Abstract
Recent reports have shown that Ca2+/calmodulin (Ca2+/CaM) signaling plays a crucial role in angiogenesis. We previously developed a new Ca2+/CaM antagonist, HBC (4-{3,5-bis-[2-(4-hydroxy-3-methoxyphenyl)ethyl]-4,5-dihydropyrazol-1-yl}benzoic acid), from a curcumin-based synthetic chemical library. Here, we investigated its anti-angiogenic activity and mode of action. HBC potently inhibited the proliferation of human umbilical vascular endothelial cells with no cytotoxicity. Furthermore, HBC blocked in vitro characteristics of angiogenesis such as tube formation and chemoinvasion, as well as neovascularization of the chorioallantoic membrane of growing chick embryos in vivo. Notably, HBC markedly inhibited expression of hypoxia-inducible factor-1α (HIF-1α) at the translational level during hypoxia, thereby reducing HIF-1 transcriptional activity and expression of its major target gene, vascular endothelial growth factor. In addition, combination treatment with HBC and various HIF-1 inhibitors, including suberoylanilide hydroxamic acid, rapamycin, and terpestacin, had greater anti-angiogenic activity than treatment with each single agent. Collectively, our findings indicate that HBC is a new anti-angiogenic agent targeting HIF that can be used to explore the biological role of Ca2+/CaM in angiogenesis.
Keywords: Anticancer Drug, Calmodulin, Drug Action, Hypoxia, Reactive Oxygen Species (ROS), Tumor
Introduction
Efficient inhibition of angiogenesis is considered a promising strategy for the treatment of angiogenesis-related diseases, including cancer, diabetic retinopathy, macular degeneration, rheumatoid arthritis, psoriasis, and hemangioma (1, 2). In particular, angiogenesis is essential for the growth, progression, and metastasis of solid tumors and is thus considered a potent pharmacological target for anticancer therapy (3, 4). Hypoxia-inducible factor-1 (HIF-1)2 plays a key role in tumor angiogenesis by regulating the expression of angiogenic factors, including VEGF (5, 6). HIF-1 is a heterodimeric protein composed of a constitutively expressed β subunit (HIF-1β) and a hypoxia-inducible α subunit (HIF-1α) (7). Under hypoxic conditions, HIF-1α protein is stabilized by inhibition of the proline hydroxylase/Von Hippel-Lindau degradation pathway and dimerizes with HIF-1β to transactivate target genes (8). HIF-1α overexpression has been implicated in many human cancers and is associated with increased vascularization, drug resistance, and poor diagnosis (9, 10). A growing number of HIF-1α inhibitors have shown potent inhibitory effects on tumor growth and angiogenesis (11).
Calmodulin (CaM) is a ubiquitous Ca2+-binding protein that mediates Ca2+-dependent signaling in eukaryotic cells (12, 13). It has no intrinsic enzymatic activity but regulates diverse cellular processes, including cell proliferation, development, motility, and secretion, by modulating the activity of a considerable number of target enzymes in a Ca2+-dependent manner (14, 15). Accumulating evidence suggests that Ca2+/CaM plays a pivotal role in tumor development (16, 17). Overexpression of Ca2+/CaM is often observed in certain tumors and many Ca2+/CaM-dependent enzymes, such as CaM kinases, protein phosphatases, phosphodiesterases, and nitric oxide synthases, are involved in tumor progression via regulation of a variety of cellular signaling pathways (18, 19). Accordingly, specific Ca2+/CaM antagonists exhibit potent anti-cancer activity (20, 21).
Recent studies have also shown that Ca2+/CaM plays a crucial role in angiogenesis (22). Ca2+/CaM activates HIF-1 and consequently induces the expression of pro-angiogenic factors such as VEGF (23, 24). Hypoxia-induced increase in intracellular Ca2+ concentration promotes both the expression and transcriptional activity of HIF-1α by modulating the activity of Ca2+/CaM-dependent enzymes, including CaM kinase and calcineurin (25, 26). Furthermore, inhibition of Ca2+/CaM by a CaM-dominant negative mutant, Ca2+/CaM antagonist, or Ca2+ chelator down-regulates HIF-1 transcriptional activity and thus suppresses angiogenesis (22, 27). Therefore, Ca2+/CaM might be a potent therapeutic target for the treatment of angiogenesis-related diseases, including cancer.
We previously developed a new curcumin derivative, 4-{3,5-bis-[2-(4-hydroxy-3-methoxyphenyl)ethyl]-4,5-dihydropyrazol-1-yl}benzoic acid (HBC), with potent anti-proliferative activity against tumor cells by inducing G0/G1 cell cycle arrest (Fig. 1A) (28). A chemical proteomics approach revealed that HBC directly binds to the C-terminal hydrophobic pocket of Ca2+/CaM in a Ca2+-dependent manner, similar to other known Ca2+/CaM antagonists such as trifluoperazine (TFP) and W7, and thus negatively modulates Ca2+/CaM-dependent proliferative signaling (29). In this study, we explored the anti-angiogenic activity of this new Ca2+/CaM antagonist. Our results identify HBC as a novel anti-angiogenic agent that down-regulates HIF-1 transcriptional activity via suppression of HIF-1α expression at the translational level. In addition, we show that HBC enhances the anti-angiogenic activity of known HIF-1 inhibitors suberoylanilide hydroxamic acid (SAHA), rapamycin, and terpestacin, suggesting that HBC might be applied in combination therapy with known inhibitors targeting HIF.
FIGURE 1.
