Abstract
Patients with hypertrophic cardiomyopathy (HC) have coronary microvascular dysfunction which is an independent predictor of adverse left ventricular remodeling, systolic dysfunction, and mortality in these patients. Whether these defects in vasomotor function are localized to the coronary arteries or whether systemic vasomotor dysfunction is present in HC patients has not yet been adequatley examined. We tested the hypothesis that patients with HC (n = 46) have altered peripheral vascular endothelial function. Subjects without coronary artery disease (CAD− n = 46) and subjects with CAD (CAD+, n = 46), served as negative and positive controls, respectively. Conduit artery endothelium-dependent vasomotion was assessed with ultrasound by measuring flow-mediated dilation (FMD) of the brachial artery. FMD was lower in HC patients compared to CAD− (p<0.05) but was similar to CAD+ patients (p=NS). In conclusion, vasomotor dysfunction in HC is not restricted to the coronary vasculature. Patients with HC have impaired peripheral conduit vessel endothelial function and the magnitude of impairment is similar to that seen in older patients with advanced CAD.
Keywords: cardiomyopathy, flow mediated dilation, vascular function
Patients with hypertrophic cardiomyopathy (HC) have abnormalities of the intramural coronary arteries characterized by structurally atypical coronary endothelial cells and thickening of the intima and/or medial layers of the vessel wall associated with decreased luminal cross-sectional area 1-4. These abnormalities of the intramural coronary vessels likely represent the primary morphologic substrate contributing to microvascular dysfunction (i.e. abnormal vasodilatory capacity) and its functional consequences, namely blunted myocardial blood flow during stress 1-4. Coronary microvascular dysfunction is an independent predictor of adverse left ventricular (LV) remodeling, systolic dysfunction, and mortality in patients with HC 5-7. Whether these defects in vasomotor function in HC are localized to the coronary arteries or whether systemic vasomotor dysfunction is present has not been adequatley examined. The primary purpose of this study is to examine vascular endothelial function in HC to better characterize systemic vascular physiology and pathophysiology in this patient population.
Methods
We prospectively evaluated 46 consecutive patients with HC without known CAD. Diagnosis of HC was based on the echocardiographic demonstration of a focal area of hypertrophied LV (wall thickness ≥ 15 mm), associated with a non-dilated cavity in the absence of another cardiac or systemic disease that could produce the magnitude of hypertrophy evident. In addition, patients without HC but with coronary artery disease (CAD+; n=46) and patients without HC or coronary artery disease (CAD−; n=46) were retrospectively selected and served as positive and negative controls, respectively. Exclusion criteria included severe valvular disease, recent myocardial infarction or unstable cardiac symptoms, congestive heart failure or left ventricular ejection fraction <40%, severe arrhythmia, coexistent aortic stenosis or Raynaud's disease. In addition, HC patients were excluded if they had a history of prior septal myectomy or alcohol septal ablation.
The presence or absence of the following cardiovascular risk factors was assessed in each patient: male sex; hypertension (taking hypertension medication or systolic blood pressure > 140 mmHg, diastolic blood pressure > 90 mmHg); hypercholesterolemia (taking lipid medication or total serum cholesterol > 200 mg/dL); diabetes mellitus (taking diabetes medication or fasting glucose levels > 126 mg/dl); smoking (having smoked at least 5 cigarettes per day within the prior month); family history of CAD (having first- or second- degree relatives with premature CAD). CAD was defined as the presence of ischemia or infarction on single-photon emission computed tomographic nuclear myocardial perfusion imaging or >50% stenosis of an epicardial coronary artery by angiography. All subjects gave written informed consent and this study was approved by the institutional review board at Tufts Medical Center.
Brachial artery diameter was assessed using high-resolution ultrasonography. Briefly, the right brachial artery was longitudinally imaged 2-cm above the antecubital fossa using a 10mHz linear array vascular ultrasound transducer (Philips, Andover, MA). Diameters were measured during end-diastole (gated with ECG R waves) using ultrasonic calipers. The average of 5 evenly spaced measures (distance between the anterior and posterior intima-blood interfaces) obtained within a 5 cm segment of the vessel was used for subsequent analysis. Following baseline arterial diameter measurement, reactive hyperemia was induced by an ischemic stimulus (rapid inflation of a blood pressure cuff around the upper arm to a supra-systolic pressure for 5 minutes). Immediately post cuff release, reactive hyperemia was confirmed by qualitatively assessing blood velocity for 10 seconds using spectral Doppler. Sixty seconds following release of the occlusion cuff, brachial diameter was once again measured as aforementioned. Responses were calculated as percentage change in brachial artery diameter from baseline and taken as a measure of conduit vessel endothelial function.
