Abstract
Objective. The present study aimed to investigate the salivary chemokine levels in patients with primary SS (pSS) and compare them with those in patients with non-SS sicca symptoms or non-sicca controls.
Methods. Unstimulated and stimulated whole saliva samples were obtained from pSS patients (n = 30) and age- and gender-matched patients with non-SS sicca (n = 30) and non-sicca healthy controls (n = 25). Salivary CCL2, CCL3, CCL4, CXCL8 and CXCL10 levels were measured using a Luminex bead-based multiplex assay.
Results. Patients with pSS had significantly different distributions of salivary CCL3 (P = 0.0001 by the Kruskal–Wallis test), CCL4 (P < 0.00001), CXLC8 (P < 0.0001) and CXCL10 (P < 0.05) levels in unstimulated saliva and all chemokine levels in stimulated saliva when compared with non-SS sicca and non-sicca controls. In comparison with chemokine production rate, the CXCL8 and CXCL10 production rates were significantly higher in pSS than in non-SS sicca controls (P < 0.01 by the Mann–Whitney test). Logistic regression analyses revealed that salivary CXCL8 (P < 0.05) and CXCL10 (P < 0.05) were the significant discriminating chemokines between the pSS and non-SS sicca groups. Although CXCL8 and CXCL10 levels were not correlated with the focus scores, CXCL8 and CXCL10 levels were significantly associated with salivary gland dysfunction.
Conclusion. These results support the notion that CXCL8 or CXCL10 chemokine plays a role in the pathogenesis of pSS.
Keywords: Sjögren’s syndrome, Sicca, Saliva, Chemokine
Introduction
SS is an autoimmune disease characterized by sicca symptoms, chronic lymphocytic infiltration of exocrine glands and the presence of anti-Ro/SSA or anti-La/SSB (or both). It has been suggested that chronic inflammation of exocrine glands depends on chemokines, which control the selective traffic and tissue homing of inflammatory cells [1]. Enhanced expression of several CCL and CXCL chemokines, including CCL2, CCL3, CCL4, CXCL8 and CXCL10, has been reported in the salivary glands of primary SS (pSS) patients or SS animal models [2–9]. However, which chemokines play a major role in the pathogenesis of pSS has not been studied. Moreover, the salivary chemokine levels have rarely been investigated in pSS patients, while inflammatory chemokine levels are expected to be elevated in fluid from inflamed glands.
Therefore, we hypothesized that the levels of chemokines playing an influential role in the pathogenesis of pSS are elevated in the saliva of pSS patients. To test this hypothesis, we investigated the levels of CCL2, CCL3, CCL4, CXCL8 and CXCL10 chemokines in saliva samples using a multi-analyte profiling bead-based assay.
Methods
Study subjects
From the pSS patients diagnosed at Seoul National University Bundang Hospital according to the criteria of the American–European Consensus Group (AECG) for SS [10], 30 female patients [median age (range), 50 (30–72) years] with both unstimulated and stimulated saliva samples available were enrolled into this study (pSS group). Thirty age-matched women [aged 50 (33–75) years] with dry mouth or dry eye (or both), who did not fulfil the AECG criteria, served as controls (non-SS sicca controls). The non-pSS sicca group comprised patients with drug-induced sicca (n = 7), burning mouth syndrome (n = 2), undifferentiated CTD (n = 1), hypothyroidism (n = 1) and primary biliary cirrhosis (n = 1); the remaining had dry eyes and mouth syndrome [11]. Twenty-five chronic gingivitis patients [aged 49.4 (30–67) years] without a medical illness were recruited as non-sicca controls. Their whole saliva was collected just after all treatment sessions.