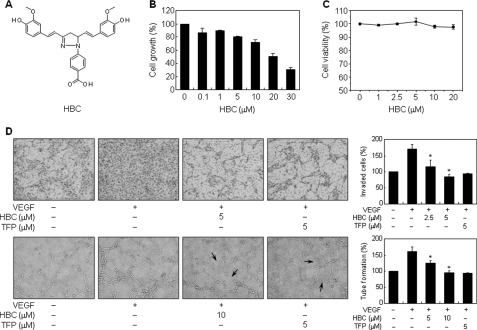
Effect of HBC on in vitro angiogenesis. A, chemical structure of HBC. B, effect of HBC on the growth of HUVECs. Cells were treated with various concentrations of HBC for 48 h, and cell growth was measured using an MTT colorimetric assay. C, effect of HBC on the viability of HUVECs. Cells were treated with HBC (0–20 μm) and incubated for 72 h. Cell viability was measured by trypan blue assay. D, effect of HBC on the invasion and tube-forming abilities of HUVECs. Serum-starved HUVECs were stimulated with VEGF (30 ng/ml) in the presence or absence of HBC or TFP. Arrows indicate inhibition of tube formation by the compounds. The basal levels of invasiveness (upper panel) and capillary tube formation (lower panel) of HUVECs that were incubated in serum-free media were normalized to 100%. *, p < 0.01 versus VEGF control. Each value represents mean ± S.E. from three independent experiments.
EXPERIMENTAL PROCEDURES
Materials
VEGF was obtained from Upstate Biotechnology. Endothelial growth medium-2 was purchased from Lonza, and RPMI 1640 medium and FBS were obtained from Invitrogen. Matrigel and Transwell plates were from Collaborative Biomedical Products and Corning Costar, respectively. TFP, RA, and ionomycin were purchased from Sigma and MG132 from A. G. Scientific. HBC was synthesized and characterized in our laboratory as described previously (28). Anti-HIF-1α antibody was purchased from BD Biosciences, and anti-HIF-2α and HIF-1β antibodies were from Novus Biologicals. Anti-phospho-p70S6K, -p70S6K, -phospho-mTOR, and -mTOR antibodies were obtained from Cell Signaling, and anti-tubulin antibody was from Upstate Biotechnology.
Cell Culture and Hypoxic Conditions
Early passages (4–8 passages) of human umbilical vascular endothelial cells (HUVECs) were grown in endothelial growth medium-2 supplemented with 10% FBS. HepG2 (human hepatocellular carcinoma) cells were grown in RPMI 1640 medium containing 10% FBS. All cells were maintained at 37 °C in a humidified 5% CO2 incubator. For hypoxic conditions, cells were incubated at a CO2 level of 5% with 1% O2 balanced with N2 in a hypoxic chamber (Forma).
Cell Growth and Viability Assay
Cell growth was measured using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay and cell viability was assessed using trypan blue staining as described previously (30).
Chemoinvasion Assay
The invasiveness of endothelial cells was determined in vitro using a Transwell chamber system with polycarbonate filter inserts with a pore size of 8.0 μm as described previously (30). Briefly, the lower side of the filter was coated with 10 μl of gelatin (1 mg/ml), and the upper side was coated with 10 μl of Matrigel (3 mg/ml). HUVECs (1 × 105 cells) were placed in the upper chamber of the filter, and HBC or TFP was added to the lower chamber in the presence of VEGF (30 ng/ml). The chamber was incubated at 37 °C for 18 h, and then the cells were fixed with methanol and stained with hematoxylin and eosin. The total number of cells that invaded the lower chamber of the filter was counted using an optical microscope (Olympus) at 100× magnification.
Capillary Tube Formation Assay
Capillary tube formation of endothelial cells in vitro was assessed as described previously (30). Briefly, HUVECs (1 × 105 cells) were inoculated on the surface of the Matrigel, and HBC or TFP was added for 6–18 h in the presence or absence of VEGF. Morphological changes of the cells and tube formation were observed under a microscope and photographed at 100× magnification using a JVC digital camera (Victor). Tube formation was quantified by counting the number of connected cells in randomly selected fields at 100× magnification and dividing that number by the total number of cells in the same field.
Chorioallantoic Membrane (CAM) Assay
Fertilized chick eggs were maintained in a humidified incubator at 37 °C for 3 days. Approximately 2 ml of egg albumin was removed with a hypodermic needle allowing the CAM and yolk sac to drop away from the shell membrane. On day 3.5 the shell was punched out and removed, and the shell membrane was peeled away. When the chick embryos were 4.5 days old, HBC-loaded Thermanox coverslips were air-dried and then applied to the CAM surface. Two days later, 2 ml of 10% fat emulsion was injected into the chorioallantois, and the CAM was observed under a microscope. Because RA is a known anti-angiogenic compound, RA was used as a positive control for anti-angiogenic responses. The response was scored as positive when CAM treated with the sample showed an avascular zone similar to that of RA-treated CAM with very few vessels compared with a control coverslip, and was calculated as the percentage of positive eggs relative to the total number of eggs tested.
Western Blot Analysis
Cell lysates were separated by 10% SDS-PAGE and transferred to polyvinylidene fluoride membranes (Millipore) using standard electroblotting procedures. Blots were blocked and immunolabeled overnight at 4 °C with primary antibodies against HIF-1α, HIF-2α, HIF-1β, phospho-p70S6K, p70S6K, phospho-mTOR, mTOR, and tubulin. Immunolabeling was detected by an enhanced chemiluminescence (ECL) kit (Amersham Biosciences) according to the manufacturer's instructions.
Transient Transfection and HRE-luciferase Reporter Assay
To assay the transcriptional activity of HIF-1, HepG2 cells were transiently cotransfected with hypoxia-response element (HRE) luciferase reporter vector (pGL3-SV40–6HRE) and an internal control reporter vector (pRL-SV40, Promega) using Lipofectamine LTX reagent (Invitrogen). Luciferase activity was analyzed using the Dual-Luciferase Reporter Assay System (Promega) in a luminometer (Tecan).
Measurement of VEGF by ELISA
The VEGF concentration in media from treated cells was determined using an ELISA kit for VEGF protein (R&D Systems) according to the manufacturer's instructions. The results were expressed as concentration of VEGF relative to the total amount of protein from each well.
