Abstract
Staphylococcus xylosus typically is described as a nonpathogenic common inhabitant of rodent skin. Reports of S. xylosus as a primary pathogen in human and veterinary medicine are scarce. Here we report 37 cases, affecting 12 strains of laboratory mice, of spontaneous infections in which S. xylosus was isolated and considered to be the primary pathogen contributing to the death or need for euthanasia of the animal. Infection with S. xylosus was the major cause of death or euthanasia in 3 strains of mice deficient in the production of phagocyte superoxide due to defects in NADPH oxidase. NADPH-oxidase–deficient mice (n = 21) were most susceptible to spontaneous S. xylosus infections. The infections were characterized by abscesses and granulomas in soft tissues, with bacterial migration to internal organs (primarily regional lymph nodes and lungs and, to a lesser degree, muscle, bone, and meninges). In contrast, 9 strains of phagocyte-superoxide–producing mice (n = 16) also had S. xylosus infections, but these were largely confined to eyelids, ocular conjunctiva, and skin and rarely involved other tissues or organs. Because exhaustive bacterial culture and isolation may not be performed routinely from mouse abscesses, S. xylosus infections may be underdiagnosed. S. xylosus should be considered in the differential diagnosis in laboratory mice with abscesses and other skin lesions. This report expands the range of mouse strains and tissues and organs susceptible to spontaneous S. xylosus infection and compares the pathology among various mice strains.
Abbreviations: CGD, chronic granulomatous disease; KO, knockout
Staphylococcus xylosus is a coagulase-negative nonmotile gram-positive coccus belonging to the Staphylococcus saprophyticus group. This bacterial group is ubiquitous in nature, persisting in soils and on surfaces.22 S. xylosus is a common commensal bacterium that generally is found inhabiting the skin and mucous membranes of a variety of mammals and occasionally humans.23 It probably colonizes the skin and surfaces by forming a biofilm.25 Although S. xylosus is considered a nonpathogenic Staphylococcus and commonly is used as a starter culture for meat products6, several reports describe opportunistic infections in animals3,4,8,13,21 and humans.7,16,36
A primary defense mechanism of phagocytic cells is superoxide, which is produced by the enzyme complex NADPH oxidase.2 Activation of the phagocyte NADPH oxidase requires coordinated assembly of 4 structural proteins: the membrane-bound flavocytochrome b558, composed of a heavy chain (gp91phox) and a light chain (p22phox), and 3 cytosolic proteins, p47phox, p67phox, and the GTP-binding regulatory protein rac.2, 27, 28, 29, 31 Defects of some NADPH oxidase components in humans results in a group of genetic disorders, known collectively as chronic granulomatous disease (CGD), that are manifested clinically by recurrent bacterial and fungal infections and tissue granuloma formation.29 Superoxide production also is thought to be involved in other diseases, including Parkinson disease, cancer, inflammatory bowel disease, hypertension, cataracts, and multiple sclerosis.17, 18, 20, 34, 35 In addition, patients with CGD are known to have excessive inflammatory responses manifesting as granulomatous enteritis, genitourinary obstruction, and cutaneous lesions.15
Several strains of mice have been genetically altered not to express the genes involved in the production of superoxide (called knockout [KO] mice).12, 26 The p47phox KO and gp91phox KO mice have defects in NADPH oxidase that cause the animals to develop a disease similar to human CGD, manifesting as an increased susceptibility to infection with bacteria and fungi and making them excellent models for the study of CGD pathogenesis and therapy.3, 12, 19, 22, 26
In the current report, we describe spontaneous infections with Staphylococcus xylosus as the main cause of morbidity and mortality in a specific pathogen-free phagocyte superoxide-deficient mouse colony model of CGD. In addition, we report spontaneous S. xylosus infections in 9 strains of mice and compare the lesions with those observed in CGD mice, which include meningitis, bone lysis, and myositis. Management practices were modified to prevent additional cases of S. xylosus infection in our CGD mice colony.
Materials and Methods
The colony comprised 3 different mouse models of CGD: B6.129S6-Cybbtm1Din/J (gp91phoxKO),26 B6.129S2-Ncf1tm1shlN14, and B6p47phox[KO]HLL (both p47phox KO),12,30 all of which lacked phagocyte superoxide production. In addition, other colonies comprised of different strains, transgenics, and knockout mice, all of which have normal production of phagocyte superoxide, were maintained and bred in the same facility. Other strains affected were: AKR/J-Lvif, which carries a segment of chromosome 10 with a null allele of a gene that inactivates some murine leukemia viruses; B6;129-Adora2atm1Dyj/J, adenosine deaminase-deficient mice causing severe combined immunodeficiency accompanied by T-cell depletion and accumulation of both intracellular and extracellular adenosine and deoxyadenosine; AKR/J-Xpr1Sxv , which carries a wild-mouse version of a chromosome 1 locus that encodes the receptor for xenotropic–polytropic murine leukemia viruses; C57BL/6NTac-[KO]FPR, with defective neutrophil chemotaxis; C57BL/6NTac-[KO]P50NF-kB-[KO]P52NF-kB, a double-knockout mouse that lacks nuclear factor proteins, causing complete block of maturation of B cells in the spleen and partial block of B cell development in bone marrow; C57BL/6J-[KO]RAG1 N10, which lacks recombination-activating gene 1 (Rag1), which controls recombination of immunoglobulin and T-cell receptor genes (as a consequence of this defect, these mice have no mature T or B cells); NOD.Cg-Prkdcscid Il2rgtm1Sug/JicTac, which mice are severe combined immunodeficient with dysfunctional cytokine production and incompetence of T, B, and NK cells; NFS/N-Xpr1Sxv Rmcf2+, which carry an endogenous retrovirus on chromosome 18 originally identified in the Asian species M. castaneus; and BALB/c, which are immunocompetent.
