Abstract
Single-dose vaccine formats can prevent clinic-level vaccine wastage but may incur higher production, medical waste disposal, and storage costs than multi-dose formats. To help guide vaccine developers, manufacturers, distributors, and purchasers, we developed a computational model to predict the potential economic impact of various single-dose versus multi-dose measles (MEA), hemophilus influenzae type B (Hib), Bacille Calmette-Guérin (BCG), yellow fever (YF), and pentavalent (DTP-HepB-Hib) vaccine formats. Lower patient demand favors fewer dose formats. The mean daily patient arrival thresholds for each vaccine format are as follows: for the MEA vaccine, 2 patients/day (below which the single-dose vial and above which the 10-dose vial are least costly); BCG vaccine, 6 patients/day (below, 10-dose vial; above, 20-dose vial); Hib vaccine, 5 patients/day (below, single-dose vial; above, 10-dose vial); YF vaccine, 33 patients/day (below, 5-dose vials; above 50-dose vial); and DTP-HepB-Hib vaccine, 5 patients/day (below, single-dose vial; above, 10-dose vial).
Keywords: vaccines, doses per vial, economics
INTRODUCTION
Vaccine manufacturers, distributors, and purchasers must understand when single dose and various multi-dose vials are optimal. Excessive vaccine wastage is a significant problem throughout the world, and much wastage occurs at the clinic level when health care workers open a multi-dose vaccine vial to immunize a patient and then cannot use the remainder of the doses before they expire. For example, if a health care worker must open a 10-dose Bacille Calmette-Guérin (BCG), yellow fever (YF), measles (MEA), or hemophilus influenzae type B (Hib) vaccine vial to immunize a patient, the health care worker must use the remaining 9 doses within the next 6 hours before the doses expire (i.e., lose their efficacy). This can be problematic when immunization demand is infrequent or unpredictable. Losing expired doses leads to additional costs and when vaccines are in short supply, depletes vaccine availability. Multi-dose vaccine vials present potential safety problems as well. Every time a needle is inserted into the same vaccine vial to draw vaccine for immunization, there is opportunity to contaminate the vaccine and subsequently, the vaccine recipient.
Single-dose vaccine formats can prevent many of these multi-dose vial problems but present drawbacks as well. With only one vaccine dose per vial or vessel, each dose remains sealed and protected until it is ready for administration, decreasing chances for wastage and contamination. Single-dose formats that include an integrated injection device (e.g., prefilled auto-disable device) offer the additional advantage of being safer and more convenient for health care workers. Because each dose needs its own container, single dose formats are typically more expensive per dose and occupy a greater volume per dose with regard to supply chain storage and medical waste disposal. The latter issue is a significant problem when storage and transport space is limited. Additionally, single dose formats have more packaging per dose, and consequently, more litter per dose—a substantial problem where adequate medical and non-medical waste disposal is limited. Single dose formats also have excess filling volume that would be spread across a greater number of doses in multi-dose formats.
The choice between single-dose formats and multi-dose vial formats is a balance between their relative benefits and drawbacks, a balance that may shift based on local circumstance. For example, if vaccine wastage is not a significant problem, the vaccine is relatively inexpensive, disposing of medical waste is difficult, and cold-chain storage capacity is constrained, multi-dose vials may be more favorable. Conversely, if the vaccine is expensive, vaccine contamination risk is high, and patients arrive to the clinic with irregular frequency, single-dose formats may be more appropriate.
To better delineate these trade-offs and understand when each type of format may be more favorable, we developed a computational model to predict the potential economic impact of utilizing a single-dose versus multi-dose vaccine format. The model determines the potential effects of each format on vaccine wastage and cost per vaccination. Sensitivity analyses explored how these measures may vary with patient arrival patterns, vaccine cost, and a vaccine vial’s shelf life once opened.
METHODS
Data Inputs
Table 1 lists the various data inputs for our model and the corresponding distributions and data sources used. Five routine childhood vaccines were included in this analysis: BCG, YF, MEA, Hib, and DTP-HepB-Hib (lyophilized and liquid). Each of these vaccines, once opened, has a shelf life of 6 hours beyond which, vaccine efficacy is no longer guaranteed. The presentations (number of doses per vial, volume, and weight), and costs for each of the vaccines were referenced from the 2009 World Health Organization (WHO) Vaccine Volume Calculator, which provides essential information on vaccines available for all countries whose EPI is assisted by WHO.[1] For vaccine presentations with missing values (including values for cost, volume, and weight), linear regression analysis extrapolated missing data points.
TABLE 1.