The whole saliva flow (WSF) rate was determined by the spitting method, before initiating any drug with immunomodulating effects and after discontinuation of any medication with anti-cholinergic or diuretic effects for at least 4-fold the drug's half-life [10]. Unstimulated whole saliva (USWS) was collected for 15 min and, after the subjects were made to chew paraffin wax for 10 s, stimulated whole saliva (SWS) was collected for 5 min. The saliva volume was measured with a micropipette after centrifugation at 22 250 g for 10 min at 4°C. Saliva samples were kept at −70°C until analysis. Schirmer’s test and Rose Bengal staining were performed by an ophthalmologist (J.Y.H.) and the oral health status was evaluated using the decayed/missing/filled surface (DMFS) index by a dentist (P.-Y.Y.) [12]. The results of salivary gland scintigraphy were available in 31/60 subjects (19/30 pSS patients and 12/30 controls) and a minor salivary gland biopsy was performed in 31/60 (15/30 pSS patients and 16/30 controls).
Laboratory measurements
In subjects with sicca symptoms, the complete blood count, Westergren ESR, CRP, liver and renal function tests, ANA, RF and anti-Ro/La were determined at the time of saliva collection. Salivary CCL2, CCL3, CCL4, CXCL8 and CXCL10 levels were analysed using a Luminex 100 system (Luminex, Austin, TX, USA) and a multi-analyte profiling bead-based assay kit (Millipore, Billerica, MA, USA) according to the manufacturers’ recommendations. This study was approved by the Institutional Review Board (B-0506/021-004) and written informed consent was obtained before enrolment.
Statistical analysis
The salivary chemokines were measured in duplicate and determined values are presented as median (range). Differences of continuous variables were analysed using the Mann–Whitney test. To adjust for multiple comparisons, the Bonferroni method was used. Dichotomous variables were compared using the chi-square test or Fisher’s exact test. Correlations were calculated using Spearman’s coefficients and, for the multivariate analysis, logistic regression was performed. P < 0.05 was considered statistically significant.
Results
The pSS group had a significantly lower WSF rate [unstimulated, 0.05 (0.003–0.17) ml/min, P < 0.00001; stimulated, 0.51 (0.03–1.60) ml/min, P < 0.05] than non-SS sicca [unstimulated, 0.11 (0.01–0.17); stimulated, 0.86 (2.10–1.94)] and non-sicca controls [unstimulated, 0.26 (0.08–0.7); stimulated, 0.90 (0.13–1.80)]. Furthermore, the pSS group had significantly higher Rose Bengal (P < 0.05) and focus (P < 0.001) scores, ESR (P = 0.001) and CRP (P < 0.05). The prevalence of anti-Ro/La (86.7 vs 3.3%, P < 0.00001) and ANA (40.0 vs 13.3%, P < 0.05) was higher in the pSS group than in the non-SS sicca controls. Non-sicca controls had no tooth decay, but the median number with tooth decay was 2 (0–9) in the non-SS sicca and 1 (0–17) in the pSS group (P < 0.00001). However, DMFS index was not different among the three groups.
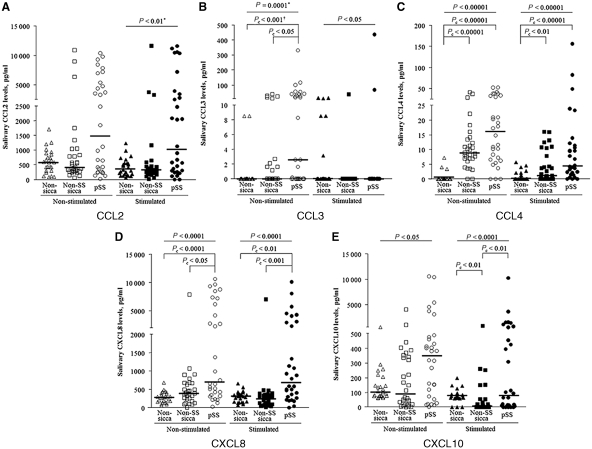
The levels of each chemokine showed significant positive correlation (each P < 0.00001) between USWS and SWS, with the exception of CCL3 levels, in total subjects. The levels of CCL3 (P = 0.0001 by the Kruskal–Wallis test), CCL4 (P < 0.00001), CXCL8 (P < 0.0001) and CXCL10 (P < 0.05) in the USWS were significantly different among the three subgroups (Fig. 1). Post hoc analysis showed that pSS had significantly higher levels of CCL3 [2.6 (0–330.9) vs 0 (0–39.6) pg/ml, corrected P (Pc) < 0.05 by the Mann–Whitney test with the Bonferroni method] and CXLC8 [708.7 (29.1–10618.8) vs 387.6 (9.1–7867.7) pg/ml, Pc < 0.05] than the