Abstract
The behavioral, biochemical, and physiologic consequences of 6 wk of environmental enrichment were evaluated in male Long Evans and Sprague–Dawley rats and compared with those of rats in standard single-housing conditions. Standard housing provided little or no social or physical stimulation whereas environmental enrichment comprised group housing for 8 h daily in a 3-story cage equipped with novel stimuli. Dependent measures included performance in the forced swim test, thresholds for brain-stimulation reward, sucrose intake and preference, determination of corticosterone levels before and after brief restraint stress, and rate of weight gain. In forced swimming tests, active behaviors (diving, swimming with struggling, and climbing) tended to dominate over passive behaviors (sinking, floating) in both groups and outbred rat stocks (especially in enriched groups) on the first day. These behaviors were replaced with maintenance behaviors such as grooming and swimming without struggling on the second exposure, with enriched Long Evans rats showing the largest decline in activity. Baseline plasma corticosterone levels were elevated in both rat stocks after 6 wk of enrichment. After restraint stress, hormone levels in enriched animals tended to peak earlier and approach or exceed baseline values more quickly than was observed in the comparable control groups. Rate of body weight gain was greater in enriched Long Evans rats than Sprague–Dawley or control rats. Our observations indicate that stock- and group-associated differences in several indices occur in association with enrichment. The data support the claim that environmental enrichment may render animals more resilient to challenges.
Abbreviation: BSR, brain-stimulation reward; EE, environmental enrichment; FST, forced swim test
Environmental enrichment (EE) paradigms are designed to enhance laboratory animals’ surroundings to encourage natural behaviors. Some enrichment paradigms also include a social component, based on the social interactions typical of the genus and species. For example, wild mice and rats generally live in colonies, whereas hamsters are known to be social with unfamiliar animals only during mating.21
Adverse environmental conditions have been shown to affect the susceptibility of animals exposed to diverse stress regimes, reflected in their behavioral,7,34 physiologic,8,25,29,36,56 and biochemical6,8,16 responses in a strain-dependent manner.7,8,16 Therefore, a diverse environment might be expected to alter their response to such stressors. A review of the literature reveals few behavioral investigations of the effects of EE on response to a stressor, and the results of biochemical studies in this context have generally been inconsistent. For example, some laboratories have reported no difference in corticosterone levels between EE- and standard-housed animals after exposure to a stressor,22,33,46 whereas others have observed a reduction in the corticosterone levels of Sprague–Dawley rats4 or even elevated levels of plasma corticosterone in enriched Wistar rats.32 These differences may be due to length of EE exposure or in-strain responsivity to stress. Therefore, the first aim of the present set of experiments was to investigate whether rat strain influences the behavioral and physiologic measures typically used to assess stress responses.
Behaviors observed during the forced swim test (FST) and sucrose intake values are known to be affected by environmental conditions.7,15,28,37 Historically, the FST has been used to assess behavioral despair, as indexed by the degree of immobility in an inescapable environment. After antidepressant administration, immobility typically is replaced by more active behaviors.41-44 EE attenuated behavioral despair in male Sprague–Dawley rats during the FST.9 In our hands, exposure to 5 min of FST generally results in more active or escape behaviors (mostly frantic swimming with struggling and climbing) and in the second 5-min test, less vigorous swimming (without struggling). This pattern is not as prominent in stressed male rats.7 In the present study, we evaluated the effects of EE on behaviors exhibited in the FST, hypothesizing that animals with experience in an enriched environment would demonstrate less swimming with struggling in the second test compared with rats living in standard housing conditions.
In the context of stress research, the presence of an anhedonic state typically is evaluated by using behavioral measures such as thresholds for brain-stimulation reward2,7,34 and sucrose intake and preference.28,37,55 The recent finding that EE also alters the behavioral profile of animals with respect to sucrose intake9 prompted us to include this measure in our study to determine the general hedonic status of animals in an enriched environment. We also evaluated rate of weight gain and corticosterone levels after 6 wk of EE, because these 2 measures are used frequently as indices of environmental challenges.3,10,58 In chronic mild stress studies, 3 to 6 wk of administration is a fairly standard regime.7,8,19,26,28,50
In summary, we conducted 2 studies using male Sprague–Dawley and Long Evans rats. In the first, we assessed weight gain and plasma corticosterone levels after 6 wk of EE. In addition to these physiologic measures, we administered weekly sucrose intake and preference tests. In the second study, thresholds for brain-stimulation reward were collected biweekly, and exposure to the FST was evaluated after 6 wk of EE.
Materials and Methods
Animals.
A total of 58 male rats (Long Evans, 28; Sprague–Dawley, 30; Rattus norvegicus) were obtained from Charles River Laboratories (St Constant, Quebec, Canada). The health status of the animals was verified by the vendor with regard to a list of excluded pathogens provided to the University of Ottawa (http://www.criver.com/en-US/ProdServ/ByType/ResModOver/health/Pages/indexbyspecies.aspx; see Area C72). A flowchart detailing the schedule of procedures, from the time of arrival to the animal facility, is presented in Figure 1. Rats from each outbred stock were separated into either control or EE groups. Upon arrival, all animals were singly housed in clear polycarbonate cages until the beginning of the experiments, at which point control animals remained singly housed and EE animals were housed in groups of 4 or 5 per large cage (0.46 m height × 0.51 m width × 0.25 m depth—approximately twice the size of a standard cage). All animals had free access to food and water and were maintained on a 12:12-h light:dark cycle with lights on at 0700 and a room temperature range of 23 ± 2 °C. Body weights were recorded weekly. All manipulations were conducted in accordance with the Canadian Council on Animal Care11 and received institutional approval.
Figure 1.
Flowchart illustrating the schedule and experimental procedures.
In the first study, after a 3-wk period of sucrose training and stabilization, 16 animals of each stock (8 per group) received 6 wk of EE, during which weekly tests of sucrose intake and preference were administered; body weight was recorded weekly. At the end of the treatment phase, trunk blood and adrenal glands were collected. Both body and adrenal weights were used as gross measures of a stress response.
In the second study, 12 Long Evans (6 per group) and 14 Sprague–Dawley (7 per group) rats received 6 wk of either control or EE treatment. Prior to treatment, all animals were implanted with a stimulating electrode in the ventral tegmental area, allowed to recuperate from surgery, and trained to lever-press for brain-stimulation reward on a continuous reinforcement schedule. Before euthanasia, all animals in this study underwent 2 trials of the FST.
