Abstract
Chronic infection with the hepatitis C virus (HCV), a noncytopathic hepatotropic RNA virus, affects over 170 million people worldwide1,2. In the majority of cases neither the early innate nor the later adaptive immune response succeeds in clearing the virus and infection becomes chronic1. Furthermore, in many patients the ineffective inflammatory response drives fibrogenesis and the development of cirrhosis3. It is critical to understand this immune pathology if preventative and curative therapies are to be developed. Chemokines are a superfamily of small proteins that promote leukocyte migration and orchestrate the immune response to viruses including HCV4. Chemokines are crucial for viral elimination but inappropriate persistence of expression in chronic hepatitis C infection can drive tissue damage and inflammation5. Here we review the role of chemokines and their receptors in HCV.
Keywords: Hepatitis C, chemokines, immune pathogenesis, lymphocyte recruitment, inflammation, fibrosis
HCV Immunopathology
HCV is a hepatotropic virus consisting of a polyprotein processed into structural proteins (core, envelope protein 1 & 2), non-structural proteins (NS2 to NS5) and a protein of unknown function (p7)1,3. The viral polymerase lacks proof-reading capability resulting in the generation of sequence diversity and quasispecies which contribute to evasion of the host immune response and chronic infection2,3,6. Although hepatocytes are the primary target of HCV the virus can interact with monocyte, lymphocyte and endothelial cells{Stamataki, 2008 23605/id}7,81. The recent description of in vitro models of HCV replication is elucidating HCV biology9 but the lack of small animal models hinders studies of disease course and outcome2.
The liver is a unique immunological environment with a dual blood supply and distinct rheological requirements for leukocyte recruitment. Although hepatic immune tolerance prevents exuberant responses to food antigen10, the liver can generate strong immune responses to infections including hepatitis A and E viruses11. In general, a vigorous intrahepatic immune response requires activation of T cells by activated dendritic cells (DCs) within secondary lymphoid tissues whereas activation within the liver by hepatocytes or endothelial cells results in tolerance12. This allows the liver to tolerate food antigens captured by endothelial cells and self-antigens on hepatocytes whilst responding appropriately to infections that cause inflammation and activation of DCs. The ability of HCV to infect hepatocytes without causing marked inflammatory damage may prevent the activation of a full immune response3,13.
Whether HCV infection is acute and self-limiting or persistent is determined early2,6. The first line of defense is provided by infected hepatocytes which secrete IFN production and downregulate RNA translation in response to infection before innate immune cells, including macrophages, DCs, natural killer (NK) and natural killer T (NKT) cells are activated to amplify secretion of type I interferons and IFN-response genes. Activation of pattern recognition receptors (PRRs), particularly Toll-like receptors (TLR), triggers chemokine secretion which amplifies leukocyte recruitment2,3,12. Innate immune activation facilitates the development of adaptive immunity through DCs which take up and process viral antigens and migrate to lymph nodes to activate naïve T cells14. Two functionally distinct subsets of DCs, myeloid (mDC) and plasmacytoid (pDC), have been implicated in HCV persistence15. pDCs secrete type-1 IFN, prime Th1 responses and activate cascades of chemokine secretion in HCV infection16. mDCs prime Th1 responses via IL-12 but produce little type-1 IFN. The frequency of IFN-α secreting intrahepatic pDCs is reduced in chronic HCV infection17 but it is unclear whether mDC function is impaired15,16. Viral clearance is associated with a vigorous, multi-specific CD4+ and CD8+ T cell response with Th1 responses dominating2,6. Regulatory T cells (Treg) are found in the HCV infected liver where they have a dualistic effect; on one hand they suppress HCV-specific CD8 T cells and promote viral replication on the other they suppress collateral inflammatory damage to reduce liver injury in chronic infection18,19.
It is unclear how HCV evades immune responses and why persistence in the liver leads to hepatitis and fibrogenesis3,13. Although HCV does not cause general immunosuppression, it compromises innate and adaptive immunity in many ways including through effects on chemokines and their receptors.
Chemokines and chemokine receptors
Chemokines are 8-12 kDa heparin-binding cytokines that coordinate the homeostatic trafficking of leukocytes and their recruitment to inflammatory sites (figure 1). Dysregulation of chemokine expression or function underlies many inflammatory diseases5. They can be divided functionally into homeostatic and inflammatory chemokines. Whereas most chemokines are inducible with inflammation, homeostatic chemokines are constitutively expressed and bring cell together within primary, secondary, and tertiary lymphoid organs to form functional microenvironments4. However, several chemokines don’t fall clearly into one category and others previously thought to be homeostatic are implicated in inflammation5. Chemokines mediate their effects through seven-transmembrane spanning receptors which signal via heterotrimeric GTP-binding proteins. Homeostatic chemokine receptors bind only one or two chemokines, for example a monogamous pair CXCR5 and CXCL13 recruit B cells to follicles in lymph nodes, whereas receptors that recruit cells to inflammatory sites often have several ligands. Chemokines and their receptors also undergo post-translational modifications which alter their function providing almost limitless options that bring exquisite specificity to the control of leukocyte homing and positioning in tissues4.
Figure 1.
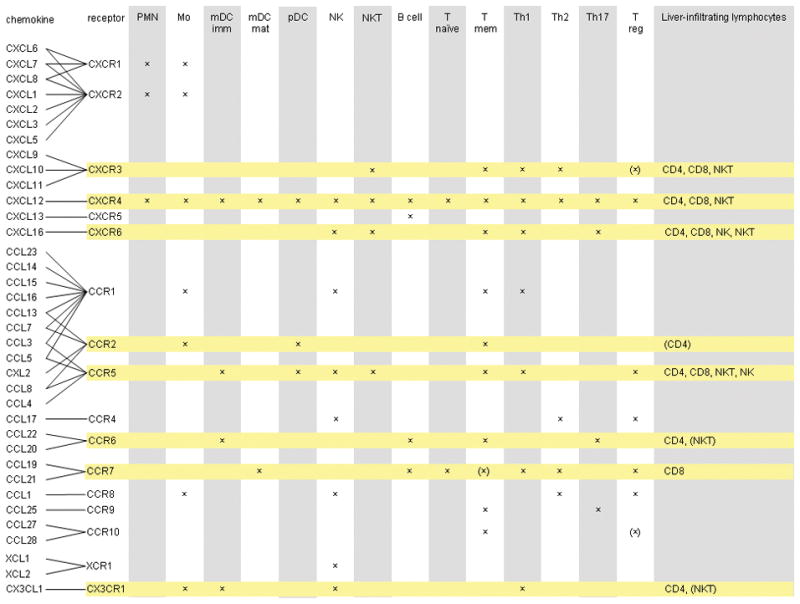
Important major human chemokines that act on cells of the lymphocyte and monocyte lineages are shown. 50 human chemokines and 18 chemokine receptors have been identified which can be divided into subfamilies based on the position of conserved cysteine residues within a conserved tetra-cysteine motif. In CC chemokines the first two consensus cysteines are next to each other; in CXC chemokines they are separated by a non-conserved amino acid. These two subfamilies account for all but three of the known chemokines the other being CX3CL1 (three intervening amino acids between the first cysteines) and XCL1 and XCL2, which lack two out of four canonical cysteines. Ligands and their receptors are connected by lines and the main leukocytes expressing receptors are shown by crosses. Receptors that are important for leukocyte recruitment to the liver are highlighted in yellow and the intrahepatic cell types expressing the receptors shown in the far right hand column. PMN: polymorphs; Mo: monocyte; mDC: myeloid DC (imm: immature and mat: mature); pDC: plasmacytoid DC; NK: Natural killer cell; NKT: Natural killer T cell; T naïve: naïve T cell; T mem: memory T cell; Th: T helper and Treg: regulatory T cells. Data are drawn from many sources including references4,5,43 and our own unpublished data on liver-infiltrating lymphocytes.