non-SS sicca controls. In addition, CCL3 (Pc < 0.001), CCL4 (Pc < 0.00001) and CXCL8 (Pc < 0.00001) levels in pSS were significantly higher than in the non-sicca controls. Unstimulated CXCL10 levels tended to be higher in pSS, but it did not reach statistical significance (uncorrected P = 0.019 vs non-SS sicca controls; uncorrected P = 0.021 vs non-sicca controls). All chemokine levels in the SWS were differently distributed among the three groups (Fig. 1). Especially, CXCL8 levels were significantly elevated in pSS [691.2 (5.4–10120.6) pg/ml], when compared with those of the non-SS sicca [242.3 (9.6–7.34.2) pg/ml, Pc < 0.001] and non-sicca controls [307.0 (70.4–657.5) pg/ml, Pc < 0.01]. The pSS group had higher CCL4 levels [4.4 (0–156.3) vs 0.0 (0–5.6) pg/ml, Pc < 0.00001] than the non-sicca controls and higher CXCL10 levels [78.9 (0–10247.1) vs 5.2 (0–772.9) pg/ml, Pc < 0.01] than the non-SS sicca controls.
Fig. 1.
Salivary chemokine levels in pSS patients, non-SS sicca patients and non-sicca controls. Bars mean the median values. *P-value calculated by the Kruskal–Wallis test; **P value calculated by the Mann–Whitney test and corrected by the Bonferroni method.
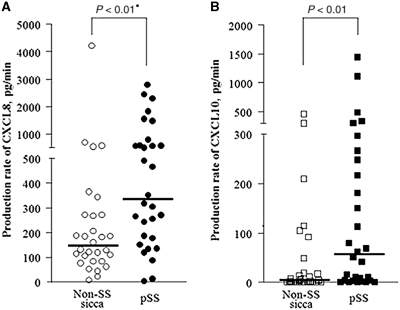
Since non-sicca controls had the highest WSF rates and did not have tooth decay, further analyses were limited to the non-SS sicca and pSS groups. To adjust for the effect of saliva volume on the chemokine concentrations, the chemokine production rate was calculated by multiplication of its concentration and WSF. The CXCL8 [334.2 (2.0–2797.0) vs 145.0 (6.4–4220.5) pg/min, P < 0.01] and CXCL10 [56.3 (0–1439.2) vs 2.9 (0–463.7) pg/min, P < 0.01] production rates were significantly higher in the pSS than in the non-SS sicca group, when saliva secretion was stimulated (Fig. 2).
Fig. 2.
Salivary production rates of CXCL8 (A) and CXCL10 (B) in pSS and non-SS sicca patients, when saliva secretion was stimulated. The production rate was calculated by multiplying salivary flow rates by chemokine concentrations. Bars mean the median values. *P-value calculated by the Mann–Whitney test.
Due to multiple bivariate correlations between the chemokine levels and complicated results in univariate analyses, logistic regression analyses were performed. When all chemokine variables were applied, the unstimulated salivary CXCL8 concentration and stimulated CXCL8 and CXCL10 production rates were significantly associated with pSS (P < 0.05). However, when chemokines in the USWS and SWS samples were separately analysed, CXCL8 concentration was found to be a significant variable in both samples (P < 0.05).
We selected to determine whether CLXL8 and CLXL10 levels, which were significant in the multiple regression analyses, were associated with the clinical manifestations. In the pSS group, the stimulated salivary CXCL8 concentrations were significantly associated with the unstimulated (ρ = −0.483, P < 0.01) and stimulated WSF (ρ = −0.451, P < 0.05) rates and significantly higher in pSS patients with unstimulated WSF rate ≤0.01 ml/min than in those with the WSF rate >0.01 ml/min (P < 0.05). Moreover, pSS patients with abnormal salivary gland scan results had significantly higher levels of stimulated CXCL10 than those without abnormal findings (P = 0.01). Even though the salivary CXCL8 and CXCL10 levels tended to be higher in patients with a focus score ≥1 than in those with a focus score <1, the grades of lymphocytic infiltration were not significantly correlated with CXCL8 and CXCL10 in the 15 pSS patients undergoing minor salivary gland biopsy. Total DMFS scores were not correlated with salivary CXCL8 and CXCL10 levels.
