Abstract
Inflammatory pathways may mediate preparation of the “metastatic soil” in the lungs. Some of these pathways—activation and/or the recruitment of certain inflammatory cells—might depend on vascular endothelial growth factor receptor 1 (VEGFR1) activity. Thus, blocking the activity of VEGFR1 (or the interaction with its ligands) has emerged as a potential anti-metastasis strategy to target not only angiogenesis and cancer cell survival and migration, but also the recruitment of tumor growth-promoting bone marrow-derived cells (BMDCs). However, inhibition of VEGFR1 activity by blocking antibodies or by genetic deletion of the tyrosine kinase domain neither prevented nor changed the rate of spontaneous metastasis formation after surgical removal of primary tumors. Thus, development of VEGFR1-targeted agents should be pursued in selected tumors (e.g., by identifying cancers that depend on VEGFR1 signaling for survival) or in specific combination therapies. Preventing metastasis will likely require identification and blockade of additional or alternative pro-inflammatory pathways that mediate the priming of the metastatic soil and the growth of micro-metastases.
Background
In 1889, Stephen Paget hypothesized that metastatic cells—the “seeds”—only grow in the secondary sites—the “soil”—with a permissive microenvironment. While this concept remains the current wisdom, many determinants of the metastatic process continue to remain elusive. As a result—while therapy of primary tumors is constantly being improved—the prevention of metastasis formation is unfortunately still an unmet goal. Metastasis remains the predominant cause of cancer-related deaths and the ultimate frontier of cancer therapy.
Metastasis is a complex multi-step process in which metastatic cells must detach from their neighbors, invade the surrounding stroma, intravasate, survive in the circulation, arrest in the vessels in the target organ where they extravasate, invade the matrix, and grow by co-opting vessels or recruiting new vessels– all these while evading the immune system (1). To overcome these hurdles, metastatic cells may express or recruit molecular players that disrupt the vasculature and facilitate tumor growth in distant sites such as the lungs (2).
Why is lung a good “soil”?
Lung capillary endothelial cells together with the alveolar epithelial cells form the blood-gas barrier, where the venous blood coming from all the tissues of the body is oxygenated. Thus, circulating cancer cells have a high likelihood to interact with lung microvasculature. But what makes the lung (or regions of it) a good “soil”? First, its pre-existing dense and highly oxygenated vasculature could facilitate early tumor growth. Second, lung tissue is rich in alveolar macrophages. These myeloid cells are the front line of cellular defense against respiratory pathogens and regulators of innate alveolar defenses against respiratory infection, by secreting pro-inflammatory cytokines. These cytokines––such as, interleukin 6 and tumor necrosis factor-alpha (TNF-α)––may increase vascular permeability, angiogenesis or tumor growth and their expression may be stimulated by distant tumors (3). In addition, myeloid cells may express VEGFR1, similar to endothelial cells and some cancer cells, and secrete proteolytic enzymes such as MMP-9 in response to VEGFR1 activation by its ligands (4). Knockout mouse models have demonstrated that host cell-derived MMP-9 and TNF-α are critical for experimental lung metastasis (3,4). Collectively, these results support the increasingly held view that cancer cells are likely to home to or be entrapped in the abundant lung microvasculature and grow as tumor nodules by “usurping components of the host innate immune system” (3).
“Pre-metastatic niche”
Hiratsuka et al. proposed the concept of lung tissue “activation” prior to metastasis (4). They showed that activation of lung endothelial cells and macrophages by primary LLC tumors enhanced lung metastasis in a model of experimental metastasis (i.e., after tumor cell infusion). Deletion of either VEGFR1-tyrosine kinase (VEGFR1-TK) or MMP-9 reduced lung metastasis. Kaplan et al. introduced the term “pre-metastatic niche” to describe areas of VEGFR1+ BMDC conglomerates and fibronectin deposition in the lungs– as a result of primary LLC tumor-released factors. LLC tumor cells preferentially homed to these “niches” and blockade of VEGFR1 prevented “niche” formation and metastasis (5).