The mice were housed in an AAALAC-accredited animal facility (National Institute of Allergy and Infectious Diseases, Bethesda, MD). The mice were individually or group-housed in sterile ventilated microisolation caging (Thoren Caging Systems, Hazleton, PA) with autoclaved hardwood bedding (SaniChip, Harlan Teklad, Madison, WI) and food (Rodent NIH31 Autoclavable NA, Zeigler Brothers, Gardners, PA) and acidified water provided ad libitum. All mice were maintained in the same animal facility but not necessarily in the same animal room; however, health status was the same for all mice in this study. Colony sentinels were tested quarterly and were free of the following agents: mouse hepatitis virus, pneumonia virus of mice, Sendai virus, Theiler murine encephalomyelitis virus, mouse rotavirus, lymphocytic choriomeningitis virus, ectromelia virus, mouse cytomegalovirus, minute virus of mice, polyoma virus, reovirus 3, mouse adenovirus, rodent parvoviruses, Mycoplasma pulmonis, and cilia-associated respiratory bacillus. Hantavirus testing was performed once a year. Endoparasite and ectoparasite examination was performed every 6 wk in sentinel animals and throughout the year on culled research animals. Mouse norovirus and Helicobacter spp. were not excluded from the animal colony; therefore, all mice were considered potentially infected with these agents. When tested, sentinel mice were routinely positive to mouse norovirus, but individual experimental mice were not tested. Necropsies of clinical cases were performed as part of the routine colony health surveillance program.
The mouse colony was maintained as part of several experimental protocols approved by the institutional animal care and use committee. All procedures and use of animals were in accordance with the Guide for the Care and Use of Laboratory Animals.10 Sick animals were euthanized with CO2 overdose according to the American Veterinary Medical Association guidelines on rodent euthanasia.1 Complete necropsies were performed immediately after euthanasia, and tissue samples from lesions and all major organs were collected and fixed in 10% neutral-buffered formalin, embedded in paraffin, sectioned at 5 µm, and stained with hematoxylin and eosin for light microscopy. In addition, samples taken from lesions and exudates were submitted (Microbiology Laboratory, NIH Division of Veterinary Resources, Bethesda, MD) for culture, isolation, and sensitivity tests.
Results
From November 2005 to July 2008, a total of 37 mice representing 12 strains were found to be infected with S. xylosus (Table 1), which was considered to be the primary pathogen contributing to the death or euthanasia of the animal. Briefly, 25 male and 12 female mice were affected. Among the 1190 phagocyte-superoxide–deficient mice in the colony, 21 (1.76%) had abscesses affecting soft tissues or cervical lymph nodes (n = 19; 90.5%) and lungs (n = 13; 61.9%), followed by meninges and bone (n = 4 each; 19.0%). Gross lesions were characterized by subcutaneous masses or swollen cervical lymph nodes containing purulent material and tan to gray masses (diameter, 1 to 2 mm) in lung parenchyma. Histologically, the masses were multiple, confluent microabscesses which sometimes were surrounded by a dense fibrous connective tissue, with large colonies of cocci in the center of the abscess (Figures 1 and 2). In 4 of 13 CGD mice, inflammatory nodules in the lung were granulomatous with primarily histiocytic cell infiltrates. The center of most of the abscesses had eosinophilic crystals or spicules occasionally admixed with basophilic round cocci (diameter, 1 to 1.5 µm;Figure 3). In animals in which the microabscesses affected bone, bone lysis and new bone formation were a result of inflammatory infiltrates extending from soft tissues (Figure 4). Histologically, 4 animals had granulomas with bacterial colonies in the meninges (Figure 5), and 5 of 21 (23.8%) CGD mice had ulcerative skin lesions with granulocytic infiltrate and fibrosis. In general, the incidence of S. xylosus infection was higher in B6p47phox[KO]HLL (8 of 98 males [8.16%] and 4 of 123 females [3.25%]) compared with B6.129S6-Cybbtm1Din/J (4 of 209 males [1.91%] and 2 of 162 females [1.23%]) and B6.129S2-Ncf1tm1shlN14 (1 of 288 males [0.34%] and 2 of 310 females [0.64%]) during the reporting period, and mostly breeders were affected.
Table 1.