Environmental enrichment.
The enrichment exposure phase occurred in 4- to 6-mo-old rats. The enriched groups were housed from 0800 to 1600 h Monday through Friday in a large 3-story cage built with grid sides for climbing (0.73 m height × 0.44 m width × 0.75 m depth—a commercial ferret cage). The cage was equipped with a metal running wheel, climbing rope, cloth hammock, and large plastic tubes in addition to small rubber and plastic toys (Tonk toys, PetSmart, ON, Canada). Food and water were available at all times in the cage. Although we did not quantify them, typical behaviors of animals in this environment include running through the tubes, climbing the grid walls, eating, drinking, lying in the tubes, lying in the hammock, and gnawing the rope.
Study 1.
Behavioral procedure: sucrose test.
Animals were first acclimatized to a 1% sucrose solution for 48 h (without water present) and then to 2 bottles containing separate solutions of 1% sucrose and water. Over a period of 3 wk, 5 baseline measures of sucrose intake and preference were collected in the following manner. Before each test, animals were food and water deprived overnight and the group housed rats placed in individual cages 1 h prior to the sucrose test. In order to reduce the potential novelty stress associated with individual placement in the cage for the sucrose test, each animal was also housed for a short period (1 h) in its ‘test’ cage during collection of body weights. Fluid intake was recorded at the end of the one hour sucrose test; note that food was not available during the test period. Preference for sucrose was determined by the ratio of sucrose (g) to total liquid intake (g) (sucrose + water) and converted to a percentage. After the collection of baseline data, sucrose tests were conducted weekly during the 6 wk of enrichment according to the above procedure.
Physiologic procedures.
Blood sampling.
Immediately after the enrichment phase, tail-blood samples were collected in an alternating fashion between the control and enriched groups over a period of 2 d to ensure that all samples were collected within the same time-frame (0800 to 1100). Briefly, the rat's tail was lanced close to its tip and blood collected on filter paper; the procedure was an adaptation of a previous method57 and is described elsewhere.28 A time-0 sample was collected, after which the animal was restrained in a plastic cone for 10 min. Subsequent blood samples were taken at 15, 30, 60, and 120 min after onset of the stressor. Filter paper was left to dry for 4 to 5 h, and then was stored in plastic bags at –20 °C until further analysis.
Corticosterone assay.
Blood samples were eluted from the filter paper by placing a single 3.2-mm punch in a glass tube and adding 100 µL Dulbecco phosphate buffered saline (containing 0.1% gelatin) to each tube. These were shaken for 1 h at 50 rpm at room temperature, refrigerated overnight, and shaken for an additional hour the following morning.57
Plasma corticosterone levels in duplicate samples were determined using a commercially available radioimmunoassay kit (ICN Biomedicals, Costa Mesa, CA). The intraassay variability was less than 10%. Total corticosterone concentration levels were determined as outlined previously.57
Statistical analyses.
All statistical analyses were carried out by using the Statistica software package (StatSoft, Tulsa, OK). We used mixed 3-factor analysis of covariance for the body weight data, with group and stock as the independent factors (2 levels each) and time as the repeated factor (6 levels). The fixed covariate was baseline body weight. Organ weights were analyzed by using a 2-factor design with treatment (2 levels) and stock (2 levels) as independent factors.
Two-way randomized group ANOVA (treatment versus stock) were conducted on the time 0 corticosterone data, which represent the hormone levels immediately after the 6 wk of EE. The effect of a subsequent stressor on the absolute corticosterone levels was investigated separately for each stock via a trend analysis. Finally, 3-way mixed ANOVA (treatment, stock, time) were conducted on the corticosterone values obtained after restraint. These results were followed by pairwise t tests with Bonferroni correction, as appropriate. Sucrose intake and preference were analyzed similarly to the weight data, with the addition of body weight at each time point designated as a running covariate.
Study 2.
Surgery.
Immediately before surgery, rats received atropine sulfate (0.05 mg/kg) to prevent excessive bronchopulmonary secretion and then were anesthetized by continuous administration of halothane (Fluotec 3, Cyprane, Keighley, UK). The oxygen flow rate was set at 1.5 L/min, and the halothane concentration set between 3% and 4% for anesthesia induction and reduced to 0.5% to 2% for maintenance; the rate was adjusted according to animals’ responses. Palpebral and foot pad reflexes to pinches were used to assess the stage of anesthesia. To avoid discomfort due to pressure from ear and incisor bars during surgery, lidocaine hydrochloride gel (Xylocaine 2%, AstraZeneca, Mississauga, Canada) was applied topically inside each rat's ears and just behind the upper incisors. The rats’ eyes were covered with ophthalmic ointment (BNP, Vetcom Inc, Upton, Canada) to prevent dryness. For pain management, oral acetaminophen (300 mg/mL; 100 to 200 mg/kg, Glebe Apothecary, Ottawa, Canada) was given for 3 d before and 3 d after surgery in the drinking water. In conjunction with treated drinking water, doses of acetaminophen rectal gel (50 mg/mL; 100 to 200 mg/kg for a 250- to 300-g rat, Glebe Apothecary, Ottawa, Canada) were given at the start of surgery and 4 to 6 h postoperatively.
Electrodes were aimed bilaterally through holes drilled in the skull dorsum at the ventral tegmental area by using the coordinates 4.8 mm posterior to bregma, 0.7 mm lateral to the midsaggital suture, and 8.0 to 8.4 mm below the skull surface.38 The electrodes were fashioned from stainless steel wire (diameter, 250 µm) and insulated with epoxylite or polyimide at the polished tip; electrodes were made inhouse or purchased (Plastics One, Roanoke, VA). A flexible stainless steel wire was wrapped around 4 stainless steel skull screws and served as the current return. The entire assembly was secured to the skull with dental acrylic.
Behavioral procedures.
Brain-stimulation reward.