Model Data Inputs
| Description (units) | Mean | Lower Limit |
Upper Limit |
Source |
|---|---|---|---|---|
| Cost per dose (US$) | ||||
| Measles vaccine | ||||
| 1 dose/vial | 0.943 | 0.849 | 1.037 | [1] |
| 5 doses/vial | 0.633 | 0.202 | 0.402 | [1] |
| 10 doses/vial | 0.246 | 0.221 | 0.271 | [1] |
| Yellow fever vaccine | ||||
| 5 doses/vial | 0.728 | 0.655 | 0.801 | [1] |
| 10 doses/vial | 0.915 | 0.824 | 1.016 | [1] |
| 20 doses/vial | 0.645 | 0.581 | 0.710 | [1] |
| 50 doses/vial | 0.510 | 0.459 | 0.561 | [1] |
| BCG vaccine | ||||
| 10 doses/vial | 0.191 | 0.172 | 0.211 | [1] |
| 20 doses/vial | 0.104 | 0.094 | 0.114 | [1] |
| Hib vaccine | ||||
| 1 dose/vial | 3.619 | 3.257 | 3.981 | [1] |
| 2 doses/vial | 3.420 | 3.078 | 3.762 | [1] |
| 10 doses/vial | 1.830 | 1.647 | 2.013 | [1] |
|
DTP-HepB-Hib (Pentavalent)
vaccine | ||||
| 1 dose/vial | 3.60 | 3.24 | 3.96 | [1] |
| 2 doses/vial | 3.50 | 3.25 | 3.75 | [1] |
| 10 doses/vial | 2.00 | 1.80 | 2.20 | [1] |
| Vaccine volume per dose (cm3) | ||||
| Measles vaccine | ||||
| 1 dose/vial | 9.30 | 8.37 | 10.23 | [1] |
| 5 doses/vial | 6.72 | 6.05 | 7.39 | [1] |
| 10 doses/vial | 3.50 | 3.15 | 3.85 | [1] |
| Yellow fever vaccine | ||||
| 5 doses/vial | 6.50 | 5.85 | 7.15 | [1] |
| 10 doses/vial | 2.50 | 2.25 | 2.75 | [1] |
| 20 doses/vial | 1.50 | 1.35 | 1.65 | [1] |
| 50 doses/vial | 0.70 | 0.63 | 0.77 | [1] |
| BCG vaccine | ||||
| 10 doses/vial | 2.20 | 1.98 | 2.42 | [1] |
| 20 doses/vial | 1.20 | 1.08 | 1.32 | [1] |
| Hib vaccine | ||||
| 1 dose/vial | 13.00 | 11.70 | 14.30 | [1] |
| 2 doses/vial | 6.00 | 5.40 | 6.60 | [1] |
| 10 doses/vial | 2.50 | 2.25 | 2.75 | [1] |
|
DTP-HepB-Hib (Pentavalent)
vaccine | ||||
| 1 dose/vial | 16.80 | 15.12 | 18.48 | [1] |
| 2 doses/vial | 11.00 | 9.90 | 12.10 | [1] |
| 10 doses/vial | 4.40 | 3.96 | 4.84 | [1] |
| Cost of medical waste disposal (2004 US$) | ||||
| Waste disposal cost per Kg | 5.737 | 1.730 | 9.070 | [8] |
| Cost of vaccine storage (US$) | ||||
| Cold storage cost per dose administered (1cm3) |
0.030 | 0.027 | 0.033 | [9, 10] |
| Weight of vaccines and vaccine accessories (g) | ||||
| One-dose vial (empty) | 1.713 | 1.542 | 1.885 | [11] |
| Two-dose vial (empty) | 1.914 | 1.723 | 2.106 | [11] |
| Five-dose vial (empty) | 2.517 | 2.265 | 2.769 | [11] |
| Ten-dose vial (empty) | 3.522 | 3.169 | 3.874 | [11] |
| Twenty-dose vial (empty) | 5.531 | 5.431 | 5.631 | [11] |
| Fifty-dose vial (empty) | 11.559 | 10. 403 | 12.715 | [11] |
| Reconstitution syringe (66.25 g/unit) | 6.625 | 5.967 | 7.293 | [1] |
Vaccine Utilization
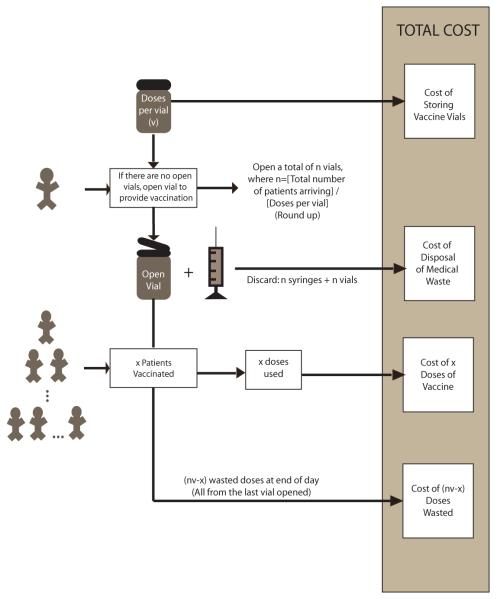
Figure 1 depicts the structure of our Excel (Microsoft Corporation, Redmond, WA) equation-based spreadsheet model, which evaluates the impact on a clinic of changing the number of doses per vial. Key variables include:
Number of vaccine doses per vial (v).
Daily demand at the clinic (d): This is the number of patients requiring immunization on a given day and draws from a Poisson distribution with meanλ. The arrival rate for this analysis was bounded between 1 and 50 patients to simulate a range of plausible clinic sizes and levels of population demand.
FIGURE 1.
