Abstract
Objectives. The purpose of this study was to examine prevalence trends of serious extra-articular manifestations (EAMs) in a data set representing both hospitalized and ambulatory patients with RA.
Methods. This retrospective cohort study used serial cross-sectional data to examine the prevalence of serious EAMs in patients with RA from 1985 to 2006 across the United States (US) Veteran’s Health Administration system. Serious EAMs included rheumatoid carditis, RA lung disease, FS and pooled EAM rates included previously reported vasculitis prevalence as queried by ICD-9 searches. Statistical analysis employed auto-regression and time series analysis using the Chow and Durbin–Watson tests to detect breakpoints and linear time-trends.
Results. Among 3 million veterans, including >35 000 RA patients annually, we noted declining RA hospitalizations emphasizing the importance of examining both the inpatient and outpatient settings to assess EAM prevalence. Individual EAM trends varied, demonstrating linear declines in FS, increases in RA lung disease and significant breakpoint declines in carditis and pooled serious EAMs. Pooled EAM prevalence dropped around 2000, from an early linear trend peak of 10% among inpatients, to <7% among both inpatients and outpatients by 2006.
Conclusions. Overall, serious EAMs of RA have declined among US veterans in both the inpatient and outpatient settings, with the exception of RA lung disease likely reflecting improved detection. Breakpoints in pooled EAM prevalence appear to demonstrate consistent, true declines in severe RA extra-articular disease around 2000. Future work should explore the relationship between temporal EAM trends and specific RA therapies including adoption of biological agents.
Keywords: Rheumatoid arthritis, Epidemiology, Respiratory, Cardiovascular, Haematopoietic, Vasculitis
Introduction
Researchers continue to debate whether serious extra-articular manifestations (EAMs) of RA are declining [1–3]. Changes in hospitalization thresholds and standards of care over time add further complexity to assessing EAM trends. Reports in the USA have not yet examined a national data set integrating both inpatient and outpatient data accounting for these complexities to examine temporal trends in RA EAMs. The purpose of this study was to examine prevalence trends of serious EAMs in the United States (US) Veteran’s Health Administration (VHA) database, a national US data set representing both hospitalized and ambulatory patients with RA. We hypothesized that severe EAMs are declining among patients with RA in the context of new treatments.
Patients and methods
This serial cross-sectional study examined the prevalence of serious EAMs of RA from 1985 to 2006 among the entire national VHA database. The VHA is the largest US single-payer health system serving 25 million veterans, caring for 4.2 million in 2001 [4]. Its unique electronic record has tracked inpatient encounters since 1985 and outpatients since 1997. Distinctive features of veterans receiving care include high male gender (>90%), advanced age (>65 years; 36%), high disability/low-income status and high smoking rates [5, 6].
After receiving ethical approval from the University of Wisconsin Health Sciences Institutional Review Board (IRB) and the William S. Middleton Veteran’s Hospital IRB and both authorized a waiver of consent, we searched nearly 10 million hospitalizations to compare EAM rates among nearly 3–6000 RA patients hospitalized annually. Separate analysis examined outpatient EAM rates, encompassing >38 000 unique RA patients annually (Table 1).
Table 1.
