Abstract
Objective
To evaluate the relationship between early CD4+ lymphocyte recovery on antiretroviral therapy (ART) and subsequent survival among low body mass index (BMI) HIV-1 infected adults.
Design
Retrospective analysis of a large programmatic cohort in Lusaka, Zambia.
Methods
We evaluated ART treated adults enrolled in care >6 months. We stratified this study population according to WHO malnutrition criteria: normal (BMI ≥18.5 kg/m2), mild (17.00-18.49), moderate (16.00-16.99), and severe (<16.0). We used Cox proportional hazards regression to estimate the subsequent risk of death associated with absolute CD4+ count change over the first 6 months on ART. To account for effect modification associated with baseline CD4+ count, a weighted summary measure was calculated.
Results
From May 2004 to February 2009, 56,612 patients initiated ART at Lusaka district clinics; of these, 33,097 (58%) were included in this analysis. The median change in 0-6 month CD4+ count in each baseline BMI strata varied from 127 to 131 cells/μL. There was a statistically significant, inverse association between baseline BMI and the post-6 month hazard for mortality only among those patients with <100 cells/μL increase in the first 6 months of ART. A CD4+ count increase of ≥100 cells/μL over the first 6 months of ART was not associated with a higher hazard for mortality, regardless of baseline BMI.
Conclusions
Low baseline BMI and attenuated CD4+ count response at 6 months had a compounding, negative impact on post-6 month survival. Specific guidelines for monitoring ART response using immunologic criteria may be warranted for low BMI patients.
Keywords: HIV; Nutrition; CD4 lymphocyte count; Antiretroviral therapy, highly active; Body mass index; Zambia; Africa
Introduction
Sub-Saharan Africa is disproportionately affected by the global epidemics of HIV-1 infection and malnutrition [1, 2]. A low body mass index (BMI; calculated as weight in kilograms divided by height in meters, squared) is a general indicator of a poor nutritional state, and a powerful, independent predictor of early mortality (i.e., within the first 90 days) after starting antiretroviral therapy (ART) [3-5]. Patients with an attenuated CD4+ lymphocyte recovery following ART initiation are at greater risk of death than are those with more robust immune reconstitution, but the influence of malnutrition on this observation is unknown [6, 7]. In this analysis, we examine the relationships between baseline BMI, 6-month CD4+ cell count change, and subsequent mortality among HIV-1 infected adults initiating ART in Lusaka, Zambia.
Methods
We analyzed data from a programmatic cohort of HIV-infected adults (>15 years of age) who initiated ART between May 1, 2004 and February 28, 2009 in the Zambian national program for HIV care and treatment. This program was implemented in the Lusaka district in April 2004 and has been described in detail elsewhere [3, 8]. Briefly, HIV-infected patients are enrolled in care and undergo a history and physical, WHO clinical staging, and a CD4+ cell count. Weight and height (i.e., the components of BMI) are recorded by a nurse at the initial visit, and weight is recorded at subsequent visits. Patients with WHO stage 4 disease; a CD4+ cell count <200 cells/μL; or WHO stage 3 disease and a CD4+ cell count <350 cells/μL are eligible for ART initiation.
The analysis cohort was limited to patients who were active in the ART program for at least 6 months, had a documented baseline BMI, and had a CD4+ cell count value recorded at baseline and at 6 months post-ART initiation. Patients who died or were lost to follow-up (i.e., more than a month overdue for their last scheduled clinical or pharmacy visit) prior to 6 months were excluded.
We stratified the analysis cohort according to the World Health Organization (WHO) categories for malnutrition: severe (BMI <16.00 kg/m2), moderate (BMI = 16.00-16.99 kg/m2), mild (BMI = 17.00-18.49 kg/m2), and non-malnourished (≥18.5 kg/m2) [9]. We further categorized patients in each BMI strata according to absolute CD4+ cell count change from baseline to 6 months (≥300, 200-299, 100-199, 0-99 cells/μL, or a CD4+ decline).