General Model Structure where: v is the number of doses per vial, n is the total number of patients arriving ÷ v (roundup), and x is the number patients vaccinated. It is assumed that vaccine vials are discarded within 6 hours of reconstitution.
Our model assumes that the clinic satisfies patient demand. So, the number of vials opened that day (n) is equal to:
| (1) |
For example, if v = 10 and d = 13 then n = 2. Every time a vaccine vial is opened, doses that are not used by the end of the day go to waste. Therefore, the number of doses wasted that day, w, is:
| (2) |
where d mod v means to use modulo arithmetic. For example, if v = 10 and d = 13 then w =7 because 13 modulo 10 equals 3 and 10 – 3 = 7. (Two vials are opened and 7 doses are wasted from the second one.)
The percent of doses wasted (wp) then is:
| (3) |
The denominator represents the total number of doses reconstituted that day. The numerator is the number of doses that are not used. Thus, when v1 = 10 and d = 13, the number of vials opened (n) is 2, the number of doses that go unused (w) is 7 and the percent wastage (wp)=7/20 or 35%. Dose utilization (u) is the complement of vial wastage, where u = 1 – wp or 65% in the case of the given example.
Calculating the expected or average vial utilization entails: (1) determining the probability, p(x), of each possible value of demand x, (2) multiplying this probability for each x by the corresponding dose utilization value (u) that results from meeting that demand x and (3) summing all of these products across all x. In other words, if p(x) = the probability that daily clinic demand equals x and u(x) = the vial utilization corresponding to a daily demand of x, then the expected vial utilization, E[U] is equal to the following:
| (4) |
where D represents the set of all possible daily demand values. Our baseline scenario assumed that demand x is distributed as a Poisson distribution.
We test three different cost scenarios that consider different combinations of the cost of the vaccines, the cost of disposing of medical waste, and the cost of storing the vaccine vials.
Cost of Vaccine Vials
The first scenario considered only the cost of the vaccine, i.e., cost of doses used and doses wasted. Table 1 shows the cost per dose for each vaccine presentation. The cost of vaccine wastage was determined as follows:
Cost of Vaccine Wastage = Cost per Dose × Number of Doses Wasted (5)
Therefore, the total cost of vaccination for each presentation is:
Total Vaccine Costs = Cost of Vaccines Used + Cost of Vaccine Wastage = (Number Vaccinated Patients × Cost per Dose) + (Cost per Dose × Number of Doses Wasted) (6)
The cost per vaccinated patient then is:
Cost per Vaccinated Patient = Total Vaccine Costs /Number of Vaccinated Patients (7)
Cost of Disposal of Vaccine Vials
As Table 1 shows, the different vial presentations result in different volumes of medical waste incurring additional costs. A second cost scenario added the costs of discarding used vaccine vials. In this segment of the analysis, the cost of disposal of reconstitution syringes was also considered, while the cost of disposal of injection syringes was not included. The cost of the injection syringe does not change as vial size changes since a new injection syringe is used for each arriving patient. The cost of the reconstitution syringe changes as the vial size changes and becomes less costly per dose as the doses per vial increases. Table 1 also shows the medical waste from each vial presentation and the associated costs. The medical waste generated is:
Total Weight of Discarded Vials = Number of Vials Used × Weight per Vial (8)
Total Weight of Discarded Reconstitution Syringes = Number of Vials Used × Weight per Syringe (9)
The cost of discarded medical waste is:
Total Cost of Medical Waste = [Total Weight of Discarded Vials + Total Weight of Discarded Reconstitution Syringes] × Cost per Kg of Medical Waste Discarded (10)
Cost of Storage of Vaccine Vials
A third cost scenario considered the storage costs for the vaccines in addition to the purchase cost of the vaccines and the medical waste disposal costs. Different presentations require different storage volumes which affects storage costs. The storage capacity needed is:
Storage Capacity = Number of Vials Used per Day × Doses per Vial × Storage Volume per Dose (11)
Therefore, the cost of storage is:
| (12) |
Sensitivity Analyses
Given that vaccine costs may vary geographically and temporally, we examined the effects of systematically varying each of the costs given in Table 1. The upper and lower limits of the range of costs considered are also given in Table 1. Additional sensitivity analyses explored the effects of ranging both disposal and storage costs up to 200% their original values. Additional simulations scenarios used a uniform distribution instead of the Poisson distribution for the patient arrivals (demand) to represent different patterns of clinic level demand. The uniform distribution had a mean of μ and a range of (e.g., if demand was 9 then demand varied uniformly from 6 to 12). The results from five hundred simulations were used for each analysis.