RT-PCR
Total cellular RNA was isolated using TRIzol reagent (Invitrogen) and then reverse transcribed by Moloney murine leukemia virus reverse transcriptase (Invitrogen) using Oligo-d(T)15 primers. To determine the expression of HIF-1α mRNA, standard PCR was performed using 5′-GCTGGCCCCAGCCGCTGGAG-3′ and 5′-GAGTGCAGGGTCAGCACTAC-3′ as primers. The PCR products were resolved by 1% agarose gel electrophoresis and visualized by ethidium bromide staining. The mRNA level of β-actin was used as an internal control.
Statistical Analysis
Results are expressed as the means ± S.E. Student's t test was used to determine statistical significance between control and test groups. A p value of <0.05 was considered statistically significant.
RESULTS
HBC Inhibits Angiogenesis Both in Vitro and in Vivo
Recent studies have shown that Ca2+/CaM is crucially involved in angiogenesis and that several Ca2+/CaM antagonists, including TFP and W-7, suppress angiogenesis (22). To explore the anti-angiogenic activity of HBC, we first investigated the effect of HBC on the growth of HUVECs using the MTT colorimetric assay. As shown in Fig. 1B, HBC potently inhibited the proliferation of HUVECs with an IC50 of 20 μm. These data indicate that HBC may exhibit anti-angiogenic activity through inhibition of endothelial cell proliferation. To further evaluate whether the suppressed proliferation by HBC is due to cytotoxic or cytostatic activity, viability assay was performed using trypan blue exclusion method. As shown in Fig. 1C, the viability of HUVECs was >95% even at 20 μm HBC treatment for 72 h. These results demonstrate that the anti-proliferative activity of HBC results from the cytostatic effect by inducing cell cycle arrest, but not cytotoxic effect.
We next investigated the effect of HBC on angiogenic phenotypes of endothelial cells, such as cell invasion and tube formation. Based on the above viability assay, in vitro angiogenesis assays were performed with optimal HBC doses that exhibited no cytotoxicity. TFP was used as a positive control for anti-angiogenic responses and VEGF was used as a chemoattractant or an angiogenic factor. Serum-starved HUVECs were stimulated by VEGF with or without HBC or TFP, and in vitro angiogenesis assays were performed. As shown in Fig. 1D, HBC and TFP potently inhibited VEGF-induced invasion and tube formation of HUVECs. Trypan blue staining was performed in parallel with in vitro angiogenesis assays, and cytotoxicity was not observed at concentrations used in this study.
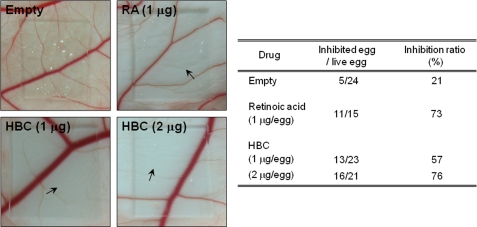
The anti-angiogenic activity of HBC was validated in vivo using CAM from growing chick embryos. HBC-loaded Thermanox coverslips were placed on the CAM surface, and neovascularized zones were observed under the microscope. RA was used as a positive control for anti-angiogenic responses. The inhibition of angiogenesis by RA was 73% (n = 15), and the inhibition of control coverslips was 21% (n = 24). HBC inhibited the neovascularization of CAM from chick embryos in a dose-dependent manner (57% at 1 μg/egg, n = 23; 76% at 2 μg/egg, n = 21) without showing any rupture or toxicity of pre-existing vessels (Fig. 2). These results demonstrate that HBC potently inhibited angiogenesis without affecting endothelial cell viability both in vitro and in vivo and suggest that the inhibitory effect of HBC on Ca2+/CaM activity may be crucial for its anti-angiogenic activity.
FIGURE 2.
Effect of HBC on in vivo angiogenesis of CAM. Fertilized chick eggs were maintained in a humidified incubator at 37 °C. At the stage of a 4.5-day embryo, HBC- or RA-loaded Thermanox coverslips were applied to the CAM surface. Two days later, the chorioallantois was observed under a microscope. When CAM that received treatment showed an avascular zone (arrows), the response was scored as positive. Calculations were based on the proportion of positive eggs relative to the total number of eggs tested.
HBC Suppresses Hypoxia-induced Activation of HIF-1
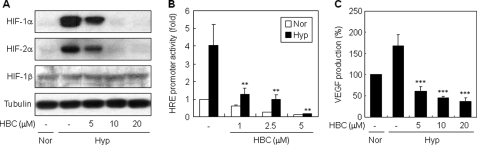
Ca2+/CaM signaling is closely associated with the activation of HIF-1, a pro-angiogenic transcription factor (25, 26). We thus investigated whether regulation of HIF-1 activity contributes to the anti-angiogenic activity of HBC. Because human hepatocellular carcinoma represents a hypervascularized tumor, and its progression requires angiogenesis (31, 32), we first examined the effect of HBC on HIF-1α stabilization in the human hepatocellular carcinoma cell line HepG2. As shown in Fig. 3A, HBC treatment of HepG2 cells reduced the hypoxia-induced accumulation of HIF-1α protein in a dose-dependent manner. HBC also inhibited HIF-2α, which has been reported to be regulated similarly to HIF-1α and to be responsible for comparable prominent angiogenic phenotypes (33). In contrast, HBC treatment did not affect the protein level of HIF-1β, indicating that HBC specifically down-regulates the α subunit of HIF-1 and HIF-2, but not HIF-1β.
FIGURE 3.