Clinical, histologic, and microbiologic characteristics of necropsied mice
| Age (mo) | Sex | Strain | Lesion | Tissues or organs involved | Granuloma? | Microbiology |
| 4 | M | B6p47phox[KO]HLL | Abscess | Head, neck, brain, and lung | Yes | S. xylosus |
| 5 | M | B6p47phox[KO]HLL | Abscess | Cervical lymph nodes, mandible | No | S. xylosus, E. coli,Micrococcus sp. |
| 7 | M | B6p47phox[KO]HLL | Abscess | Lung | No | S. xylosus |
| 9 | M | B6p47phox[KO]HLL | Abscess | Cervical lymph nodes, maxilla, lung | No | S. xylosus |
| 10 | M | B6p47phox[KO]HLL | Abscess | Lymph nodes, lung, limb skin and bone | No | S. xylosus |
| 12 | M | B6p47phox[KO]HLL | Abscess | Lung | No | S. xylosus |
| 16 | M | B6p47phox[KO]HLL | Abscess | Cervical lymph nodes, mandible | No | S. xylosus |
| 6 | F | B6p47phox[KO]HLL | Abscess | Skin, lung, brain | No | S. xylosus |
| 7 | F | B6p47phox[KO]HLL | Abscess | Cervical lymph nodes, maxilla, lung | No | S. xylosus |
| 7 | F | B6p47phox[KO]HLL | Dermatitis | Muzzle skin | Yes | S. xylosus |
| 7 | F | B6p47phox[KO]HLL | Dermatitis | Muzzle skin | Yes | S. xylosus |
| 2 | M | B6.129S6-Cybbtm1Din/J | Abscess | Head, brain, lung | Yes | S. xylosus, Enterococcus fecalis,Micrococcus sp., Bacillus sp. |
| 8 | M | B6.129S6-Cybbtm1Din/J | Abscess | Muzzle, skull | Yes | S. xylosus |
| 12 | M | B6.129S6-Cybbtm1Din/J | Abscess | Maxilla, lymph node, lung | No | S. xylosus |
| 12 | M | B6.129S6-Cybbtm1Din/J | Abscess | Maxilla, lymph node, lung, brain | No | S. xylosus |
| 8 | F | B6.129S6-Cybbtm1Din/J | Abscess | Skull, leg, lymph node | Yes | S. xylosus |
| 19 | F | B6.129S6-Cybbtm1Din/J | Abscess | Neck, lung | Yes | S. xylosus |
| 7 | M | AKR/J-Lvif | Conjunctivitis, blepharitis, dermatitis | Eyelid and conjunctiva, perineum | No | S. xylosus,Corynebacterium pseudotuberculosis, Lactobacillus sp., Pasteurella sp. |
| 9 | M | AKR/J-Lvif | Conjunctivitis, blepharitis | Eyelid and conjunctiva | No | S. xylosus,S. epidermidis |
| 9 | M | AKR/J-Lvif | Conjunctivitis, blepharitis | Eyelid and conjunctiva | No | S. xylosus, S. sciuri,Enterococcus sp., Candida sp., Stenotrophomonas maltophilia |
| 12 | M | AKR/J-Lvif | Conjunctivitis, blepharitis | Eyelid and conjunctiva | No | S. xylosus |
| 12 | M | AKR/J-Lvif | Conjunctivitis, blepharitis | Eyelid and conjunctiva | No | S. xylosus |
| 6 | F | AKR/J-Lvif | Skin/mucosal ulcers | Perineum, anus, rectum, vagina | No | S. xylosus, E. coli,Enterococcus sp. |
| 21 | M | B6.129S2-Ncf1tm1shlN14 | Abscess | Lung, pelvic muscles | No | S. xylosus |
| 12 | F | B6.129S2-Ncf1tm1shlN14 | Abscess | Leg muscles and bone | No | S. xylosus |
| 19 | F | B6.129S2-Ncf1tm1shlN14 | Dermatitis | Skin | No | S. xylosus |
| 19 | M | B6;129-Adora2atm1Dyj/J | Conjunctivitis, blepharitis | Eyelid and conjunctiva | No | S. xylosus |
| 19 | M | B6;129-Adora2atm1Dyj/J | Conjunctivitis, blepharitis | Eyelid and conjunctiva | No | S. xylosus, Micrococcus sp. |
| 2 | M | BALB/c | Conjunctivitis, dermatitis | Eye conjunctiva, skin | No | S. xylosus, Aerococcus sp., Pasteurella sp. |
| 7 | M | BALB/c | Dermatitis | Skin | Yes | S. xylosus |
| 12 | M | NOD.Cg-Prkdcscid Il2rgtm1Sug/JicTac | Adenitis | Preputial gland | No | S. xylosus, Raoultella ornithinolytica |
| 1 | M | C57BL/6Ncr-[KO]P47phoxN14 | Abscess | Maxilla, lung | Yes | S. xylosus |
| 24 | M | NFS/N-Xpr1Sxv Rmcf2+ | Vesiculitis | Seminal vesicle, urethra | No | S. xylosus, E. coli,Enterobacter sp. |
| 1 | F | C57BL/6NTac-[KO]P50NF-kB-[KO]P52NF-kB | Dermatitis | Skin | No | S. xylosus, S. sciuri |
| 7 | F | C57BL/6J-[KO]RAG1 N10 | Dermatitis | Skin | No | S. xylosus |
| 7 | M | AKR/J-Xpr1Sxv | Conjunctivitis, blepharitis | Eyelid and conjunctiva | No | S. xylosus |
| 1 | F | C57BL/6NTac-[KO]FPR | Blepharitis, keratitis | Eyelid, cornea | No | S. xylosus |
M, male; F,female
Figure 1.