Annual veteran patient totals in respective outpatient and inpatient settings including annual prevalence (shown in parentheses) of serious EAMs
| Outpatient | Inpatient | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Total veteran outpatients, n | RA outpatients | FS | Pleuritis/ILD | Carditis | Pooled EAMsa | RA inpatients | FS | Pleuritis/ILD | Carditis | Pooled EAMsa |
| 2006 | 5 078 432 | 43 347 (0.85) | 131 (0.30) | 426 (0.98) | 277 (0.64) | 2274 (5.25) | 3369 (0.92) | 19 (0.56) | 130 (3.86) | 10 (0.30) | 208 (6.17) |
| 2005 | 4 998 714 | 43 670 (0.87) | 131 (0.30) | 401 (0.92) | 255 (0.58) | 2157 (4.94) | 3249 (0.88) | 24 (0.74) | 143 (4.40) | 10 (0.31) | 218 (6.71) |
| 2004 | 4 871 136 | 43 655 (0.90) | 125 (0.29) | 351 (0.80) | 330 (0.76) | 2134 (4.89) | 3189 (0.87) | 27 (0.85) | 124 (3.89) | 16 (0.50) | 218 (6.84) |
| 2003 | 4 692 694 | 44 560 (0.95) | 140 (0.31) | 298 (0.67) | 430 (0.96) | 2024 (4.54) | 3218 (0.90) | 27 (0.84) | 119 (3.70) | 18 (0.56) | 205 (6.37) |
| 2002 | 4 434 671 | 44 129 (1.00) | 138 (0.31) | 325 (0.74) | 480 (1.09) | 2091 (4.74) | 3112 (0.87) | 19 (0.61) | 125 (4.02) | 23 (0.74) | 211 (6.78) |
| 2001 | 4 052 934 | 41 791 (1.03 | 168 (0.40) | 261 (0.62) | 538 (1.29) | 2146 (5.14)* | 3139 (0.88) | 30 (0.96) | 119 (3.79) | 22 (0.70) | 219 (6.98) |
| 2000 | 3 640 811 | 38 961 (1.07) | 134 (0.34) | 222 (0.57) | 593 (1.52)* | 2549 (6.54) | 3031 (0.86) | 30 (0.99) | 95 (3.13) | 22 (0.73) | 246 (8.12)* |
| 1999 | 3 390 527 | 38 746 (1.14) | 146 (0.38) | 238 (0.61) | 941 (2.43) | 2955 (7.63) | 3151 (0.88) | 3 (0.98) | 129 (4.09) | 32 (1.02) | 291 (9.24) |
| 1998 | 3 235 212 | 37 813 (1.17) | 139 (0.37) | 210 (0.56) | 879 (2.32) | 2814 (7.44) | 3192 (0.86) | 35 (1.10) | 135 (4.23) | 25 (0.78) | 324 (10.15) |
| 1997 | 2 956 905 | 37 878 (1.28) | 150 (0.40) | 212 (0.56) | 965 (2.55) | 2912 (7.69) | 3397 (0.84) | 50 (1.47) | 128 (3.77) | 33 (0.97) | 351 (10.33) |
Inpatient data for the period 1985–1996 are not shown, whereas inpatient data available from then on are shown. To summarize inpatient data for the period 1985–1996: RA ranged 4136–5832 persons, pooled EAMs ranged 376–585; individual EAM peaks: FS n = 123 (1986), pleuritis/ILD n = 163 (1991), carditis n = 60 (1987). Values are presented as n (%). aVasculitis annual totals previously published [5] are not shown, but included in pooled EAM totals. *Significant breakpoint (P < 0.01) in linear time-trend analysis from time-period ending at the preceding year.
Serious EAMs in this study were defined to include FS, rheumatoid lung disease, carditis, and pooled rates also included rheumatoid vasculitis, as described in a previous report [7]. These entities were selected as most serious, correlating with increased mortality risk [8]. Cases were identified searching International Classification of Diseases, Ninth Edition (ICD-9) codes representing the top 10–13 corresponding diagnoses per encounter for: RA (714.0); FS (714.1); RA lung disease including interstitial lung disease (ILD) and pleurisy (714.8, 515.0, 517.8, 511.0 or 511.9, combined with a simultaneous 714.0 code indicating RA); carditis (714.2); or vasculitis as previously published [2, 7]. FS and RA carditis were designated by RA-specific ICD codes. Simultaneous RA diagnoses were required to accompany codes for designation of RA lung disease and drug-induced ILD codes were intentionally excluded.
Individual EAM prevalence derived from dividing the number of unique individuals with an EAM by the number of unique RA inpatients and outpatients seen annually. Statistical analysis employed auto-regression and time series analysis. Step-wise Chow tests were used for breakpoint detection and Durbin–Watson analysis assisted selection of appropriate auto-regression models that were all ultimately linear. Briefly, Chow analysis sought to test a single linear regression line null vs two separate regression lines representing a significant trend breakpoint during an interval. If model performance improved when separate interval regression lines were fit, then breakpoint significance was tested.
Results
The VHA population maintained high age, male representation and increasing enrolment over all years. Mean age was 67 years among RA inpatients and 65 years among outpatients—consistently 5 years older than the total VHA cohort. Among inpatients and outpatients 92–96% of veterans with RA were male. All veterans, including RA patients, were less likely to be hospitalized after 1995, consistent with initiatives emphasizing outpatient chronic disease management.
