly organized laminar structure that captures, integrates, and transmits visual signals to other parts of the central nervous system for further processing. Six neuronal cell types and Muller glia in the retina originate from pools of multipotent progenitor cells in a conserved sequential order (9, 10). The determination of specific cell fate and subsequent differentiation is dictated primarily by intrinsic control mechanisms; however, extrinsic signals modulate key steps in the developmental pathway (9–13). Rod and cone photoreceptors have a unique and specialized function and initiate the phototransduction process by converting photons into electrical signal (14). Differentiation and homeostasis of photoreceptors are tightly controlled by a set of key transcriptional regulatory proteins, which include nuclear receptors (such as RORβ (15), thyroid hormone receptor β2 (TRβ2) (16), and NR2E3 (17–21)), homeodomain proteins (such as orthodenticle homeobox 2 (OTX2) (22) and CRX (cone-rod homeobox) (23, 24), signal transducers (including STAT3 (25), PIAS3 (26), glycogen synthase kinase 3 (GSK3) (27)), and NRL, a basic motif-leucine zipper (bZIP) protein of Maf subfamily (28).
The bZIP transcription factor NRL is a key regulator of rod versus cone photoreceptor cell fate in mammalian retina (28, 29). Targeted deletion of Nrl in mice leads to a retina with only cones that primarily express S-opsin (28), whereas ectopic expression of NRL in photoreceptor precursors leads to a rod-only retina (29). NRL expression is detected soon after the final mitosis and drives a photoreceptor precursor toward rod cell fate (30). NRL interacts with a number of transcription factors (including CRX, NR2E3, and SP4) and activates the expression of many rod-specific genes (23, 31–34). NRL is also the major regulator of NR2E3, an orphan nuclear receptor, and together these two proteins repress cone gene expression (19–21, 35). Loss of Nr2e3 in mice results in rod photoreceptors that express cone genes and eventually degenerate (17, 18, 36, 37). CRX is another important modulator of photoreceptor maturation (38). Rods and cones do not fully differentiate in the Crx knock-out retina and lack outer segments (24). RORβ and OTX2 control photoreceptor differentiation as well but act upstream of NRL and CRX in the transcriptional regulatory hierarchy (15, 22).
Differentiation of rod photoreceptors proceeds in a stepwise manner during the development of mammalian retina. In rodents, although some rods are born as early as embryonic day 12, a majority of rods are generated postnatally (9, 30, 39, 40). Interestingly, the expression of rod-specific visual pigment protein, rhodopsin, reveals a substantial “delay” with two distinct phases (41) despite the presence of key activator proteins, NRL, CRX, and NR2E3 (40). One can hypothesize that additional signals/factors are needed to modify the activity of one or more of these regulators and/or to stabilize the assembly of “enhanceosome” complex (42) before the transcription of photoreceptor-specific genes can be initiated. Recruitment of histone acetyl transferases by CRX is implicated in rod gene transcription (43). Recently, PIAS3, an E3-SUMO ligase, has been shown to interact with CRX and NR2E3 and play a significant role in rod differentiation by sumoylating the NR2E3 protein (26). Chromatin remodeling and post-translational modifications can therefore contribute to photoreceptor development by modulating cell type-specific transcription.
To gain insights into the role of extrinsic signaling molecules in guiding retinal development and homeostasis, we are exploring the impact of post-translational modifications on the transcriptional regulatory function of NRL. We have previously reported multiple phosphorylated isoforms of NRL (44) and demonstrated that a number of human retinopathy mutations in NRL alter its phosphorylation state and activity in vitro (45–47). Here, we show that the NRL protein is disumoylated in vivo and in vitro and that sumoylation of NRL modifies its activity toward two distinct target promoters, Nr2e3 and rhodopsin. Although PIAS3 is part of a multiprotein complex with NRL, it does not appear to be the primary mediator of NRL sumoylation. Our studies further strengthen the growing role of post-translational mechanisms in influencing photoreceptor development and function.
EXPERIMENTAL PROCEDURES
Mice
Nrl−/− mice on C57Bl/6J background were used for in vivo electroporation experiments. All animal studies followed approved institutional protocols.
Antibodies
The following antibodies were used: anti-NRL polyclonal antibody (44); rhodopsin monoclonal antibody, Rho4D2 (Dr. R. Molday, University of British Columbia, Vancouver, British Columbia, Canada); anti-PIAS3 and anti-FLAG monoclonal antibodies (Sigma); anti-SUMO1 monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA); anti-cone arrestin polyclonal antibody (Chemicon, Billerica, MA); anti-rabbit and anti-mouse light chain specific horseradish peroxidase-conjugated anti-IgG antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA); and goat-anti-rabbit and anti-mouse antibodies conjugated with Alexa Fluor 488, 568, and 633 (Molecular Probes, Invitrogen).