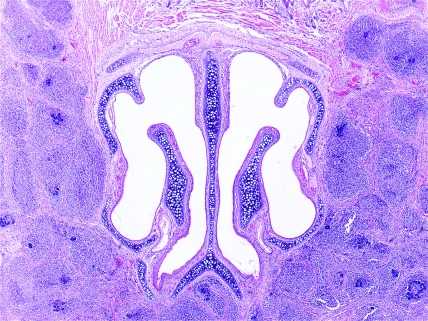
B6p47phox[KO]HLL. Phagocyte superoxide-deficient mouse, nose. Transverse section showing multiple microabscesses surrounded by fibrosis affecting the masseter muscles. Hematoxylin and eosin stain; magnification, ×40.
Figure 2.
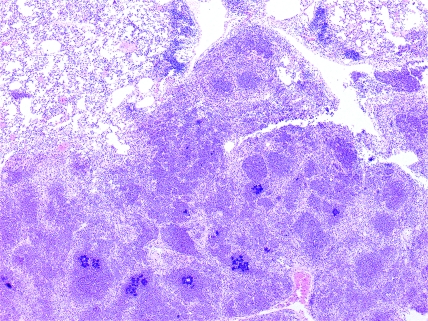
B6p47phox[KO]HLL. Phagocyte superoxide-deficient mouse, lung. Low-power photomicrograph showing multiple coalescing microabscesses. Hematoxylin and eosin stain; magnification, ×40.
Figure 3.
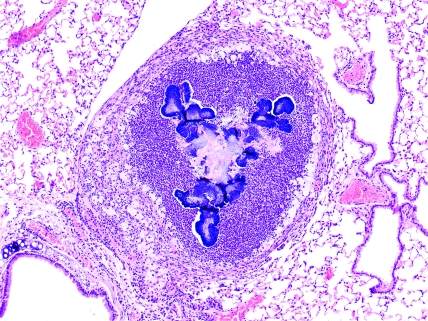
B6p47phox[KO]HLL. Phagocyte superoxide-deficient mouse, lung. Eosinophilic crystals and spicules admixed with basophilic cocci in the center of a granuloma. Hematoxylin and eosin stain; magnification, ×100.
Figure 4.
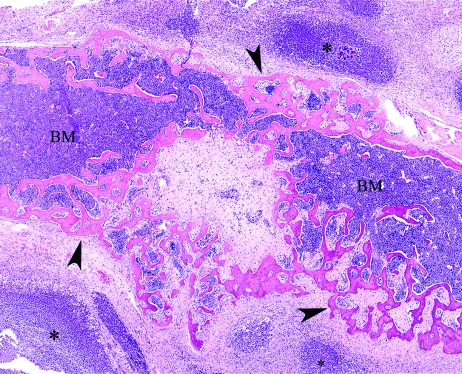
B6.129S2-Ncf1tm1shlN14. Phagocyte-superoxide–deficient mouse, front leg. Chronic suppurative cellulitis, with bone lysis and new bone formation (arrowheads) associated with Staphylococcus xylosus abscesses (asterisk). BM, bone marrow. Hematoxylin and eosin stain; magnification, ×40.
Figure 5.
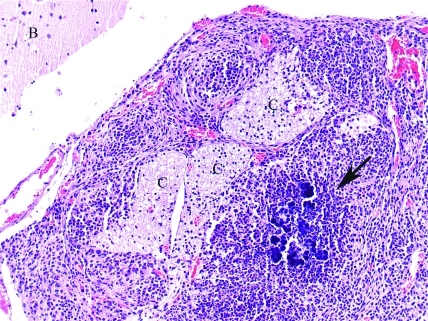
B6.129S6-Cybbtm1Din/J. Phagocyte-superoxide–deficient mouse, brain (B) and meninges. Granuloma containing cocci colonies (arrow) in the meninges next to cranial nerves (C). Hematoxylin and eosin stain; magnification, ×200.
In mice with normal phagocyte superoxide production (AKR/J-Lvif−, B6;129-Adora2atm1Dyj/J, BALB/c, AKR/J-Xpr1Sxv, C57BL/6NTac-[KO]FPR, C57BL/6NTac-[KO]P50NF-kB-[KO]P52NF-kB, C57BL/6J-[KO]RAG1 N10, NOD.Cg-Prkdcscid Il2rgtm1Sug/JicTac, and NFS/N-Xpr1Sxv Rmcf2+), the lesions mainly affected eyelids or ocular conjunctiva (10 of 16; 62.5%) and, to a lesser degree, caused dermatitis (7 of 16; 43.7%). Eyelids and conjunctiva of affected animals were swollen and irritated and had creamy yellow exudates around the margins. Some mice also had exophthalmus. Microscopically, the eyelids were ulcerated with serocellular crust formation of the lid margin and superficial colonization with colonies of cocci. Some cases demonstrated suppurative keratitis whereas more severely affected animals had histiocytic and lymphocytic infiltrates at the mucocutaneous junction with folliculitis, melanin drop-off, and rare thrombi in small vessels in adjacent skin.