Chemokines and leukocyte trafficking
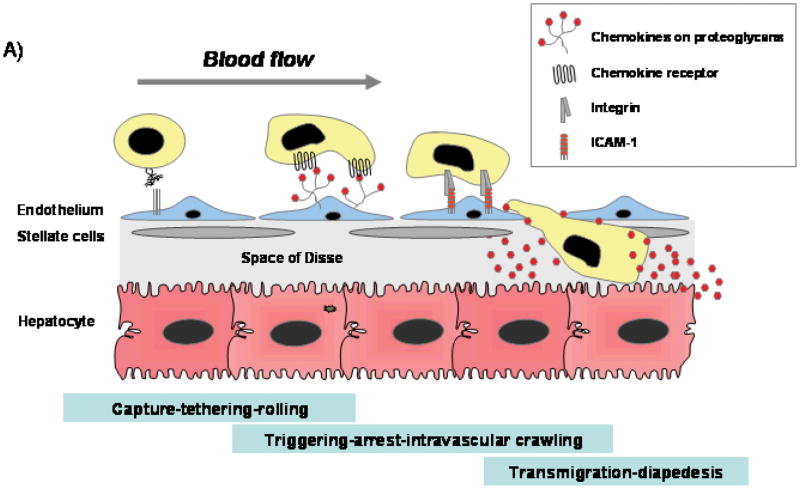
The process of leukocyte extravasation into tissue involves at least four stages (figures 2,4 and 5)20. An initial carbohydrate-dependent ‘tether’ brings the flowing cell into contact with the vessel wall and a second rolling step mediated by selectins slows the leukocyte, allowing interactions with the endothelium. In the liver, rolling is attenuated, as a consequence of low levels of shear stress in hepatic sinusoids and there is little role for selectins21. In the subsequent ‘triggering’ step signaling from leukocyte chemokine receptors triggers conformational activation of integrins and binding to endothelial ligands such as ICAM-1. During this stage the leukocyte arrests on the vessel wall and then migrates on the endothelium looking for the signals that drive transendothelial migration into tissue. Integrin-mediated arrest depends on a rapid in situ increase in integrin affinity in response to chemokines bound to the endothelial glycocalyx. The signaling complexes activated by chemokines are found in pre-assembled complexes that differ between leukocytes, thereby providing a degree of cell specificity22.
Figure 2. Chemokines are critical factors in regulating lymphocytes recruitment from blood into the liver.
A) The process of leukocyte extravasation into tissue involves at least four stages20. Cells are captured by carbohydrate-dependent ‘tethering’ which brings the flowing cell into contact with the vessel wall allowing interactions with the endothelium. In the subsequent ‘triggering’ stage chemokines (immobiised on endothelial proteoglycans activate chemokine receptors on leukocytes resulting in activation of integrin binding to endothelial ligands such as ICAM-1 and VCAM-1. During this stage the leukocyte arrests on the vessel wall and then migrates on the endothelium looking for the signals that drive transendothelial migration into tissue. Chemokines are also involved in transmigration during which leukocytes migrate across endothelium and enter tissue. Once in tissue, the cell follows chemokine gradients to sites of infection using chemokine-mediated changes in the actin cytoskeleton to propel migration23.
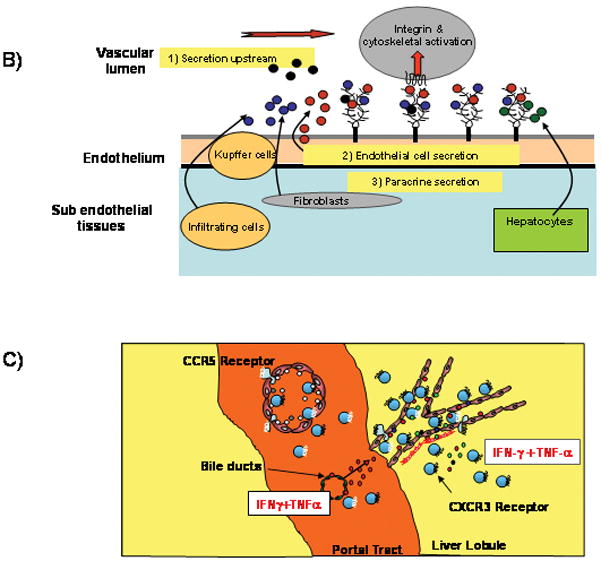
B) Chemokines are immobilized on the endothelium by proteoglycans in the glycocalyx that present chemokines to flowing leukocytes. The endothelium can secrete chemokines itself or present chemokines secreted by underlying cells in tissue including hepatocytes, stellate cells, Kupffer cells and infiltrating leukocytes; or capture chemokines secreted upstream by other structures such as cholangiocytes.
C) The CXCR3 ligands CXCL9-11 dominate in the recruitment of CXCR3 expressing effector lymphocytes via sinusoidal endothelium whereas CCR5 ligands are more important for recruitment via portal vascular endothelium. The critical trigger for CXCR3 ligand induction in the infected liver is IFNγ acting synergistically with proinflammatory cytokines such as TNFα secreted by multiple cells in response to viral infection and injury.
Figure 4.
