Abstract
Objectives. Musculoskeletal pain is reported commonly; however, the extent to which pain in individual body areas reflects the severity of site-specific pathology or a more generalized propensity to feel pain is uncertain. We used a classical twin design to examine the pattern of pain reporting at different body sites among monozygotic (MZ) and dizygotic (DZ) twins to assess its heritability and to examine evidence for a common underlying propensity to report musculoskeletal pain.
Methods. A well-characterized sample of female twins (TwinsUK cohort) was sent a questionnaire to determine their experience of pain in the neck and back, elbow, knee, thigh, hands or feet. The genetic contribution to pain reporting was assessed through univariate and multivariate analyses.
Results. Pain was reported with a prevalence of 17–46%, depending on the anatomical site. Univariate analysis indicated an underlying heritability for pain reporting at all sites of 28–71%. Pain reporting at different sites was modestly but uniformly correlated; a single factor accounted for 95% of the overall variance in pain reporting. The correlation for scores on this factor was 0.46 in MZ twins and 0.23 in DZ twins, corresponding to a ‘pain reporting factor’ heritability of 46% (95% CI 40%, 52%).
Conclusions. A single genetic factor underlies the propensity to report body pain at different musculoskeletal sites. These findings, which contrast with those for radiographic OA that is determined by genetic factors specific to each anatomical site, will inform the future search for therapeutic targets to treat pain in chronic degenerative diseases.
Keywords: Osteoarthritis, Pain, Gene, Twin, Cholesky, Factor analysis, Multivariate, Pain reporting
Introduction
The extent to which musculoskeletal pain reflects pathological processes specific to individual joints or a more generalized propensity to feel pain is unclear. Data from both clinical and laboratory studies support a role for genetic factors in manifesting the experience of pain in man [1]. In this study, we aimed to determine whether reported musculoskeletal pain specifically is heritable and, if so, whether there is a discernable pattern of genetic influence underlying pain experienced at multiple body sites.
Methods
A questionnaire was sent to study participants taken from the TwinsUK cohort (www.twinsuk.ac.uk) asking subjects ‘if they had experienced pain for the most part of the last month in the neck and back, or at the elbow, knee, thigh, hand or foot’. This was contemporaneous with the radiological study of OA reported previously [2]. The similarity in twin responses in monozygotic (MZ) and dizygotic (DZ) groups was assessed by casewise concordance. The extent to which genetic factors contributed to the occurrence of each pain trait was assessed through path modelling initially in univariate analyses that considered each trait in turn. The pattern of association among traits was first examined by inspecting the matrix of phenotypic correlations. This was further explored by applying a Cholesky decomposition to the data implemented in Mx [3]. This method uses the observed pattern of genetic and environmental correlation among twins to apportion phenotypic trait correlation into components attributable to shared additive genetic factors, shared factors in the common family environment of the twins and shared factors in the unique environment of each twin. Based on the observed pattern of correlations, a phenotypic factor score was constructed that captured the variation in all traits; its heritability was estimated in a univariate analysis. All subjects gave written informed consent according to the Declaration of Helsinki, and the study was approved by the St Thomas’ Hospital ethics committee.
Results
The questionnaire was sent to 9036 twins of whom 57% responded. The sample for analysis comprised 991 complete MZ and 1074 complete DZ pairs, with mean age for MZ twins of 50.4 years (range 18–82 years) and for DZ twins of 50.7 years (range 19–82 years). Fifteen per cent of the respondents were male and 18% had been included in the earlier study of radiographic OA. The prevalence and casewise concordance for pain reported at the six body sites are shown in Table 1. Pain at these sites was reported commonly with a prevalence of 17–46%. For all sites there was a significantly higher casewise concordance in MZ compared with DZ twins indicating the presence of a genetic influence.
Table 1.