After a minimum of 7 d recovery from surgery, the rats were trained to press a lever to self-administer brain stimulation on a continuous-reinforcement schedule. All tests were conducted in a wood-and-polycarbonate box measuring 28 cm × 38 cm × 44 cm. A rodent lever protruded from a side wall approximately 3 cm above the floor. A constant-current amplifier35 and an integrated circuit pulse generator built inhouse supplied the electrical stimulation. The current was monitored continuously on an oscilloscope by reading the voltage drop across a 1-kΩ precision resistor in series with the rat. Each depression of the lever produced a 500-ms train of rectangular, monophasic, cathodal pulses that were 100 µs in duration, with a train duration of either 300 or 500 ms.
Rats were introduced to the threshold procedure once lever pressing was deemed reliable (when experimenter-assisted shaping was no longer required). Rate-frequency functions were determined by using a descending method of limits, starting with a frequency value that supported a high rate of responding and descending by 0.10 common log units at the start of each 60-s trial until a value for which little or no responding was obtained. The current was held constant and ranged from 200 to 630 µA across animals. The beginning of each trial was signaled by 3 priming stimulations, which were set at the same stimulation parameters as the subsequent 60-s trials.
A frequency threshold, defined as the value that supported half the maximal response rate, was determined for each curve; this value was interpolated from the rate-frequency function. Four rate-frequency functions were generated during each session. The first was considered a warm-up; the thresholds based on the last 3 iterations were averaged to produce a single threshold value per session per current tested. Thresholds were deemed stable for each rat when they did not vary by more than 0.1 log10 units for 3 consecutive test days. After a single baseline session, the animals were exposed to 6 wk of EE, and threshold tests were conducted biweekly.
Forced swim test.
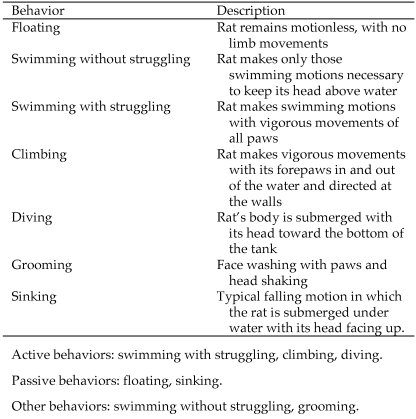
At the conclusion of the 6-wk enrichment phase, rats in the second group were administered the FST. The rats were immersed in an opaque acrylic cylinder (height, 62.5 cm; diameter, 32.5 cm) filled with water (24 °C) to a depth of 48.5 cm. Trial duration was 5 min, after which rats were removed from the cylinder, towel dried, and returned to a heated cage. The next day, the test was repeated at the same time, between 0800 and 1000. All sessions were videotaped (Sony Color Video Camera, Sony, New York, NY) and then scored by 2 blinded observers, who determined whether the predominant behavior was active, passive, or other (Figure 2). To facilitate the analyses and comparison of our data with those of others, we grouped the 7 behaviors described in Figure 2 into clusters of active (swimming with struggling, climbing, and diving), passive (floating and sinking), and other (swimming without struggling and grooming, which are neither escape nor despair behaviors). Interrater reliability was greater than 95%. Scoring was completed with the aid of customized software (Stewart Software, University of Ottawa, Ottawa, Canada) that recorded the frequency and duration of behaviors in 1-min bins.
Figure 2.
A description of each of the behaviors scored during the 5-min FST.
Histology.
After completion of all behavioral tests, rats received a lethal dose of sodium pentobarbital (65 mg/kg IP; Somnotol, MTC Pharmaceuticals, Cambridge, Canada). They were perfused intracardially with saline followed by buffered 10% formalin containing 10% sucrose. Brains were removed and stored in the formalin solution at 6 °C for a minimum of 24 h. Coronal sections were placed on gelatin-coated slides and stained with cresyl violet to locate the electrode tips by using the Paxinos and Watson38 atlas.
Statistical analysis.
The response rate and frequency thresholds for brain-stimulation reward were evaluated by using mixed 3-factor analysis of covariance. Group and stock were the independent factors (2 levels each) and time the repeated factor (12 levels); baseline values served as the fixed covariate. Given interindividual differences in the train duration and currents used, frequency thresholds were transformed to charge values to better compare the total amount of stimulation delivered across animals. A mean charge value was calculated for each rat by using the formula:
where Q is the charge in µC, I is the current in µA, N is the required number of pulses in the stimulation train, and d is the pulse duration in s.23 These data were analyzed in the same manner as those pertaining to the rate and frequency thresholds described earlier.
Each cluster of swimming behavior (active, passive, or other) was analyzed in a mixed 3-factor ANOVA design with group and stock as the independent factors and day as the repeated factor. The Hundt–Felt correction for violation of the assumption of sphericity was applied to repeated factors with more than 2 levels49 as an adjustment to the degrees of freedom.
Results
Study 1.
Weight.
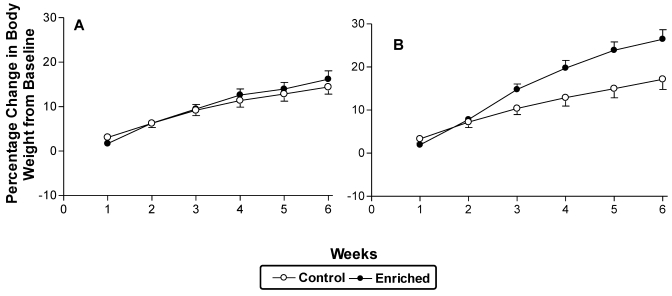
Panels A and B of Figure 3 show the percentage change in body weight from baseline in study 1. The 6-wk EE treatment was associated with a significant (F2,41 = 7.87; P < 0.01) interaction between stock and time, with Long Evans and Sprague–Dawley rats showing 18% and 12% increases in body weight, respectively, over the entire treatment phase. The treatment×time interaction was due to a difference (F2,41 = 3.67; P < 0.05) in the rate of weight gain (enriched, 15%; control, 10%) between enriched and control rats over the course of the 6-wk study period. Stock was not a significant factor in the interaction. Differences in baseline weights between groups of individual stocks were not significant.
Figure 3.
Percentage change in body weight (mean ± SEM) over the course of 6 wk of treatment. (A) Sprague–Dawley rats. (B) Long Evans rats. Six weeks of enrichment gave rise to significant (P < 0.05) strain × time and treatment × time interactions.
Corticosterone.