RESULTS
Vaccine Utilization
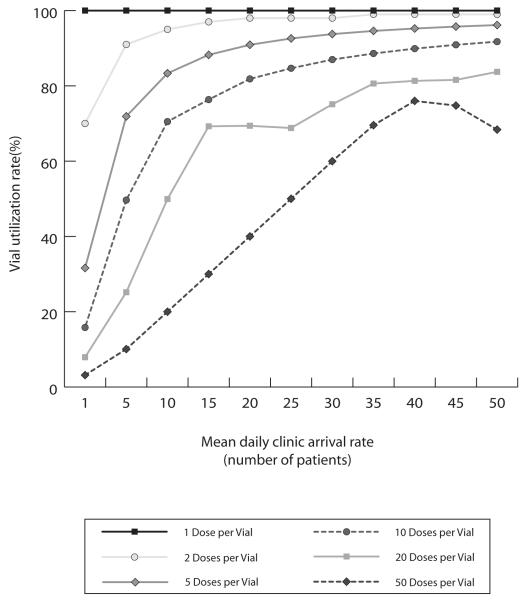
Figure 2 shows how percentage of doses wasted (i.e., number of doses wasted/total number of doses) varies by the patient arrival rate. Each of the bands represents a different vial presentation. As the patient arrival rate increases, the wastage rate for the multi-dose presentations decreases while the wastage rate for the single dose presentation remains constant. As the fifty-dose vial is rather large compared to the other vial sizes displayed, its representative curve has a different shape. This is seen because the utilization at lower mean daily patient arrival rates for the fifty-dose vial is much lower than smaller vial size formats.
FIGURE 2.
Vial Utilization Rate by Clinic Demand and Doses per Vial
Cost of Vaccine Wastage Alone
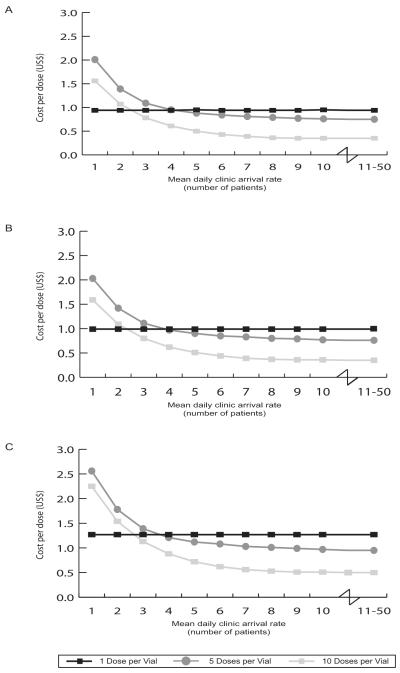
Figure 3a shows how measles vaccine cost per vaccinated patient varies by the patient arrival rate when the mean cost values in Table 1 are used. When the mean daily patient arrival rate is between 1 and 2 patients the single-dose vaccine format is the least costly option, costing $0.94 per vaccinated individual. When the mean arrival rate exceeds 2 people per day, the 10-dose measles vaccine format becomes the most economic choice, costing from $0.27 to $0.78 per vaccinated individual. Interestingly, the 5-dose measles vaccine vial size is never the least costly option (even when exploring mean daily patient arrival rates of up to 50 people per day).
FIGURE 3(a-c).
Average Costs per Dose (by Clinic Demand and Doses per Vial) for:
(a) Measles Vaccine
(b) Vaccine and Disposal, and
(c) Vaccine, Disposal and Storage
Table 2 delineates how the least costly option varies with daily mean patient arrival rate for the different vaccines. As can be seen, for the YF vaccine, a mean daily arrival rate of 33 people is the threshold above which the 50-dose supersedes the 5-dose as the least costly YF vaccine dose format. The 10-dose and 20-dose YF vaccine formats are never the optimal economic choice. For the BCG vaccine, a mean of 6 patients per day is the cut point between the 10-dose BCG vaccine format and the 20-dose format being most favorable. However, the 20-dose format costs are only slightly higher when the mean number of patients per day is less than 6. The single-dose DTP-HepB-Hib vaccine vial is the least costly option until the patient arrival rates exceeds 5 patients per day at which point, the 10-dose vial size becomes least costly. The 2-dose DTP-HepB-Hib vaccine vial size is never the most cost-effective option.
TABLE 2.
Average Cost per Dose of Vaccine, Vaccine and Disposal, Vaccine and Disposal and Storage
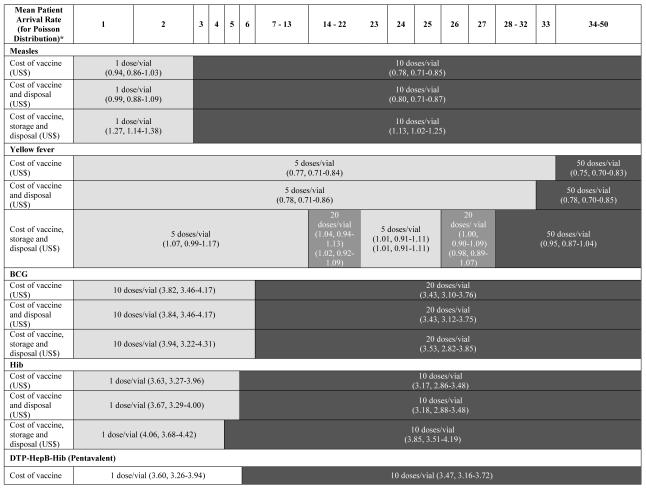
|
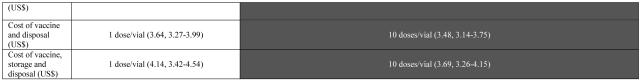
|
Note: Each vial size is represented by the mean, minimum, and maximum cost for a given scenario (cost of vaccine, cost of vaccine and disposal, and cost of vaccine, storage and disposal) as generated from a series of five hundred simulations.