Cardiac dimensions and ejection fraction were assessed using standard 2-dimensional echocardiographic techniques. Presence and magnitude of left ventricular outflow tract (LVOT) obstruction was assessed as previously described at rest, with Valsalva maneuver and during exercise8. LVOT obstruction was defined as a peak instantaneous outflow gradient of ≥ 30 mmHg by continuous-wave Doppler echocardiography8. Systolic anterior motion (SAM) and mitral regurgitation were assessed semi-quantitatively (scale 0-4) as previously described 8.
All data are reported as means ± standard error. Group differences were assessed using ANOVA with the Tukey method for post hoc comparisons. ANCOVA was performed with variables known to influence FMD entered as co-variates. Chi-square tests were used to compare categorical variables. Pearson's/Spearman's correlation coefficients were used to assess relationships between variables of interest. Significance was set at p < 0.05. All data analysis was carried out using Statistical Package for the Social Sciences (SPSS, v 16.0, SPSS, Inc., Chicago, IL).
Results
HC and CAD− controls did not differ in age, body mass index, gender, and prevalence of cardiovascular risk factors (Table 1). Compared with HC and CAD− controls, patients with CAD+ were older, with a greater prevalence of hypertension, hyperlipidemia and diabetes mellitus (Table 1, p<0.05). HC patient characteristics are presented in Table 2.
Table 1.
Patient characteristics.
| Variable | HC (n = 46) |
CAD− (n = 46) |
CAD+ (n = 46) |
|---|---|---|---|
| Age (years) | 46 ± 2‡ | 48 ± 2‡ | 56 ± 1 |
| Body mass index (kg/m2) | 28 ± 1 | 29 ± 1 | 30 ± 1 |
| Male | 22 (48%) | 20 (43%) | 24 (52%) |
| Hypertension | 14 (30%)‡ | 16 (35%)‡ | 26 (57%) |
| Hyperlipidemia | 19 (41%)‡ | 14 (30%)‡ | 31 (67%) |
| Diabetes mellitus | 3 (7%)‡ | 3 (7%)‡ | 17 (37%) |
| Smoker | 10 (22%) | 8 (17%) | 15 (33%) |
| Family history of coronary artery disease |
14 (30%) | 16 (35%) | 22 (48%) |
Significantly different from CAD− (p<0.05).
Significantly different from CAD+ (p<0.05).
Data are presented as mean ± SEM or number of patients (percentage).
Table 2.
Hypertrophic cardiomyopathy patient characteristics (n=46).
| Variable | Value ± SEM |
|---|---|
| Ejection fraction (%) | 64 ± 1 |
| Maximum left ventricular thickness (mm) | 20.5 ± 0.7 |
| Left ventricular end diastolic dimension (mm) | 40.4 ± 1.0 |
| Left atrial size (mm) | 39.6 ± 1.2 |
| Systolic anterior motion (scale 0-4) | 2.2 ± 0.2 |
| Mitral regurgitation (scale 0-4) | 1.5 ± 0.1 |
| Family history of hypertrophic cardiomyopathy | 19 (41%) |
| Resting gradient | 18 (39%) |
| Exercise gradient | 24 (52%) |
| New York Heart Association class | |
| I | 26 (56%) |
| II | 10 (22%) |
| III | 10 (22%) |
| Implantable cardioverter defibrillator | 19 (41%) |
| Medications | |
| ß-blocker | 29 (63%) |
| Calcium channel blocker | 14 (30%) |
| Diuretic | 7 (15%) |
| Angiotensin converting enzyme inhibitor/ Angiotensin receptor blocker |
4 (9%) |
| Anti-arrhythmic | 3 (6%) |
| Statin | 11 (24%) |
Data are presented as mean ± SEM or number of patients (percentage).