Plasmid Construction and Mutagenesis
Human wild-type (WT) NRL cDNA (714 nucleotides) was subcloned into pcDNA4c His/Max C vector (Invitrogen) (46), and mutants (NRL-K20R, K24R, K161R, K168R, K179R, K216R, and K20R/K24R) were generated using the QuikChange XL site-directed mutagenesis kit (Stratagene, La Jolla, CA). For in vivo electroporation, WT-NRL and NRL-K20R/K24R mutant were subcloned at EcoRI-NotI sites of the Ub-GFP vector after removing GFP (53). The ubiquitin (Ub) promoter used in this vector is transcriptionally active in all retinal cell types. The WT and mutant NRL proteins were expressed in Escherichia coli using pGex4T vector (GE Healthcare), containing an N-terminal glutathione S-transferase (GST) fusion tag under the control of the “tac” promoter. PIAS3 cDNA was subcloned into EcoRI-NotI sites of the pcDNA3.1/V5-His C vector (Invitrogen). pTag-FLAG-SUMO was a generous gift from Dr. Shiming Chen (Washington University, St. Louis, MO).
Recombinant GST-NRL and in Vitro Sumoylation Assay
For in vitro sumoylation assays, GST-NRL protein was expressed in bacteria using standard protocols and eluted from a GSTrap FF Sepharose column (GE Healthcare) with 50 mm Tris-HCl, pH 8.0, containing 20 mm reduced glutathione. This protocol yielded ∼95% pure protein as assessed by SDS-PAGE analysis. Purified GST-WT-NRL or NRL mutants (0.5 μg) were incubated at 30 °C for 3 h with E1 activating enzyme, E2 conjugating enzyme, and SUMO protein using the SUMOlink kit (Active Motif, Carlsbad, CA). Sumoylated proteins were assayed by SDS-PAGE and immunoblotting.
Cell Culture and Transfection
HEK-293 and HEK293T cells were cultured in Dulbecco's modified Eagle's medium containing 10% fetal calf serum, 100 units/ml penicillin G, and 100 μg/ml streptomycin. Cells at 80% confluence were transiently transfected with FuGENE 6 (Roche Applied Science).
Dual-Luciferase Assay
HEK-293 cells were seeded in 24-well plates (4 × 104/well) and co-transfected with 0.1 μg of bovine rhodopsin promoter driving firefly luciferase (pBR130-luc (46)), 0.1 μg of pcDNA4-CRX (46) and/or pcDNA4c-NR2E3 (46), 0.01–0.3 μg of WT-NRL or NRL mutants, and 0.001 μg of Renilla reporter pRL-TK (Promega, Madison, WI). Empty pcDNA4c was used to adjust the total amount of transfected DNA. Cells were harvested 48 h after transfections and lysed in 100 μl of passive lysis buffer (Promega). Firefly and Renilla luciferase activities were determined using the Dual-Luciferase reporter assay system (Promega) and measured with the modulus microplate luminometer (Turner BioSystems, Sunnyvale, CA). Renilla luciferase activity was used as an internal control for transfection efficiency. All experiments were repeated three times. An analysis of variance test was performed for statistical analysis, and p value of < 0.05 was considered significant.
Immunoprecipitation and Immunoblotting
Transfected HEK293T cells were harvested after 48 h and lysed by sonication in radioimmunoprecipitation buffer supplemented with 20 mm N-ethylmaleimide (Sigma) and protease inhibitor (Roche Applied Science). Supernatants were used either for immunoprecipitation or for immunoblot analysis. For immunoprecipitation, lysates were incubated with anti-NRL antibody for 6 h at 4 °C, and immunoprecipitate was collected on protein A-Sepharose beads for 1 h (GE Healthcare). Beads were washed in lysis buffer and boiled in 2× SDS-PAGE loading buffer (Invitrogen). Proteins were resolved by SDS-PAGE under reducing conditions and transferred to nitrocellulose membrane (Invitrogen). After 1 h of blocking with 5% skim milk in phosphate-buffered saline, 0.1% Tween 20 (PBT), the membrane was incubated overnight at 4 °C with antibody in PBT, 5% skim milk. After three washes in PBT, the membrane was then incubated with secondary antibody coupled to horseradish peroxidase for 1 h in PBT, 5% skim milk. After three washes in PBT, proteins were visualized by enhanced chemiluminescence plus (Thermo Scientific).
In Vivo Electroporation
Retina of P0 Nrl−/− pups was electroporated in vivo as described (53). Briefly, an equal amount of Ub-WT-NRL or Ub-NRL-K20R/K24R plasmid was mixed with Ub-GFP, and 0.2 μl (concentration, 1 μg/μl) was injected subretinally. Square electric pulses (80 V, 1 Hz, five pulses) were applied across the heads of pups with an ECM830 square wave electroporator using 10-mm diameter BTX Tweezertrode electrodes (Holliston, MA). Eyeballs were harvested at P21 for analysis.
Immunohistochemistry
Cryosections were probed with specific antibodies as described (66) and visualized using an Olympus FluoView FV1000 confocal laser scanning unit and Olympus BX61WI upright microscope (Olympus America Inc., Center Valley, PA).
RESULTS
Putative Sumoylation Sites Are Highly Conserved in Maf Subfamily Proteins
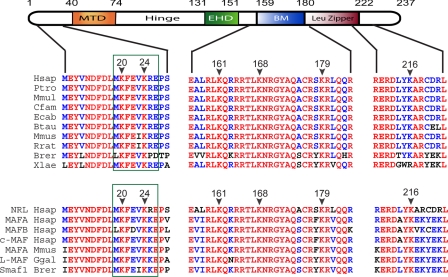
SUMO (small ubiquitin modifier) is an 8-kDa protein that can be linked covalently to target proteins, usually at a lysine residue within the consensus sequence ψKX(E/D) (Ψ = hydrophobic residue) (48, 49). The human NRL protein contains six lysine residues that are evolutionarily conserved in other vertebrates and in Maf proteins (Fig. 1). SUMOplot analysis predicts two high probability sumoylation sites in NRL at Lys-20 and Lys-24 (p > 0.80 and p > 0.93, respectively), close to the minimal transactivation domain (50).