Pain prevalence and casewise concordance at different sites and heritability under an AE model
| Prevalence, % | Cc MZ (95% CI) | Cc DZ (95% CI) |
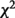 [MZ−DZ] (P) [MZ−DZ] (P) |
h2 (95% CI) | |
|---|---|---|---|---|---|
| Neck | 46 | 0.61 (0.57, 0.65) | 0.55 (0.51, 0.59) | 4.71 (0.03) | 0.44 (0.35, 0.53) |
| Back | 38 | 0.52 (0.48, 0.57) | 0.44 (0.40, 0.49) | 5.87 (0.02) | 0.34 (0.25, 0.43) |
| Elbow | 19 | 0.39 (0.32, 0.45) | 0.26 (0.20, 0.32) | 8.02 (<0.01) | 0.38 (0.28, 0.49) |
| Knee | 38 | 0.51 (0.46, 0.55) | 0.43 (0.39, 0.48) | 5.76 (0.02) | 0.28 (0.19, 0.37) |
| Thigh | 17 | 0.36 (0.29, 0.43) | 0.25 (0.19, 0.32) | 4.86 (0.03) | 0.36 (0.25, 0.48) |
| Hand | 28 | 0.50 (0.44, 0.55) | 0.38 (0.33, 0.43) | 9.26 (<0.01) | 0.44 (0.34, 0.53) |
| Foot | 24 | 0.45 (0.39, 0.51) | 0.31 (0.26, 0.37) | 11.27 (<0.01) | 0.39 (0.29, 0.50) |
AE model contains additive genetic (A) and unique environmental (E) factors only. Cc MZ: casewise concordance for MZ twins; Cc DZ: casewise concordance for DZ twins; 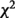 [MZ−DZ] (P): chi squared test for the difference between Cc MZ and Cc DZ (P-value); h2: heritability.
[MZ−DZ] (P): chi squared test for the difference between Cc MZ and Cc DZ (P-value); h2: heritability.
Univariate analysis gave estimates for site-specific joint pain (adjusted for age) of between 28% and 44% (Table 1). The phenotypic correlations for pain reported at different anatomical sites are shown in Table 2. These showed a modest but largely uniform correlation across all traits. In the subset with radiographs, there was a weak correlation (r < 0.1) between pain and knee OA and hand OA. As would be expected, these data generated a similarly uniform pattern of genetic and environmental correlation when the Cholesky decomposition was applied.
Table 2.
Phenotypic within-individual tetrachoric correlation in site-specific pain
| Neck | Back | Elbow | Knee | Thigh | Hand | Foot | |
|---|---|---|---|---|---|---|---|
| Neck | 1.000 | ||||||
| Back | 0.439 | 1.000 | |||||
| Elbow | 0.503 | 0.341 | 1.000 | ||||
| Knee | 0.338 | 0.320 | 0.388 | 1.000 | |||
| Thigh | 0.402 | 0.472 | 0.423 | 0.465 | 1.000 | ||
| Hand | 0.441 | 0.312 | 0.533 | 0.380 | 0.347 | 1.000 | |
| Foot | 0.336 | 0.344 | 0.394 | 0.369 | 0.487 | 0.472 | 1.000 |
Pain reporting factor score
Factor analysis based on the raw data pain variables showed only one factor with an eigenvalue of >1. The scoring coefficients on this were of approximately equal weight for all seven variables (neck 0.28, back 0.19, elbow 0.21, knee 0.18, thigh 0.19, hand 0.21 and foot 0.18). This ‘common pain factor’ explained >95% of the variance in the data. The intra-class correlation for the common pain factor among MZ twins was 0.46 (95% CI 0.40, 0.51) and among DZ twins was 0.23 (95% CI 0.17, 0.31). That the intra-class correlation is greater in MZ than in DZ twins indicates that the variable captured genetic variation in the phenotypes. Heritability for this common pain factor was estimated to be 46% (95% CI 40%, 52%).
Discussion
Our results show considerable clinical overlap between pain reporting at different sites, and indicate that a single, genetically determined, common pain factor accounts for the tendency to report musculoskeletal pain. This is in stark contrast to our OA findings from a subset of the same group of twins [4], which reveal that radiographic OA at different anatomical sites is determined by genetic factors that are specific to the individual joint site [2].
The interpretation of these findings needs to take into account the relatively low response rate to the questionnaire. However, there were no systematic differences between responders and non-responders with respect to age and gender and, of importance with respect to interpreting the genetic influence, there were no detectable differences between MZ and DZ twins. Our questionnaire contained a commonly used format for capturing the report of pain in community-based studies. It was not our aim to address the nature of the pain in further detail or its causes.