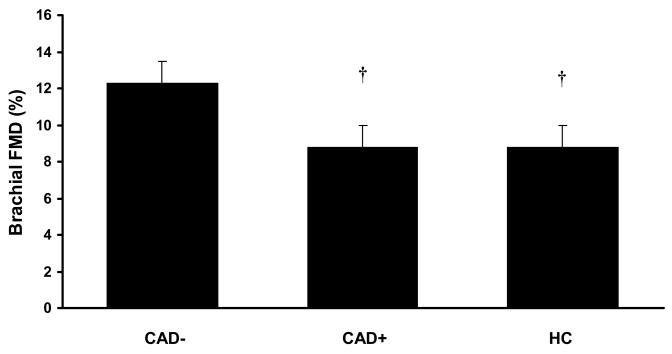
FMD was impaired in patients with HC compared with CAD− controls (Figure 1, p<0.05), to levels similar to that in CAD+ subjects (Figure 1, p=NS). After adjusting for potential confounders that were different between groups (age, prevalence of hypertension, hyperlipidemia, diabetes mellitus), FMD was still similarly impaired in HC and CAD+ compared to CAD− controls (adjusted means: HC, 8.4 ± 0.9 %; CAD+, 9.1 ± 0.9 CAD−, 12.3 ± 0.9 %; p<0.05).
Figure 1.
Flow mediated dilation (%) in patients with HC. FMD is lower in HC and CAD+ compared with CAD−. † Significantly different from CAD− (p<0.05).
There was no association between FMD and age in HC (r = 0.10, p=NS). There was a significant inverse association between FMD and age in control patients (r = −0.35, p<0.05). There was no association between FMD and absolute number of CV risk factors in HC (r = −0.12, p=NS). There was a significant inverse association between FMD and absolute number of CV risk factors in control patients (r = −0.22, p<0.05). Medication use had no effect on FMD in HC patients. FMD was not different in patients with HC taking beta-blockers (p>0.05), calcium channel blockers (p>0.05) ACE-I/ARBs (p>0.05), diuretics (p>0.05) or statins (p>0.05) versus HC patients not taking these agents.
In the HC cohort, 24 patients had evidence of LVOT obstruction with an average resting gradient for all patients of 30±6 mmHg. Patient with LVOT obstruction were older than those without obstruction (51±3 vs. 39±4 yrs, p<0.05). Patients with and without obstruction did not differ in cardiovascular risk factors, gender, body mass index, or cardiac structures (LV end diastolic dimension, LV wall thickness, left atrial size). FMD was similar between HC with obstruction vs. without obstruction (9.2±1.4 vs. 8. 3±1.1 %, p=NS). FMD was not correlated with absolute resting LVOT gradient (r = 0.045, p=NS). Adjusting for age had no effect on the lack of group difference in FMD (p>0.05).
Discussion
The novel findings of this investigation are: 1) patient with HC have reduced peripheral vascular endothelial function compared with CAD− control patients and the level of impairment is similar to that seen in CAD+ patients; 2) peripheral vascular endothelial impairment with HC is not related to the presence or magnitude of LVOT obstruction. Our findings suggest that vascular endothelial dysfunction in HC is systemic and may not be influenced by other attributes of the disease pathology, namely LVOT obstruction.
Patients with HC have coronary vascular dysfunction contributing to low coronary flow reserve, ischemia, systolic dysfunction, fibrosis, LV remodeling and ultimately death. The extent to which vascular dysfunction with HC is systemic has been sparsely examined. Previous studies in small select HC patient populations have reported heightened forearm resistance vessel vasoconstriction and blunted resistance vessel vasodilation to pharmacological and physiological perturbations9-12. Patients with HC also have elevated levels of plasma biomarkers associated with endothelial dysfunction such as C-reactive protein, interleukin-6, soluble CD40 ligand, tissue factor pathway inhibitor, soluble thrombomodulin, beta-thromboglobulin, asymmetric dimethylarginine (ADMA, the endogenous inhibitor of nitric oxide) and endothelin-1 13-15. Our finding of reduced conduit vessel FMD in a larger HC cohort is consistent with the notion that there is global vascular endothelial dysfunction in patients with HC.
Peripheral vascular endothelial dysfunction is associated with coronary endothelial dysfunction 16 and predicts future cardiovascular events 17-19. A novel finding of the present study was that the degree of impairment of peripheral vascular endothelial function in HC is similar in magnitude to the degree of impairment in CAD+. It has been previously established that this level of impairment in CAD+ holds important prognostic implications 20. Whether the vascular endothelial dysfunction witnessed in patients with HC also carries with it prognostic utility will require further study.