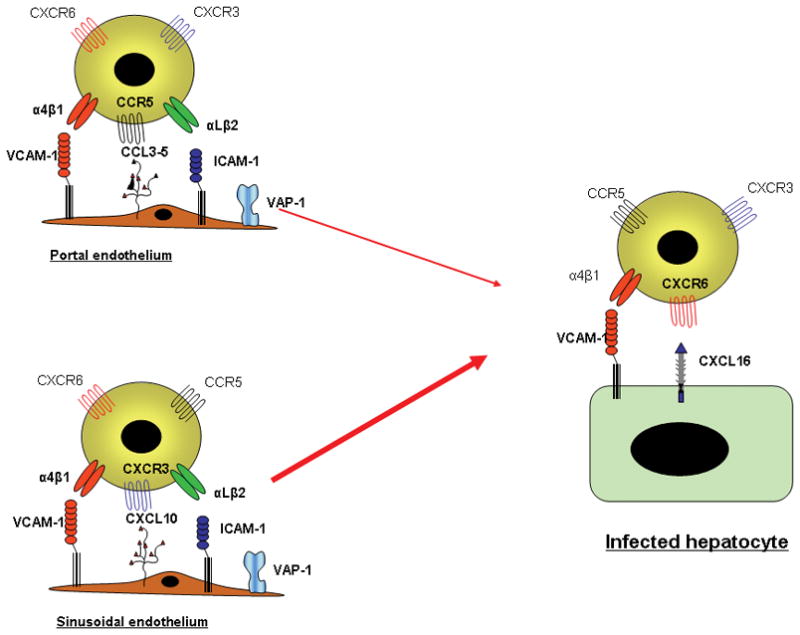
Distinct chemokines are involved in the recruitment and positioning of effectors cells in the HCV infected liver. Recruitment via portal vascular endothelium involved CCR5 ligands and the adhesion molecules ICAM-1, VCAM-1 and VAP-on liver endothelium which together promote adhesion and transmigration into tissue. Recruitment via the sinusoids appears to be dependent on CXCR3 rather than CCR5. The chemokine signals are displayed on the endothelial glycocalyx as described above. The mechanisms of migration through the subendothelial tissues are poorly understood but probably involve interactions with underlying matrix and fibroblasts. Localization at infected hepatocytes involves integrin-mediated adhesion to the hepatocytes triggered by chemokines upregulated on infected cells including the transmembrane chemokines CXCL16, shown in this example, and fractalkine.
Figure 5. A sketch of the liver micro-architecture from sinusoidal space (top) to matrix between hepatocytes (bottom) is shown.
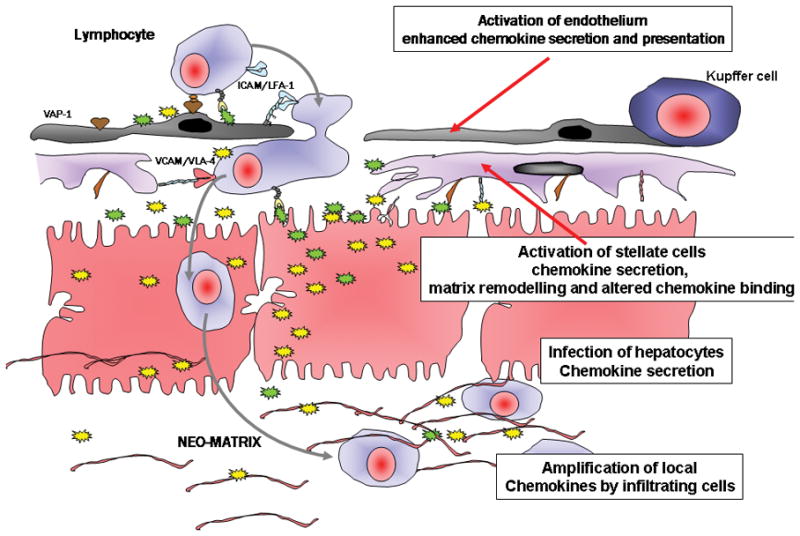
Direct effects of viral infection of hepatocytes or interactions between viral particles or proteins and liver cells may trigger chemokine secretion (yellow and green stars) and leukocyte recruitment. Lymphocytes are seen crossing the sinsuoidal endothelium using the adhesion molecules VAP-1, ICAM-1 and VCAM-1 and then interacting with hepatic stellate cells (purple) in the Space of Disse using VCAM-1 to promote motility into the underlying tissue. Multiple chemokines are involved at each stage secreted as part of local paracrine and autocrine networks induced by viral infection. Chemokines are retained in the infected liver by binding to the endothelial glycocalyx and cell matrix (pink filaments). Viral proteins and the effects of PAMPs during viral infection can activate endothelium and induce local chemokine secretion; viral proteins also activate stellate cells to secrete chemokines which can then be retained in matrix or transcytosed to the endothelial surface to promote recruitment. Infection of hepatocytes themselves leads to activation of chemokine transcription and secretion; finally viral proteins or particles can interact with infiltrating leukocytes to modulate the secretion of and response to chemokines.
During diapedesis, leukocytes migrate across endothelium and basement membrane to enter tissue20. Hepatic sinusoids lack tight junctions and a basement membrane and little is known about how leukocytes migrate through sinusoidal endothelium, nor how they cross the space of Disse21. Once in tissue, the cell follows chemokine gradients to sites of infection using chemokine-mediated changes in the actin cytoskeleton to propel migration23.
Transcytosis and presentation on the endothelial glycocalyx localizes chemokines to specific sites in the vasculature where they undergo post-translational modification and activation/deactivation by ectoenzymes such as CD264,24. Furthermore, a group of promiscuous, non-signalling, chemokine receptors termed interceptors, act as a sump to remove chemokines or promote their disposal via lymphatics25 (figure 2).
Chemokines and the immune response
Chemokines are secreted early after infection in response to activation of PRRs on epithelial, stromal and immune cells. These chemokines recruit the first wave of immune cells including neutrophils, monocytes, NK and NK T cells all of which express inflammatory chemokine receptors. Chemokines also recruit DCs which provide the link between innate and adaptive immunity. In response to pathogens DCs take up antigen and increase expression of CCR7 which promotes migration via lymphatics into lymph nodes26,27. CCR7 is also expressed on naïve T and B cells and the presence of its ligands CCL21 (on high endothelial venules and lymphatics) and CCL19 (within lymph node stroma) brings naïve lymphocytes and DCs together in the T-cell zone to allow immune activation. B lymphocytes, also express CXCR5 allowing them to respond to another chemokine CXCL13, the expression of which is restricted to follicles28. A subset of T cells upregulate CXCR5 promoting their migration to follicles where they provide help to activated B cells.
T-cell activation in lymph nodes expands antigen-specific effector cells which are imprinted with receptors that direct their homing back to tissue. In the gut and skin lymphocytes are imprinted with tissue-specific homing receptors29. It is not clear whether liver-specific homing receptors exist, although the inflammatory chemokine receptors CXCR3, CCR5 and CXCR6 are strongly associated with infiltration into the inflamed liver30–32. The activation state of the DC and the local cytokine milieu determine whether Th1, Th2 or Th17 effector cells or Treg are generated. Differential expression of chemokine receptors between lymphocyte subsets determines where and when they are recruited to tissue. Thus Th2 cells express CCR4 and CCR8 whereas Th1 cell preferentially express CCR5 and CXCR3 and Th17 cells express CCR6 and CXCR633,34. Treg in lymphoid tissues and blood express CCR4, CCR5 and CCR6 whereas those in the liver express CXCR3 and in some situations CXCR6 and CCR1035. All subsets display shared receptors consistent with the requirement for cells to be recruited to many tissues under different conditions36.