We compared the median 0-6 month CD4+ change between the BMI categories using a Wilcoxon rank sum test. We calculated the adjusted hazard of death from 6 months onward among patients in each dually-stratified BMI and CD4+ change group using Cox proportional hazards regression. Models were adjusted for age, gender, baseline hemoglobin, WHO clinical stage, the presence of active tuberculosis (TB), initial ART regimen, and adherence (calculated as the medication possession ratio from pharmacy refill data) [10]. To account for effect modification associated with baseline CD4+ cell count [7], a weighted summary measure was determined by calculating separate hazard ratios across 5 different baseline CD4+ categories: <100, 100–199, 200–299, 300–399, and ≥400 cells/μL. For the post 6-month mortality analyses, patients were censored at the time of voluntary withdrawal from the program or when classified as lost to follow-up.
All available patient data through February 28, 2009 were considered. Statistical analyses were performed using SAS version 9.1 (SAS Institute, Cary, North Carolina, USA). The study was approved by the relevant ethical review committees.
Results
Between May 1, 2004 and February 28, 2009, 56,612 patients initiated ART at Lusaka district clinics, 48,916 (86%) of whom had a baseline BMI and CD4+ cell count measurement documented. A further 15,819 (28%) patients were excluded from the analyses: 3,549 (6%) died, 5,599 (10%) were lost to follow-up prior to 6 months, and 6,617 (12%) were missing a 6 month CD4+ cell count value. Patients who had died or were lost to follow-up prior to six months had a lower median BMI compared to those in the analysis cohort (18.6 vs. 20.1 kg/m2; p<0.01), lower median baseline CD4+ count (115 vs. 142 cells/μL; p<0.01), lower median hemoglobin (10.2 vs. 11.0; p<0.01) and a higher prevalence of WHO stage 4 disease (11.6% vs. 7.3%; p<0.01). Likewise, those without a 6 month CD4+ cell count had a lower BMI compared to the analysis cohort (19.7 vs. 20.1 kg/m2; p<0.01), baseline CD4+ cell count (138 vs. 142; p<0.05), hemoglobin (10.8 vs. 11.0; p<0.01), and a higher prevalence of WHO stage 4 disease (9.0% vs. 7.3%; p<0.01).
Overall, 33,097 (58%) patients remained active in the ART program after 6 months and had a documented baseline and 6-month CD4+ cell count. Compared to patients in the BMI ≥18.5 kg/m2 strata, those in the BMI <16 kg/m2 strata were younger (33 versus 35 years), and had a lower median baseline CD4+ cell count (92 versus 151 cells/μL), lower median hemoglobin (9.9 versus 11.2 g/dL), and a higher prevalence of WHO stage 4 disease (13% versus 6%).
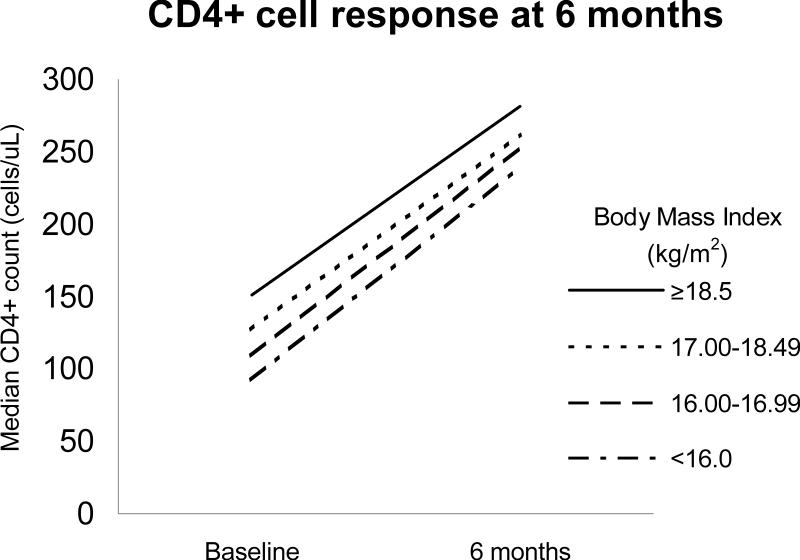
The median change in CD4+ cell count at 6 months ranged from 127 to 131 cells/μL, despite lower median baseline CD4+ values in the lower BMI strata (Figure). There was no statistically significant difference between the BMI 16.00-16.99 kg/m2 and 17.00-18.49 kg/m2 groups compared to BMI >18.5 kg/m2, while there was a statistically, but not clinically, significant difference for <16.0 kg/m2 group (p<0.05).