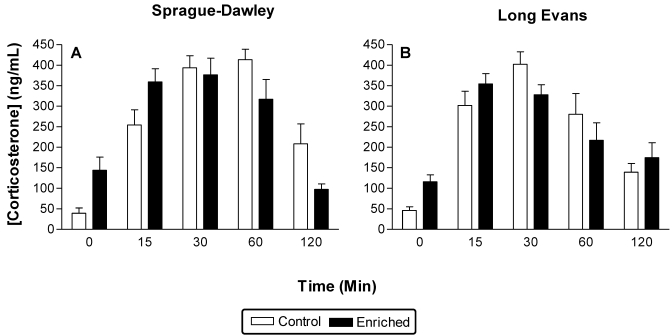
Figure 4 displays the corticosterone levels in each group before (time 0) and after (time 15 to 120) restraint. Significant (F1,28 = 19.44; P = 0.001) differences were found at time 0, that is, before the restraint stressor was applied, after 6 wk of treatment. No other baseline differences were observed. The quadratic trend analyses conducted on all time points (0 to 120 min) separately for stock were significant (P < 0.001 in all cases).
Figure 4.
Absolute plasma corticosterone levels (mean ± SEM) that were obtained in response to enriched or standard housing conditions. Data were collected immediately after enrichment (time 0) and 15 to 120 min after brief restraint. Both strains of rats demonstrated group differences at time 0 after 6 wk of enrichment. After restraint, significant time and time×treatment effects were found.
Two main patterns were characteristic after restraint. First, the baseline corticosterone levels in enriched Sprague–Dawley and Long Evans groups are elevated relative to their control groups. Second, corticosterone values in the EE groups return to baseline levels more quickly than in the associated control groups. For example, hormone levels in both Sprague–Dawley and Long Evans control groups at 2 h after restraint are still 3 to 4 times higher than before restraint. In contrast, for the enriched Sprague–Dawley groups, values at 120 min after restraint are about 30% below that of baseline, and for enriched Long Evans groups, roughly 50% above baseline.
Analysis on these data based on all time points revealed significant effects of time (F4, 80 = 47.3; P < 0.001) and time×treatment (F4,80 = 6.8; P = 0.00009). The time × treatment effect developed because enriched groups, collapsed across stock, showed a more rapid and earlier peak (15 to 30 min) in corticosterone than did the comparable control group (peak at 30 to 60 min). Planned pair-wise comparisons on baseline data were significant in both stocks (Sprague–Dawley, P = 0.0008; Long Evans, P = 0.02). Similarly, the comparison between time 0 and time 120 min after restraint was significant in Sprague–Dawley control animals (P = 0.002).
Organ weights.
Enrichment and stock did not significantly affect adrenal gland weights. Adrenal gland weights (mean ± SEM) were: control Sprague–Dawley, 0.048 ± 0.002g; EE Sprague–Dawley, 0.048 ± 0.003 g; control Long Evans, 0.047 ± 0.003 g; and EE Long Evans, 0.049 ± 0.001 g.
Sucrose consumption.
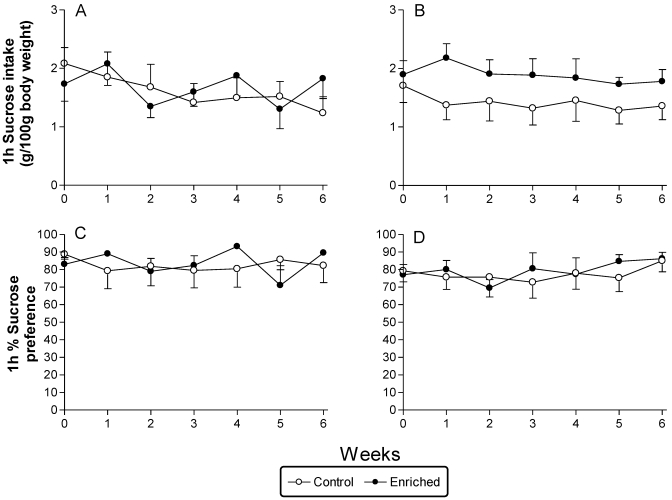
Sucrose intake and preference data are depicted in Figure 5. No overall effects of treatment were found for either of the 2 measures, with or without body weight included as a covariate.
Figure 5.
Sucrose intake and preference data (mean ± SEM) associated with 6-wk EE. (A and C) Sprague–Dawley rats. (B and D) Long Evans rats.
Study 2.
Brain-stimulation reward.
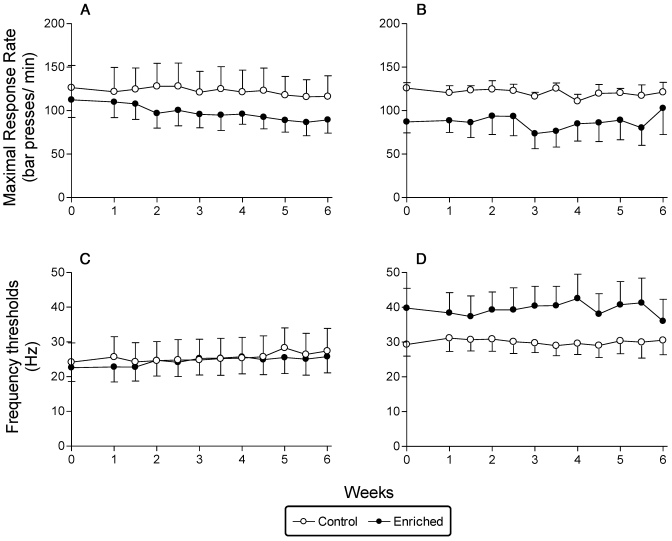
Histologic analyses verified that all electrode tips were positioned in the medial forebrain bundle, with most tips in or near the ventral tegmental area.38 EE had no effect on maximal response rates (Figure 6 A), frequency threshold data (Figure 6 B), or charge values.
Figure 6.
(A, B) Average maximal response rates (bar presses per min) for (A) Sprague–Dawley and (B) Long Evans rats obtained during the 6 wk of EE. (C, D) Mean (± SEM) frequency threshold per group; (C) Sprague–Dawley rats. (D) Long Evans rats.
FST.