Regulation of HIF-1 activity by HBC. A, effect of HBC on HIF-1 protein accumulation. HepG2 cells were pretreated with HBC for 30 min at the indicated concentrations and then exposed to 1% O2 for 4 h. HIF-1α, HIF-2α, HIF-1β, and tubulin protein levels were measured by Western blot analysis. B, effect of HBC on HIF-1 transcriptional activity. HepG2 cells transiently co-transfected with pGL3-SV40–6HRE and pRL-SV40 were pretreated with HBC for 30 min at the indicated concentrations and then exposed to 1% O2 for 16 h. The ratio of firefly:Renilla luciferase activity was determined, and the result from cells incubated in normoxic conditions in the absence of HBC was normalized to 1. **, p < 0.005 versus hypoxic control. C, effect of HBC on VEGF expression. HepG2 cells were pretreated with HBC for 30 min at the indicated concentrations and then exposed to 1% O2 for 16 h. The concentration of VEGF protein in the culture supernatant was determined by ELISA specific for VEGF. ***, p < 0.05 versus hypoxic control. Each value represents mean ± S.E. from three independent experiments.
HIF-1 is a central transcription factor that binds to a number of genes via HREs and activates their transcription (9). We thus tested the effect of HBC on HIF-1 transcriptional activity using a reporter gene assay. HepG2 cells were transiently transfected with a construct containing the luciferase gene under control of the HRE promoter. Consistent with the inhibition of HIF-1α accumulation, HBC treatment blocked the hypoxia-induced transcriptional activity of HIF-1 in a dose-dependent manner (Fig. 3B). To further evaluate the effect of HBC on HIF-1 transcriptional activity, we measured levels of VEGF protein secreted by HepG2 cells. VEGF is a major target gene of HIF-1 and plays a critical role in hypoxia-induced angiogenesis (9). As expected, HBC significantly decreased VEGF production induced by hypoxia (Fig. 3C). Collectively, these results suggest that the anti-angiogenic activity of HBC may be related to the regulation of HIF-1 activity.
HBC Down-regulates Ca2+-dependent Activation of HIF-1
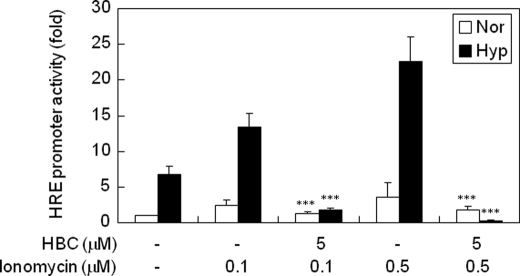
Hypoxia induces a significant increase in intracellular Ca2+ levels in both tumor and endothelial cells, and this elevation of Ca2+ concentration subsequently leads to up-regulation of HIF-1 (34, 35). To determine whether down-regulation of HIF-1 activity by HBC involves calcium signaling, we examined the effect of HBC on ionomycin-induced transcriptional activity of HIF-1 using the HIF-1-dependent luciferase reporter gene assay. Ionomycin is a calcium ionophore that is capable of bringing extracellular calcium ions into the cytosol. The increase in the intracellular calcium concentration can activate calmodulin via binding to EF-hand motifs and inducing the conformational change of calmodulin (36, 37). Once activated, Ca2+/CaM can activate a number of its client proteins, including calmodulin-dependent kinases and other enzymes that lead to up-regulation of HIF-1 (22, 25, 26). Consequently, similar to hypoxia, ionomycin increases intracellular calcium level and in turn up-regulates HIF-1 protein level and its transcription factor activity (27). However, the activation of HIF-1 by increasing intracellular calcium level can be completely abrogated by the treatment with calmodulin antagonist (W7), calmodulin-dependent kinase inhibitor (KN93), or intracellular calcium chelator (1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis (acetoxymethyl ester)) (25, 27). These results strongly suggest that inhibition of calmodulin is sufficient to block HIF-1 activity. As shown in Fig. 4, ionomycin increased HIF-1 transcriptional activity in a dose-dependent manner under both normoxic and hypoxic conditions. However, HBC markedly blocked the ionomycin-induced transcriptional activity of HIF-1, demonstrating that HBC suppresses HIF-1 activation by antagonizing Ca2+/CaM functions.
FIGURE 4.
Effect of HBC on ionomycin-induced transcriptional activity of HIF-1. HepG2 cells transiently co-transfected with pGL3-SV40–6HRE and pRL-SV40 were pretreated with ionomycin (0.1 and 0.5 μm) for 3 h. The cells were then exposed to 1% O2 for 16 h in the presence of HBC and analyzed for luciferase activity. ***, p < 0.05 versus ionomycin control. Each value represents mean ± S.E. from three independent experiments.
HBC Inhibits HIF-1α Expression at the Translational Level
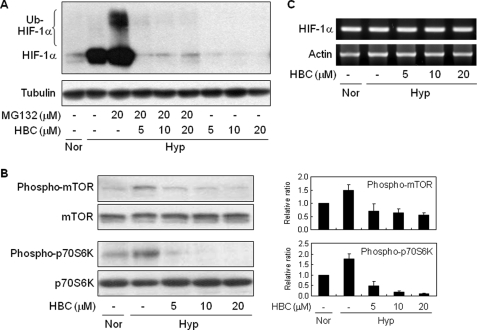
To further evaluate the mechanism by which HBC treatment decreases HIF-1α protein level and its transcriptional activity, we investigated whether HBC affects HIF-1α accumulation when the HIF-1α protein degradation pathway is blocked. HepG2 cells were treated with HBC in the presence or absence of MG132, a specific proteasome inhibitor that blocks ubiquitin-dependent HIF-1α degradation. As expected, treatment with MG132 resulted in an increase of HIF-1α protein levels, including its ubiquitinated species, in hypoxic conditions (Fig. 5A). However, MG132 did not prevent the HBC-mediated reduction in HIF-1α protein levels, suggesting that HBC may affect a step upstream of the HIF-1α protein degradation pathway such as HIF-1α transcription or translation.
FIGURE 5.