FS decline
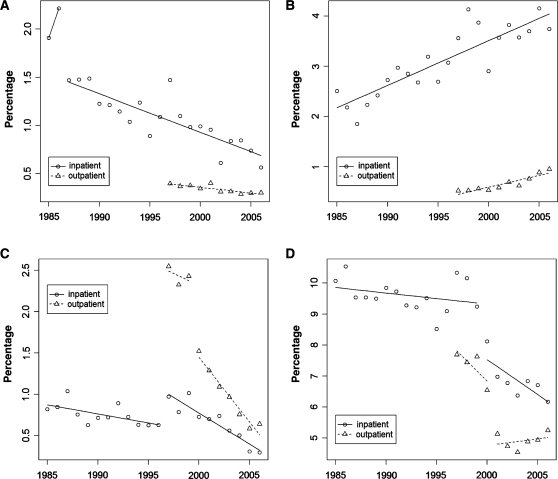
Beyond an initial step down in FS after 1986, regression demonstrated a significant linear decline in prevalence (Table 1 and Fig. 1A). Among inpatients with RA, FS decreased from 22 to 6 cases per 1000 (roughly >1.5 to 0.5%). From the first outpatient year, 1997, through 2006 the prevalence declined modestly from 4 to 3 per 1000 ambulatory RA patients. Nevertheless, the 25% decrease among RA outpatients and >3-fold decrease among RA inpatients demonstrated significant declines in the prevalence of FS (P = 0.003 and P = 0.000).
Fig. 1.
Individual serious EAM prevalence versus total RA cases by year (x-axis), circles for inpatient and triangles for outpatient point prevalence. Note linear declining trend for (A) FS vs linear increase in (B) RA lung disease cases. Breakpoints noted in (C) rheumatoid carditis demonstrated between 2000 and 2001 among outpatients, and similar breakpoints demonstrating declines in (D) pooled serious EAMs demonstrated between 2000 and 2001 in both the inpatient and outpatient settings.
RA lung disease increasingly reported
RA lung disease representing pleurisy and rheumatoid ILD were the only serious EAMs increasingly reported over the study (Table 1 and Fig. 1B). Specifically, inpatient RA lung disease in the 1980s ranged from ∼19 (2%) to 22 (4%) cases per 1000, and outpatient prevalence rose between 1997 and 2006. ILD, again specifically excluding drug-induced cases, was noted in <1% of patients. Linear trends for RA lung disease reached significance (P < 0.001), though ILD was infrequently captured.
Drop in prevalence of rheumatoid carditis
Rheumatoid carditis demonstrated a significant change (P < 0.0003) in time-trend analysis (Table 1 and Fig. 1C). Outpatient carditis declined from 24 to 15 cases per 1000 (37%) between 1999 and 2000 (P < 0.0001). Inpatient RA carditis trends were more complex, starting with a transient increase in the late 1990s followed by a significant linear decline (P < 0.000). Subsequent declines resulted in a prevalence of <6 per 1000 among both inpatients and outpatients from a previous outpatient peak of >25 per 1000 (>2.5%).
Decreased pooled serious EAMs
The pooled prevalence of serious EAMs also declined with breakpoints between 1999 and 2000 among inpatients, and between 2000 and 2001 among outpatients (Table 1 and Fig. 1D). Pooled events peaked at ∼10% for hospitalized RA patients in the late 1980s. Inpatient EAM events drifted down to 92 cases per 1000 in 1999, dropped to 81 in 2000 and 62 in recent years. Combined EAMs among outpatients also significantly declined, dropping more than one-third from 77 to 50 cases per 1000, just 1 year later between 2000 and 2001 (P < 0.0028).
Discussion
This study examined a large US veteran population with RA, including both inpatients and outpatients, demonstrating declines in overall serious EAMs. Only RA lung disease increased from 1985 to 2006. The demonstration of simultaneous declines in pooled inpatient and outpatient EAMs around 2000 raises causal questions including whether widespread use of DMARDs or the introduction of biological therapies may have decreased EAMs.