T-cell priming also generates long-lived CCR7+ central memory T cells that circulate through lymphoid tissues and CCR7− effector memory cells which home to inflamed tissues37. CCR7 has regulatory properties, being required for the activation of Treg and for the emigration of CCR7+ effector cells out of tissue via lymphatics during resolution of inflammation27,38. Thus chemokines are critical for the initiation, maintenance and resolution of immune responses implying they will be central to disease pathogenesis in HCV infection.
Chemokines in HCV pathogenesis
Given the lack of animal models of HCV infection, understanding disease pathogenesis relies on observational studies in infected humans and in vitro experiments. Expression studies using infected liver tissue implicate specific chemokines in HCV pathogenesis39–44 and these observations are supported by reports linking chemokine gene polymorphisms to altered susceptibility and progression of disease45,46. Here we review the role of chemokines and their cognate receptors in the different stages of HCV infection.
Chemokines in the innate immune response against HCV
Viral infection of hepatocytes activates chemokine secretion40,41 resulting in the recruitment of innate immune cells including NK and NKT cells which sustain local IFNγ production47. HCV core protein48, NS4A, NS4B and NS5A49,50 can all induce chemokine secretion in vitro and some of these chemokines subvert the anti-viral immune response. For example CXCL8 secretion increases as a consequence of transactivation of the CXCL8 promoter by HCV proteins in hepatic stellate cells (HSCs)51, hepatocytes49 and infected macrophages or endothelial cells52. The resulting increased CXCL8 levels promote immune evasion, by inhibiting IFN antiviral activity53 However, IL-8 increases expression of the death inducing receptor TRAIL-R2 on hepatocytes which may sensitise them to cytotoxicity mediated by TRAIL-expressing cytotoxic T cells54. HCV proteins and full length virus can also inhibit CCL3, CCL5, CXCL8 and CXCL10 induction by other viruses such as the Sendai Virus55.
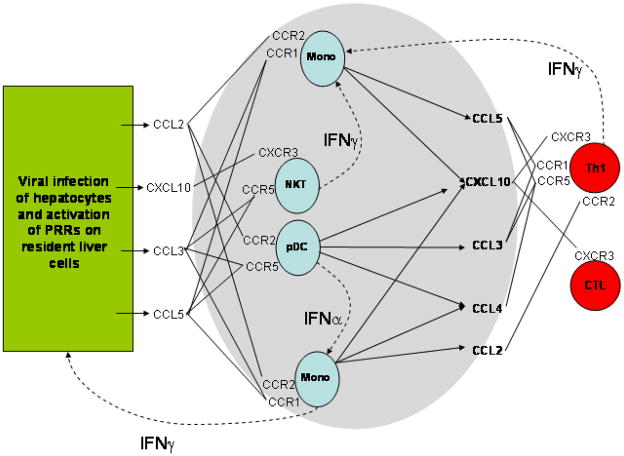
NK and NKT cells are present at high frequencies in the liver56 and express chemokine receptors associated with tissue infiltration43. CXCR6 and its ligand CXCL16 support the recruitment and survival of NKT cells57 allowing them to sustain high local levels of IFNγ thereby promoting the recruitment of Th1 cells58. The existence of intrahepatic chemokine cascades has been demonstrated in murine cytomegalovirus (MCMV)59 and it is likely that similar mechanisms shape the immune/inflammatory response in HCV. MCMV stimulates local IFN secretion which triggers CCL2 release by Kupffer Cells (KCs). This leads to the recruitment of CCL3-secreting monocytes that recruit NK cells which in turn secrete IFNγ thereby triggering macrophage secretion of CXCL9 and CCL3 and the recruitment of CD4+ T cells. The secretion of IL-12 and IL-18 by macrophages and KCs results in a local Th1 response and because Th1 cells secrete IFNγ, they create a feedback loop which promotes further T cell recruitment. Such a cascade is difficult to demonstrate in humans but in chimpanzees the early IFNα response and upregulation of chemokines correlates with viral elimination suggesting it is crucial in determining outcome60 (figure 3). Thus in the early phase of HCV infection chemokines, in particular CXCL8, CXCL16, CCL2 and CCL3, promote recruitment of innate immune cells to the liver including DCs which then initiate the adaptive immune response.
Figure 3.
Complex networks of autocrine and paracrine interactions involving many cell types determine the chemokine milieu within the HCV infected liver and thereby determine which leukocytes are recruited. Early events are a consequence of viral infection of hepatocytes and activation of PRR on resident liver cells. This triggers the secretion of chemokines including CCL2; CXCL10; CCL3 and CCL5 which recruit the first wave in innate immune cells including NK cells, NK T cells, monocytes (mono) and plasmacytoid DCs (pDC). These infiltrating cells amplify and broaden chemokine secretion by secreting interferons. Infiltrating monocytes and NKT cells secrete large amounts of IFNγ which further stimulates resident liver cells to secrete chemokines including CXCL10 as well as providing autocrine stimulation of monocytes to secrete CXCL10 and CCL5. pDCs secrete CCL3 and also large amounts of IFNα which acts on monocytes to induce them to secrete CCL2, CCL4 and CXCL10. Effector T cells including Th1 cells are recruited in response to CXCL10 and secrete more IFNγ helping to sustain the Th1 polarized local environment.
Chemokines and the trafficking of dendritic cells; the link between innate and adaptive immunity
Secretion of chemokines by KCs and infected hepatocytes enhances the recruitment of DC precursors. In the inflamed liver DCs become activated, take up antigen and migrate via the space of Disse and portal tracts to draining lymph nodes where they activate adaptive immunity61. The signals that recruit mDC precursors into the liver include chemokines, particularly CX3CL1, displayed on sinusoidal endothelium61(Aspinall A and Adams DH unpublished). pDCs express the inflammatory chemokine receptors CCR2, CCR5 and CXCR3 which direct them to the inflamed HCV-infected liver. There they secrete type-1 IFNs, TNFα, CCL3, CCL4 and CXCL10 and induce CCL2 secretion by other cell types as part of a cascade that amplifies leukocyte recruitment16.
HCV can interfere with DC trafficking to prevent efficient antigen presentation. Nattermann reported that HCV-induced secretion of CCL5 attracts CCR5+ immature DCs to the liver where they are rendered unresponsive to CCR7 ligands as a consequence of HCVE2 binding to CD81. The inability to use CCR7 to migrate into lymphatics traps DCs within the liver and thereby delays the establishment of an effective immune response in the crucial initial phase of infection62.
Adaptive immune response and T cell recruitment in HCV
Th1 immune responses dominate in the HCV infected liver30,63 and intrahepatic T cells express chemokine receptors associated with Th1 responses including CXCR3, CXCR6, CCR1 and CCR530,32,42,43. The distribution of chemokines within the liver compartmentalizes recruitment to different anatomical sites; CCR5 recruits lymphocytes to portal tracts whereas CXCR3 is essential for recruitment into the parenchyma via sinusoids and CXCR6 localizes cells to infected hepatocytes31,64–66 (figure 2 and 4). In other viral infections the CXCL8 receptor CXCR1 has been found on populations of virus-specific cytolytic CD8 T cells67 and we detect CXCR1+ cells within the HCV infected liver suggesting that the CXCL8/CXCR1 axis may be important for recruitment of HCV specific cytotoxic T cells.