The skin lesions in mice with normal production of phagocyte superoxide were characterized by ulcerations with underlying dermal and subcutaneous granulation tissue and neutrophilic infiltrate. In a C57BL/6J-[KO]RAG1 N10, there was necrotizing and vesicular dermatitis with a mixed inflammatory infiltrate. One AKR/J-Lvif mouse had perineal ulceration that affected the skin, anus, rectum, and vagina and formed a fistula into the adjacent skeletal muscle. Large colonies of coccoid bacteria were present on the fistula surface and invading the muscle. The adjacent soft tissues contained abundant granulation tissue (Figure 6). A C57BL/6NTac-[KO]P50NF-kB-[KO]P52NF-kB mouse had flaky skin on the tail with ulceration, which was characterized by suppurative dermatitis with multifocal ulceration, acanthosis, hyperkeratosis, and intracorneal bacterial colonization with cocci. A NOD.Cg-Prkdcscid Il2rgtm1Sug/JicTac mouse presented preputial gland thickening with a yellowish content. Microscopically, large numbers of cocci and bacilli were observed within the preputial gland but without an obvious inflammatory response. However NOD.Cg-Prkdcscid Il2rgtm1Sug/JicTac mice are severe combined immunodeficient,11 thereby explaining the lack inflammatory infiltrate. A NFS/N-Xpr1Sxv Rmcf2+ mouse had enlarged seminal vesicles containing a white opaque creamy and yellow-tinged creamy fluid. Multifocal to coalescing areas of fibrosis and fibroplasia of the seminal vesicle wall was noted, along with mucosal ulceration and a lymphocytic infiltrate with a large focus of plump macrophages containing amphophilic globular bodies. S. xylosus was isolated from all of these mice.
Figure 6.
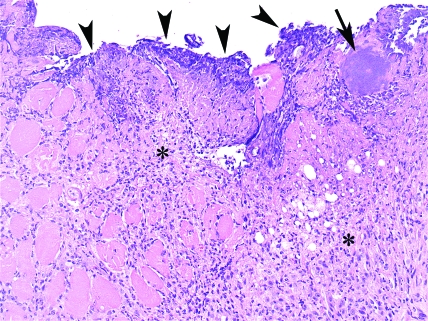
AKR/J-Lvif mouse, skeletal muscle; fistula. Photomicrograph showing colonies of coccoid bacteria on the fistula surface (arrowheads) and deeper in the tissue (arrow). Note the abundant granulation tissue and fibrosis with mild neutrophilic infiltrate and edema on the adjacent tissue (asterisk). Hematoxylin and eosin stain; magnification, ×200.
Granuloma formation was most common in gp91−/− mice (4 of 6; 66.6%) followed by p47−/− mice (4 of 15; 26.7%). Of 37 cases, S. xylosus was the only organism isolated from 27, and the remaining 10 had, in addition to S. xylosus, one or more other bacteria (Table 1). Colonies of cocci were observed on histologic examination in 26 of the 37 cases (70.27%). Sensitivity tests performed on 5 S. xylosus isolates from clinical cases revealed that the organism was susceptible to 13 commonly used antibiotics, including trimethoprim–sulfamethoxazole (Table 2). To manage the S. xylosus outbreaks in the CGD mice colony, management practices were modified and included replacing acidified drinking water with antibiotic (trimethoprim–sulfamethoxazole)-containing water and replacing woodchip bedding (SaniChip, Harlan Teklad) with paper-based bedding (Diamond Soft, Harlan Teklad). After instituting these changes, no additional cases of S. xylosus infection occurred in our CGD colony.
Table 2.