*Daily patient arrival rate assumes a Poisson distribution with this mean patient arrival rate.
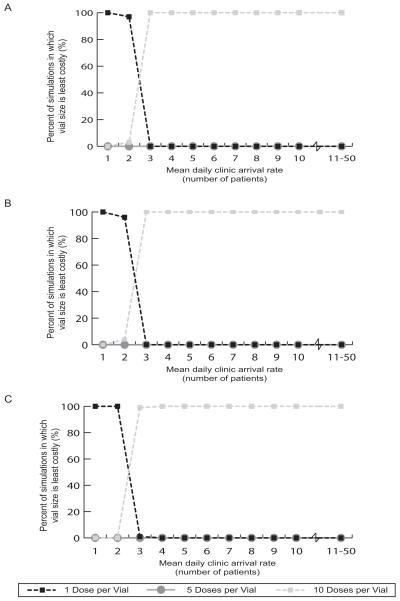
Simulation studies were conducted to determine the robustness of the results shown in Figure 3 and Table 2. These simulations let the cost values given in Table 1 vary between the lower and upper limits. All of the cost values were varied simultaneously. Figure 4a shows how the acceptability curves for measles vaccine vary with mean daily patient arrival rate. These curves show the percentage of simulations for which each vaccine format is the most economical choice. When a mean of 1 or 2 patients arrive per day, the 1-dose measles vaccine is the least costly (i.e., cost per vaccinated patient) option in 97%-100% of the simulations. For mean daily patient arrival rates between 3 and 50, the 10-dose measles vial size is the most cost-effective vial size option in 100% of the simulations.
FIGURE 4(a-c).
Acceptability Curves for Average Costs per Dose (by Clinic Demand and Doses per Vial) for:
(a) Measles Vaccine,
(b) Vaccine and Disposal, and
(c) Vaccine, Disposal and Storage
The acceptability curves for the other vaccine types (not shown) confirm the findings in Table 2. For example, when the mean daily patient arrival rate ranges from 1 to 33, the 5-dose YF vaccine is the least costly option between 46% and 100% of the simulations. The 50-dose vial presentation becomes the most economic choice in over 50% of the simulations when mean daily patient arrival rates exceed 33.
The Cost of Vaccine Wastage plus Vaccine Vial Disposal
Figure 3b shows the effects of adding the cost of vaccine vial disposal to the costs of the measles vaccine. As can be seen, while this does increase the cost per vaccinated patient, it does not significantly change the most economic choice for different mean daily patient arrival rates. These results confirm that the cost of vaccine wastage far outweighs any added cost from having to dispose of more vaccine vials with different vaccine formats.
Similarly, as highlighted in Table 2, the relative costs of the different YF, BCG, and Hib vaccine presentations do not change significantly. The mean daily patient arrival thresholds at which 20-dose BCG vaccine and the 10-dose Hib vaccine become favorable remain at 7 and 6 respectively. The threshold for YF changes slightly, with the 5-dose YF vaccine being the least costly option when mean daily patient arrival is 32 or below and the 50-dose for higher arrival rates.
Acceptability curves further confirm these findings. Figure 4b demonstrates that for mean daily patient arrival rates of 1 and 2 patients, the 1-dose measles vial size is the most cost effective option 100% of the time. For mean daily patient arrival rates from 3 to 50 patients, the 10-dose vial size is the most cost effective option between 99% and 100% of the time. Acceptability curves for the other vaccines (not pictured) also support the findings in Table 2.
For mean daily patient arrival rates from 1 to 13 patients, the 5-dose YF vial size is the most cost effective option between 69% and 100% of the time. For mean daily patient arrival rates from 14 to 22 patients, the 20-dose YF vial size is the most cost effective option between 50% and 87% of the time. For mean daily patient arrival rates from 22 to 25 patients, the 5-dose YF vial size is the most cost effective option between 50% and 59% of the time. For mean daily patient arrival rates of 26 and 27 patients, the 20-dose vial size is the most cost effective option 46% and 41% of the time respectively. For mean daily patient arrival rates from 28 to 50 patients, the 50-dose YF vial size is the most cost effective option between 46% and 100% of the time.
For mean daily patient arrival rates between 1 and 6 patients, the 10-dose BCG vial is the most cost effective option between 79% and 94% of the time. For mean daily patient arrival rates from 7 to 50 patients, the 20-dose BCG vial is the most cost effective option between 54% and 100% of the time.
For mean daily patient arrival rates from 1 to 4 patients, the 1-dose Hib vial size is the most cost effective option between 57% and 100% of the time. For mean daily patient arrival rates from 5 to 50 patients, the 10-dose Hib vial size is the most cost effective option between 62% and 100% of the time. The 2-dose Hib vial size is never the most cost effective option.
Finally, for mean daily patient arrival rates from 1 to 5, the sinle-dose DTP-HepB-Hib vial size is the most cost-effective option between 56% and 100% of the time. For mean daily patient arrival rates between 6 and 50 patients, the 10-dose DTP-HepB-Hib vial size becomes the most cost effective option between 95% and 100% of the time.