Inhibition of HIF-1α protein synthesis by HBC. A, effect of HBC on HIF-1α protein degradation. HepG2 cells were exposed to 1% O2 for 4 h in the presence of MG132 (20 μm) or HBC (5, 10, and 20 μm) as indicated, and HIF-1α and tubulin protein levels were analyzed by Western blot. B, effect of HBC on phosphorylation of mTOR and p70S6K. HepG2 cells were pretreated with HBC for 30 min at the indicated concentrations and then exposed to 1% O2 for 30 min. Protein levels were detected by Western blot analysis using specific antibodies. Ratios of phosphorylated to unphosphorylated mTOR or p70S6K were determined by densitometry. The phosphorylation ratio for the normoxic control was normalized to 1. Each value represents mean ± S.E. from three independent experiments. C, HepG2 cells were pretreated with HBC (5, 10, and 20 μm) for 30 min and then exposed to 1% O2 for 4 h. HIF-1α mRNA levels were determined by RT-PCR.
The PI3K/Akt/mTOR/p70S6K pathway is implicated in the regulation of HIF-1α expression at the translational level (38, 39). Moreover, several evidences demonstrated that the increase of intracellular calcium level by hypoxia or ionomycin is sufficient for activation of the kinases (40, 41) To address the role of this pathway in the HBC-induced decrease in HIF-1α protein levels, we investigated the effect of HBC on phosphorylation of mTOR and its downstream effector, p70S6K. HBC treatment of HepG2 cells markedly suppressed the hypoxia-induced phosphorylation of both proteins (Fig. 5B). However, HBC had no effect on HIF-1α mRNA expression (Fig. 5C), suggesting that HBC inhibits HIF-1α expression at the level of translation via blocking of Ca2+/CaM function, but not transcription.
HBC Enhances the Anti-angiogenic Effect of HIF-1 Inhibitors
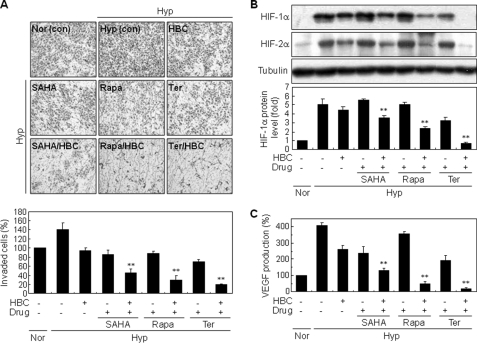
Inhibition of HIF-1 is an attractive therapeutic strategy to target tumor angiogenesis (9). Although a number of anti-cancer agents inhibit HIF-1 through diverse molecular mechanisms, single treatment with these inhibitors may have limited activity on drug-resistant solid tumors and consequently combination therapies might be required (11). We therefore evaluated whether HBC increased the anti-angiogenic activity of various anti-cancer agents known to inhibit HIF-1 by different mechanisms. For this study, we assessed the effect of combination treatment with HBC and the histone deacetylase (HDAC) inhibitor SAHA, the mTOR inhibitor rapamycin, or a new mitochondria Complex III ubiquinol-cytochrome c reductase binding protein (UQCRB) inhibitor terpestacin (42–44). To determine the anti-angiogenic activity of the inhibitors, a chemoinvasion assay was conducted using endothelial cells. As shown in Fig. 6A, hypoxia significantly induced invasion of HUVECs compared with normoxia. Single treatment with the HIF-1 inhibitors modestly reduced this hypoxia-induced invasion of HUVECs, whereas the addition of HBC to each of the inhibitors significantly increased their anti-angiogenic activities (1.9-fold for SAHA, 2.9-fold for rapamycin, and 3.5-fold for terpestacin, relative to single agent treatment).
FIGURE 6.
Enhanced anti-angiogenic effect of combined treatment with HBC and HIF-1 inhibitors. A, effect of a combination of HBC and various HIF-1 inhibitors on the invasiveness of HUVECs. Serum-starved HUVECs were exposed to 1% O2 in the presence or absence of HBC (0.5 μm), SAHA (2.5 μm), rapamycin (5 μm), and terpestacin (6 μm) as indicated. The basal level of invasiveness for normoxic controls was normalized to 100%. B, effect of combined treatment of HBC and HIF-1 inhibitors on the accumulation of HIF-α proteins. HepG2 cells were exposed to 1% O2 for 16 h in the presence or absence of HBC (5 μm), SAHA (5 μm), rapamycin (10 μm), and terpestacin (25 μm) as indicated. HIF-1α, HIF-2α, and tubulin protein levels were analyzed by Western blot. HIF-1α protein levels were further determined by densitometry. C, effect of combined treatment with HBC and HIF-1 inhibitors on VEGF expression. HepG2 cells were exposed to 1% O2 for 16 h in the presence or absence of HBC (5 μm), SAHA (5 μm), rapamycin (10 μm), and terpestacin (25 μm) as indicated. The concentration of VEGF protein in culture supernatant was determined using a VEGF-specific ELISA. **, p < 0.005 versus single agent treatment. Each value represents mean ± S.E. from three independent experiments.
To elucidate whether the enhanced anti-angiogenic effect of the HBC combination treatment was caused by the corresponding down-regulation of HIF-1α expression, we analyzed the effect of the inhibitors alone or in combination with HBC on HIF-1α protein accumulation and subsequent VEGF expression. Western blot analysis showed that co-treatment with the inhibitors and HBC caused a remarkable increase in HIF-1α suppression in HepG2 cells compared with single agent treatment (Fig. 6B). We also found that the protein levels of HIF-2α were significantly reduced by combined treatment with HBC and the HIF-1 inhibitors. In accordance with these data, the combined treatment also resulted in a greater inhibition of VEGF expression induced by hypoxia (Fig. 6C). Taken together, these results suggest that HBC can be applied to combination therapy targeting HIF and may contribute to overcoming the chemoresistance of solid tumors (Fig. 7).
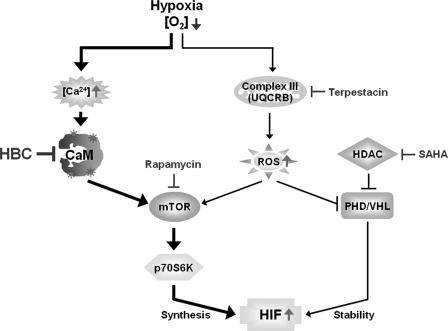
FIGURE 7.