Discussion
The characteristic histological feature of pSS is mononuclear cell infiltration into the exocrine glands, and recently, attention has been drawn to the role of chemokines in the pathogenesis of SS. It has been shown that ductal epithelia of the SS minor salivary glands have significantly increased CCL3, CCL4, CCL5 and CXCL8 levels [2] and that CXCL9, CXCL10 and CXCL11 are up-regulated in the minor salivary glands of SS patients [4, 7]. In addition, CXCL13, CCL21 and CXCL12 expression has been reported in the acinar and ductal epithelia of SS patients [8]. Furthermore, it has been reported that transcript levels of CCL2 were elevated in the minor salivary glands of SS patients [9].
The present study showed that salivary CCL3, CCL4, CXCL8 and CXCL10 levels were significantly higher in the pSS group, compared with the non-sicca or non-SS sicca controls. Logistic regression analyses confirmed that salivary CXCL8 and CXCL10 were the significantly elevated chemokines in pSS patients. Moreover, in the present study, salivary CXCL8 levels were significantly associated with WSF rate, and CXCL10 levels were associated with abnormal salivary gland scan results.
In previous studies, CXCL8 was reported to be up-regulated in the salivary gland ductal epithelium, conjunctival epithelium, airway mucosa and tear film of SS patients [2, 13–15]. Furthermore, a recent study revealed that CXCL8 can be induced by IL-17 and IL-18 in salivary gland cells [16]. Immunohistochemical studies have revealed up-regulated CXCL10 expression in the minor salivary glands of SS patients [4, 17]. In non-obese diabetic mice, an animal model of SS, CXCL10 expression precedes inflammatory cell infiltration in the lacrimal gland [18]. It was reported that NH2-terminal-truncated CXCL10 antagonistic analogues significantly reduced sialadenitis in MRL/lpr mice, another SS animal model [19]. The findings of significantly higher salivary CXCL8 and CXCL10 levels in our study are in agreement with those presented in the aforementioned reports, suggesting that CXCL8 and CXCL10 contribute more significantly to the pathogenesis of pSS than CCL2, CCL3 and CCL4.
However, we could not find a significant association between these chemokine levels and focus scores. CXCL8 and CXCL10 are inflammatory rather than homoeostatic chemokines, and ectopic lymphoid follicle formation may not be dependent on these chemokines. Although we could not obtain detailed information about disease duration from all patients, most of them are likely in the late phase because there tends to be a significant delay before seeking medical attention and they had significant salivary gland dysfunction [20]. Therefore, one cannot exclude the possibility that reduction in saliva flow had contributed to the increase in concentrations of chemokines. However, when we calculated chemokine production rates, the CXCL8 and CXCL10 production rates were found to be elevated in pSS. Recent animal studies revealed that gland inflammation and dysfunction may be discordant [21]. In this study, salivary CXCL8 and CXCL10 levels were found to be associated with salivary gland dysfunction. Therefore, an exact role of CXCL8 or CXCL10 in salivary dysfunction remains to be elucidated.
Some limitations must be considered when extending our results to general cases. We used whole saliva sample rather than one obtained by the cannulation method. It is undisputed that salivary chemokines originate from non-salivary gland cells and CXCL8 and CXCL10 are known to be secreted from gingival fibroblasts, oral keratinocytes and oral epithelial cells [22–24]. On this background, it could be considered that periodontal factors might contribute to the increase in salivary CXCL8 and/or CXCL10 levels. However, recent studies have demonstrated that SS patients do not show an increased prevalence of periodontal diseases [25, 26]. In our study, the oral health status did not differ between the pSS and non-SS sicca groups. In addition, the sample size was not large and there was substantial overlap between chemokine levels in the pSS and non-SS sicca groups. Due to the pleiotropism and redundancy of the chemokines and the small sample size, interpretation of the results can be challenging.