Linear decline in FS after definition change in 1980s
The linear decline in cases of FS since 1987 confirms informal impressions of clinicians who now rarely encounter this syndrome. The early Felty breakpoint after 1986 (Fig. 1A) likely reflects reclassification of large granular lymphoma (LGL) in 1985 [9]. Patients with RA have 3-fold increased risk of lymphoma [10] and this T-cell lymphoma would have been classified as FS before defining the LGL subcategory. Nevertheless, declines in reported FS in the VHA persisted beyond the introduction of this new diagnostic category, suggesting that FS has in fact declined beyond the reclassification.
Few prior reports on FS prevalence exist. Classic papers cite a <1% risk, and one series reported mean RA duration of 12 years before onset [11]. A study examining California hospitalization claims previously reported declining splenectomy to treat FS [2]. However, evolving thresholds for hospitalization and standard treatments including splenectomy, may have affected case identification in that study. Here, we examined a data source including outpatient data to overcome limitations of lone inpatient claims, demonstrating more conclusively that FS has declined.
RA carditis
RA carditis declined around 2000, after an initial slight increase. One may question whether inpatient carditis transiently increased around 1997 due to (i) increased diagnosis, (ii) decreased cure or (iii) chance. Increased inpatient echocardiograms in the mid-1990s may have increased diagnosis given an 87% US increase in use in 2001 vs 1993 [12]. Declining ‘cure’ rates may have occurred with less corticosteroid use in the 1990s. When comparing the prevalence of carditis in this study with prior reports, one must acknowledge imaging indication bias. An 8% prevalence of pericardial effusion was seen among severe RA patients screened by echo [13], while an autopsy series reported effusions in 30% [14]. In our cross-sectional sample, the detected prevalence of rheumatoid carditis remained <2.5%, recently <1%, though the screening rate was unknown.
RA lung disease
RA lung disease was the only serious EAM that increased over time. The linear increase most likely reflects improved diagnostic sensitivity. Comparative studies report RA lung disease ranging from <12% using X-ray to 30–80% using high-resolution CT, though again, estimating prevalence based upon routine imaging may reflect detection bias [15, 16]. Certainly, CT has improved resolution and become more utilized (US rates 5 million in 1985, 20 million in 1995 and 62 million in 2005) likely leading to higher detection [17]. Comorbidity and medications may also contribute to selection bias. In 1996, 16% of inpatient admission diagnoses lists indicated the presence of comorbid COPD [18], and although coded medication ILD was excluded, either may have increased screening or misclassification. It also remains possible that medications may have actually increased ILD as with MTX or case reports with anti-TNF-α agents [19–21].
Declining pooled serious EAM rates
Pooled prevalence of EAMs declined among US veterans with RA, consistent with our hypothesis and the prior hospital-based study. This contrasts with the community study reporting no change in EAMs. The discrepancy likely reflects design differences and limitations including limited final cohort follow-up in the US community study. The hospital claims study raised questions regarding changing hospitalization thresholds and standard treatments. To our knowledge, our study is the first study examining EAMs in a national US data set representing both RA inpatients and outpatients.
In our analysis, EAM declines clustered around 2000 for pooled events. We should point out that an alternative model accounting for possible piecewise time series correlation amplifies the breakpoint decline but may shift it up to 3 years earlier. Nevertheless, the consistent finding of a breakpoint and relative timing provide an opportunity for generating causal hypotheses. Pharmacotherapy for RA has evolved considerably over the examined period. Previous reports suggest that EAMs frequently occur after prolonged RA [11, 22]. Using this assumption, one may speculate that EAM declines reflect over a decade of MTX and combination therapies or that adoption of biologics in 1999 may have rapidly decreased EAM prevalence. Lastly, given the historically high prevalence of tobacco use in the US veterans [23], it is also possible that declines reflect VHA tobacco cessation efforts. Future work should examine the relationship of tobacco and pharmacotherapy trends with EAMs.