Targeting HIF activation pathway for cancer therapy. HBC functions as an anti-angiogenic agent by inhibiting HIF synthesis at the translational level during hypoxia. The compound can be also applied to combination therapy with anti-cancer agents targeting HIF by different mode of action.
DISCUSSION
Targeting angiogenesis has become an attractive strategy to treat cancer growth and metastasis, as well as other disease states associated with angiogenesis. In the current study, we demonstrated that the new Ca2+/CaM antagonist HBC is a potent anti-angiogenic agent that exerts its activity by inhibiting the pro-angiogenic transcription factor HIF-1. Our results show that HBC inhibits angiogenesis without affecting endothelial viability both in vitro and in vivo and down-regulates HIF-1 transcriptional activity via suppression of HIF-1α expression at the translational level. We also found that HBC markedly reduces protein levels of HIF-2α in HepG2 cells (Fig. 3A). Similar to HIF-1α, HIF-2α is stabilized during hypoxia and forms a heterodimer with HIF-1β that transactivates angiogenic factors such as VEGF, Tie-2, and KDR/Flk-1 (33). Recent studies have shown that HIF-2α overexpression in human cancer cells increases tumor growth, angiogenesis, and metastasis (45). Interestingly, combined inhibition of both HIF-1α and HIF-2α enhances the decrease in tumor angiogenesis compared with inhibition by either factor alone through the regulation of multiple angiogenic pathways (46). Based on these findings, we propose that HBC is a novel angiogenesis inhibitor that targets both HIF-1 and HIF-2.
The level of HIF-1α protein during hypoxia depends on the relative rates of protein degradation and synthesis. We found that HBC inhibits HIF-1α accumulation in HepG2 cells, but affected neither HIF-1α protein degradation pathway nor HIF-1α transcription (Fig. 5, A and C). The key serine/threonine protein kinase mTOR regulates HIF-1α protein translation by phosphorylation of downstream effectors, such as p70S6K and 4E-BP1 (9, 39). We showed that HBC blocked the hypoxia-induced phosphorylation of mTOR and p70S6K, suggesting that the compound inhibits HIF-1α expression at the level of translation (Fig. 5B). In addition, a recent study has shown that calcium signaling stimulates translation of HIF-α proteins, including HIF-1α and HIF-2α, through the mTOR pathway in hypoxic conditions (40). Nonetheless, additional signaling pathways besides mTOR signaling may be involved in the inhibitory effect of HBC on HIF-1α expression. We thus investigated whether HBC affects activity of ERK, another serine/threonine kinase that is known to control HIF-1α protein synthesis (9). However, HBC does not cause a significant down-regulation of ERK phosphorylation during hypoxia but instead induces prolonged phosphorylation of ERK, as shown in our previous study (29). These results suggest that HBC suppresses the synthesis of HIF-α proteins by blocking mTOR signaling, but not the ERK pathway.
HIF-1α is frequently overexpressed in response to intratumoral hypoxia and genetic alterations in oncogenes or tumor-suppressor genes, and this overexpression is associated with increased resistance to chemotherapy and treatment failure of widespread tumors (9, 10). Therefore, inhibition of HIF-1α activity is a promising strategy for the treatment of solid tumors through an anti-angiogenic effect. HDAC and mTOR inhibitors are known to inhibit tumor angiogenesis through post-transcriptional modulation of HIF-1α (11, 42, 43). Despite their antitumor activity, these inhibitors show limited effectiveness for cancer treatment as single agents; however, combination treatment with mTOR and HDAC inhibitors significantly decreases in vivo tumor growth and angiogenesis compared with single agent treatment by enhancing the reduction in HIF-1α protein expression (47). Likewise, recent studies have proved that blocking the HIF-1α pathway by different mechanisms can improve antitumor and antiangiogenic activities of targeted therapies (47–49). Thus, we hypothesized that combination treatment of the Ca2+/CaM antagonist HBC and HIF-1 inhibitors, including the HDAC inhibitor SAHA, the mTOR inhibitor rapamycin, and the mitochondria Complex III UQCRB inhibitor terpestacin, would have greater anti-angiogenic activity than treatment with each as a single agent. Our results showed that HBC potentiates the anti-angiogenic activity of the HIF-1 inhibitors and demonstrated that this effect is associated with increased reduction of the expression of HIF-1α and its target gene VEGF (Fig. 6). In particular, among the HIF-1 inhibitors tested, terpestacin exhibited the greatest anti-angiogenic activity in combination with HBC. Terpestacin blocks hypoxia-induced ROS generation by targeting UQCRB of mitochondrial Complex III, thereby resulting in suppression of HIF-1 activation (44, 50). It is conceivable that combined treatment of terpestacin and HBC may inhibit HIF-1α activity at multiple levels, including synthesis, stability, translocation, and transcriptional activity, by blocking both ROS and calcium signaling in hypoxic conditions. Furthermore, addition of HBC to the HIF-1 inhibitors led to a significant inhibition of protein expression of HIF-2α, further suggesting that HBC might be a powerful anti-angiogenic agent for combination strategy targeting HIF (Fig. 7).
Although HIF inhibition by HBC is associated with modulation of Ca2+/CaM function (Fig. 4), we have not yet identified the signaling event linking Ca2+/CaM and HIF. Recent studies have revealed that several target proteins of Ca2+/CaM, including CaM kinase II, calcineurin, and actin, activate the expression and transcriptional activity of HIF-1α (22, 25, 26). Further studies to elucidate the role of HBC in regulation of the Ca2+/CaM signaling pathway will help to define the mechanism underlying the HIF inhibitory activity of HBC.