CXCR3 and lymphocyte recruitment
Expression of CXCR3 is closely linked to Th1 function and its ligands CXCL9, CXCL10 and CXCL11 are induced by the Th1 cytokines IFNγ and TNFα. Many studies using different approaches have shown that CXCR3 ligands are increased in blood and liver of HCV infected patients68,69. Successful antiviral therapy is associated with an increase in circulating CXCR3+CD8+ T cells and a reduction in CXCL10 and CXCL9 levels in blood. Furthermore, high levels of CXCL10 reduce the probability of sustained virological response to therapy68–70. A polymorphism which results in a deletion in the CXCL11 promoter and reduced CXCL11 expression is more frequent in HCV patients compared with controls71 consistent with a role for CXCR3 in viral clearance as well as liver injury. The sources of CXCR3 ligands in HCV infection include hepatocytes, HSC and sinusoidal endothelium30,72 which express CXCL9-11 on stimulation with IFNγ and TNFα. These and other proinflammatory cytokines are released by KCs in response to infection and amplified by the initial wave of infiltrating pDC, NK and NK T cells. An additional contribution comes from activated CD4+ T cells which release CXCR3 ligands after interacting with HCV antigens in hepatocytes73. This provides a feedback loop in which antigen-specific cells maintain the expression of the chemokines required for effector cell recruitment (figure 3 and 5).
Hepatic CXCR3 ligands are increased in many liver diseases suggesting they play a generic role in effector cell recruitment to the inflamed liver66. Endothelial CXCR3 ligands drive transendothelial migration into tissue and can be secreted by the endothelium itself, by neighboring cells (and transcytosed to the endothelium) or by “upstream” cells (and captured from the slow-flowing sinusoidal blood by proteoglycans within the endothelial glycocalyx)66. Hepatocytes promote lymphocyte recruitment by increasing endothelial chemokine secretion and adhesion molecule expression74. Thus hepatocyte stimulation by HCV infection together with IFNγand TNFα derived from KCs may provide a paracrine amplification signal to increase lymphocyte recruitment via sinusoidal endothelium in chronic HCV75.
In MCMV, CXCR3 ligands recruit antigen-specific T lymphocytes to the liver76 but it is difficult to ascertain what proportion of lymphocytes recruited to the liver in HCV are viral-specific as opposed to bystander cells. Bystander cells contribute to tissue injury in several ways. They express CD40 ligand allowing them to activate CD40 on hepatocytes leading to NFkB-dependent chemokine secretion77. Both antigen-specific and bystander cells express CXCR3 and use this receptor to enter the liver78,79. Treg also use CXCR3 to enter tissue, although other signals may determine where they migrate to within the inflamed liver and hence where they mediate their anti-inflammatory effects35. Thus, complex networks have evolved to induce CXCR3 ligands in the infected liver. These recruit anti-viral effector cells which could promote viral clearance in early disease, but in chronic infection their persistence results in continuing effector cell recruitment and collateral liver injury. The fact that CXCR3+ effector cells drive damage makes CXCR3 a potential therapeutic target in chronic infection. Although, whether the anti-inflammatory effects would be outweighed by enhanced viral proliferation remains to be seen (figure 5).
Leukocyte recruitment mediated by CCR5 & CCR1 in HCV
CCR2 and CCR5 are characteristic of memory T cells5 and CD8 T cells expressing these receptors are enriched in the liver in HCV30,42,43. The receptors share chemokine ligands; CCR5 interacts with CCL3, CCL4, CCL5 and CCL8; and CCR2 interacts with CCL2, CCL13, CCL7 and CCL8 all of which have been detected in the liver30,80. CCR5 ligands are strongly expressed on portal endothelium81 and in murine models of graft versus host disease CCR5 and CCL3 support effector cell recruitment to portal tracts64. The complexity of chemokine networks is illustrated by the finding that mice lacking CCR5 are more susceptible to Con A-induced hepatitis and exhibit extensive inflammation mediated by CCR1+ effectors65. Thus, under some conditions, CCR5 recruits anti-inflammatory as well as effector cells.
In HCV infection a subset of CD8 T cells co-express CCR5 and the inhibitory NKG2A receptor. These T cells are attracted to the liver in response to CCR5 ligands induced by HCV E2 antigen. However engagement of the NKG2A receptor results in their inactivation demonstrating another mechanism by which HCV can manipulate chemokine networks to subvert effective immune responses80. The consequences of HCV on T cells can be unpredictable; for example although HCV-E2 binding to CD81 on CD8 T cells increases CCL5 secretion this causes autocrine desensitization of CCR5 and a loss of migratory capacity82. These chemokine-mediated effects may be enhanced by the ability of the virus to interfere with cell motility directly83 suggesting that disabling cell migration is an important mechanism of immune subversion for HCV.
Gene association studies are cited to support the importance of CCR5 in HCV pathogenesis. However the evidence is not clearcut and although some studies report that polymorphisms in CCL5 or CCR5 influence HCV pathogenesis others have not confirmed these findings45,84. For example, Woitas et al. reported an increased frequency of the CCR5δ 32 polymorphism (associated with reduced CCR5 function) in patients with HCV85 but another study showed no association and concluded that the original findings were explained by an overrepresentation of this mutation amongst HIV-seronegative individuals with HCV86. The same study did detect an association between hepatic inflammation and the CCL5 promoter polymorphism 403–A which results in over-expression of CCL5. However, patients with the polymorphism had less inflammation than controls, a finding which was replicated in a second independent study87. Analysis of the Irish cohort of women infected with HCV contaminated anti-D after childbirth found no significant relationship between CCR2 or CCL5 polymorphisms and disease outcome or severity; although heterozygosity for the CCR5δ32 mutation was associated with spontaneous viral clearance and reduced hepatic inflammation in patients with specific HLA types88. CCR5/CCL5 may affect response to treatment because haplotypes carrying mutations associated with reduced CCL5 secretion are more frequent in non-responders when compared with sustained responders89.
CXCR6, CXCL16 and leukocyte localization at epithelial surfaces in the liver
CXCR6 is expressed on CD4 and CD8 T cells, NK and NKT cells in the HCV infected liver and its ligand CXCL16 is upregulated as a transmembrane protein on inflamed bile ducts and hepatocytes31. The engagement of CXCR6 on T cells by cholangiocyte CXCL16 promotes β1 integrin-dependent adhesion which may position and retain effector cells in the HCV-infected liver. A recent study reports a unique subset of HCV-specific CXCR6+ liver-infiltrating CD8 T cells in HCV. These cells may have distinct effector functions because they express the C-type lectin CD161 which is expressed by NKT cells and Th17 cells90,91.