Antibiotic susceptibility of Staphylococcus xylosus isolates from 5 clinical cases
| Antibiotic | Growth inhibition (mm) |
| Ampicillin | 29–42 |
| Chloramphenicol | 20–24 |
| Clindamycin | 23–28 |
| Cephalothin | 26–42 |
| Ceftriaxone | 21–30 |
| Erythromycin | 23–26 |
| Kanamycin | 22–27 |
| Penicillin G | 29-43 |
| Streptomycin | 20 |
| Trimethoprim–sulfamethoxazole | 23–26 |
| Tetracycline | 20–28 |
| Vancomycin | 16–20 |
| Oxacillin | 15–21 |
Discussion
Staphylococcus xylosus is considered a common skin commensal of rodents and other mammals.23 Reports of S. xylosus as a primary pathogen in human and veterinary medicine are scarce. Acute pyelonephritis,36 endocarditis,7 and septicemia16 have been reported to occur in humans. In domestic animals, S. xylosus has been isolated from mastitis in dairy cows21 and ewes.8 In laboratory animals, S. xylosus has been associated with nasal dermatitis in gerbils.32 However, despite the large number of mice used in research compared with other species, only 5 reports describe spontaneous infections due to S. xylosus in mice. One outbreak of S. xylosus caused severe necrotizing dermatitis and high mortality in an athymic nude mice colony.4 Another report12 described 2 cases of S. xylosus infections affecting the periauricular soft tissue, plantar surface of the feet, and lung in a p47phox knockout mouse colony. A subsequent study13 reported similar lesions affecting feet, tail, lymph nodes, and lung due to spontaneous S. xylosus infections in p47phox-deficient mice, with a lower incidence in animals that had received prophylactic γ-interferon. In addition, S. xylosus was occasionally isolated from subcutaneous abscesses and, in one case, from lung in a gp91−/− Cybb mouse colony.3 S. xylosus was isolated from multiple ulcerated skin lesions in a C57BL/6J-Nos2tm1Lau mouse colony (mice with deletion of the nitric oxide synthase gene).37 Recently pure cultures of S. xylosus have been inoculated experimentally into artificially created skin lesions on the tails of SJL/J mice to determine pathogenicity.33 The intradermal tail inoculations produced focal to multifocal erythema, hyperemia, edema, ulcerative dermatitis, and scar tissue formation. Bacterial colonies were observed in and around the artificially created wounds.33 However, the infection was confined to the inoculated area, with no lesions observed in other organs, the pattern expected in immunocompetent mice.
We found that most of the spontaneous infections in our CGD mice were due to S. xylosus and not to S. aureus, which is a common cause of skin infections in animals and humans.14 Mixed infections occasionally were observed, but S. xylosus was, in most cases, the only organism isolated. S. aureus was not isolated from any of these cases. This situation is in contrast to previous reports from other investigators, in which S. xylosus occurred only occasionally and was not the main cause of morbidity or mortality in the CGD mouse colony.3,12 In addition, to our knowledge, S. xylosus has not been reported previously as a cause of myositis, bone lysis, and meningitis in CGD or other mouse strains, as we describe here. These lesions highlight the importance of S. xylosus as an emerging and serious pathogen in laboratory mice, particularly in immunodeficient strains. Abscesses observed in CGD mice are also sometimes referred to as botryomycosis. The eosinophilic material admixed with colonies of bacteria is known as Splendore–Hoeppli material and is thought to be composed of antigen–antibody complexes.24 Alternatively, the spicules and crystals observed in the abscesses may be composed of Ym1 protein as described in p47phox-deficient mice.9
Mice deficient in phagocyte superoxide may develop severe disease after S. xylosus infection characterized by soft tissue abscesses and granulomas, as part of the expected phenotype, with extension to regional lymph nodes, lungs, and, to a lesser degree, muscle, bone, and meninges. In contrast, in mice with normal production of phagocyte superoxide, the infections tend to be superficial and are confined to eyelids, ocular conjunctiva and skin and rarely affect other tissues or organs. Some skin infections may have been secondary to idiopathic ulcerative dermatitis in mice with a C57BL/6 background. In any case, mice with normal production of phagocyte superoxide but with other immune deficiencies are occasionally susceptible to develop superficial lesions in which S. xylosus is the primary pathogen or at least contributes to the pathogenesis of the inflammation. Although 37 cases in a 32-mo period in a rodent barrier colony with an estimated daily census around 20,000 mice may appear to be negligible, the majority of cases occurred in the CGD rodent model colony, which contains an average of only 25 breeder pairs, making infection with S. xylosus the exclusive cause of morbidity or mortality in our CGD rodent breeding colony during that period.
The S. xylosus isolates from our colony were sensitive to most common antibiotics. This result is in contrast to reported infections by drug-resistant strains which is common in domestic animals and humans.5,16, 38 The woodchip bedding in our CGD mice colony was replaced with paper-based bedding due to our concern that the sharp edges of the woodchip bedding material may have caused microabrasions in the skin or conjunctiva of the animals, leading to subsequent infection by S. xylosus. In addition to hematogenous or lymphatic spread, pneumonia might have been a result of cocci migrating from the nasal cavity to the lungs, and meningitis might have been a result of active bacterial migration or extension from muzzle lesions possibly through cranial nerves. Bone lysis occurred due to the action of the bacterial infection and the resultant inflammatory response against the infection.
In conclusion, S. xylosus is an important pathogen in mice with defective production of phagocyte superoxide and in other immunocompromised strains. Because bacterial culture and isolation are not performed routinely from mice with abscesses in some animal facilities, S. xylosus infections are likely to be underdiagnosed compared with the more typical pathogen, S. aureus. S. xylosus should be considered in the differential diagnosis in mice, especially immunodeficient animals, with abscesses and other skin lesions. This report expands the range of mice strains and tissues and organs susceptible to spontaneous S. xylosus infections and notes differences in the pathology of the infection between strains.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute of Allergy and Infectious Diseases, Comparative Medicine Branch; the Office of Research Support; and an NIAID contract to SoBran. We thank the Division of Veterinary Resources Pathology and Microbiology Laboratories, NIH, for pathology and microbiologic support. We also thank Dr Christine Kozak for letting us use the AKR/J-Lvif−, AKR/J-Xpr1Sxv, and NFS/N-Xpr1Sxv Rmcf2+ mice in this report and for comments on the preliminary manuscript, and we thank Dr Carmen Michaud for photographic assistance.