The overall post-6 month mortality rate was 1.79 deaths per 100 person-years, and was greater in the lower baseline BMI strata: 1.52 deaths per 100 person-years for the BMI ≥18.5 kg/m2 group, 2.20 for BMI 17.00-18.49 kg/m2, 2.40 for BMI 16.00-16.99 kg/m2, and 3.17 for BMI <16.0 kg/m2.
After dually-stratifying the cohort according to BMI and the relative 0-6 month CD4+ cell count change, we calculated the relative hazard of death among patients surviving beyond 6 months on ART (TABLE). Those patients with a baseline BMI ≥18.5 kg/m2 and a CD4+ change of ≥300 cells/μL constituted the reference group. Among patients with a 0-6 month CD4+ change of <100 cells/μL or a CD4+ decline, the associated hazard generally increased as BMI decreased. Patients with a BMI <16 kg/m2 and a CD4+ increase of 0-99 cells/μL at 6 months had a nearly 4-fold increased hazard of death compared to the reference group (adjusted hazard ratio [AHR] 3.93; 95%CI 2.66 – 5.80), while those with a CD4+ decline had an approximate 6-fold increased hazard (AHR 6.08; 95%CI 3.59 – 10.32). A CD4+ change of ≥100 cells/μL over the first 6 months of ART was not associated with a higher hazard for mortality compared to the reference group, regardless of baseline BMI.
Table.
Adjusted hazard of death after 6 months on antiretroviral therapy (95% CI); dually-stratified by body mass index and 0-6 month CD4+ count change
| 0-6 month CD4+ change (Δ cells/μL) | ||||||
|---|---|---|---|---|---|---|
| ≥300 cell increase | 200-299 cell increase | 100-199 cell increase | 0-99 cell increase | CD4 decline | ||
| Body Mass Index (kg/m2) |
≥18.5 (n=23,505) |
Reference, 1.0 [n=2876]* |
1.13 (0.80 - 1.60) [3818] |
1.10 (0.80 - 1.51) [7338] |
1.67 (1.23 - 2.26) [6850] |
2.60 (1.84 - 3.67) [2623] |
|
17.00-18.49 (n=5,351) |
0.76 (0.40 - 1.46) [652] |
1.15 (0.70 - 1.88) [886] |
1.33 (0.90 - 1.96) [1743] |
1.88 (1.28 - 2.76) [1468] |
4.50 (2.98 - 6.78) [602] |
|
|
16.00-16.99 (n=2,163) |
1.19 (0.57 - 2.50) [256] |
1.01 (0.48 - 2.12) [348] |
1.05 (0.60 - 1.82) [704] |
2.21 (1.40 - 3.47) [639] |
5.28 (3.09 - 9.03) [216] |
|
|
<16.0 (n=2,078) |
1.13 (0.53 - 2.40) [283] |
0.59 (0.23 - 1.48) [337] |
1.21 (0.73 - 2.02) [670] |
3.93 (2.66 - 5.80) [600] |
6.08 (3.59 - 10.32) [188] |
|
Number of patients in each body mass index and CD4+ change group
We performed the same regression analysis within each BMI strata, using those patients with a CD4+ cell count increase ≥300 cells/μL as the reference (data not shown). A 6-month CD4+ decline was significantly associated with subsequent mortality in all BMI categories, while a CD4+ change of 0-99 cells/μL was significantly associated with subsequent mortality in all BMI categories except 16.00-16.99 kg/m2.
Discussion
In a large, programmatic ART cohort in sub-Saharan Africa, a low baseline BMI and an attenuated CD4+ cell response at 6 months had a compounding, negative impact on post-6 month survival. A threshold CD4+ increase of ≥100 cells/μL appeared to normalize the subsequent hazard for mortality across the BMI strata. These findings suggest that immunologic criteria may readily identify patients at risk of poorer long-term outcomes on ART, especially among those with low BMI at treatment initiation.