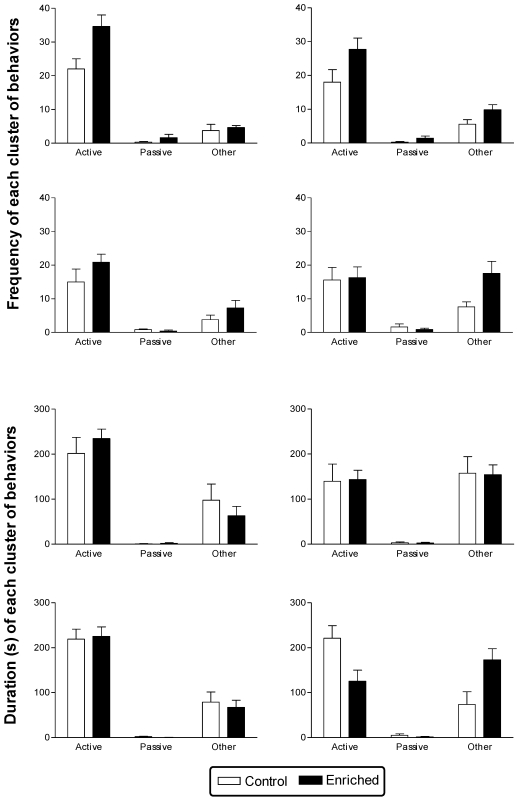
Data from the FST after 6 wk of EE are shown in Figure 7. Analysis of the frequency associated with each behavioral cluster indicates overall that Sprague–Dawley rats were more active than Long Evans rats (F1,22 = 9.53; P < 0.01). Enriched animals showed a greater increase (131%) in the frequency of other behaviors from the first to the second test day as compared with control rats (71%; F1,22 = 6.74; P < 0.05). This difference was evident for both swimming without struggling (F1,22 = 6.22; P < 0.05) and grooming (F1,22 = 4.78; P < 0.05); frequency of grooming was higher (F1,22 = 4.36; P < 0.05) overall in EE rats than the controls. Grooming frequency was higher (F1,22 = 5.14; P < 0.05) on the second day versus the first day predominantly in the Long Evans animals, with an overall difference in grooming between stocks; values from Long Evans rats greater than those of Sprague–Dawley (F1,22 = 10.51; P < 0.005). The frequency of passive behaviors was unaltered by treatment.
Figure 7.
Performance (mean ± SEM) of the enriched and control Sprague–Dawley and Long Evans rats in FST. The behaviors were classified as active, passive, or other (see Figure 2 for descriptions). Frequency is plotted in the top 2 rows and duration in the bottom 2 rows, with Sprague–Dawley data above the Long Evans data. FST were conducted on 2 consecutive days (day 1, left; day 2, right). In the frequency data, strain-associated differences were found in each behavioral cluster and a treatment×day interaction in the analysis of other behaviors. Duration data showed a significant effect of treatment in active and other behaviors and a day×treatment interaction in other behaviors.
Analysis of duration (Figure 7) revealed that the decrease in the overall duration of active behaviors was greater (39%) in the EE group than in the control group (17%; F1,22 = 7.02; P < 0.05). This pattern was reversed in the cluster of other behaviors, with the time spent performing other behaviors on the second day of testing dramatically increased in EE rats (F1,22 = 8.28; P < 0.01): enriched rats showed a 151% increase compared with 36% for control animals. Further analysis of each behavior in the other-behavior cluster revealed this pattern in the swimming without struggling behavior (F1,22 = 8.23; P < 0.01) whereas duration of grooming was unaffected by EE but was higher (F1,22 = 12.85; P < 0.005) on day 2 in the Long Evans rats only. The time spent engaged in passive behaviors was unchanged. This overall pattern was most evident in the Long Evans rats.
Discussion
The purpose of these studies was to evaluate the effects of EE on several indices—rate of weight gain, adrenal gland weight, and plasma corticosterone levels after enrichment and in response to an acute stressor, sucrose intake and preference, and brain-stimulation reward and FST behaviors—in Long Evans and Sprague–Dawley rats. The 6-wk enrichment period was associated with an increased rate of body weight gain, higher basal plasma corticosterone levels, and a faster recovery (return to baseline levels) of corticosterone after stress (brief restraint). As compared with control rats, EE rats showed less struggling behavior on the second day of FST.
The effects of EE on physiologic and biochemical indices appear to be generally inconsistent in the literature. For example, group-housed Wistar rats and isolated controls showed no difference in weight gain,54 whereas physical and social enrichment reduced weight gain in Sprague–Dawley rats.39 In mice, either no difference5,24 or an increase45 in body weight relative to control animals has been reported. Similarly, corticosterone levels have been reported as unchanged, elevated, or reduced as a result of EE.5,17,31,32 Some of these differences may be related to the specific EE protocol or the strain or stock used. In our hands, the differences between standard-housed and enriched animals were only evident after at least the third week of enrichment (Figure 3).
EE also increased baseline (time 0) plasma corticosterone levels in both stocks of rats (Figure 4). Their biochemical responses to acute restraint also differed from those of the control groups, in that the levels in enriched rats returned to baseline values more quickly than those of the control groups. Peak corticosterone levels were similar between EE and control animals; therefore, the higher baseline levels in EE animals suggests a quicker return to baseline levels in this group after acute restraint stress.
Sucrose consumption, brain-stimulation reward, and FST behaviors were measured in separate 6-wk EE groups. The FST typically is used as a model for screening antidepressants, but the procedure itself is a powerful stressor.13 A few studies have reported that , enrichment does not affect mobility and immobility times in the FST.14,27 However, assessing additional behaviors30 revealed that enrichment increases the duration of swimming (fast and slow) and diving, with an accompanying reduction in the time spent immobile or struggling.
The present study assessed the effects of enrichment on 7 FST behaviors (Figure 2), but for ease of analysis and comparison with data in the literature, these were grouped into 3 categories. EE decreased the time spent in active behaviors from the first to second day of forced swim relative to that of control animals (Figure 5). Active behaviors were replaced by either swimming without struggling or grooming on the second day, that is, an increase in the duration and frequency of other behaviors in enriched animals. Enrichment did not affect passive behaviors, which are most commonly used to assess changes in the FST. Although our shorter pretest of 5 min may account for this difference, other authors1 report no behavioral differences between 5-, 10-, and 15-min tests. In addition, our group previously validated the length of the 5-min trial as adequate for identifying changes in swim behaviors.7 Taken together with the physiologic and biochemical data, we suggest that the EE experience provided the animals with the ability to better habituate to a different environment.