Limitations
Our findings are limited by the cross-sectional design, ICD-coding limitations and demographics of the Veteran population. The study aimed to capture only the prevalence of severe EAMs, and one must use caution drawing conclusions regarding EAM incidence. ICD-9 codes were used throughout the study period in the VHA, though not routinely audited for payment. A previous report cited a lack of specificity for a single RA code at one VHA facility [24], and strictly validated administrative search algorithms require more than one RA code in 24 months [25]. Annual cross-sectional searches did not facilitate 24-month review, likely inflating the RA denominator, but would not predict a change over time. A previously validated algorithm was used for RA vasculitis [2], though no validated algorithms exist for other EAMs. Although validation was not feasible in this study, it will be important for future work. One may also question whether deaths would have differentially identified EAM cases. Identical data sources were searched reflecting outpatient encounter or inpatient discharge diagnoses, regardless of survival. The finding of parallel trends in inpatient and outpatient encounters suggests that fatalities did not inflate EAM identification.
Patients were predominantly male, older and tobacco use was likely higher than most RA cohorts [5], which may also limit extrapolating results. Likewise, the diagnostic data set did not contain RA duration, RF or cyclic citrullinated peptide antibody status, and we did not collect comorbidity or demographics for multivariate analysis. Classic reports have noted higher EAM rates among males and smokers [8, 22], perhaps making this an ‘at-risk’ group likely to detect changes. The obvious advantage of this study was the inclusion of large numbers of patients in a national health system studied over many years in both the inpatient and outpatient settings.
In summary, pooled serious EAMs of RA have declined among US veterans in both the inpatient and outpatient settings. The timing of declines remained consistent among inpatients and outpatients and correlates with timing noted in prior reports [2, 3]. Simultaneous breakpoints in the prevalence of serious EAMs of RA demonstrated in this study suggest true declines in serious RA extra-articular disease. Declines in EAMs add credence to theories that aggressive disease-modifying treatments are changing the natural history of RA.
Acknowledgements
The authors would like to thank Jennifer Wiegel for assisting in editing and manuscript preparation. C.M.B. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: The project was supported by 1KL2RR025012-01, awarded through the University of Wisconsin Institute of Clinical Research KL2 Scholar Program under 1UL1RR025011 the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Turesson C, McClelland RL, Christianson TJ, Matteson EL. No decrease over time in the incidence of vasculitis or other extraarticular manifestations in rheumatoid arthritis: results from a community-based study. Arthritis Rheum. 2004;50:3729–31. doi: 10.1002/art.20590. [DOI] [PubMed] [Google Scholar]
- 2.Ward MM. Decreases in rates of hospitalizations for manifestations of severe rheumatoid arthritis, 1983–2001. Arthritis Rheum. 2004;50:1122–31. doi: 10.1002/art.20158. [DOI] [PubMed] [Google Scholar]
- 3.Watts RA, Mooney J, Lane SE, Scott DG. Rheumatoid vasculitis: becoming extinct? Rheumatology. 2004;43:920–3. doi: 10.1093/rheumatology/keh210. [DOI] [PubMed] [Google Scholar]
- 4.Department of Veterans Affairs Office of Public Affairs. Media relations fact sheet. Washington, DC: Department of Veterans Affairs; 2002. [Google Scholar]
- 5.Bray RM, Marsden ME, Peterson MR. Standardized comparisons of the use of alcohol, drugs, and cigarettes among military personnel and civilians. Am J Public Health. 1991;81:865–9. doi: 10.2105/ajph.81.7.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roman J, Perez RL. COPD in VA hospitals. Clin Cornerstone. 2003;5:37–44. doi: 10.1016/s1098-3597(03)90007-x. [DOI] [PubMed] [Google Scholar]