FIGURE 1.
A schematic of the human NRL protein with the sequence alignment of NRL orthologs (upper panel) and MAF family proteins (lower panel). Alignments were performed using AlignX from VectorNTI (Invitrogen). Amino acids conserved in all orthologs and across MAF family members are indicated in red, and less conserved residues are shown in blue. Arrowheads indicate lysine residues with their position in the human NRL protein. Sumoylation sites predicted with high probability are framed by a green rectangle. MTD, minimal transactivation domain; Hinge, hinge domain; EHD, extended homology domain; BM, basic motif; Leu Zipper, leucine zipper; Hsap, Homo sapiens (human); Ptro, Pan troglodyte (chimpanzee); Mmul, Macaca mulatta (rhesus monkey); Cfam, Canis familiaris (dog); Ecab, Equus caballus (horse); Btau, Bos taurus (cow); Mmus, Mus musculus (mouse); Rrat, Rattus norvegicus (rat); Brer, Brachydanio rerio (zebrafish); Xlae, Xenopus laevis (Xenopus); Ggal, Gallus gallus (chicken).
NRL Is Disumoylated at Lys-20 Residue
To examine NRL sumoylation in vivo, we performed immunoprecipitation from adult mouse retinal extract using an anti-NRL antibody (44) followed by immunoblot analysis with anti-SUMO1 antibody. Detection of a single protein of ∼50 kDa (instead of phosphorylated NRL isoforms between 29 and 35 kDa (44)) is indicative of at least one sumoylated NRL isoform presumably with two linked SUMO1 molecules in the mature retina (Fig. 2A). The reverse immunoprecipitation experiment (immunoprecipitation of all sumoylated proteins from adult retina with anti-SUMO1 antibody followed by immunoblotting with NRL antibody) further confirmed the disumoylation of NRL (Fig. 2B).
FIGURE 2.
Sumoylation of NRL in vivo and in vitro. A and B, adult mouse retina extracts were immunoprecipitated with anti-NRL IgG (A) or anti-SUMO1 (B) followed by immunoblotting with anti-SUMO1 antibody or anti-NRL IgG, respectively. The arrow indicates sumoylated NRL. IP, immunoprecipitation. C, p53 and GST control experiments in the presence of WT or mutant SUMO1. p53, used as a positive control, was conjugated with SUMO1 and not by mutant SUMO1 under our assay conditions. D and E, purified GST-tagged WT or mutant NRL proteins were sumoylated in vitro with E1 and E2 ligases in the presence of SUMO1 or mutated SUMO1 protein. D shows the immunoblot probed with anti-SUMO1, and E shows the immunoblot probed with anti-NRL antibodies. Arrowheads show sumoylated p53 control, and arrows indicate sumoylated NRL. F, HEK293T cells were co-transfected with plasmids expressing WT-NRL or lysine mutants with or without FLAG-SUMO1. Cell extracts were immunoprecipitated with anti-NRL IgG and immunoblotted with anti-FLAG antibody. The arrowhead shows sumoylated NRL. G, HEK293T cells were transfected with plasmids expressing WT-NRL, NRL-S50T, and NRL-K20R/K24R. After immunoprecipitation of cell extracts with anti-NRL IgG, immunoblot was probed with anti-FLAG antibody. H, HEK293T cells were co-transfected with plasmids expressing WT-NRL, NRL-K20R/K24R, and NRL-S50T with FLAG-SUMO1. Immunoblots of cell extracts were probed with anti-NRL IgG.
To validate and identify sumoylation sites in NRL, we performed in vitro sumoylation assays using E. coli-expressed GST-tagged WT and mutant NRL proteins. In this assay, p53 is used as a positive control and shows an expected molecular mass of 65 kDa (Fig. 2, C and D). WT-NRL and NRL-K24R proteins are sumoylated with SUMO1, E1, and E2 ligase; however, no sumoylation is detected with NRL-K20R and NRL-K20R/K24R mutants (Fig. 2, D and E). Consistent with in vivo data (Fig. 2, A and B), we observe a sumoylated GST-tagged WT-NRL protein of 70 KDa (Fig. 2D), indicating the addition of two SUMO1 proteins (note that the non-sumoylated GST-NRL is 52 kDa). Other observed bands correspond to SUMO1-conjugated E1 and E2 enzyme and are not detected in the samples with mutant SUMO1. Mutations in other lysine residues of NRL (K161R, K168R, K179R, and K216R) do not affect sumoylation in our assay conditions (data not shown). These results (Fig. 2, A–E) provide strong evidence for the presence of least one Lys-20 disumoylated isoform of NRL in vivo.