Accumulation of CV risk factors contributes to a deterioration of endothelial function in various clinical cohorts and this was seen in our CAD+ and CAD− patients. However, in the present study, FMD was not associated with cardiovascular risk factor burden in patients with HC. Endothelial function has also been shown to deteriorate with advancing age 21 as seen in our CAD+ and CAD− patients, but this too was not evident in HC patients. Therefore the pathogenesis of endothelial dysfunction in HC does not appear to be due to the accumulation of CV atherosclerotic risk factors with aging as may occur with CAD− and CAD+ patients.
Prognosis in HC patients is worse when LV outflow tract obstruction is present and recent studies suggest that more than half of patients with HC have obstruction 8,22. Moreover many of the morbidities associated with HC have been attributed to presence of LVOT obstruction. To our knowledge, this is the first study to examine the impact of LVOT obstruction on peripheral vascular endothelial function in HC. The presence and/or magnitude of outflow gradient has been associated with von Willebrand factor dysfunction (a glycoprotein synthesized by endothelial cells necessary for normal hemostasis), elevated ADMA and increased inflammation13-15. Thus, it has been suggested that LVOT obstruction contributes to endothelial damage via the creation of high sheer forces on the vascular wall 23. Although patients with HC have higher circulating biomarkers of endothelial damage, we noted that in vivo peripheral vasomotor reactivity was not influenced by the presence or magnitude of obstruction suggesting that factors unrelated to obstruction are responsible for noted differences.
Limitations to this study should be noted. Clinical correlates of endothelial dysfunction in HC were not investigated. Whether low FMD in HC carries with it the same clinical significance as is seen in other cohorts needs to be demonstrated empirically. Endothelial-independent vasodilation was not assessed. Thus, it remains plausible that patients with HC may have a primary defect in smooth muscle vasomotor dysfunction contributing to blunted dilation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None
References
- 1.Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002;287:1308–1320. doi: 10.1001/jama.287.10.1308. [DOI] [PubMed] [Google Scholar]
- 2.Cannon RO, 3rd, Rosing DR, Maron BJ, Leon MB, Bonow RO, Watson RM, Epstein SE. Myocardial ischemia in patients with hypertrophic cardiomyopathy: contribution of inadequate vasodilator reserve and elevated left ventricular filling pressures. Circulation. 1985;71:234–243. doi: 10.1161/01.cir.71.2.234. [DOI] [PubMed] [Google Scholar]
- 3.Petersen SE, Jerosch-Herold M, Hudsmith LE, Robson MD, Francis JM, Doll HA, Selvanayagam JB, Neubauer S, Watkins H. Evidence for microvascular dysfunction in hypertrophic cardiomyopathy: new insights from multiparametric magnetic resonance imaging. Circulation. 2007;115:2418–2425. doi: 10.1161/CIRCULATIONAHA.106.657023. [DOI] [PubMed] [Google Scholar]
- 4.Dimitrow PP, Krzanowski M, Nizankowski R, Szczeklik A, Dubiel JS. Comparison of the effect of verapamil and propranolol on response of coronary vasomotion to cold pressor test in symptomatic patients with hypertrophic cardiomyopathy. Cardiovasc Drugs Ther. 2000;14:643–650. doi: 10.1023/a:1007871032421. [DOI] [PubMed] [Google Scholar]
- 5.Cecchi F, Olivotto I, Gistri R, Lorenzoni R, Chiriatti G, Camici PG. Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N Engl J Med. 2003;349:1027–1035. doi: 10.1056/NEJMoa025050. [DOI] [PubMed] [Google Scholar]
- 6.Olivotto I, Cecchi F, Gistri R, Lorenzoni R, Chiriatti G, Girolami F, Torricelli F, Camici PG. Relevance of coronary microvascular flow impairment to long-term remodeling and systolic dysfunction in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2006;47:1043–1048. doi: 10.1016/j.jacc.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 7.Krams R, Kofflard MJ, Duncker DJ, Von Birgelen C, Carlier S, Kliffen M, ten Cate FJ, Serruys PW. Decreased coronary flow reserve in hypertrophic cardiomyopathy is related to remodeling of the coronary microcirculation. Circulation. 1998;97:230–233. doi: 10.1161/01.cir.97.3.230. [DOI] [PubMed] [Google Scholar]