Other chemokines may also be involved in retaining T cells within the liver. These include CXCL1292,93 and the transmembrane chemokine CX3CL1 (fractalkine), both of which are expressed on inflamed bile ducts94. The fractalkine receptor CX3CR1 is expressed by Th1 cells and NK cells and may help to retain these cells at sites of epithelial inflammation or infection. We detected another epithelial chemokine, CCL28, on cholangiocytes in severak liver diseases including HCV35. However, a high proportion of the liver-infiltrating cells that expressed CCR10, the CCL28 receptor, were functional FoxP3+CD4+ regulatory T cells. When compared with blood Treg the CCR10+ liver-derived Treg express high levels of CXCR3 and low levels of CCR7 consistent with a tissue-infiltrating phenotype. This led us to propose that these cells use CXCR3 to enter the liver and then localize to inflamed bile ducts using CCR10. The role of Treg in HCV infection is controversial. High numbers of Treg in the HCV-infected liver suggest an active role in suppressing anti-viral immune responses although in established infection with chronic hepatitis they may suppress collateral damage18,95.
In summary, the chemokine receptors CCR1, CCR5, CXCR1, CXCR3 and CXCR6 may all contribute to the recruitment of an effective anti-viral immune response. But in chronic infection they recruit damaging effector cells that perpetuate liver injury without effectively clearing the virus.
Homeostatic chemokines, lymphoid follicles and lymphocyte egress from the liver
Homeostatic chemokines are upregulated at sites of chronic inflammation where they promote the formation of lymphoid follicles that have features of secondary lymphoid tissues96. Such structures express CCL19, CCL21 and CXCL13 resulting in the recruitment of CCR7+ T cells and CXCR5+ B cells and their compartmentalisation into T and B cells areas. Hepatic lymphoid follicles are seen in some patients with chronic HCV infection and can be sites for aberrant antibody production which in some cases drives the development of type II mixed cryoglobulinaemia97. The vasculitic lesions of cryoglobulinaemia in nerves and skin are characterized by up-regulation of CCL3, CCL4 and CXCL10 and infiltration by CXCR3+ Th1 cells and CCR5+ monocytes. Thus the same proinflammatory chemokines are implicated in the vasculitic complications of cryoglobulinaemia and hepatitis98.
CCR7+ T cells have been detected in livers from patients with HCV97 but most if not all are CD62Llow and LFA-1high characteristic of memory rather than naïve cells32. Because CCL19 and CCL21 are expressed on sinusoids and lymphatic vessels in portal tracts99 we suggest that CCR7 promotes the exit of T cells from the liver via lymphatics to draining lymph nodes where they are re-stimulated by antigen. The reduced numbers of intrahepatic CCR7+ memory T cells in chronic HCV infection may reflect a defect in this pathway32.
Chemokines and fibrosis
Chemokines are involved in fibrosis both indirectly by recruiting inflammatory cells that drive fibrogenesis and also by direct effects on HSC100. HSC, which play a central role in fibrogenesis following their transition to myofibroblasts, express chemokines and chemokine receptors allowing them to both contribute to the local chemokine milieu as well as responding to it101,102. CCL2 secreted by HSC recruits CCR2+ macrophages and T cells and levels are strongly associated with fibrogenesis. Inhibiting CCL2 reduces progression of fibrosis in vivo101. CCL3, which binds CCR1 and CCR5, is secreted by macrophages and epithelial cells and is profibrotic in several animal models. Studies using CCR1 and CCR5-deficient mice show how chemokines can have a broader influence on inflammation and fibrogenesis. Lack of CCR1 or CCR5 is associated with decreased IL-4 and IL-13 expression suggesting a link between chemokine signaling and the secretion of profibrotic cytokines. Because IL-4 and IL-13 can induce CCL3 and CCL2 expression a positive feedback loop may promote and sustain expression of both profibrotic Th2 cytokines and chemokines during fibrogenesis103.
HSC also respond to activation by chemokines. CXCR3-binding chemokines stimulate PI3-kinase-dependent migration and proliferation of HSC demonstrating how chemokines can directly promote myofibroblast activation and scar formation104. Thus, chemokines in the liver modulate the progression of fibrosis through several interlinked mechanisms. The involvement of such mechanisms in HCV infection is supported by the association of the CCR5δ32 mutation and a polymorphism in CCL8 (Q46K) with the severity of fibrosis in HCV infection87.
Conclusions
Chemokines are critical regulators of immunity and inflammation in all phases of HCV infection. They function within cytokine cascades that regulate the immune response to the virus. However in chronic infection their persistent expression can drive chronic inflammation in the absence of effective anti-viral immunity leading to liver injury and cirrhosis. The complex roles played by different chemokines during distinct stages of HCV infection may explain some of the conflicting findings from studies analyzing the impact of chemokine gene mutations on HCV pathogenesis. It also means that targeting chemokines therapeutically is complex. Although blocking chemokines that drive inflammation and fibrogenesis may be beneficial such approaches run the risk of inhibiting the anti-viral immune response allowing unchecked viral replication. Future research will need to focus on the precise role of specific chemokines at each stage of HCV infection. In particular we need to know the intrahepatic source of chemokines, what triggers their production and which cells are responding at different stages of HCV infection. This will elucidate which chemokine networks promote immune cells recruitment and viral elimination as opposed to those that drive collateral liver damage through inflammation and scar formation. In addition we need to understand better how HCV dysregulates chemokine expression and function to subvert anti-viral immune responses. Such information is critical if chemokines are to be targeted therapeutically in HCV infection.
Acknowledgments
Grant support
The work is supported by grants from the Wellcome Trust, European Commission (QLG1-CT-1999-00295); the Medical Research Council G0300101 and the National Institutes of Health 5RO1AA014257.