To further establish the sumoylation of NRL, we co-expressed WT and mutant (K20R, K24R, and K20R/K24R) NRL proteins in HEK293T cells with a FLAG-tagged SUMO1 construct (Fig. 2F). Immunoprecipitation of transfected cell extracts with anti-NRL antibody followed by immunoblot analysis with anti-FLAG antibody reveals WT and NRL-K24R proteins of 55 kDa that correspond to the addition of two FLAG-SUMO1 molecules (Fig. 2F). NRL-K20R and NRL-K20R/K24R mutants do not show any sumoylation, further demonstrating that WT-NRL is disumoylated at the Lys-20 residue. Immunocytochemical analysis of transfected HEK293T cells reveals that lysine mutations do not alter the nuclear localization of NRL (data not shown).
Because sumoylation can depend on the phosphorylation state of the target protein (51, 52), we examined the consequence of the S50T mutation, which affects NRL phosphorylation (45, 46). Sumoylation is not altered in the NRL-S50T mutant (Fig. 2G). Furthermore, K20R/K24R mutation in NRL (used as a control in this assay) does not affect the phosphorylation state (Fig. 2H).
Sumoylation Modulates the Transcriptional Activity of NRL on Rho and Nr2e3 Promoters
We then investigated the effect of K20R, K24R, and K20R/K24R mutations on transcriptional activation of two known photoreceptor-specific target promoters of NRL-rhodopsin (Rho) (32) and Nr2e3 (35). We performed Dual-Luciferase promoter activity assays to compare the transactivation ability of the WT and mutant NRL (either alone or with CRX and NR2E3 (18, 23, 35)) (Fig. 3). The NRL-K20R and NRL-K20R/K24R mutants, but not the NRL-K24R mutant, show statistically significant reduction of the Rho promoter activation when compared with WT-NRL (Fig. 3A). Correspondingly, similar and significant decrease in the induction of Rho promoter is observed when the NRL-K20R or NRL-K20R/K24R mutant is co-expressed with CRX and NR2E3 (Fig. 3A). Interestingly, all three NRL mutants (K20R, K24R, and K20R/K24R) exhibit significantly lesser transactivation of the Nr2e3 promoter when compared with WT-NRL (Fig. 3B). These data suggest that sumoylation fine-tunes the activity of NRL to activate promoters of specific target genes that contribute to rod development and function.
FIGURE 3.
Modulation of transcriptional regulatory activity of NRL by sumoylation. HEK293 cells were co-transfected with a construct containing bovine Rho (A) or mouse Nr2e3 promoter (B) driving firefly luciferase reporter gene simultaneously with increasing concentrations (0.01–0.3 μg) of WT- or mutant NRL expression constructs, either alone or in association with CRX and NR2E3. -Fold change is relative to the mock expression vector control. The blue box indicates NRL-responsive element (NRE). Error bars show S.E. Asterisks indicate p value <0.05. Black lines and red, blue, and green lines and asterisks correspond to WT-NRL, NRL-K20R, NRL-K24R, and NRL-K20R/K24R, respectively.
NRL Sumoylation Is Required for Normal Rod Differentiation
As expression of NRL can rescue the Nrl−/− phenotype in transgenic mice (29), we adopted an in vivo electroporation (53) assay to investigate the role of NRL sumoylation in the context of photoreceptor development. We performed in vivo transfection of newborn Nrl−/− mouse retina by electroporation using WT-NRL or NRL-K20R/K24R mutant construct and assessed photoreceptor development 3 weeks later (Fig. 4). Transfected retinal cells were monitored by co-injecting ubiquitin-GFP construct, and the untransfected portion of the retina was used as control (Fig. 4A). Following electroporation of WT-NRL at P0, rhodopsin expression is observed at P21 only in transfected GFP-positive cells but not in the untransfected region of the Nrl−/− mouse retina (Fig. 4, A and B). Consistent with the established role of NRL, transfected cells expressing rhodopsin did not express cone arrestin, a cone-specific marker that is highly expressed in Nrl−/− retina. Electroporation of the NRL-K20R/K24R mutant, however, results in a clearly distinct phenotype (Fig. 4, C and D). Rhodopsin expression is observed in lesser numbers of NRL-K20R/K24R-transfected cells (58 ± 7%) when compared with WT-NRL (85 ± 3%), and cone arrestin expression is also detected in some of the rhodopsin-positive cells transfected with the NRL-K20R/K24R mutant. The phenotype of Nrl−/− cells electroporated with NRL-K20R/K24R resembled that of the hybrid photoreceptors expressing both rod and cone markers in the rd7 mutant mouse (36), where Nr2e3 function is abolished (54). Cells in the inner nuclear layer transfected with WT or mutant NRL construct (as revealed by Ub-GFP expression) do not show the expression of rod-specific genes (such as rhodopsin) (Fig. 4, B and C).
FIGURE 4.