In addition, we observed that HBC reduces calcium concentration in the cells through the previous study (29). A literature demonstrated that calmodulin antagonists such as TFP and W7 can reduce intracellular calcium concentration via inhibition of calmodulin function. This effect seems to be a feedback effect between calcium concentration and calmodulin activity (51). Therefore, the reduction of calcium concentration by HBC is presumably attributable to inhibition of calmodulin function, as observed with TFP and W7. Consequently, this effect of HBC may contribute to a down-regulation of HIF-1.
In conclusion, we show that HBC is a promising anti-angiogenic agent that targets both HIF-1 and HIF-2 and might be used to explore the biological role of Ca2+/CaM in angiogenesis. Our study also reveals a potential application of HBC in combination therapies for cancer treatment. In addition to HIF-1 inhibitors, combined treatment of HBC with anticancer drugs targeting VEGF or its receptor, such as bevacizumab and sunitinib, may improve therapeutic efficacy by blocking the activation of compensatory pro-angiogenic pathways in response to treatment with a single agent.
Acknowledgment
We are grateful to Dr. Kyu-Won Kim for providing the HRE luciferase reporter vector.
This work was supported by grants from the National R&D Program for Cancer Control (0620350), Ministry of Health & Welfare, the Translational Research Center for Protein Function Control, NRF (2009-0083522), the National Research Foundation of Korea Grant funded by the Korean Government (MEST) (2009-0092964 and 2010-0017984), and from the Brain Korea 21 project, Republic of Korea.
- HIF-1
- hypoxia-inducible factor 1
- CaM
- calmodulin
- HUVEC
- human umbilical vascular endothelial cell
- SAHA
- suberoylanilide hydroxamic acid
- TFP
- trifluoperazine
- MG132
- carboxybenzyl-leucyl-leucyl-leucinal
- p70S6K
- 70-kDa ribosomal protein S6 kinase
- mTOR
- mammalian target of rapamycin
- CAM
- chorioallantoic membrane
- KDR/Flk-1
- kinase insert domain receptor/fetal liver kinase-1
- HDAC
- histone deacetylase
- UQCRB
- ubiquinol-cytochrome c reductase-binding protein
- HBC
- 4-{3,5-bis-[2-(4-hydroxy-3-methoxyphenyl)ethyl]-4,5-dihydropyrazol-1-yl}benzoic acid
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- HRE
- hypoxia-response element.
REFERENCES
- 1.Folkman J. (2001) Semin. Oncol. 28, 536–542 [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P., Jain R. K. (2000) Nature 407, 249–257 [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D., Folkman J. (1996) Cell 86, 353–364 [DOI] [PubMed] [Google Scholar]
- 4.André T., Chastre E., Kotelevets L., Vaillant J. C., Louvet C., Balosso J., Le Gall E., Prévot S., Gespach C. (1998) Rev. Med. Interne. 19, 904–913 [DOI] [PubMed] [Google Scholar]
- 5.Carmeliet P., Dor Y., Herbert J. M., Fukumura D., Brusselmans K., Dewerchin M., Neeman M., Bono F., Abramovitch R., Maxwell P., Koch C. J., Ratcliffe P., Moons L., Jain R. K., Collen D., Keshert E. (1998) Nature 394, 485–490 [DOI] [PubMed] [Google Scholar]
- 6.Forsythe J. A., Jiang B. H., Iyer N. V., Agani F., Leung S. W., Koos R. D., Semenza G. L. (1996) Mol. Cell. Biol. 16, 4604–4613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang G. L., Jiang B. H., Rue E. A., Semenza G. L. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 5510–5514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schofield C. J., Ratcliffe P. J. (2004) Nat. Rev. Mol. Cell Biol. 5, 343–354 [DOI] [PubMed] [Google Scholar]
- 9.Semenza G. L. (2003) Nat. Rev. Cancer. 3, 721–732 [DOI] [PubMed] [Google Scholar]
- 10.Unruh A., Ressel A., Mohamed H. G., Johnson R. S., Nadrowitz R., Richter E., Katschinski D. M., Wenger R. H. (2003) Oncogene 22, 3213–3220 [DOI] [PubMed] [Google Scholar]
- 11.Semenza G. L. (2007) Drug Discov. Today 12, 853–859 [DOI] [PubMed] [Google Scholar]
- 12.Cheung W. Y. (1980) Science 207, 19–27 [DOI] [PubMed] [Google Scholar]
- 13.Means A. R., Dedman J. R. (1980) Nature 285, 73–77 [DOI] [PubMed] [Google Scholar]
- 14.Veigl M. L., Vanaman T. C., Sedwick W. D. (1984) Biochim. Biophys. Acta 738, 21–48 [DOI] [PubMed] [Google Scholar]
- 15.Stull J. T. (2001) J. Biol. Chem. 276, 2311–2312 [DOI] [PubMed] [Google Scholar]
- 16.Hait W. N., Lazo J. S. (1986) J. Clin. Oncol. 4, 994–1012 [DOI] [PubMed] [Google Scholar]
- 17.Wei J. W., Morris H. P., Hickie R. A. (1982) Cancer Res. 42, 2571–2574 [PubMed] [Google Scholar]
- 18.Rodriguez-Mora O. G., LaHair M. M., McCubrey J. A., Franklin R. A. (2005) Cancer Res. 65, 5408–5416 [DOI] [PubMed] [Google Scholar]
- 19.Das S. B., Sharma R. K. (2005) Oncol. Rep. 14, 1059–1063 [PubMed] [Google Scholar]