VEGF pathway and tumor progression
VEGF pathway plays critical roles in tumor growth by diverse mechanisms. Among VEGF family members, VEGF and placental growth factor (PlGF) have been well characterized as modulators of angiogenesis in many tumors (6,7). These growth factors bind to VEGF receptors on endothelial cells and promote their proliferation, survival, migration and tube formation. VEGF binds to VEGFR1, VEGFR2 and neuropilins (NRPs), while PlGF only binds VEGFR1 and NRP-1 and NRP-2 (8). Knockout of VEGF, VEGFR1, VEGFR2 or both NRP-1 and NRP-2 induce embryonic lethality, while PlGF deficiency impairs pathological angiogenesis by attenuating the response to VEGF (6,7,9). VEGF binds with higher affinity to VEGFR1 than to VEGFR2, but the latter has stronger tyrosine kinase activity and is widely assumed as the mediator of the pro-angiogenic activities of VEGF (6,7). Surprisingly, unlike VEGFR1 deficiency, deletion of the tyrosine kinase domain of VEGFR1 (in flt1TK−/− mice) does not lead to embryonic lethality or vascular phenotypes—suggesting that VEGFR1 acts as a “decoy” for pro-angiogenic factors during development (10). Nevertheless, VEGFR1 and VEGFR2 could crosstalk inter– and intramolecularly (11). These complex phenotypes warrant studies of individual receptor pathways in metastasis formation.
How is VEGFR1 activity involved in tumor metastasis?
Certain cancer cells may rely on VEGFR1 activity for survival––e.g., epidermoid tumors (12)––but the potential role of autocrine VEGFR1 signaling in cancer cells during metastasis remains to be elucidated. On the other hand, VEGF family members could also act as cytokines and affect VEGFR1+ and/or VEGFR2+ hematopoietic cell populations (7). The role of inflammatory cells in tumor progression is also under intense investigation, because there is increasing evidence that certain myeloid cells (pro-angiogenic M2-like macrophages and immature myeloid cell populations) promote carcinogenesis, invasion and metastasis (13–15). In this context, the mechanisms by which VEGFR1 facilitates tumor growth and dissemination remain controversial (5,16). This is highly relevant for clinical development of agents targeting VEGFR1 alone or along with VEGFR2 and/or VEGFR3 (e.g., VEGFR1-specific antibody, tyrosine kinase inhibitors), or targeting VEGFR1 ligands (VEGF, PlGF; e.g., bevacizumab, aflibercept, PlGF-specific antibody). These strategies, i.e., targeting the ligand(s) versus the receptor(s), may lead to different effects on tumor growth and metastasis, and might depend on agent specificity, dose, circulation half-life, and potency of VEGF blockade. As a result of these complexities, here onwards we will focus our discussion only on the specific role of VEGFR1 activity blockade in the early steps of lung metastasis formation in cancers that do not express VEGFR1.
VEGFR1 expression may be constitutive, or could be induced along with VEGF and PlGF expression by hypoxia, which often accompanies tumor growth (7). Interestingly, a recent study showed that hypoxia-induced lysyl oxidase released by tumors mediates myeloid BMDC recruitment to lungs during metastasis formation (17). Collectively, these studies supported the notion that lung metastasis formation depends on inflammatory pathways, which include BMDC accumulation and/or activation in lung tissue. VEGFR1 activity may be critical in this process. However, an intriguing question remains whether blockade of VEGFR1 alone in neoadjuvant setting (i.e., prior to tumor removal) can prevent metastasis formation.