Partial rescue of the Nrl−/− phenotype and reduced expression of Nr2e3 by NRL-K20R/K24R sumoylation mutant. A, representative retinal photographs of P21 Nrl−/− mouse retinas: unelectroporated region. onl, outer nuclear layer; inl, inner nuclear layer; gcl, ganglion cell layer. B, C, E, F, and G, electroporated at P0 with Ub-GFP and either Ub-WT-NRL (B, E, and F) or Ub-NRL-K20R/K24R (C and G). A–C, GFP is green, cone arrestin is red, and rhodopsin is gray. White and yellow arrows show GFP+ electroporated cells with or without rhodopsin staining, respectively. Lower panels in B and C show higher magnification images with arrowheads indicating GFP- and rhodopsin-positive photoreceptors. Cone arrestin signal is observed in these cells only with NRL-K20R/K24R mutant. Scale bar: 20 μm. D, quantification of GFP-positive cells expressing rhodopsin after electroporation of WT-NRL (gray) or NRL-K20R/K24R (white). Error bars show S.E. from seven independent electroporated retinas for each construct. **, p < 0.01 by Student's t test. E–G, GFP is green, and NR2E3 immunostaining is red. White and yellow arrows show GFP+ electroporated cells with or without NR2E3 staining, respectively. E and F show different regions of the retina electroporated with WT-NRL. Scale bar: 20 μm. H, quantification of GFP-positive cells in ONL expressing NR2E3 after electroporation of WT-NRL (gray) or NRL-K20R/K24R (white). Error bars show S.E. from four independent electroporated retinas for each construct. **, p < 0.01 by Student's t test.
As the NRL-K20R/K24R sumoylation mutant resulted in decreased activation of the Nr2e3 promoter in vitro (Fig. 3B) and produced photoreceptors expressing both rod and cone genes (as in rd7 mouse) in electroporation assays (Fig. 4C), we examined whether NRL sumoylation is required for appropriate Nr2e3 expression in vivo. Nr2e3, a direct target of NRL (35) is not expressed in Nrl−/− mice (28), as illustrated in the untransfected region of the retina that is used as a control (GFP-negative cells) in electroporation experiments (Fig. 4E). When WT-NRL is expressed, 89 ± 3% of the GFP-positive cells in the outer nuclear layer strongly express NR2E3; however, the NRL-K20R/K24R mutant results in only 42 ± 6% cells that show weak NR2E3 immunoreactivity (Fig. 4, F–H). These data demonstrate that sumoylated NRL is a stronger transcriptional activator of Nr2e3 promoter.
Is PIAS3 Involved in NRL Sumoylation?
As PIAS3 participates in sumoylation of NR2E3 (26), we investigated whether it can sumoylate NRL. We show that PIAS3 is indeed expressed in rod photoreceptors, isolated from the Nrl-GFP mouse retinas (30) that express GFP specifically in rods under the control of Nrl promoter (Fig. 5A). NRL and PIAS3 can be co-immunoprecipitated from P4, P10, and adult retinal extracts (Fig. 5B), suggesting their presence in a protein complex. However, although NRL is sumoylated in transfected HEK293T cells (Fig. 2, C and D), PIAS3 is undetectable in these cells by immunoblot analysis (Fig. 5C). Furthermore, co-expression with PIAS3 does not alter NRL sumoylation pattern in transfected cells (Fig. 5D). It therefore appears that PIAS3 may not be the primary E3-SUMO ligase involved in NRL sumoylation.
FIGURE 5.
A, expression of Pias3 in rod photoreceptors. Dissociated cells from adult Nrl-GFP mouse retina were stained with anti-PIAS3-antibody and 4′,6-diamidino-2-phenylindole (DAPI). Arrows indicate colocalization of PIAS3 (red) in GFP-positive rods (green). B, co-immunoprecipitation of PIAS3 and NRL from retinal extracts. Immunoblots of anti-NRL immunoprecipitated (IP) proteins from P0, P4, P10, and adult (Ad) retina were probed with anti-PIAS3 antibody. Normal rabbit IgG served as negative control. C, immunoblot analysis of mock- and PIAS3-transfected HEK293T extracts with anti-PIAS3 antibody. D, conjugation of SUMO1 to NRL in the transfected cells in the absence or presence of PIAS3. HEK293T cell extracts transfected with WT-NRL, FLAG-SUMO and PIAS3 were immunoprecipitated with anti-NRL, and immunoblots were probed with anti-NRL or anti-FLAG antibody. Black arrows indicate sumoylated NRL protein.
DISCUSSION
Selective covalent linkage of SUMO moieties can alter the function of conjugated proteins (5, 55, 56). Sumoylation of transcription factors was originally associated with repression of gene expression (57), but like other reversible PTMs, SUMO modification can have diverse physiological consequences. Our studies demonstrate that at least one NRL protein isoform is disumoylated in vivo at Lys-20, and this modification has a positive impact on transcriptional activation of Nr2e3 and rhodopsin expression. We propose that NRL sumoylation affects the assembly and/or stability of specific enhanceosome complexes that are needed for high level expression of critical rod-specific genes.