Few prior studies investigated the relationship of BMI, immune recovery and survival. An analysis from Singapore found no association between BMI and the magnitude of CD4+ cell recovery, but a low BMI was a significant independent predictor of death for several years following ART initiation [11]. A report from Cote d'Ivoire compared patients with a BMI above and below 18.5 kg/m2, and found no difference in the proportion who failed to gain at least 50 cells/μL at 6 months following ART initiation [12]. However, in a study from South Africa, a BMI in the lowest quartile (<17.1 kg/m2) was associated with a failure to achieve a CD4+ count ≥200 cells/μL at 12 months, but data on relative CD4+ cell change was not provided [13]. To our knowledge, this study is the first large cohort analysis of BMI, early CD4+ cell recovery and subsequent mortality in a resource-limited setting.
It is important to note that our findings do not address causality. It is unclear if a poor early immune response is a proximate cause of subsequent higher mortality, or if additional factors may confound the association. Malnutrition due to insufficient protein and energy intake is independently associated with immunosuppression, particularly antigen-specific responses, which could retard immune reconstitution [14-16]. A low BMI may result from a combination of HIV-associated wasting and chronic inadequate energy intake, and the latter may persist despite ART treatment in the absence of sufficient food. Indeed, early weight gain is associated with improved 3 and 6 month outcomes on ART [17, 18], but our analysis did not account for time-varying changes in BMI.
The primary limitation of our study was missing data: 10% of patients were lost to follow-up prior to 6 months and 12% of were missing 6 month CD4+ cell count data. The observed loss rates are similar to reports from other programmatic cohorts in sub-Saharan Africa [19]. CD4+ cell count monitoring of patients on ART should occur every six months according to national program guidelines, and missing 6-month CD4+ values could be due to clinician error, refusal of phlebotomy, or lost, damaged, or insufficient specimens, among other causes. The effect of incomplete viral suppression on CD4+ response could not be assessed as routine HIV-1 viral load monitoring was not available in our program. Our analysis adjusted for medication adherence as determined by pharmacy refill data, but actual patient compliance could not be determined. Finally, the available data did not permit model adjustment for the presence of occult secondary infections and co-morbid conditions (with the exception of low hemoglobin and active TB [i.e., on anti-TB treatment]).
This analysis indicates the importance of a robust CD4+ cell count response among all patients starting ART, but especially among those with low BMI. Clinicians in the Zambian ART program are encouraged to prescribe multivitamins to patients with advanced disease or potentially poor nutrition status, but currently there are no specific algorithms to guide the care of those with low BMI. The World Food Programme and other organizations have implemented limited initiatives targeting food insecure HIV-infected adults at some public-sector clinics, however no formal, integrated nutritional rehabilitation or supplementation programs exist at this time [20]. Given the geographical overlap of the HIV and malnutrition epidemics in sub-Saharan Africa, the success of ART programs depends in part on improving the outcomes of these particularly vulnerable patients.
Acknowledgments
The authors would like to acknowledge the Zambian Ministry of Health for consistent support of operations research in the national HIV care and treatment program.
Sources of funding:
Investigator salary or trainee support is provided by the Fogarty International Center (R24-TW007988, K01-TW06670) and a Clinical Scientist Development Award from the Doris Duke Charitable Foundation (2007061). No conflicts of interest were reported by any author.
Footnotes
There are no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.UNAIDS . Report on the global AIDS epidemic. Geneva: 2008. [April 14, 2010]. Available at: http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/2008_Global_report.asp. [Google Scholar]
- 2.Food and Agricultural Organization of the United Nations . The State of Food Insecurity in the World 2008. FAO; Rome: 2008. [April 14, 2010]. Available at: ftp://ftp.fao.org/docrep/fao/011/i0291e/i0291e00.pdf. [Google Scholar]