Differential role of VEGFR1 activity in tumor growth and pre-metastatic niche
Despite inherent limitations, metastasis is often studied in mice either by systemic infusion of metastatic cells or by implanting metastatic primary tumors (followed or not by surgical removal). The latter strategy allows studies of neoadjuvant (pre-surgery) or adjuvant (post-surgery) therapies. We used this model to interrogate the potential mediation of spontaneous metastasis by VEGFR1 activity. Blockade of VEGFR1 activity in LLC or B16 melanomas––cancers that do not depend on VEGFR1 for survival––did not substantially affect their in vivo growth (16). Based on the prediction from previous studies (5,18), both BMDCs and metastatic cancer cells had seeded the lungs at the time of primary tumor resection in these models. Thus, we tested if continuous (neoadjuvant and adjuvant) inhibition of VEGFR1 activity with antibodies or in flt1TK−/−/C57BL/6 mice could prevent metastasis formation. In contrast to previous reports (5), blocking VEGFR1 signaling by either method did not significantly change the number of BMDCs/CD11b+ cells in the lungs of mice prior to macro-metastases formation in LLC and B16 melanoma models (16)(Figure 1). These inflammatory BMDCs were present in comparable numbers in lungs from healthy mice, and most likely represented resident pulmonary alveolar macrophages (19). Nevertheless, once the macro-metastases formed, the blockade of VEGFR1 led to a partial decrease in BMDC infiltration inside and around the growing metastatic nodules– in a tumor-dependent manner (16,19). Thus, signaling pathways additional to VEGFR1 are likely involved in BMDC infiltration in growing tumors and metastases (19). This conclusion is consistent with the finding that Gr1+CD11b+ BMDCs are recruited by tumors in spite of VEGF blockade and modulate resistance to anti-VEGF therapy in these tumor models (20). This is also consistent with the finding that once the tumor cells have seeded the distant site, VEGFR1 blockade, along with VEGFR2 and VEGFR3 inhibition, has little effect on metastatic growth in lymph nodes (21). These preclinical findings have direct implications for the treatment of localized cancers.
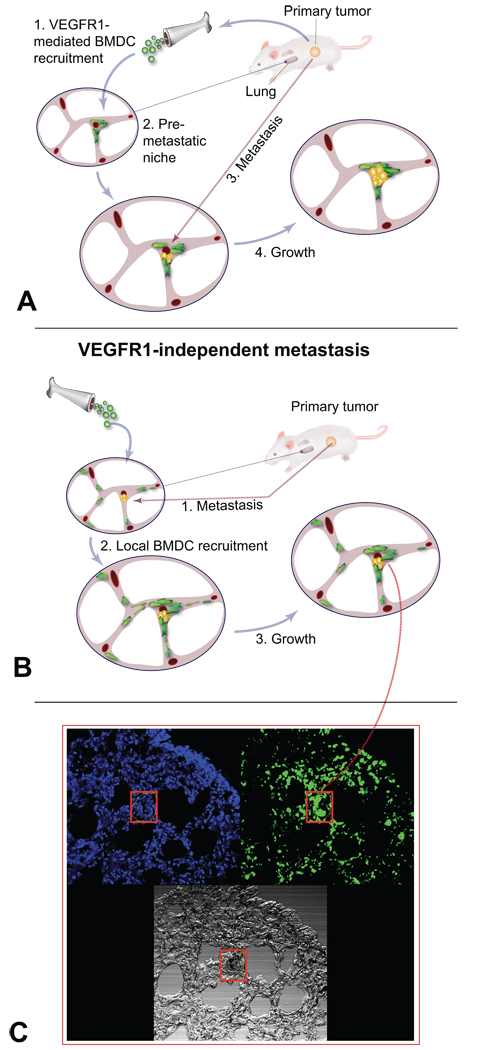
Figure 1. Mechanisms of "pre-metastatic" lung activation.