The transcriptional regulatory function of Maf subfamily proteins is modulated by PTMs, including phosphorylation and sumoylation (58–61). MAF-A sumoylation occurs at Lys-32, which corresponds to the non-sumoylated Lys-24 in NRL (Fig. 1). The sumoylation of MAF-A reduces its transcriptional activity particularly on the Ins gene without affecting its nuclear localization. Sumoylation of NRL, reported here, occurs on Lys-20, corresponding to Lys-28 in MAF-A, and does not affect its nuclear localization; however, unlike MAF-A, sumoylation of NRL positively impacts transcriptional activation of at least two downstream target genes. Notably, sumoylation of NR2E3 is necessary for its ability to repress cone gene expression (26). Interestingly, NRL-K24 exhibits the highest likelihood of sumoylation based on in silico analysis but does not appear to be sumoylated in vitro or in transfected cells. However, we are unable to discriminate from our assays whether two SUMO1 proteins are added on Lys-20 exclusively or whether in some instances one SUMO1 is linked to Lys-20 and another to Lys-24. Interestingly, the Lys-24 mutation does not alter Rho promoter activation yet shows an effect on the Nr2e3 promoter. It is possible that NRL is post-translationally modified at Lys-24 in a specific developmental context, but this requires Lys-20 sumoylation. Our data suggest that NRL disumoylation on Lys-20 is necessary for precise Rho promoter activation, but sumoylation at both Lys-20 and Lys-24 may be required for Nr2e3 promoter activity. Distinct transcription factor PTMs may therefore exhibit different target specificity.
The sumoylated form of NRL promotes Rho and Nr2e3 promoter activation to a greater extent than the non-sumoylated form. In vivo expression of the NRL-K20R/K24R mutant protein was not able to rescue the rod differentiation defects in Nrl−/− retina because of low NR2E3 expression, resulting in incomplete inhibition of cone genes. However, we cannot rule out a direct repressor effect of sumoylated NRL on cone genes. Equally significantly, the NRL sumoylation mutant exhibited reduced activation of rhodopsin promoter either alone or with CRX and NR2E3 in transfected cells and by in vivo electroporation assay. As NRL is a major transcriptional activator of most, if not all, rod genes, we propose that sumoylation of NRL is utilized as a mechanism to produce quantitatively precise expression of specific genes during development.
Recent studies have linked sumoylation to oxidative stress and neurodegeneration (62). Daily renewal of outer segments puts an extreme stress on photoreceptor metabolic machinery, and any misregulation can lead to photoreceptor dysfunction and retinal degeneration. Continuous high expression of NRL in mature photoreceptors suggests its importance in rod homeostasis. Sumoylation and phosphorylation appear to be independent PTMs for controlling NRL activity. It is possible that the two participate in a transient shift between different NRL isoforms that may have unique gene regulatory functions. Circadian or light-induced changes in rod gene expression can be rapidly accomplished by tweaking the levels of sumoylation (and other PTMs) of NRL and to maintain homeostasis. We note that the circadian regulation of BMAL1 activity in the liver is mediated by sumoylation (63).
In contrast to ubiquitination, E3 ligase activity is not mandatory for protein sumoylation in vitro (7, 64), but it enhances it, as in the case of RORα (65). PIAS3 sumoylates NR2E3 (26) and interacts with NRL, but the addition of SUMO moiety on NRL is independent of PIAS3 at least in transfected cells. Our studies suggest a redundancy of SUMO ligases and that another E3 ligase may be involved in fine-tuning NRL activity by sumoylation. Although PIAS3 can sumoylate distinct transcription factors in enhanceosome complexes, additional investigations (such as conditional Pias3-knock-out) are necessary to decipher in vivo relevance of PIAS3 in NRL sumoylation and photoreceptor development.
Elucidation of gene regulatory networks that determine neuronal cell fate and function will require integration of signaling molecules to regulation of specific transcriptional target genes. Post-translational modifications, such as sumoylation, are critical components in delineating such networks. Together with a recent report (26), our study provides significant insights into the role of sumoylation in modulating the regulatory function of transcription factors that control photoreceptor differentiation and homeostasis.
Acknowledgments
We are grateful to Tiziana Cogliati for critical reading of the manuscript, Shiming Chen, Seth Blackshaw, Atsuhiro Kanda, and Hong Cheng for providing vectors, and Nadean Brown for NR2E3 immunostaining protocol.
This work was supported, in whole or in part, by a National Institutes of Health Grant through the NEI.
- PTM
- post-translational modification
- NRL
- neural retina leucine zipper
- SUMO
- small ubiquitin-like modifier
- RAR
- retinoic acid receptor
- MAF
- musculoaponeurotic fibrosarcoma
- RORβ
- RAR-related orphan receptor β
- NR2E3
- nuclear receptor subfamily 2, group E
- CRX
- cone-rod homeobox
- STAT
- signal transducer and activator of transcription
- PIAS
- protein inhibitor of activated STAT
- WT
- wild type
- Ub
- ubiquitin
- GFP
- green fluorescent protein
- GST
- glutathione S-transferase
- P
- postnatal day.