- 20.Schuller H. M., Correa E., Orloff M., Reznik G. K. (1990) Cancer Res. 50, 1645–1649 [PubMed] [Google Scholar]
- 21.Schüller H. M., Orloff M., Reznik G. K. (1991) Carcinogenesis 12, 2301–2303 [DOI] [PubMed] [Google Scholar]
- 22.Shen W. G., Peng W. X., Dai G., Xu J. F., Zhang Y., Li C. J. (2007) Cell Biol. Int. 31, 126–134 [DOI] [PubMed] [Google Scholar]
- 23.Salnikow K., Kluz T., Costa M., Piquemal D., Demidenko Z. N., Xie K., Blagosklonny M. V. (2002) Mol. Cell. Biol. 22, 1734–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukhopadhyay D., Akbarali H. I. (1996) Biochem. Biophys. Res. Commun. 229, 733–738 [DOI] [PubMed] [Google Scholar]
- 25.Yuan G., Nanduri J., Bhasker C. R., Semenza G. L., Prabhakar N. R. (2005) J. Biol. Chem. 280, 4321–4328 [DOI] [PubMed] [Google Scholar]
- 26.Liu Y. V., Hubbi M. E., Pan F., McDonald K. R., Mansharamani M., Cole R. N., Liu J. O., Semenza G. L. (2007) J. Biol. Chem. 282, 37064–37073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mottet D., Michel G., Renard P., Ninane N., Raes M., Michiels C. (2003) J. Cell. Physiol. 194, 30–44 [DOI] [PubMed] [Google Scholar]
- 28.Shim J. S., Kim D. H., Jung H. J., Kim J. H., Lim D., Lee S. K., Kim K. W., Ahn J. W., Yoo J. S., Rho J. R., Shin J., Kwon H. J. (2002) Bioorg. Med. Chem. 10, 2987–2992 [DOI] [PubMed] [Google Scholar]
- 29.Shim J. S., Lee J., Park H. J., Park S. J., Kwon H. J. (2004) Chem. Biol. 11, 1455–1463 [DOI] [PubMed] [Google Scholar]
- 30.Jeon K. S., Na H. J., Kim Y. M., Kwon H. J. (2005) Biochem. Biophys. Res. Commun. 330, 1268–1274 [DOI] [PubMed] [Google Scholar]
- 31.Pang R., Poon R. T. (2006) Cancer Lett. 242, 151–167 [DOI] [PubMed] [Google Scholar]
- 32.Torimura T., Sata M., Ueno T., Kin M., Tsuji R., Suzaku K., Hashimoto O., Sugawara H., Tanikawa K. (1998) Hum. Pathol. 29, 986–991 [DOI] [PubMed] [Google Scholar]
- 33.Takeda N., Maemura K., Imai Y., Harada T., Kawanami D., Nojiri T., Manabe I., Nagai R. (2004) Circ. Res. 95, 146–153 [DOI] [PubMed] [Google Scholar]
- 34.Arnould T., Michiels C., Alexandre I., Remacle J. (1992) J. Cell. Physiol. 152, 215–221 [DOI] [PubMed] [Google Scholar]
- 35.Seta K. A., Yuan Y., Spicer Z., Lu G., Bedard J., Ferguson T. K., Pathrose P., Cole-Strauss A., Kaufhold A., Millhorn D. E. (2004) Cell Calcium 36, 331–340 [DOI] [PubMed] [Google Scholar]
- 36.Nakayama S., Kretsinger R. H. (1994) Annu. Rev. Biophys. Biomol. Struct. 23, 473–507 [DOI] [PubMed] [Google Scholar]
- 37.Zhang M., Tanaka T., Ikura M. (1995) Nat. Struct. Biol. 2, 758–767 [DOI] [PubMed] [Google Scholar]
- 38.García-Maceira P., Mateo J. (2009) Oncogene 28, 313–324 [DOI] [PubMed] [Google Scholar]
- 39.Mamane Y., Petroulakis E., LeBacquer O., Sonenberg N. (2006) Oncogene 25, 6416–6422 [DOI] [PubMed] [Google Scholar]
- 40.Hui A. S., Bauer A. L., Striet J. B., Schnell P. O., Czyzyk-Krzeska M. F. (2006) FASEB J. 20, 466–475 [DOI] [PubMed] [Google Scholar]
- 41.Tang X., Wang L., Proud C. G., Downes C. P. (2003) Biochem. J. 374, 137–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kong X., Lin Z., Liang D., Fath D., Sang N., Caro J. (2006) Mol. Cell. Biol. 26, 2019–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hudson C. C., Liu M., Chiang G. G., Otterness D. M., Loomis D. C., Kaper F., Giaccia A. J., Abraham R. T. (2002) Mol. Cell. Biol. 22, 7004–7014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jung H. J., Shim J. S., Lee J., Song Y. M., Park K. C., Choi S. H., Kim N. D., Yoon J. H., Mungai P. T., Schumacker P. T., Kwon H. J. (2010) J. Biol. Chem. 285, 11584–11595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bangoura G., Yang L. Y., Huang G. W., Wang W. (2004) World J. Gastroenterol. 10, 525–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burkitt K., Chun S. Y., Dang D. T., Dang L. H. (2009) Mol. Cancer Ther. 8, 1148–1156 [DOI] [PubMed] [Google Scholar]
- 47.Verheul H. M., Salumbides B., Van Erp K., Hammers H., Qian D. Z., Sanni T., Atadja P., Pili R. (2008) Clin. Cancer Res. 14, 3589–3597 [DOI] [PubMed] [Google Scholar]
- 48.Pencreach E., Guérin E., Nicolet C., Lelong-Rebel I., Voegeli A. C., Oudet P., Larsen A. K., Gaub M. P., Guenot D. (2009) Clin. Cancer Res. 15, 1297–1307 [DOI] [PubMed] [Google Scholar]
- 49.Huang X. Z., Wang J., Huang C., Chen Y. Y., Shi G. Y., Hu Q. S., Yi J. (2008) Cancer Biol. Ther. 7, 468–475 [DOI] [PubMed] [Google Scholar]
- 50.Jung H. J., Lee H. B., Kim C. J., Rho J. R., Shin J., Kwon H. J. (2003) J. Antibiot. (Tokyo) 56, 492–496 [DOI] [PubMed] [Google Scholar]
- 51.Capuozzo E., Verginelli D., Crifò C., Salerno C. (1997) Biochim. Biophys. Acta 1357, 123–127 [DOI] [PubMed] [Google Scholar]