References
- 1.American Veterinary Medical Association. [Internet] 2007. AVMA guidelines on euthanasia. [Cited 30 Aug 2007]. Available at http://www.avma.org/issues/animal_welfare/euthanasia.pdf
- 2.Babior BM. 1999. NADPH oxidase: an update. Blood 93:1464–1476 [PubMed] [Google Scholar]
- 3.Bingel SA. 2002. Pathology of a mouse model of x-linked chronic granulomatous disease. Contemp Top Lab Anim Sci 41:33–38 [PubMed] [Google Scholar]
- 4.Bradfield JF, Wagner JE, Boivin GP, Steffen EK, Russell RJ. 1993. Epizootic fatal dermatitis in athymic nude mice due to Staphylococcus xylosus. Lab Anim Sci 43:111–113 [PubMed] [Google Scholar]
- 5.Burriel AR. 1997. Resistance of coagulase-negative staphylococci isolated from sheep to various antimicrobial agents. Res Vet Sci 63:189–190 [DOI] [PubMed] [Google Scholar]
- 6.Casaburi A, Di Monaco R, Cavella S, Toldrá F, Ercolini D, Villani F. 2008. Proteolytic and lipolytic starter cultures and their effect on traditional fermented sausages ripening and sensory traits. Food Microbiol 25:335–347 [DOI] [PubMed] [Google Scholar]
- 7.Conrad SA, West BC. 1984. Endocarditis caused by Staphylococcus xylosus associated with intravenous drug abuse. J Infect Dis 149:826–827 [DOI] [PubMed] [Google Scholar]
- 8.Fthenakis GC, Marples RR, Richardson JF, Jones JE. 1994. Some properties of coagulase-negative staphylococci isolated from cases of ovine mastitis. Epidemiol Infect 112:171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harbord M, Novelli M, Canas B, Power D, Davis C, Godovac-Zimmermann J, Roes J, Segal AW. 2002. Ym1 is a neutrophil granule protein that crystallizes in p47phox-deficient mice. J Biol Chem 277:5468–5475 [DOI] [PubMed] [Google Scholar]
- 10.Institute for Laboratory Animal Research 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; [PubMed] [Google Scholar]
- 11.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, Heike T, Nakahata T. 2002. NOD/SCID/γcnull mouse: an excellent recipient mouse model for engraftment of human cells. Blood 100:3175–3182 [DOI] [PubMed] [Google Scholar]
- 12.Jackson SH, Gallin JI, Holland SM. 1995. The p47phox mouse knockout model of chronic granulomatous disease. J Exp Med 182:751–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson SH, Miller GF, Segal BH, Mardiney M, 3rd, Domachowske JB, Gallin JI, Holland SM. 2001. IFNγ is effective in reducing infections in the mouse model of chronic granulomatous disease (CGD). J Interferon Cytokine Res 21:567–573 [DOI] [PubMed] [Google Scholar]
- 14.Jones TC, Hunt RD, King NW. 1997. Veterinary pathology, 6th ed Philadelphia (PA): Lippincott, Williams, and Wilkins [Google Scholar]
- 15.Kang EM, Malech HL. 2009. Advances in treatment for chronic granulomatous disease. Immunol Res.43:77–84. [DOI] [PubMed] [Google Scholar]
- 16.Koksal F, Yasar H, Samasti M. 2009. Antibiotic resistance patterns of coagulase-negative staphylococcus strains isolated from blood cultures of septicemic patients in Turkey. Microbiol Res 164:404–410 [DOI] [PubMed] [Google Scholar]
- 17.Krause KH. 2007. Aging: a revisited theory based on free radicals generated by NOX family NADPH oxidases. Exp Gerontol 42:256–262 [DOI] [PubMed] [Google Scholar]
- 18.Kuwano Y, Tominaga K, Kawahara T, Sasaki H, Takeo K, Nishida K, Masuda K, Kawai T, Teshima-Kondo S, Rokutan K. 2008. Tumor necrosis factor α activates transcription of the NADPH oxidase organizer 1 (NOXO1) gene and upregulates superoxide production in colon epithelial cells. Free Radic Biol Med 45:1642–1652 [DOI] [PubMed] [Google Scholar]
- 19.Lacy SH, Gardner DJ, Olson LC, Ding L, Holland SM, Bryant MA. 2003. Disseminated trichosporonosis in a murine model of chronic granulomatous disease. Comp Med 53:303–308 [PubMed] [Google Scholar]