In conclusion, this study showed that pSS patients had a different salivary profile of inflammatory chemokines, and CXCL8 and CXCL10 were the most distinct chemokines between pSS and non-pSS sicca patients. These results support the notion that CXCL8 and CXCL10 play a role in the pathogenesis of pSS.

Acknowledgements
Funding: This work was supported by the Seoul National University Bundang Hospital, Korea (grant no. 03-05-004); and the National Institutes of Health (grant no. DE017561 to R.H.S.).
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Amft N, Bowman SJ. Chemokines and cell trafficking in Sjögren’s syndrome. Scand J Immunol. 2001;54:62–9. doi: 10.1046/j.1365-3083.2001.00970.x. [DOI] [PubMed] [Google Scholar]
- 2.Cuello C, Palladinetti P, Tedla N, et al. Chemokine expression and leucocyte infiltration in Sjögren’s syndrome. Br J Rheumatol. 1998;37:779–83. doi: 10.1093/rheumatology/37.7.779. [DOI] [PubMed] [Google Scholar]
- 3.Xanthou G, Polihronis M, Tzioufas AG, Paikos S, Sideras P, Moutsopoulos HM. “Lymphoid” chemokine messenger RNA expression by epithelial cells in the chronic inflammatory lesion of the salivary glands of Sjögren’s syndrome patients: possible participation in lymphoid structure formation. Arthritis Rheum. 2001;44:408–18. doi: 10.1002/1529-0131(200102)44:2<408::AID-ANR60>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 4.Ogawa N, Ping L, Zhenjun L, Takada Y, Sugai S. Involvement of the interferon-gamma-induced T cell-attracting chemokines, interferon-gamma-inducible 10-kD protein (CXCL10) and monokine induced by interferon-gamma (CXCL9), in the salivary gland lesions of patients with Sjögren’s syndrome. Arthritis Rheum. 2002;46:2730–41. doi: 10.1002/art.10577. [DOI] [PubMed] [Google Scholar]
- 5.Matin K, Salam MA, Akhter J, Hanada N, Senpuku H. Role of stromal-cell derived factor-1 in the development of autoimmune diseases in non-obese diabetic mice. Immunology. 2002;107:222–32. doi: 10.1046/j.1365-2567.2002.01478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akpek EK, Jabs DA, Gérard HC, et al. Chemokines in autoimmune lacrimal gland disease in MRL/MpJ mice. Invest Ophthalmol Vis Sci. 2004;45:185–90. doi: 10.1167/iovs.03-0812. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa N, Kawanami T, Shimoyama K, Ping L, Sugai S. Expression of interferon-inducible T cell alpha chemoattractant (CXCL11) in the salivary glands of patients with Sjögren’s syndrome. Clin Immunol. 2004;112:235–8. doi: 10.1016/j.clim.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Barone F, Bombardieri M, Manzo A, et al. Association of CXCL13 and CCL21 expression with the progressive organization of lymphoid-like structures in Sjögren’s syndrome. Arthritis Rheum. 2005;52:1773–84. doi: 10.1002/art.21062. [DOI] [PubMed] [Google Scholar]
- 9.Egerer T, Martinez-Gamboa L, Dankof A, et al. Tissue-specific up-regulation of the proteasome subunit beta5i (LMP7) in Sjögren’s syndrome. Arthritis Rheum. 2006;54:1501–8. doi: 10.1002/art.21782. [DOI] [PubMed] [Google Scholar]
- 10.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price EJ, Venables PJ. Dry eyes and mouth syndrome – a subgroup of patients presenting with sicca symptoms. Rheumatology. 2002;41:416–22. doi: 10.1093/rheumatology/41.4.416. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Oral health survey: basic methods. 3rd edition. Geneva: 1987. [Google Scholar]