- 3.Stringer JS, Zulu I, Levy J, Stringer EM, Mwango A, Chi BH, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296:782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 4.Johannessen A, Naman E, Ngowi BJ, Sandvik L, Matee MI, Aglen HE, et al. Predictors of mortality in HIV-infected patients starting antiretroviral therapy in a rural hospital in Tanzania. BMC Infect Dis. 2008;8:52. doi: 10.1186/1471-2334-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zachariah R, Fitzgerald M, Massaquoi M, Pasulani O, Arnould L, Makombe S, Harries AD. Risk factors for high early mortality in patients on antiretroviral treatment in a rural district of Malawi. AIDS. 2006;20:2355–2360. doi: 10.1097/QAD.0b013e32801086b0. [DOI] [PubMed] [Google Scholar]
- 6.Floridia M, Fragola V, Galluzzo CM, Giannini G, Pirillo MF, Andreotti M, et al. HIV-related morbidity and mortality in patients starting protease inhibitors in very advanced HIV disease (CD4 count of < 50 cells/microL): an analysis of 338 clinical events from a randomized clinical trial. HIV Med. 2002;3:75–84. doi: 10.1046/j.1468-1293.2002.00104.x. [DOI] [PubMed] [Google Scholar]
- 7.Chi BH, Giganti M, Mulenga PL, Limbada M, Reid SE, Mutale W, et al. CD4+ Response and Subsequent Risk of Death Among Patients on Antiretroviral Therapy in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2009 doi: 10.1097/QAI.0b013e3181ab6d8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, Chintu N, Stringer EM, Chi BH, et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA. 2007;298:1888–1899. doi: 10.1001/jama.298.16.1888. [DOI] [PubMed] [Google Scholar]
- 9.United Nations Administrative Committee on Coordination Sub-Committee on Nutrition . Fourth Report on the World Nutrition Situation. United Nations ACC/SCN; Geneva, Switzerland: 2000. [Google Scholar]
- 10.Goldman JD, Cantrell RA, Mulenga LB, Tambatamba BC, Reid SE, Levy JW, et al. Simple adherence assessments to predict virologic failure among HIV-infected adults with discordant immunologic and clinical responses to antiretroviral therapy. AIDS Res Hum Retroviruses. 2008;24:1031–1035. doi: 10.1089/aid.2008.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paton NI, Sangeetha S, Earnest A, Bellamy R. The impact of malnutrition on survival and the CD4 count response in HIV-infected patients starting antiretroviral therapy. HIV Med. 2006;7:323–330. doi: 10.1111/j.1468-1293.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 12.Toure S, Kouadio B, Seyler C, Traore M, Dakoury-Dogbo N, Duvignac J, et al. Rapid scaling-up of antiretroviral therapy in 10,000 adults in Cote d'Ivoire: 2-year outcomes and determinants. AIDS. 2008;22:873–882. doi: 10.1097/QAD.0b013e3282f768f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barth RE, van der Meer JT, Hoepelman AI, Schrooders PA, van de Vijver DA, Geelen SP, et al. Effectiveness of highly active antiretroviral therapy administered by general practitioners in rural South Africa. Eur J Clin Microbiol Infect Dis. 2008;27:977–984. doi: 10.1007/s10096-008-0534-2. [DOI] [PubMed] [Google Scholar]
- 14.Keusch G. Malnutrition and the thymus gland. In: Cunningham-Rundles S, editor. Nutrient Modulation of the Immune Response. Marcel Dekker, Inc.; New York, NY: 1993. pp. 283–299. [Google Scholar]
- 15.Gershwin M, Beach R, Hurley L. Nutrition and Immunity. Academic Press; New York, NY: 1984. [Google Scholar]
- 16.Chandra RK. 1990 McCollum Award lecture. Nutrition and immunity: lessons from the past and new insights into the future. Am J Clin Nutr 1991. 53:1087–1101. doi: 10.1093/ajcn/53.5.1087. [DOI] [PubMed] [Google Scholar]
- 17.Koethe JR, Lukusa A, Giganti MJ, Chi BH, Nyirenda CK, Limbada MI, et al. Association between weight gain and clinical outcomes among malnourished adults initiating antiretroviral therapy in Lusaka, Zambia. J Acquir Immune Defic Syndr. 53:507–513. doi: 10.1097/QAI.0b013e3181b32baf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madec Y, Szumilin E, Genevier C, Ferradini L, Balkan S, Pujades M, et al. Weight gain at 3 months of antiretroviral therapy is strongly associated with survival: evidence from two developing countries. AIDS. 2009;27:853–861. doi: 10.1097/QAD.0b013e32832913ee. [DOI] [PubMed] [Google Scholar]
- 19.Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. 2007;4:e298. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cantrell RA, Sinkala M, Megazinni K, Lawson-Marriott S, Washington S, Chi BH, et al. A pilot study of food supplementation to improve adherence to antiretroviral therapy among food-insecure adults in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2008;49:190–195. doi: 10.1097/QAI.0b013e31818455d2. [DOI] [PMC free article] [PubMed] [Google Scholar]