REFERENCES
- 1.Levine M., Tjian R. (2003) Nature 424, 147–151 [DOI] [PubMed] [Google Scholar]
- 2.Levine M., Davidson E. H. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 4936–4942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunter T. (2007) Mol. Cell 28, 730–738 [DOI] [PubMed] [Google Scholar]
- 4.Sims R. J., 3rd, Reinberg D. (2008) Nat. Rev. Mol. Cell Biol. 9, 815–820 [DOI] [PubMed] [Google Scholar]
- 5.Ulrich H. D. (2008) Mol. Cell 32, 301–305 [DOI] [PubMed] [Google Scholar]
- 6.Yang X. J., Seto E. (2008) Mol. Cell 31, 449–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weissman A. M. (2001) Nat. Rev. Mol. Cell Biol. 2, 169–178 [DOI] [PubMed] [Google Scholar]
- 8.Seet B. T., Dikic I., Zhou M. M., Pawson T. (2006) Nat. Rev. Mol. Cell Biol. 7, 473–483 [DOI] [PubMed] [Google Scholar]
- 9.Livesey F. J., Cepko C. L. (2001) Nat. Rev. Neurosci. 2, 109–118 [DOI] [PubMed] [Google Scholar]
- 10.Marquardt T., Gruss P. (2002) Trends Neurosci. 25, 32–38 [DOI] [PubMed] [Google Scholar]
- 11.Cayouette M., Barres B. A., Raff M. (2003) Neuron 40, 897–904 [DOI] [PubMed] [Google Scholar]
- 12.Bradford R. L., Wang C., Zack D. J., Adler R. (2005) Dev. Biol. 286, 31–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levine E. M., Fuhrmann S., Reh T. A. (2000) Cell Mol. Life Sci. 57, 224–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo D. G., Xue T., Yau K. W. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 9855–9862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia L., Oh E. C., Ng L., Srinivas M., Brooks M., Swaroop A., Forrest D. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 17534–17539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng L., Hurley J. B., Dierks B., Srinivas M., Saltó C., Vennström B., Reh T. A., Forrest D. (2001) Nat. Genet. 27, 94–98 [DOI] [PubMed] [Google Scholar]
- 17.Haider N. B., Jacobson S. G., Cideciyan A. V., Swiderski R., Streb L. M., Searby C., Beck G., Hockey R., Hanna D. B., Gorman S., Duhl D., Carmi R., Bennett J., Weleber R. G., Fishman G. A., Wright A. F., Stone E. M., Sheffield V. C. (2000) Nat. Genet. 24, 127–131 [DOI] [PubMed] [Google Scholar]
- 18.Cheng H., Khanna H., Oh E. C., Hicks D., Mitton K. P., Swaroop A. (2004) Hum. Mol. Genet. 13, 1563–1575 [DOI] [PubMed] [Google Scholar]
- 19.Cheng H., Aleman T. S., Cideciyan A. V., Khanna R., Jacobson S. G., Swaroop A. (2006) Hum. Mol. Genet. 15, 2588–2602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J., Rattner A., Nathans J. (2005) J. Neurosci. 25, 118–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng G. H., Ahmad O., Ahmad F., Liu J., Chen S. (2005) Hum. Mol. Genet. 14, 747–764 [DOI] [PubMed] [Google Scholar]
- 22.Nishida A., Furukawa A., Koike C., Tano Y., Aizawa S., Matsuo I., Furukawa T. (2003) Nat. Neurosci. 6, 1255–1263 [DOI] [PubMed] [Google Scholar]
- 23.Chen S., Wang Q. L., Nie Z., Sun H., Lennon G., Copeland N. G., Gilbert D. J., Jenkins N. A., Zack D. J. (1997) Neuron 19, 1017–1030 [DOI] [PubMed] [Google Scholar]
- 24.Furukawa T., Morrow E. M., Cepko C. L. (1997) Cell 91, 531–541 [DOI] [PubMed] [Google Scholar]
- 25.Rhee K. D., Goureau O., Chen S., Yang X. J. (2004) J. Neurosci. 24, 9779–9788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onishi A., Peng G. H., Hsu C., Alexis U., Chen S., Blackshaw S. (2009) Neuron 61, 234–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore K. B., Schneider M. L., Vetter M. L. (2002) Neuron 34, 183–195 [DOI] [PubMed] [Google Scholar]
- 28.Mears A. J., Kondo M., Swain P. K., Takada Y., Bush R. A., Saunders T. L., Sieving P. A., Swaroop A. (2001) Nat. Genet. 29, 447–452 [DOI] [PubMed] [Google Scholar]
- 29.Oh E. C., Khan N., Novelli E., Khanna H., Strettoi E., Swaroop A. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 1679–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akimoto M., Cheng H., Zhu D., Brzezinski J. A., Khanna R., Filippova E., Oh E. C., Jing Y., Linares J. L., Brooks M., Zareparsi S., Mears A. J., Hero A., Glaser T., Swaroop A. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 3890–3895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshida S., Mears A. J., Friedman J. S., Carter T., He S., Oh E., Jing Y., Farjo R., Fleury G., Barlow C., Hero A. O., Swaroop A. (2004) Hum. Mol. Genet. 13, 1487–1503 [DOI] [PubMed] [Google Scholar]
- 32.Rehemtulla A., Warwar R., Kumar R., Ji X., Zack D. J., Swaroop A. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 191–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pittler S. J., Zhang Y., Chen S., Mears A. J., Zack D. J., Ren Z., Swain P. K., Yao S., Swaroop A., White J. B. (2004) J. Biol. Chem. 279, 19800–19807 [DOI] [PubMed] [Google Scholar]