With regard to the effect of enrichment on reward-related behavior, we found no difference in any of the assessed brain-stimulation reward measures (Figure 6) or in the sucrose consumption or preference results between enriched and isolated control animals (Figure 5). Other authors20 previously reported increased preference for and volume of saccharin intake after EE, although only in rats with low saccharin preference. Related paradigms investigating reward anticipation also show an effect of enrichment. For example, group-housed animals show a higher incentive value for sucrose52,54 and social contact.51 Conversely, compared with their control counterparts, socially housed animals that are also exposed to physical enrichment demonstrate a lower anticipatory response for a sucrose reward.53 Others have shown recently that animals reared in EE are less sensitive to the rewarding effects of heroin, as evidenced by a reduction in conditioned place preference in these animals, whereas behavioral sensitization to heroin is unaffected by long-term EE.18 Overall, EE in adult animals appears to alter their sensitivity to rewards, although the sucrose test may not be the most sensitive marker in this context and brain-stimulation reward thresholds may be too robust to be altered by manipulations other than drug challenges. In the chronic mild stress paradigm, many investigators have failed to observe significant changes in the reward indices of brain-stimulation reward and intake or preference for a weakly sweet solution.2,7,15,28,37,47
Ideally, behavioral assessment of rodents should be conducted during their active phase; however, in most studies, including those that evaluate the effects of enrichment, these assessments are performed during the light hours, or resting phase of the animals.12,18,32,40 All laboratory animals are subjected to the normal day-to-day activity that occurs in a vivarium, and these disturbances may alter the circadian rhythm of corticosterone secretion, among other effects. However, the control group, our reference condition, is exposed to the same influences. Although the absolute values associated with the various measures would have likely been different had the study been conducted during the dark (or active) phase of the rats, it is difficult to ascertain whether the proportional differences between groups would also have been affected.
Because environmental enrichment has varied effects on physiologic, biochemical, and behavioral measures, one questions how these differences can be reconciled in order to make the best use of this paradigm. We and others find elevated plasma glucocorticoids in enriched animals.5,31,32 However, this may not necessarily reflect a negative condition. Stress has been defined as a nonspecific response by the organism to a significant disturbance.48 In other words, the same physiologic reactions are initiated by a variety of physical and psychologic stimuli, whether they are of a positive (eustress) or negative (distress) nature. Therefore, taken together with the increased rate of body weight gain and lack of change in adrenal gland weight, one could interpret the biochemical patterns we observed as a positive condition, given that this increase in glucocorticoids may allow the organism to better adapt to new situations. In our hands, EE rats readily entered the enrichment cage, appeared calm, and did not display the avoidance to handling that is typical of stressed rats,7,8,28 suggesting that the enrichment experience was a positive one. However, to assess the effects of enrichment more comprehensively, future studies by our group will perform preference tests of standard and enriched rats and carefully qualify group dynamics (for example, dominant–subordinate hierarchy) both before and during enrichment.
In conclusion, enrichment experience in adulthood has measurable effects on several physiologic and behavioral indices in rats. The chronic novelty and accrued sensory and cognitive experiences associated with an enrichment paradigm may allow animals to be more resilient in the face of subsequent challenges. Therefore, rearing animals in an enriched environment rather than isolation might be a more appropriate basal condition on which to assess the effects of subsequent manipulations, particularly when trying to model human psychopathologies in animals. Ethical concerns alone suggest that standard housing conditions should include social and physical enrichment; empirical evidence indicates that the social and physical environment has a significant influence on research results.
Acknowledgments
This project was funded by the Natural Science and Engineering Research Council of Canada. In addition, we thank Ms Ayalah Hutchins and Ms Sylvie Emond for their technical assistance.
References
- 1.Armario A, Gavalda A, Marti O. 1988. Forced swimming test in rats: effects of desipramine administration and the period of exposure to the test on struggling behavior, swimming, immobility, and defecation rate. Eur J Pharmacol 158:207–212 [DOI] [PubMed] [Google Scholar]
- 2.Baker SL, Kentner AC, Konkle ATM, Santa-Maria Barbagallo L, Bielajew C. 2006. Behavioral and physiological effects of chronic mild stress in female rats. Physiol Behav 87:314–322 [DOI] [PubMed] [Google Scholar]
- 3.Bean K, Nemelka K, Canchola P, Hacker S, Sturdivant RX, Rico PJ. 2008. Effects of housing density on Long Evans and Fischer 344 rats. Lab Anim (NY) 37:421–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belz EE, Kennell JS, Czambel K, Rubin RT, Rhodes ME. 2003. Environmental enrichment lowers stress-responsive hormones in singly housed male and female rats. Pharmacol Biochem Behav 76:481–486 [DOI] [PubMed] [Google Scholar]