The Costs of Vaccine Wastage and Vaccine Vial Disposal and Vaccine Vial Storage
Figure 3c (for measles vaccine) and Table 2 (for all of the vaccines) show that adding the cost of storage for the vaccines does not have a significant impact on the optimal dose format for each daily patient arrival rate. Figure 4c depicts the acceptability curves for the measles vaccine when factoring in the costs of vaccine wastage, vaccine vial disposal, and vaccine vial storage. Note the results in Figure 4c are very similar to the results in Figure 4b.
As Table 2 illustrates, the BCG and DTP-HepB-Hib vaccine threshold do not change when the cost of vial storage is added, while the Hib vaccine threshold decreases slightly. By contrast, the YF vaccine patterns change, as the 20-dose format becomes favorable under certain conditions. For mean daily patient arrival rates from 1 to 13 patients, the 5-dose YF vial size is most cost effective between 68% and 100% of the time. For mean daily patient arrival rates from 14 to 21 patients, the 20-dose YF vial size is most cost effective between 52% and 86% of the time. For mean daily patient arrival rates from 22 to 25 patients, the 5-dose YF vial size is most cost effective between 51% and 58% of the time. For mean daily patient arrival rates of 26 and 27 patients, the 20-dose vial size is most cost effective 47% and 44% of the time respectively. For mean daily patient arrival rates from 28 to 50 patients, the 50-dose YF vial size is most cost effective between 54% and 100% of the time.
Nearly all of the above mentioned results were robust to increases in storage and disposal costs (doubling both disposal and storage costs simultaneously had some minor effects on the thresholds of yellow fever vaccine), demonstrating that vaccine cost is primarily driving the optimal dose presentation at the clinic level.
Projected Annual Savings when Switching to the Least Costly Format
Table 3 projects the potential annual cost savings of switching from the most costly to the least costly vaccine format for different mean daily patient arrival rates and vaccination days per week (1 day to 5 days) over a one year period. For all vaccine types, the cost savings over one year is greatest when both the mean daily patient arrival rate and the number of vaccination days per week are highest.
TABLE 3.
Potential Annual Savings (US$) when Switching to the Least Costly Format
| Vaccine | Mean Daily Patient Arrival Rate (for a Poisson Distributi on)* |
Number of Immunization Days per Week | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Measles | 1 | 50 | 100 | 150 | 200 | 250 |
| 10 | 302 | 605 | 907 | 1,210 | 1,512 | |
|
| ||||||
| 25 | 828 | 1,656 | 2,484 | 3,312 | 4,140 | |
| 50 | 1,728 | 3,456 | 5,184 | 6,912 | 8,640 | |
|
| ||||||
| Yellow Fever | 1 | 664 | 1,328 | 1,992 | 2,655 | 3,319 |
| 10 | 802 | 1,603 | 2,405 | 3,206 | 4,008 | |
|
| ||||||
| 25 | 348 | 696 | 1,044 | 1,392 | 1,740 | |
| 50 | 600 | 1,200 | 1,800 | 2,400 | 3,000 | |
|
| ||||||
| BCG | 1 | 58 | 115 | 173 | 230 | 288 |
| 10 | 346 | 691 | 1,037 | 1,382 | 1,728 | |
|
| ||||||
| 25 | 1,044 | 2,088 | 3,132 | 4,176 | 5,220 | |
| 50 | 2,328 | 4,656 | 6,984 | 9,312 | 11,640 | |
|
| ||||||
| Hib | 1 | 380 | 759 | 1,139 | 1,519 | 1,898 |
| 10 | 514 | 1,027 | 1,541 | 2,054 | 2,568 | |
|
| ||||||
| 25 | 1,800 | 3,600 | 5,400 | 7,200 | 9,000 | |
| 50 | 4,008 | 8,016 | 12,024 | 16,032 | 20,040 | |
|
| ||||||
|
DTP-HepB-
Hib |
1 | 486 | 972 | 1,459 | 1,945 | 2,431 |
| 10 | 588 | 1,175 | 1,763 | 2,350 | 2,938 | |
|
| ||||||
| 25 | 2,093 | 4,186 | 6,279 | 8,372 | 10,465 | |
| 50 | 4,732 | 9,464 | 14,196 | 18,928 | 23,660 | |
Daily patient arrival rate assumes a Poisson distribution with this mean patient arrival rate.
Varying the Patient Demand Distribution
Changing the patient demand distribution from a Poisson to a uniform distribution generated essentially identical vial size breakpoints.
DISCUSSION
Our results underscore the importance and potential economic impact of altering the number of vaccine doses in a vial. Such information can guide the development and production of new vaccine formats in the future. It can also assist purchasers in determining how many of each format to procure for their populations. Those who handle ordering/purchasing vaccines for a clinic can match the expected (e.g., historical) patient arrival rates with the thresholds established by our model. Finally, our study results may help vaccine distributors better understand how and where to allocate their stocks of different vaccine formats.