Abbreviations
- APC(s)
antigen presenting cell(s)
- DC(s)
dendritic cell(s)
- CMV
Cytomegalovirus
- HCC
hepatocellular carcinoma
- IFN
interferon
- LPS
lipopolysaccharide
- MCMV
Mouse Cytomegalovirus
- NK cells
Natural killer cells
- PAMPs
pathogen associated molecular patterns
- PRRs
pattern recognition receptors
- NKT cells
Natural killer T cells
- TLR(s)
Toll-like receptors
References
- 1.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 2.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–229. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 3.Dustin LB, Rice CM. Flying under the radar: the immunobiology of hepatitis C. Annu Rev Immunol. 2007;25:71–99. doi: 10.1146/annurev.immunol.25.022106.141602. [DOI] [PubMed] [Google Scholar]
- 4.Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- 5.Viola A, Luster AD. Chemokines and their receptors: drug targets in immunity and inflammation. Annu Rev Pharmacol Toxicol. 2008;48:171–197. doi: 10.1146/annurev.pharmtox.48.121806.154841. [DOI] [PubMed] [Google Scholar]
- 6.Bowen DG, Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005;436:946–952. doi: 10.1038/nature04079. [DOI] [PubMed] [Google Scholar]
- 7.Lai WK, Sun PJ, Zhang J, Jennings A, Lalor PF, Hubscher S, McKeating JA, Adams DH. Expression of DC-SIGN and DC-SIGNR on Human Sinusoidal Endothelium: A Role for Capturing Hepatitis C Virus Particles. Am J Pathol. 2006;169:200–208. doi: 10.2353/ajpath.2006.051191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pham TN, King D, Macparland SA, McGrath JS, Reddy SB, Bursey FR, Michalak TI. Hepatitis C virus replicates in the same immune cell subsets in chronic hepatitis C and occult infection. Gastroenterology. 2008;134:812–822. doi: 10.1053/j.gastro.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Lindenbach BD, Meuleman P, Ploss A, Vanwolleghem T, Syder AJ, McKeating JA, Lanford RE, Feinstone SM, Major ME, Leroux-Roels G, Rice CM. Cell culture-grown hepatitis C virus is infectious in vivo and can be recultured in vitro. Proc Natl Acad Sci U S A. 2006;103:3805–3809. doi: 10.1073/pnas.0511218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crispe IN, Giannandrea M, Klein I, John B, Sampson B, Wuensch S. Cellular and molecular mechanisms of liver tolerance. Immunol Rev. 2006;213:101–118. doi: 10.1111/j.1600-065X.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 11.Knolle PA, Limmer A. Neighborhood politics: the immunoregulatory function of organ-resident liver endothelial cells. Trends Immunol. 2001;22:432–437. doi: 10.1016/s1471-4906(01)01957-3. [DOI] [PubMed] [Google Scholar]
- 12.Bowen DG, McCaughan GW, Bertolino P. Intrahepatic immunity: a tale of two sites? Trends Immunol. 2005;26:512–517. doi: 10.1016/j.it.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Willberg C, Barnes E, Klenerman P. HCV immunology--death and the maiden T cell. Cell Death Differ. 2003;10 (Suppl 1):S39–S47. doi: 10.1038/sj.cdd.4401122. [DOI] [PubMed] [Google Scholar]
- 14.Kudo S, Matsuno K, Ezaki T, Ogawa M. A novel migration pathway for rat dendritic cells from the blood: hepatic sinusoids-lymph translocation. J Exp Med. 1997;185:777–784. doi: 10.1084/jem.185.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szabo G, Dolganiuc A. Subversion of plasmacytoid and myeloid dendritic cell functions in chronic HCV infection. Immunobiology. 2005;210:237–247. doi: 10.1016/j.imbio.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 16.Decalf J, Fernandes S, Longman R, Ahloulay M, Audat F, Lefrerre F, Rice CM, Pol S, Albert ML. Plasmacytoid dendritic cells initiate a complex chemokine and cytokine network and are a viable drug target in chronic HCV patients. J Exp Med. 2007;204:2423–2437. doi: 10.1084/jem.20070814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai WK, Curbishley SM, Goddard S, Alabraba E, Shaw J, Youster J, McKeating J, Adams DH. Hepatitis C is associated with perturbation of intrahepatic myeloid and plasmacytoid dendritic cell function. J Hepatol. 2007;47:338–347. doi: 10.1016/j.jhep.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 18.Godkin A, Ng WF, Gallagher K, Betts G, Thomas HC, Lechler RI. Expansion of hepatitis C-specific CD4+CD25+ regulatory T cells after viral clearance: a mechanism to limit collateral damage? J Allergy Clin Immunol. 2008;121:1277–1284. doi: 10.1016/j.jaci.2008.01.070. [DOI] [PubMed] [Google Scholar]
- 19.Rushbrook SM, Ward SM, Unitt E, Vowler SL, Lucas M, Klenerman P, Alexander GJ. Regulatory T cells suppress in vitro proliferation of virus-specific CD8+ T cells during persistent hepatitis C virus infection. J Virol. 2005;79:7852–7859. doi: 10.1128/JVI.79.12.7852-7859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 21.Lee WY, Kubes P. Leukocyte adhesion in the liver: distinct adhesion paradigm from other organs. J Hepatol. 2008;48:504–512. doi: 10.1016/j.jhep.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Alon R, Dustin ML. Force as a facilitator of integrin conformational changes during leukocyte arrest on blood vessels and antigen-presenting cells. Immunity. 2007;26:17–27. doi: 10.1016/j.immuni.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Lammermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, Hirsch K, Keller M, Forster R, Critchley DR, Fassler R, Sixt M. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- 24.McCaughan GW, Gorrell MD, Bishop GA, Abbott CA, Shackel NA, McGuinness PH, Levy MT, Sharland AF, Bowen DG, Yu D, Slaitini L, Church WB, Napoli J. Molecular pathogenesis of liver disease: an approach to hepatic inflammation, cirrhosis and liver transplant tolerance. Immunol Rev. 2000;174:172–191. doi: 10.1034/j.1600-0528.2002.017420.x. [DOI] [PubMed] [Google Scholar]
- 25.Colditz IG, Schneider MA, Pruenster M, Rot A. Chemokines at large: in-vivo mechanisms of their transport, presentation and clearance. Thromb Haemost. 2007;97:688–693. [PubMed] [Google Scholar]
- 26.Reis e Sousa Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476–483. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 27.Forster R, valos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 28.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 29.Mora JR, von Andrian UH. T-cell homing specificity and plasticity: new concepts and future challenges. Trends Immunol. 2006;27:235–243. doi: 10.1016/j.it.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Shields PL, Morland CM, Salmon M, Qin S, Hubscher SG, Adams DH. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J Immunol. 1999;163:6236–6243. [PubMed] [Google Scholar]
- 31.Heydtmann M, Lalor PF, Eksteen JA, Hubscher SG, Briskin M, Adams DH. CXC chemokine ligand 16 promotes integrin-mediated adhesion of liver-infiltrating lymphocytes to cholangiocytes and hepatocytes within the inflamed human liver. J Immunol. 2005;174:1055–1062. doi: 10.4049/jimmunol.174.2.1055. [DOI] [PubMed] [Google Scholar]