- 5.Benaroya-Milshtein N, Hollander N, Apter A, Kukulansky T, Raz N, Wilf A, Yaniv I, Pick CG. 2004. Environmental enrichment in mice decreases anxiety, attenuates stress responses, and enhances natural killer cell activity. Eur J Neurosci 20:1341–1347 [DOI] [PubMed] [Google Scholar]
- 6.Bhatnagar S, Dallman M. 1998. Neuroanatomical basis for facilitation of hypothalamic–pituitary–adrenal responses to a novel stressor after chronic stress. Neuroscience 84:1025–1039 [DOI] [PubMed] [Google Scholar]
- 7.Bielajew C, Konkle ATM, Kentner AC, Baker SL, Stewart A, Hutchins AA, Santa-Maria Barbagallo L, Fouriezos G. 2003. Strain- and gender-specific effects in the forced swim test: effects of previous stress exposure. Stress 6:269–280 [DOI] [PubMed] [Google Scholar]
- 8.Bielajew C, Konkle ATM, Merali Z. 2002. The effects of chronic mild stress on male Sprague–Dawley and Long Evans rats: I. Biochemical and physiological analyses. Behav Brain Res 136:583–592 [DOI] [PubMed] [Google Scholar]
- 9.Brenes Sáenz JC, Villagra OR, Fornaguera Trías J. 2006. Factor analysis of forced swimming test, sucrose preference test, and open-field test on enriched, social, and isolated reared rats. Behav Brain Res 169:57–65 [DOI] [PubMed] [Google Scholar]
- 10.Burton CL, Chatterjee D, Chatterjee-Chakraborty M, Lovic V, Grella SL, Steiner M, Fleming AS. 2007. Prenatal restraint stress and motherless rearing disrupts expression of plasticity markers and stress-induced corticosterone release in adult female Sprague–Dawley rats. Brain Res 1158:28–38 [DOI] [PubMed] [Google Scholar]
- 11.Canadian Council on Animal Care 1993. Guide to the care and use of experimental animals, vol 1, 2nd ed. Ottawa (Canada): Canadian Council on Animal Care [Google Scholar]
- 12.Chauvet C, Lardeux V, Golrdberg SR, Jaber M, Solinas M. 2009. Environmental enrichment reduces cocaine seeking and reinstatement induced by cues and stress but not by cocaine. Neuropsychopharmacology 34:2767–2778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connor TJ, Kelly JP, Leonard BE. 1997. Forced swim test-induced neurochemical, endocrine, and immune changes in the rat. Pharmacol Biochem Behav 58:961–967 [DOI] [PubMed] [Google Scholar]
- 14.Cui M, Yang Y, Yang J, Zhang J, Han H, Ma W, Li H, Mao R, Xu L, Hao W, Cao J. 2006. Enriched environment experience overcomes the memory deficits and depressive-like behavior induced by early life stress. Neurosci Lett 404:208–212 [DOI] [PubMed] [Google Scholar]
- 15.D'Aquila PS, Newton J, Willner P. 1997. Dirunal variation in the effect of chronic mild stress on sucrose intake and preference. Physiol Behav 62:421–426 [DOI] [PubMed] [Google Scholar]
- 16.Dhabhar FS, McEwen BS, Spencer RL. 1997. Adaptation to prolonged or repeated stress-comparison between rat strains showing intrinsic differences in reactivity to acute stress. Neuroendocrinology 65:360–368 [DOI] [PubMed] [Google Scholar]
- 17.Dronjak S, Gavrilovic L, Filipovic D, Radojcic MB. 2004. Immobilization and cold stress affect sympathoadrenomedullary system and pituitary–adrenocortical axis of rats exposed to long-term isolation and crowding. Physiol Behav 81:409–415 [DOI] [PubMed] [Google Scholar]
- 18.El Rawas R, Thiriet N, Lardeaux V, Jaber M, Solinas M. 2009. Environmental enrichment decreases the rewarding but not the activating effects of heroin. Psychopharmacology (Berl) 203:561–570 [DOI] [PubMed] [Google Scholar]
- 19.Elizalde N, Gil-Bea FJ, Ramírez MJ, Aisa B, Lasheras B, Del Rio J, Tordera RM. 2008. Long-lasting behavioral effects and recognition memory deficit induced by chronic mild stress in mice: effect of antidepressant treatment. Psychopharmacology (Berl) 199:1–14 [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Teruel A, Driscoll P, Gil L, Aguilar R, Tobena A, Escorihuela RM. 2002. Enduring effects of environmental enrichment on novelty seeking and saccharin and ethanol intake in two rat lines (RHA/Verh and RLA/Verh) differing in incentive-seeking behavior. Pharmacol Biochem Behav 73:225–231 [DOI] [PubMed] [Google Scholar]
- 21.Field KJ, Sibold AL. 1999. The laboratory hamster and gerbil. Boca Raton (FL): CRC Press [Google Scholar]
- 22.Francis DD, Diorio J, Plotsky PM, Meaney J. 2002. Environmental enrichment reverses the effects of maternal separation on stress reactivity. J Neurosci 22:7840–7843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallistel CR. 1978. Self-stimulation in the rat: quantitative characteristics of the reward pathway. J Comp Physiol Psychol 92:977–998 [DOI] [PubMed] [Google Scholar]
- 24.Haemisch A, Voss T, Gartner K. 1994. Effects of environmental enrichment on aggressive behavior, dominance hierarchies, and endocrine states in male DBA/2J mice. Physiol Behav 56:1041–1048 [DOI] [PubMed] [Google Scholar]
- 25.Harris RBS, Zhou J, Youngblood BD, Smagin GN, Ryan DH. 1997. Failure to change exploration or saccharin preference in rats exposed to chronic mild stress. Physiol Behav 63:91–100 [DOI] [PubMed] [Google Scholar]
- 26.Jans LA, Blokland A. 2008. Influence of chronic mild stress on the behavioural effects of acute tryptophan depletion induced by a gelatin-based mixture. Behav Pharmacol 19:706–715 [DOI] [PubMed] [Google Scholar]
- 27.Koh S, Magid R, Chung H, Stine CD, Wilson DN. 2007. Depressive behavior and selective downregulation of serotonin receptor expression after early-life seizures: reversal by environmental enrichment. Epilepsy Behav 10:26–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Konkle ATM, Baker SL, Kentner AC, Barbagallo LS, Merali Z, Bielajew C. 2003. Evaluation of the effects of chronic mild stressors on hedonic and physiological responses: sex and strain compared. Brain Res 992:227–238 [DOI] [PubMed] [Google Scholar]
- 29.Kubera M, Basta-Kaim A, Holan V, Simbirtsev A, Roman A, Pigareva N, Prokopieva E, Sham J. 1998. Effect of mild chronic stress, as a model of depression, on the immunoreactivity of C57BL/6 mice. Int J Immunopharmacol 20:781–789 [DOI] [PubMed] [Google Scholar]