The findings are consistent with the notion that pain experienced at multiple anatomical sites is heritable. There is an emerging literature on the heritable basis of FM and chronic widespread pain [5], both of which are currently the subject of intense genetic scrutiny in genetic association studies [6, 7]. It is clear that pain reporting is intimately linked to mood, in particular depression, and the genetics of psychology and coping mechanisms are beginning to be unravelled [8]. While the present work requires independent validation, if a single genetic factor is confirmed to account for pain at different anatomical sites, then a search for such a genetic variant would be a reasonable next step.
This study demonstrates a clear distinction in the genetic contribution to the pattern of pain reporting at different joint sites and degenerative change. That radiographic OA and pain reporting appear to be aetiologically distinct is not surprising. In population studies, there is a well-recognized lack of correlation between radiographic change and clinical pain [9]: this has been taken as an indication of the lack of sensitivity of plain films to capture the early processes involved in joint degeneration. There is a trend to use clinical measures such as pain as a substitute for radiographic change. Our results indicate that this process may lead to very different conclusions in studies aimed at examining the genetic basis of degenerative diseases. Arguably, however, pain reporting has greater validity as it represents the symptoms with which patients present to their physicians. It must be recognized that the genetic architecture underlying radiographic OA and joint pain reporting are very different so one should not be used as a surrogate for the other. The identification of the genetic variants underlying the common pain factor would shed further light on the anatomical pathways involved in joint/soft tissue pain perception and the results would have clear and important implications for the therapeutic management of pain in OA and other musculoskeletal conditions in future.

Acknowledgements
Funding: We are indebted to the Wellcome Trust and ARC for supporting this work. Funding to pay the Open Access publication charges for this article was provided by The Wellcome Trust.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Norbury TA, MacGregor AJ, Urwin J, Spector TD, McMahon SB. Heritability of responses to painful stimuli in women: a classical twin study. Brain. 2007;130:3041–9. doi: 10.1093/brain/awm233. [DOI] [PubMed] [Google Scholar]
- 2.MacGregor AJ, Li Q, Spector TD, Williams FM. The genetic influence on radiographic osteoarthritis is site specific at the hand, hip and knee. Rheumatology. 2009;48:277–80. doi: 10.1093/rheumatology/ken475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neale MC, Boker SM, Xie G, Maes HH. Mx: statistical modeling. 7th edition. Richmond: Department of Psychiatry; 2006. [Google Scholar]
- 4.Spector TD, Williams FM. The UK adult twin registry (TwinsUK) Twin Res Hum Genet. 2006;9:899–906. doi: 10.1375/183242706779462462. [DOI] [PubMed] [Google Scholar]
- 5.Kato K, Sullivan PF, Evengard B, Pedersen NL. Importance of genetic influences on chronic widespread pain. Arthritis Rheum. 2006;54:1682–6. doi: 10.1002/art.21798. [DOI] [PubMed] [Google Scholar]
- 6.Holliday KL, Nicholl BI, Macfarlane GJ, Thomson W, Davies KA, McBeth J. Genetic variation in the hypothalamic-pituitary-adrenal stress axis influences susceptibility to musculoskeletal pain: results from the EPIFUND study. Ann Rheum Dis. 2010;69:556–60. doi: 10.1136/ard.2009.116137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Limer KL, Nicholl BI, Thomson W, McBeth J. Exploring the genetic susceptibility of chronic widespread pain: the tender points in genetic association studies. Rheumatology. 2008;47:572–7. doi: 10.1093/rheumatology/ken027. [DOI] [PubMed] [Google Scholar]
- 8.Bradley LA. Pathophysiology of fibromyalgia. Am J Med. 2009;122(Suppl. 12):S22–30. doi: 10.1016/j.amjmed.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wood LR, Peat G, Thomas E, Duncan R. The contribution of selected non-articular conditions to knee pain severity and associated disability in older adults. Osteoarthr Cartil. 2008;16:647–53. doi: 10.1016/j.joca.2007.10.007. [DOI] [PubMed] [Google Scholar]