A, It has been proposed that tumor-derived factors create pre-metastatic “niches" by recruiting bone marrow-derived cells (BMDCs), which facilitate homing and growth of metastatic cells. B, Alternatively, paracrine interactions may occur between lung resident VEGFR1+ endothelial cells and resident myeloid BMDCs––e.g., macrophages––that "activate" the normal lung tissue in response to tumor-derived factors. (Drawings in A, B courtesy of Dr. Lance L. Munn.) C, Nonetheless, metastasis formation can occur independently of VEGFR1 activity in lung stromal cells. Once metastases become macroscopic, VEGFR1 activity modulates the recruitment of local/circulating myeloid BMDCs to the growing metastatic nodules. Green, green fluorescence protein (GFP)-positive BMDCs; blue, DAPI nuclear counterstaining of lung cells; black in red box, melanotic B16 melanoma metastases formed in lungs after primary tumor resection despite VEGFR1 blockade. Images in C are 512 µm across (courtesy of Dr. Michelle R. Dawson).
Clinical implications
While there are currently no clinical efficacy data for VEGFR1-specific antibodies, agents that target directly VEGFR1 activity or its ligand VEGF have been approved for patients with several types of advanced, metastatic cancers. More recently, anti-VEGF agents are being increasingly tested in pre-operative (neoadjuvant) setting in patients with localized tumors with no detectable metastases. In this context, understanding the role of VEGF/VEGFR1 blockade in progression to metastatic disease will be most relevant. But beyond reconciling inconsistencies between preclinical models on the role of VEGFR1 activity, an even greater challenge is gauging the relevance of these experimental results for the clinical use of anti-VEGF agents. At the time of diagnosis, metastatic cells have most likely seeded in distant organs in many patients. Thus, treatment strategies need to be directed at targeting micrometastases and not just prevention of further metastatic dissemination. One validated strategy to combat these, particularly in locally advanced cancers, is neoadjuvant or adjuvant cytotoxic therapy. Therefore, integration of anti-VEGF therapies in neoadjuvant protocols will likely occur in combination with cytotoxics, making impossible the determination of the specific role of VEGF or VEGFR1 blockade. There are currently no mature data available on neoadjuvant anti-VEGF therapy with cytotoxics from randomized studies. In a phase II study of neoadjuvant bevacizumab with chemoradiation for rectal cancer, local control was 92%, and actuarial 5-year disease-free survival was 69% – outcomes comparable to chemoradiation alone. However, the overall survival rate in the bevacizumab-treated cohort (95%) compared favorably to chemoradiation alone (22). Future studies should establish whether VEGF and/or VEGFR1 blockade has favorable impact on metastatic progression in specific cancers.
Unanswered questions and future directions
In light of the data discussed here, a number of critical questions have become a priority for preclinical research on metastasis.
How is the distant stroma activated by metastatic primary tumors?
Are the mechanisms of activation tumor– and organ-specific?
Could these pathways be targeted to benefit patients by preventing metastasis?
Is systemic inflammation a generic/non-specific promoter of metastasis/tumor growth or is it operating only in the context of lung metastasis formation by activation of specific inflammatory pathways? If so, why?
What are the mechanisms by which the “activated soil” promotes metastatic cell seeding and survival?
Are these pathways activated in cancer patients?
If anti-VEGF therapy is used as neoadjuvant therapy, what pathways are systemically and intra-tumorally upregulated?
Of note, we recently found that neoadjuvant bevacizumab treatment upregulated the chemokine stromal-derived factor 1 alpha (SDF1α) and its receptor CXCR4, and CXCL6 in rectal cancer in patients. Interestingly, circulating SDF1α levels during therapy associated with tumor progression to metastasis after a three-year follow-up (23).
Closing remarks
Recent reports show that spontaneous formation of lung metastases may be independent of VEGFR1 activity in host cells. This indicates that other pathways––activated in lung-resident stromal cells prior to metastatic dissemination––may promote BMDC infiltration in lung metastases. Identifying these pathways will lead to the development of agents targeting their activation, which might optimize anti-VEGF therapy, to prevent or delay metastasis formation and growth.