- 30.Magalhaes A, Summavielle T, Tavares MA, de Sousa L. 2004. Effects of postnatal cocaine exposure and environmental enrichment on rat behavior in a forced swim test. Ann N Y Acad Sci 1025:619–629 [DOI] [PubMed] [Google Scholar]
- 31.Marashi V, Barnekow A, Ossendorf E, Sachser N. 2003. Effects of different forms of environmental enrichment on behavioral, endocrinological, and immunological parameters in male mice. Horm Behav 43:281–292 [DOI] [PubMed] [Google Scholar]
- 32.Moncek F, Duncko B, Johansson B, Zezova D. 2004. Effect of environmental enrichment on stress related systems in rats. J Neuroendocrinol 16:423–431 [DOI] [PubMed] [Google Scholar]
- 33.Morley-Fletcher S, Rea M, Maccari S, Laviola G. 2003. Environmental enrichment during adolescence reverses the effects of prenatal stress on play behaviour and HPA axis reactivity in rats. Eur J Neurosci 18:3367–3374 [DOI] [PubMed] [Google Scholar]
- 34.Moreau JL, Schershlicht R, Jenck F, Martin JR. 1995. Chronic mild stress-induced anhedonia model of depression: sleep abnormalities and curative effects of electroshock treatment. Behav Pharmacol 6:682–687 [PubMed] [Google Scholar]
- 35.Mundl WJ. 1980. A constant-current amplifier. Physiol Behav 24:991–993 [DOI] [PubMed] [Google Scholar]
- 36.Muscat R, Towell A, Willner P. 1988. Changes in dopamine autoreceptor sensitivity in an animal model of depression. Psychopharmacology (Berl) 94:545–550 [DOI] [PubMed] [Google Scholar]
- 37.Nielsen CK, Arnt J, Sanchez C. 2000. Intracranial self-stimulation and sucrose intake differ as hedonic measures following chronic mild stress: interstrain and interindividual differences. Behav Brain Res 107:21–33 [DOI] [PubMed] [Google Scholar]
- 38.Paxinos G, Watson C. 1998. The rat brain in stereotaxic coordinates, 3rd ed. New York (NY): Academic Press [Google Scholar]
- 39.Pena Y, Prunell M, Dimitsantos V, Nadal R, Escorihuela RM. 2006. Environmental enrichment effects in social investigation in rats are gender dependent. Behav Brain Res 174:181–187 [DOI] [PubMed] [Google Scholar]
- 40.Pena Y, Prunell M, Rotllant D, Armario A, Escorihuela RM. 2009. Enduring effects of environmental enrichment from weaning to adulthood on pituitary–adrenal function, prepulse inhibition, and learning in male and female rats. Psychoneuroendocrinology 34:1390–1404 [DOI] [PubMed] [Google Scholar]
- 41.Porsolt RD, Anton G, Blavet N, Jalfer M. 1978. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol 47:379–391 [DOI] [PubMed] [Google Scholar]
- 42.Porsolt RD, Bertin A, Jalfre M. 1977. Behavioural despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229:327–336 [PubMed] [Google Scholar]
- 43.Porsolt RD, Bertin A, Jalfre M. 1978. Behavioural despair in rats and mice: strain differences and the effects of imipramine. Eur J Pharmacol 51:291–294 [DOI] [PubMed] [Google Scholar]
- 44.Porsolt RD, Le Pinchon M, Jalfre M. 1977. Depression: a new animal model sensitive to antidepressant treatments. Nature 266:730–732 [DOI] [PubMed] [Google Scholar]
- 45.Roy V, Belzung C, Delarue C, Chapillon P. 2001. Environmental enrichment in BALB/c mice: effects in classical tests of anxiety and exposure to a predatory odor. Physiol Behav 74:313–320 [DOI] [PubMed] [Google Scholar]
- 46.Schrijver NCA, Bahr NI, Weiss IC, Wurbel H. 2002. Dissociable effects of isolation rearing and environmental enrichment on exploration, spatial learning and HPA activity in adult rats. Pharmacol Biochem Behav 73:209–224 [DOI] [PubMed] [Google Scholar]
- 47.Schweizer MC, Henniger MSH, Sillaber I. 2009. Chronic mild stress (CMS) in mice: of anhedonia, “anomalous anxiolysis” and activity. PLoS One 4:e4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Selye H. 1956. The stress of life. New York (NY): McGraw-Hill Book Company [Google Scholar]
- 49.Tabachnick BG, Fidell LS. 2006. Experimental designs using ANOVA. Florence (KY): Cengage Learning [Google Scholar]
- 50.Trzctńska M, Tonkiss J, Galler JR. 1999. Influence of prenatal protein malnutrition on behavioral reactivity to stress in adult rats. Stress 3:71–83 [DOI] [PubMed] [Google Scholar]
- 51.van den Berg CL, Hol T, Van Ree JM, Spruijt BM, Everts H, Koolhaas JM. 1999. Play is indispensable for an adequate development of coping with social challenges in the rat. Dev Psychobiol 34:129–138 [PubMed] [Google Scholar]
- 52.van den Berg CL, Pijlman FT, Koning HA, Diergaarde L, Van Ree JM, Spruijt BM. 1999. Isolation changes the incentive value of sucrose and social behaviour in juvenile and adult rats. Behav Brain Res 106:133–142 [DOI] [PubMed] [Google Scholar]
- 53.van der Harst JE, Baars AM, Spruijt BM. 2003. Standard housed rats are more sensitive to rewards than enriched housed rats as reflected by their anticipatory behaviour. Behav Brain Res 142:151–156 [DOI] [PubMed] [Google Scholar]
- 54.von Frijtag JC, Reijmers LG, Van der Harst JE, Leus IE, Van den Bos R, Spruijt BM. 2000. Defeat followed by individual housing results in long-term impaired reward- and cognition-related behaviours in rats. Behav Brain Res 117:137–146 [DOI] [PubMed] [Google Scholar]
- 55.Willner P. 2005. Chronic mild stress (CMS) revisited: consistency and behavioural–neurobiological concordance in the effects of CMS. Neuropsychobiology 52:90–110 [DOI] [PubMed] [Google Scholar]
- 56.Willner P, Lappas S, Cheeta S, Muscat R. 1994. Reversal of stress-induced anhedonia by the dopamine receptor agonist pramipexole. Psychopharmacology 115:454–462 [DOI] [PubMed] [Google Scholar]
- 57.Worthman CM, Stallings JF. 1997. Hormone measures in finger-prick blood spot samples: new field methods for reproductive endocrinology. Am J Phys Anthropol 104:1–21 [DOI] [PubMed] [Google Scholar]
- 58.Zhu ZH, Wang BR, Tan QR, Duan XL, Kuang F, Xu Z, Ju G. 2006. Effects of water restrictions on the physiological parameters, psychological behavior, and brain c-Fos expression in rats. Neurosci Bull 22:144–150 [PubMed] [Google Scholar]