- 7.Bartels C, Bell C, Rosenthal A, Shinki K, Bridges A. Decline in rheumatoid vasculitis prevalence among US veterans: a retrospective cross-sectional study. Arthritis Rheum. 2009;60:2553–7. doi: 10.1002/art.24775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turesson C, O’Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Occurrence of extraarticular disease manifestations is associated with excess mortality in a community based cohort of patients with rheumatoid arthritis. J Rheumatol. 2002;29:62–7. [PubMed] [Google Scholar]
- 9.Loughran TP, Jr, Kadin ME, Starkebaum G, et al. Leukemia of large granular lymphocytes: association with clonal chromosomal abnormalities and autoimmune neutropenia, thrombocytopenia, and hemolytic anemia. Ann Intern Med. 1985;102:169–75. doi: 10.7326/0003-4819-102-2-169. [DOI] [PubMed] [Google Scholar]
- 10.Isomaki HA, Hakulinen T, Joutsenlahti U. Excess risk of lymphomas, leukemia and myeloma in patients with rheumatoid arthritis. J Chronic Dis. 1978;31:691–6. doi: 10.1016/0021-9681(78)90071-1. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg J, Pinals RS. Felty syndrome. Semin Arthritis Rheum. 1980;10:52–65. doi: 10.1016/0049-0172(80)90014-1. [DOI] [PubMed] [Google Scholar]
- 12.Levin DC, Rao VM, Maitino AJ, Parker L, Sunshine JH. Comparative increases in utilization rates of ultrasound examinations among radiologists, cardiologists, and other physicians from 1993 to 2001. J Am Coll Radiol. 2004;1:549–52. doi: 10.1016/j.jacr.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 13.Wislowska M, Sypula S, Kowalik I. Echocardiographic findings and 24-h electrocardiographic holter monitoring in patients with nodular and non-nodular rheumatoid arthritis. Rheumatol Int. 1999;18:163–9. doi: 10.1007/s002960050079. [DOI] [PubMed] [Google Scholar]
- 14.Kirk J, Cosh J. The pericarditis of rheumatoid arthritis. Q J Med. 1969;38:397–423. [PubMed] [Google Scholar]
- 15.Dawson JK, Fewins HE, Desmond J, Lynch MP, Graham DR. Fibrosing alveolitis in patients with rheumatoid arthritis as assessed by high resolution computed tomography, chest radiography and pulmonary function tests. Thorax. 2001;56:622–7. doi: 10.1136/thorax.56.8.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gochuico BR, Avila NA, Chow CK, et al. Progressive preclinical interstitial lung disease in rheumatoid arthritis. Arch Intern Med. 2008;168:159–66. doi: 10.1001/archinternmed.2007.59. [DOI] [PubMed] [Google Scholar]
- 17.Brenner DJ, Hall EJ. Computed tomography–an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–84. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 18.Roman J, Perez RL. COPD in VA hospitals. Clin Cornerstone. 2003;5:37–44. doi: 10.1016/s1098-3597(03)90007-x. [DOI] [PubMed] [Google Scholar]
- 19.Kremer JM, Alarcon GS, Weinblatt ME, et al. Clinical, laboratory, radiographic and histopathologic features of methotrexate-associated lung injury in patients with rheumatoid arthritis: a multicenter study with literature review. Arthritis Rheum. 1997;40:1829–37. doi: 10.1002/art.1780401016. [DOI] [PubMed] [Google Scholar]
- 20.Lindsay K, Melsom R, Jacob BK, Mestry N. Acute progression of interstitial lung disease: a complication of etanercept particularly in the presence of rheumatoid lung and methotrexate treatment. Rheumatology. 2006;45:1048–9. doi: 10.1093/rheumatology/kel090. [DOI] [PubMed] [Google Scholar]
- 21.Tournadre A, Ledoux-Eberst J, Poujol D, Dubost JJ, Ristori JM, Soubrier M. Exacerbation of interstitial lung disease during etanercept therapy: two cases. Joint Bone Spine. 2008;75:215–8. doi: 10.1016/j.jbspin.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 22.Cimmino MA, Salvarani C, Macchioni P, et al. Extra-articular manifestations in 587 italian patients with rheumatoid arthritis. Rheumatol Int. 2000;19:213–7. doi: 10.1007/pl00006853. [DOI] [PubMed] [Google Scholar]
- 23.Spigel S. Smoking among veterans. [(6 November 2009, date last accessed)];OLR Research Report. 2007 http://www.cga.ct.gov/2007/rpt/2007-R-0534.htm. [Google Scholar]
- 24.Singh JA, Holmgren AR, Noorbaloochi S. Accuracy of veterans administration databases for a diagnosis of rheumatoid arthritis. Arthritis Rheum. 2004;51:952–7. doi: 10.1002/art.20827. [DOI] [PubMed] [Google Scholar]
- 25.Katz JN, Barrett J, Liang MH, et al. Sensitivity and positive predictive value of medicare part b physician claims for rheumatologic diagnoses and procedures. Arthritis Rheum. 1997;40:1594–600. doi: 10.1002/art.1780400908. [DOI] [PubMed] [Google Scholar]