- 34.Lerner L. E., Gribanova Y. E., Ji M., Knox B. E., Farber D. B. (2001) J. Biol. Chem. 276, 34999–35007 [DOI] [PubMed] [Google Scholar]
- 35.Oh E. C., Cheng H., Hao H., Jia L., Khan N. W., Swaroop A. (2008) Brain Res. 1236, 16–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corbo J. C., Cepko C. L. (2005) PLoS Genet. 1, e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacobson S. G., Sumaroka A., Aleman T. S., Cideciyan A. V., Schwartz S. B., Roman A. J., McInnes R. R., Sheffield V. C., Stone E. M., Swaroop A., Wright A. F. (2004) Hum. Mol. Genet. 13, 1893–1902 [DOI] [PubMed] [Google Scholar]
- 38.Hennig A. K., Peng G. H., Chen S. (2008) Brain Res. 1192, 114–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carter-Dawson L. D., LaVail M. M. (1979) J. Comp. Neurol. 188, 263–272 [DOI] [PubMed] [Google Scholar]
- 40.Nasonkin I., Cogliati T., Swaroop A. (2010) in Encyclopedia of the Eye (Dartt D. A. ed) Vol. 3, pp. 332–339, Academic Press, Oxford [Google Scholar]
- 41.Morrow E. M., Belliveau M. J., Cepko C. L. (1998) J. Neurosci. 18, 3738–3748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carey M. (1998) Cell 92, 5–8 [DOI] [PubMed] [Google Scholar]
- 43.Peng G. H., Chen S. (2007) Hum. Mol. Genet. 16, 2433–2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swain P. K., Hicks D., Mears A. J., Apel I. J., Smith J. E., John S. K., Hendrickson A., Milam A. H., Swaroop A. (2001) J. Biol. Chem. 276, 36824–36830 [DOI] [PubMed] [Google Scholar]
- 45.Bessant D. A., Payne A. M., Mitton K. P., Wang Q. L., Swain P. K., Plant C., Bird A. C., Zack D. J., Swaroop A., Bhattacharya S. S. (1999) Nat. Genet. 21, 355–356 [DOI] [PubMed] [Google Scholar]
- 46.Kanda A., Friedman J. S., Nishiguchi K. M., Swaroop A. (2007) Hum. Mutat 28, 589–598 [DOI] [PubMed] [Google Scholar]
- 47.Nishiguchi K. M., Friedman J. S., Sandberg M. A., Swaroop A., Berson E. L., Dryja T. P. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 17819–17824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kerscher O., Felberbaum R., Hochstrasser M. (2006) Annu. Rev. Cell Dev. Biol. 22, 159–180 [DOI] [PubMed] [Google Scholar]
- 49.Sampson D. A., Wang M., Matunis M. J. (2001) J. Biol. Chem. 276, 21664–21669 [DOI] [PubMed] [Google Scholar]
- 50.Friedman J. S., Khanna H., Swain P. K., Denicola R., Cheng H., Mitton K. P., Weber C. H., Hicks D., Swaroop A. (2004) J. Biol. Chem. 279, 47233–47241 [DOI] [PubMed] [Google Scholar]
- 51.Kang J., Gocke C. B., Yu H. (2006) BMC Biochem. 7, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grégoire S., Tremblay A. M., Xiao L., Yang Q., Ma K., Nie J., Mao Z., Wu Z., Giguère V., Yang X. J. (2006) J. Biol. Chem. 281, 4423–4433 [DOI] [PubMed] [Google Scholar]
- 53.Matsuda T., Cepko C. L. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 16–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen J., Rattner A., Nathans J. (2006) Hum. Mol. Genet. 15, 2146–2156 [DOI] [PubMed] [Google Scholar]
- 55.Hay R. T. (2005) Mol. Cell 18, 1–12 [DOI] [PubMed] [Google Scholar]
- 56.Hunter T., Sun H. (2008) Ernst Schering Found. Symp. Proc. 1, 1–16 [DOI] [PubMed] [Google Scholar]
- 57.Verger A., Perdomo J., Crossley M. (2003) EMBO Rep 4, 137–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Benkhelifa S., Provot S., Nabais E., Eychène A., Calothy G., Felder-Schmittbuhl M. P. (2001) Mol. Cell. Biol. 21, 4441–4452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rocques N., Abou Zeid N., Sii-Felice K., Lecoin L., Felder-Schmittbuhl M. P., Eychène A., Pouponnot C. (2007) Mol. Cell 28, 584–597 [DOI] [PubMed] [Google Scholar]
- 60.Shao C., Cobb M. H. (2009) J. Biol. Chem. 284, 3117–3124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leavenworth J. W., Ma X., Mo Y. Y., Pauza M. E. (2009) J. Immunol. 183, 1110–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dorval V., Fraser P. E. (2007) Biochim. Biophys. Acta 1773, 694–706 [DOI] [PubMed] [Google Scholar]
- 63.Cardone L., Hirayama J., Giordano F., Tamaru T., Palvimo J. J., Sassone-Corsi P. (2005) Science 309, 1390–1394 [DOI] [PubMed] [Google Scholar]
- 64.Rytinki M. M., Kaikkonen S., Pehkonen P., Jääskeläinen T., Palvimo J. J. (2009) Cell Mol. Life Sci. 66, 3029–3041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hwang E. J., Lee J. M., Jeong J., Park J. H., Yang Y., Lim J. S., Kim J. H., Baek S. H., Kim K. I. (2009) Biochem. Biophys. Res. Commun. 378, 513–517 [DOI] [PubMed] [Google Scholar]
- 66.Roger J., Brajeul V., Thomasseau S., Hienola A., Sahel J. A., Guillonneau X., Goureau O. (2006) Dev. Biol. 298, 527–539 [DOI] [PubMed] [Google Scholar]