- 8.Maron MS, Olivotto I, Zenovich AG, Link MS, Pandian NG, Kuvin JT, Nistri S, Cecchi F, Udelson JE, Maron BJ. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation. 2006;114:2232–2239. doi: 10.1161/CIRCULATIONAHA.106.644682. [DOI] [PubMed] [Google Scholar]
- 9.Dimitrow PP, Krzanowski M, Surdacki A, Nizankowski R, Szczeklik A, Dubiel JS. Impaired response of the forearm resistance but not conductance vessels to reactive hyperemia in hypertrophic cardiomyopathy. Angiology. 1999;50:267–272. doi: 10.1177/000331979905000401. [DOI] [PubMed] [Google Scholar]
- 10.Imaizumi T, Takeshita A, Yamamoto K, Nakamura M, Sueishi K. Limited maximal vasodilator capacity of forearm resistance vessels in patients with hypertrophic cardiomyopathy. Heart Vessels. 1990;5:159–165. doi: 10.1007/BF02059911. [DOI] [PubMed] [Google Scholar]
- 11.Kawano S, Iida K, Nishi I, Iwasaki Y, Masumi T, Sugishita Y, Yamaguchi I. Impaired peripheral vasoconstriction in response to alpha-adrenergic stimulation in patients with idiopathic hypertrophic cardiomyopathy. Jpn Circ J. 1998;62:903–908. doi: 10.1253/jcj.62.903. [DOI] [PubMed] [Google Scholar]
- 12.Pedrinelli R, Spessot M, Chiriatti G, Gistri R, Salvadori P, Catapano G, Panarace G, Papi L, L'Abbate A, Camici PG. Evidence for a systemic defect of resistance-sized arterioles in hypertrophic cardiomyopathy. Coron Artery Dis. 1993;4:67–72. doi: 10.1097/00019501-199301000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Dimitrow PP, Undas A, Bober M, Tracz W, Dubiel JS. Plasma biomarkers of endothelial dysfunction in patients with hypertrophic cardiomyopathy. Pharmacol Rep. 2007;59:715–720. [PubMed] [Google Scholar]
- 14.Dimitrow PP, Undas A, Bober M, Tracz W, Dubiel JS. Obstructive hypertrophic cardiomyopathy is associated with enhanced thrombin generation and platelet activation. Heart. 2008;94:e21. doi: 10.1136/hrt.2007.126896. [DOI] [PubMed] [Google Scholar]
- 15.Hasegawa K, Fujiwara H, Koshiji M, Inada T, Ohtani S, Doyama K, Tanaka M, Matsumori A, Fujiwara T, Shirakami G, Hosoda K, Nakao K, Sasayama S. Endothelin-1 and its receptor in hypertrophic cardiomyopathy. Hypertension. 1996;27:259–264. doi: 10.1161/01.hyp.27.2.259. [DOI] [PubMed] [Google Scholar]
- 16.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 17.Guazzi M, Reina G, Gripari P, Tumminello G, Vicenzi M, Arena R. Prognostic value of flow-mediated dilatation following myocardial infarction. Int J Cardiol. 2009;132:45–50. doi: 10.1016/j.ijcard.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 18.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 19.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive Value of Brachial Flow-Mediated Dilation for Incident Cardiovascular Events in a Population-Based Study. The Multi-Ethnic Study of Atherosclerosis. Circulation. 2009 doi: 10.1161/CIRCULATIONAHA.109.864801. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuvin JT, Patel AR, Sliney KA, Pandian NG, Rand WM, Udelson JE, Karas RH. Peripheral vascular endothelial function testing as a noninvasive indicator of coronary artery disease. J Am Coll Cardiol. 2001;38:1843–1849. doi: 10.1016/s0735-1097(01)01657-6. [DOI] [PubMed] [Google Scholar]
- 21.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 22.Maron MS, Olivotto I, Betocchi S, Casey SA, Lesser JR, Losi MA, Cecchi F, Maron BJ. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003;348:295–303. doi: 10.1056/NEJMoa021332. [DOI] [PubMed] [Google Scholar]
- 23.Le Tourneau T, Susen S, Caron C, Millaire A, Marechaux S, Polge AS, Vincentelli A, Mouquet F, Ennezat PV, Lamblin N, de Groote P, Van Belle E, Deklunder G, Goudemand J, Bauters C, Jude B. Functional impairment of von Willebrand factor in hypertrophic cardiomyopathy: relation to rest and exercise obstruction. Circulation. 2008;118:1550–1557. doi: 10.1161/CIRCULATIONAHA.108.786681. [DOI] [PubMed] [Google Scholar]