Open vial waste is a significant cause of vaccine wastage at the clinic level, as our study highlights. Optimal vial size for a location depends on mean daily patient arrival rates as well as available resources for vaccine storage and disposal. In some settings, both single and multi-dose vials may be deemed appropriate by in-country health care managers. Some vial sizes are less expensive than others; however, without proper monitoring of vaccine wastage, there is a risk that costs of vaccine wastage will be greater than the resources saved in using multi-dose vial formats.
As our model suggests, the optimal number of doses per vial may vary by vaccine, geographically, and patient demand. Lower patient demand favors fewer dose formats, while higher demand favors greater dose formats. In other words, when patient volume is low, the value of lost vaccine doses outweighs the potential gains from squeezing more doses into each vaccine vial; reducing the number of vials needed which can decrease material costs, medical waste, and storage needs. Therefore, it may make sense to tailor the appropriate format to the anticipated population served. Clinics may compare their local circumstances with the benchmarks in our model to make such a choice. Moreover, fewer dose formats may be more conducive to lower volume routine immunizations and greater dose formats to mass immunization campaigns.
By using a Poisson distribution for the patient arrival rate, our model incorporated the fluctuations that may occur in a clinic from day-to-day as well as those that may occur from clinic-to-clinic. So, a decision maker at an individual clinic could construct a distribution of his or her clinic’s patient arrival rate and then match it with the benchmarks that our model provides. A regional or national level decision maker (who often must choose only one vial size to serve a wide variety of clinics) could construct a distribution of patient arrival rates for all the clinics in a region and then match them with the benchmarks in our model. Table 3 can help decision makers understand the potential cost of not more specifically tailoring vial size to individual clinics and their circumstances. Our model emphasizes the importance of better understanding and forecasting patient arrival rates. Of note, the model does focus on costs and decision-making at the clinic level (i.e., the periphery of the vaccine supply chain). While sensitivity analyses explored the effects of ranging vaccine storage costs, fully examining the countrywide supply chain impact of vial presentation requires a more extensive representation of country’s entire supply chain, which our Vaccine Modeling Initiative (VMI), funded by the Bill and Melinda Gates Foundation, is currently developing for countries in West Africa and Southeast Asia.
The model helps generate the following heuristic: (1) determine the distribution of patient (those requiring immunization) arrival rates, including a mean and a spread (e.g., standard deviation) and then (2) match this distribution with the benchmarks established by our model, which will then identify the optimal dose-presentation. For clinics that serve a wide variety of patients (i.e., including many patients who will not be immunized), the decision maker can focus exclusively on the anticipated arrival patterns for those requiring immunization. For example if 10% of a general clinic are children of the age requiring routine immunization, then the decision maker can create a distribution of the arrival rate of this specific 10%.
Our model delineates the major trade-offs that occur when varying the number of doses per vial. Several previous studies have focused on some of these trade-offs.[2-13] With the exception of the YF vaccine, per dose vaccine production costs increase as the number of doses per vial increases. Fewer dose formats incur higher filling costs, vaccine overfill adjustments, storage requirements, medical waste, and packaging costs.[3] On average, manufacturing and packaging costs are 2.5 times greater for single-dose formats than 10-dose formats.[3] Conversely, single-dose formats reduce open vial wastage.[3]
Traditionally, very high multi-dose (e.g., 50 doses/vial) presentations have not been part of routine immunizations and instead have been reserved for immunization campaigns. Our model did include these vial presentations to probe theoretically their potential utility in routine settings. However, one must keep in mind that such high multi-dose presentations may have issues that limit such use, such as safety issues (i.e., having to repeatedly use needles to draw vaccine doses from the same vial) for health care workers and patients.
Developing this model has been part of the VMI, which has involved working with public health officials at the WHO and various countries in West Africa and Southeast Asia on modeling vaccine distribution. Their ongoing input has contributed to the continuing updating and improvement of our vaccine distribution and administration models and tools. As each model and tool reaches its maturation, we will make them freely available to relevant decision makers.
Limitations
Every computational model is a simplification of real life. No model can fully represent every single factor or outcome that may affect a decision. While our model focuses on the key advantages and disadvantages of various dose formats, single dose formats have some additional potential advantages (e.g., easier tracking and increased safety) and disadvantages (e.g., more complex manufacturing processes) were not captured. Rather than make decisions, models generate information intended to inform decision makers by elucidating relationships and factors that may not be readily apparent. Our model depends on the strength of its assumptions and data sources. For example, the model assumed that health care workers would be able to access the full complement of doses in a vial, which is often not the case; a health care worker may only be able to obtain eight or nine doses from a ten-dose vial. However, accounting for the cost of such unused doses would only further favor fewer dose formats under low patient volume conditions. Additionally, the results of multiple sensitivity analyses confirmed the robustness of our model.
Our model also does not capture an additional issue: multi-dose presentations could trigger missed opportunities for immunization, i.e., a vaccinator may hesitate opening a next vial near the end of a clinic session in order to avoid wasting the remaining doses.