- 13.Pflugfelder SC, Jones D, Ji Z, Afonso A, Monroy D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjögren’s syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999;19:201–11. doi: 10.1076/ceyr.19.3.201.5309. [DOI] [PubMed] [Google Scholar]
- 14.Amin K, Lúdvíksdóttir D, Janson C, et al. Bronchial hyper-responsiveness. Inflammation and structural changes in the airways of patients with primary Sjögren’s syndrome. Respir Med. 2001;95:904–10. doi: 10.1053/rmed.2001.1174. [DOI] [PubMed] [Google Scholar]
- 15.Zywalewska-Górna N, Mrugacz M, Bakunowicz-Lazarczyk A. The evaluation of chosen cytokines in induction of ocular changes in Sjögren’s syndrome of dry eye. Klin Oczna. 2007;109:435–7. [PubMed] [Google Scholar]
- 16.Sakai A, Sugawara Y, Kuroishi T, Sasano T, Sugawara S. Identification of IL-18 and Th17 cells in salivary glands of patients with Sjögren’s syndrome, and amplification of IL-17-mediated secretion of inflammatory cytokines from salivary gland cells by IL-18. J Immunol. 2008;181:2898–906. doi: 10.4049/jimmunol.181.4.2898. [DOI] [PubMed] [Google Scholar]
- 17.Wakamatsu E, Matsumoto I, Yasukochi T, et al. Overexpression of phosphorylated STAT-1alpha in the labial salivary glands of patients with Sjögren’s syndrome. Arthritis Rheum. 2006;54:3476–84. doi: 10.1002/art.22176. [DOI] [PubMed] [Google Scholar]
- 18.Törnwall J, Lane TE, Fox RI, Fox HS. T cell attractant chemokine expression initiates lacrimal gland destruction in nonobese diabetic mice. Lab Invest. 1999;79:1719–26. [PubMed] [Google Scholar]
- 19.Hasegawa H, Inoue A, Kohno M, et al. Antagonist of interferon-inducible protein 10/CXCL10 ameliorates the progression of autoimmune sialadenitis in MRL/lpr mice. Arthritis Rheum. 2006;54:1174–83. doi: 10.1002/art.21745. [DOI] [PubMed] [Google Scholar]
- 20.Manthorpe R, Asmussen K, Oxholm P. Primary Sjögren’s syndrome: diagnostic criteria, clinical features, and disease activity. J Rheumatol. 1997;24(Suppl. 50):8–11. [PubMed] [Google Scholar]
- 21.Nikolov NP, Illei GG. Pathogenesis of Sjögren’s syndrome. Curr Opin Rheumatol. 2009;21:465–70. doi: 10.1097/BOR.0b013e32832eba21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang PL, Ohura K, Fujii T, et al. DNA microarray analysis of human gingival fibroblasts from healthy and inflammatory gingival tissues. Biochem Biophys Res Commun. 2003;305:970–3. doi: 10.1016/s0006-291x(03)00821-0. [DOI] [PubMed] [Google Scholar]
- 23.Sakai A, Ohshima M, Sugano N, Otsuka K, Ito K. Profiling the cytokines in gingival crevicular fluid using a cytokine antibody array. J Periodontol. 2006;77:856–64. doi: 10.1902/jop.2006.050340. [DOI] [PubMed] [Google Scholar]
- 24.Milward MR, Chapple IL, Wright HJ, Millard JL, Matthews JB, Cooper PR. Differential activation of NF-kappaB and gene expression in oral epithelial cells by periodontal pathogens. Clin Exp Immunol. 2007;148:307–24. doi: 10.1111/j.1365-2249.2007.03342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jorkjend L, Johansson A, Johansson AK, Bergenholtz A. Periodontitis, caries and salivary factors in Sjögren’s syndrome patients compared to sex- and age-matched controls. J Oral Rehabil. 2003;30:369–78. doi: 10.1046/j.1365-2842.2003.01088.x. [DOI] [PubMed] [Google Scholar]
- 26.Kuru B, McCullough MJ, Yilmaz S, Porter SR. Clinical and microbiological studies of periodontal disease in Sjögren syndrome patients. J Clin Periodontol. 2002;29:92–102. doi: 10.1034/j.1600-051x.2002.290202.x. [DOI] [PubMed] [Google Scholar]