Acknowledgements
The work of the authors is supported in part by the NIH grants P01-CA80124, R01-CA115767, R01-CA85140, R01-CA126642, T32-CA73479 and R21-CA139168. The authors thank Drs. MR Dawson, P Carmeliet, LL Munn and CG Willett for useful input and discussions.
References
- 1.Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 3.Kim S, Takahashi H, Lin WW, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hiratsuka S, Nakamura K, Iwai S, et al. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell. 2002;2:289–300. doi: 10.1016/s1535-6108(02)00153-8. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 7.Fischer C, Mazzone M, Jonckx B, Carmeliet P. FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat Rev Cancer. 2008;8:942–956. doi: 10.1038/nrc2524. [DOI] [PubMed] [Google Scholar]
- 8.Miao HQ, Klagsbrun M. Neuropilin is a mediator of angiogenesis. Cancer Metastasis Rev. 2000;19:29–37. doi: 10.1023/a:1026579711033. [DOI] [PubMed] [Google Scholar]
- 9.Takashima S, Kitakaze M, Asakura M, et al. Targeting of both mouse neuropilin-1 and neuropilin-2 genes severely impairs developmental yolk sac and embryonic angiogenesis. Proc Natl Acad Sci U S A. 2002;99:3657–3662. doi: 10.1073/pnas.022017899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci U S A. 1998;95:9349–9354. doi: 10.1073/pnas.95.16.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Autiero M, Waltenberger J, Communi D, et al. Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat Med. 2003;9:936–943. doi: 10.1038/nm884. [DOI] [PubMed] [Google Scholar]
- 12.Lichtenberger BM, Tan PK, Niederleithner H, Ferrara N, Petzelbauer P, Sibilia M. Autocrine VEGF signaling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell. 140:268–279. doi: 10.1016/j.cell.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 13.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 14.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 15.Kitamura T, Kometani K, Hashida H, et al. SMAD4-deficient intestinal tumors recruit CCR1+ myeloid cells that promote invasion. Nat Genet. 2007;39:467–475. doi: 10.1038/ng1997. [DOI] [PubMed] [Google Scholar]
- 16.Dawson MR, Duda DG, Fukumura D, Jain RK. VEGFR1-activity-independent metastasis formation. Nature. 2009;461:E4–E5. doi: 10.1038/nature08254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erler JT, Bennewith KL, Cox TR, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duda DG, Cohen KS, Kozin SV, et al. Evidence for incorporation of bone marrow-derived endothelial cells into perfused blood vessels in tumors. Blood. 2006;107:2774–2776. doi: 10.1182/blood-2005-08-3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dawson MR, Duda DG, Chae SS, Fukumura D, Jain RK. VEGFR1 activity modulates myeloid cell infiltration in growing lung metastases but is not required for spontaneous metastasis formation. PLoS One. 2009;4:e6525. doi: 10.1371/journal.pone.0006525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shojaei F, Wu Wu, Malik AK, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 21.Padera TP, Kuo AH, Hoshida T, et al. Differential response of primary tumor versus lymphatic metastasis to VEGFR-2 and VEGFR-3 kinase inhibitors cediranib and vandetanib. Mol Cancer Ther. 2008;7:2272–2279. doi: 10.1158/1535-7163.MCT-08-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willett CG, Duda DG, Ancukiewicz M, et al. A study of neoadjuvant bevacizumab with standard chemoradiation in locally advanced rectal cancer. The Oncologist. 2010 doi: 10.1634/theoncologist.2010-0030. In revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu L, Duda DG, di Tomaso E, et al. Direct evidence that bevacizumab, an anti-VEGF antibody, up-regulates SDF1alpha, CXCR4, CXCL6, and neuropilin 1 in tumors from patients with rectal cancer. Cancer Res. 2009;69:7905–7910. doi: 10.1158/0008-5472.CAN-09-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]