Our analyses did not include the cost of contamination. Every time a health care worker handles a multi-dose vial to obtain a vaccine dose, there is a risk of contaminating the remaining doses. As the number of doses per vial increases, the number of times a given vial is accessed and therefore the risk and attendant expected cost of contamination increases. This is especially true in vaccines that do not contain preservatives such as thimerosal. Vaccine contamination can lead to injection site infections such as cellulitis and abscesses or rarely, in more severe cases, systemic infections that could lead to hospital admission or death. However, the risk of contamination has not been well established.[4, 8, 14-16] Moreover, such costs would only further support the use of fewer dose formats in lower patient volume situation.
Conclusions
Single-dose vaccine formats can prevent clinic-level vaccine wastage but may incur higher production, medical waste disposal, and storage costs per vaccinated individual than multi-dose formats. Our computational model predicted the potential economic impact of utilizing various single-dose versus multi-dose measles, Hib, BCG, and YF vaccine formats. Lower patient demand favors fewer dose formats (i.e., the cost of vaccine wastage often may outweigh that of added medical waste and storage requirements), while higher demand favors greater dose formats.
ACKNOWLEDGEMENTS
This study was supported by the Vaccine Modeling Initiative (VMI), funded by the Bill and Melinda Gates Foundation and the National Institute of General Medical Sciences Models of Infectious Agent Study (MIDAS) grant 1U54GM088491-0109. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].World Health Organization Vaccine Volume Calculator. 2009 cited 2010 February 20, 2010]; Immunization service delivery and accelerated disease control]. Available from: http://www.who.int/immunization_delivery/systems_policy/logistics/en/index4.html.
- [2].Parmar D, Baruwa EM, Zuber P, Kone S. Impact of wastage on single and multidose vaccine vials: Implications for introducing pneumococcal vaccines in developing countries. Hum Vaccin. 2010 Mar 21;6(3) doi: 10.4161/hv.6.3.10397. [DOI] [PubMed] [Google Scholar]
- [3].Drain PK, Nelson CM, Lloyd JS. Single-dose versus multi-dose vaccine vials for immunization programmes in developing countries. Bull World Health Organ. 2003;81(10):726–31. [PMC free article] [PubMed] [Google Scholar]
- [4].Gooding J, Millage A, Rye AK, Lacroix R. The cost and safety of multidose use of palivizumab vials. Clin Pediatr (Phila) 2008 Mar;47(2):160–3. doi: 10.1177/0009922807306994. [DOI] [PubMed] [Google Scholar]
- [5].Griffiths UK, Korczak VS, Ayalew D, Yigzaw A. Incremental system costs of introducing combined DTwP-hepatitis B-Hib vaccine into national immunization services in Ethiopia. Vaccine. 2009 Feb 25;27(9):1426–32. doi: 10.1016/j.vaccine.2008.12.037. [DOI] [PubMed] [Google Scholar]
- [6].Howard SM. Use of multidose vials. Infect Control. 1983 Sep-Oct;4(5):358–60. doi: 10.1017/s019594170005966x. [DOI] [PubMed] [Google Scholar]
- [7].Longfield R, Longfield J, Smith LP, Hyams KC, Strohmer ME. Multidose medication vial sterility: an in-use study and a review of the literature. Infect Control. 1984 Apr;5(4):165–9. doi: 10.1017/s0195941700059154. [DOI] [PubMed] [Google Scholar]
- [8].Paparella S. The risks associated with the use of multidose vials. J Emerg Nurs. 2006 Oct;32(5):428–30. doi: 10.1016/j.jen.2006.05.016. [DOI] [PubMed] [Google Scholar]
- [9].Schreuder B, Arentsen H, Matosse M. How important are airfreight rates and vaccine packaging in cost-saving efforts for the Expanded Programme on Immunization? Bull World Health Organ. 1997;75(4):315–21. [PMC free article] [PubMed] [Google Scholar]
- [10].Sheth NK, Post GT, Wisniewski TR, Uttech BV. Multidose vials versus singledose vials: a study in sterility and cost-effectiveness. J Clin Microbiol. 1983 Feb;17(2):377–9. doi: 10.1128/jcm.17.2.377-379.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].World Health Organization WHO policy statement . The use of opened multi-dose vials of vaccine in subsequent immunization sessions. Geneva, Switzerland: 2000. [Google Scholar]
- [12].World Health Organization . Management of solid health-care waste at primary health-care centres: A decision-making guide. Geneva, Switzerland: 2005. [Google Scholar]
- [13].World Health Organization . Management of waste from injection activities at district level: Guidelines for district health managers. Geneva, Switzerland: 2006. [Google Scholar]
- [14].Dicko M, Oni AQ, Ganivet S, Kone S, Pierre L, Jacquet B. Safety of immunization injections in Africa: not simply a problem of logistics. Bull World Health Organ. 2000;78(2):163–9. [PMC free article] [PubMed] [Google Scholar]
- [15].Mattner F, Gastmeier P. Bacterial contamination of multiple-dose vials: a prevalence study. Am J Infect Control. 2004 Feb;32(1):12–6. doi: 10.1016/j.ajic.2003.06.004. [DOI] [PubMed] [Google Scholar]
- [16].Simonsen L, Kane A, Lloyd J, Zaffran M, Kane M. Unsafe injections in the developing world and transmission of bloodborne pathogens: a review. Bull World Health Organ. 1999;77(10):789–800. [PMC free article] [PubMed] [Google Scholar]