- 20.Liu Y, Hao W, Letiembre M, Walter S, Kulanga M, Neumann H, Fassbender K. 2006. Suppression of microglial inflammatory activity by myelin phagocytosis: role of p47phox-mediated generation of reactive oxygen species. J Neurosci 26:12904–12913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miedzobrodzki J, Naidu AS, Watts JL, Ciborowski P, Palm K, Wadström T. 1989. Effect of milk on fibronectin and collagen type I binding to Staphylococcus aureus and coagulase-negative staphylococci isolated from bovine mastitis. J Clin Microbiol 27:540–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreno-Carranza B, Gentsch M, Stein S, Schambach A, Santilli G, Rudolf E, Ryser MF, Haria S, Thrasher AJ, Baum C, Brenner S, Grez M. 2009. Transgene optimization significantly improves SIN vector titers, gp91(phox) expression, and reconstitution of superoxide production in X-CGD cells. Gene Ther 16:111–118 [DOI] [PubMed] [Google Scholar]
- 23.Nagase N, Sasaki A, Yamashita K, Shimizu A, Wakita Y, Kitai S, Kawano J. 2002. Isolation and species distribution of staphylococci from animal and human skin. J Vet Med Sci 64:245–250 [DOI] [PubMed] [Google Scholar]
- 24.Percy DH, Barthold SW. 2001. Pathology of laboratory rodents and rabbits, 2nd ed Ames (IA): Iowa State University Press [Google Scholar]
- 25.Planchon S, Gaillard-Martinie B, Dordet-Frisoni E, Bellon-Fontaine MN, Leroy S, Labadie J, Hébraud M, Talon R. 2006. Formation of biofilm by Staphyloccus xylosus. Int J Food Microbiol 109:88–96 [DOI] [PubMed] [Google Scholar]
- 26.Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, Orkin SH, Doerschuk CM, Dinauer MC. 1995. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet 9:202–209 [DOI] [PubMed] [Google Scholar]
- 27.Rae J, Noack D, Heyworth PG, Ellis BA, Curnutte JT, Cross AR. 2000. Molecular analysis of 9 new families with chronic granulomatous disease caused mutations in CYBA, the gene encoding p22phox. Blood 96:1106–1112 [PubMed] [Google Scholar]
- 28.Roos D, de Boer M, Kuribayashi F, Meischl C, Weening RS, Segal AW, Åhlin A, Nemet K, Hossle LP, Bernatowska-Matuszkiewicz E, Middleton-Price H. 1996. Mutations in the X-linked and autosomal recessive forms of chronic granulomatous disease. Blood 87:1663–1681 [PubMed] [Google Scholar]
- 29.Roos D, Kuijpers TW, Curnutte JT. 2007. Chronic granulomatous disease, p 525–549 : Ochs HD, Smith CIE, Puck JM. Primary immunodeficiency diseases, a molecular and genetic approach, 2nd ed New York (NY): Oxford University Press [Google Scholar]
- 30.Sadikot RT, Zeng H, Yull FE, Li B, Cheng DS, Kernodle DS, Jansen ED, Contag CH, Segal BH, Holland SM, Blackwell TS, Christman JW. 2004. p47phox deficiency impairs NFκB activation and host defense in Pseudomonas pneumonia. J Immunol 172:1801–1808 [DOI] [PubMed] [Google Scholar]
- 31.Segal BH, Leto TL, Gallin JI, Malech HL, Holland SM. 2000. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine (Baltimore) 79:170–200 [DOI] [PubMed] [Google Scholar]
- 32.Solomon HF, Dixon DM, Pouch W. 1990. A survey of staphylococci isolated from the laboratory gerbil. Lab Anim Sci 40:316–318 [PubMed] [Google Scholar]
- 33.Thornton VB, Davis JA, St Clair MB, Cole MN. 2003. Inoculation of Staphylococcus xylosus into SJL/J mice to determine pathogenicity. Contemp Top Lab Anim Sci 42:49–52 [PubMed] [Google Scholar]
- 34.Tian N, Moore RS, Phillips WE, Lin L, Braddy S, Pryor JS, Stockstill RL, Hughson MD, Manning RD., Jr 2008. NADPH oxidase contributes to renal damage and dysfunction in Dahl salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol 295:R1858–R1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tieu K, Ischiropoulos H, Przedborski S. 2003. Nitric oxide and reactive oxygen species in Parkinson disease. IUBMB Life 55:329–335 [DOI] [PubMed] [Google Scholar]
- 36.Tselenis-Kotsowilis AD, Koliomichalis MP, Papavassiliou JT. 1982. Acute pyelonephritis caused by Staphylococcus xylosus. J Clin Microbiol 16:593–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Won YS, Kwon HJ, Oh GT, Kim BH, Lee CH, Park YH, Hyun BH, Choi YK. 2002. Identification of Staphylococcus xylosus isolated from C57BL/6J-Nos2tm1Lau mice with dermatitis. Microbiol Immunol 46:629–632 [DOI] [PubMed] [Google Scholar]
- 38.Yasuda R, Kawano J, Onda H, Takagi M, Shimizu A, Anzai T. 2000. Methicillin-resistant coagulase-negative staphylococci isolated from healthy horses in Japan. Am J Vet Res 61:1451–1455 [DOI] [PubMed] [Google Scholar]