- 32.Heydtmann M, Hardie D, Shields PL, Faint J, Buckley CD, Campbell JJ, Salmon M, Adams DH. Detailed analysis of intrahepatic CD8 T cells in the normal and hepatitis C-infected liver reveals differences in specific populations of memory cells with distinct homing phenotypes. J Immunol. 2006;177:729–738. doi: 10.4049/jimmunol.177.1.729. [DOI] [PubMed] [Google Scholar]
- 33.costa-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 34.Singh SP, Zhang HH, Foley JF, Hedrick MN, Farber JM. Human T cells that are able to produce IL-17 express the chemokine receptor CCR6. J Immunol. 2008;180:214–221. doi: 10.4049/jimmunol.180.1.214. [DOI] [PubMed] [Google Scholar]
- 35.Eksteen B, Miles A, Curbishley SM, Tselepis C, Grant AJ, Walker LS, Adams DH. Epithelial Inflammation Is Associated with CCL28 Production and the Recruitment of Regulatory T Cells Expressing CCR10. J Immunol. 2006;177:593–603. doi: 10.4049/jimmunol.177.1.593. [DOI] [PubMed] [Google Scholar]
- 36.Lim HW, Lee J, Hillsamer P, Kim CH. Human Th17 cells share major trafficking receptors with both polarized effector T cells and FOXP3+ regulatory T cells. J Immunol. 2008;180:122–129. doi: 10.4049/jimmunol.180.1.122. [DOI] [PubMed] [Google Scholar]
- 37.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions [see comments] Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 38.Debes GF, Arnold CN, Young AJ, Krautwald S, Lipp M, Hay JB, Butcher EC. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat Immunol. 2005;6:889–894. doi: 10.1038/ni1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Apolinario A, Majano PL, Alvarez-Perez E, Saez A, Lozano C, Vargas J, Garcia-Monzon C. Increased expression of T cell chemokines and their receptors in chronic hepatitis C: relationship with the histological activity of liver disease. Am J Gastroenterol. 2002;97:2861–2870. doi: 10.1111/j.1572-0241.2002.07054.x. [DOI] [PubMed] [Google Scholar]
- 40.Harvey CE, Post JJ, Palladinetti P, Freeman AJ, Ffrench RA, Kumar RK, Marinos G, Lloyd AR. Expression of the chemokine IP-10 (CXCL10) by hepatocytes in chronic hepatitis C virus infection correlates with histological severity and lobular inflammation. J Leukoc Biol. 2003;74:360–369. doi: 10.1189/jlb.0303093. [DOI] [PubMed] [Google Scholar]
- 41.Helbig KJ, Ruszkiewicz A, Semendric L, Harley HA, McColl SR, Beard MR. Expression of the CXCR3 ligand I-TAC by hepatocytes in chronic hepatitis C and its correlation with hepatic inflammation. Hepatology. 2004;39:1220–1229. doi: 10.1002/hep.20167. [DOI] [PubMed] [Google Scholar]
- 42.Leroy V, Vigan I, Mosnier JF, Dufeu-Duchesne T, Pernollet M, Zarski JP, Marche PN, Jouvin-Marche E. Phenotypic and functional characterization of intrahepatic T lymphocytes during chronic hepatitis C. Hepatology. 2003;38:829–841. doi: 10.1053/jhep.2003.50410. [DOI] [PubMed] [Google Scholar]
- 43.Boisvert J, Kunkel EJ, Campbell JJ, Keeffe EB, Butcher EC, Greenberg HB. Liver-infiltrating lymphocytes in end-stage hepatitis C virus: subsets, activation status, and chemokine receptor phenotypes. J Hepatol. 2003;38:67–75. doi: 10.1016/s0168-8278(02)00328-8. [DOI] [PubMed] [Google Scholar]
- 44.Wang J, Holmes TH, Cheung R, Greenberg HB, He XS. Expression of chemokine receptors on intrahepatic and peripheral lymphocytes in chronic hepatitis C infection: its relationship to liver inflammation. J Infect Dis. 2004;190:989–997. doi: 10.1086/423283. [DOI] [PubMed] [Google Scholar]
- 45.Asselah T, Bieche I, Paradis V, Bedossa P, Vidaud M, Marcellin P. Genetics, genomics, and proteomics: implications for the diagnosis and the treatment of chronic hepatitis C. Semin Liver Dis. 2007;27:13–27. doi: 10.1055/s-2006-960168. [DOI] [PubMed] [Google Scholar]
- 46.Wald O, Weiss ID, Galun E, Peled A. Chemokines in hepatitis C virus infection: pathogenesis, prognosis and therapeutics. Cytokine. 2007;39:50–62. doi: 10.1016/j.cyto.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 47.Guidotti LG, Chisari FV. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu Rev Immunol. 2001;19:65–91. doi: 10.1146/annurev.immunol.19.1.65. [DOI] [PubMed] [Google Scholar]
- 48.Hoshida Y, Kato N, Yoshida H, Wang Y, Tanaka M, Goto T, Otsuka M, Taniguchi H, Moriyama M, Imazeki F, Yokosuka O, Kawabe T, Shiratori Y, Omata M. Hepatitis C virus core protein and hepatitis activity are associated through transactivation of interleukin-8. J Infect Dis. 2005;192:266–275. doi: 10.1086/430924. [DOI] [PubMed] [Google Scholar]
- 49.Kadoya H, Nagano-Fujii M, Deng L, Nakazono N, Hotta H. Nonstructural proteins 4A and 4B of hepatitis C virus transactivate the interleukin 8 promoter. Microbiol Immunol. 2005;49:265–273. doi: 10.1111/j.1348-0421.2005.tb03728.x. [DOI] [PubMed] [Google Scholar]
- 50.Girard S, Vossman E, Misek DE, Podevin P, Hanash S, Brechot C, Beretta L. Hepatitis C virus NS5A-regulated gene expression and signaling revealed via microarray and comparative promoter analyses. Hepatology. 2004;40:708–718. doi: 10.1002/hep.20371. [DOI] [PubMed] [Google Scholar]
- 51.Bataller R, Paik YH, Lindquist JN, Lemasters JJ, Brenner DA. Hepatitis C virus core and nonstructural proteins induce fibrogenic effects in hepatic stellate cells. Gastroenterology. 2004;126:529–540. doi: 10.1053/j.gastro.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 52.Balasubramanian A, Munshi N, Koziel MJ, Hu Z, Liang TJ, Groopman JE, Ganju RK. Structural proteins of Hepatitis C virus induce interleukin 8 production and apoptosis in human endothelial cells. J gen Virol. 2005;86:3291–3301. doi: 10.1099/vir.0.81056-0. [DOI] [PubMed] [Google Scholar]
- 53.Polyak SJ, Khabar KS, Rezeiq M, Gretch DR. Elevated levels of interleukin-8 in serum are associated with hepatitis C virus infection and resistance to interferon therapy. J Virol. 2001;75:6209–6211. doi: 10.1128/JVI.75.13.6209-6211.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dunn C, Brunetto M, Reynolds G, Christophides T, Kennedy PT, Lampertico P, Das A, Lopes AR, Borrow P, Williams K, Humphreys E, Afford S, Adams DH, Bertoletti A, Maini MK. Cytokines induced during chronic hepatitis B virus infection promote a pathway for NK cell-mediated liver damage. J Exp Med. 2007;204:667–680. doi: 10.1084/jem.20061287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaukinen P, Sillanpaa M, Kotenko S, Lin R, Hiscott J, Melen K, Julkunen I. Hepatitis C virus NS2 and NS3/4A proteins are potent inhibitors of host cell cytokine/chemokine gene expression. Virol J. 2006;3:66. doi: 10.1186/1743-422X-3-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doherty DG, Norris S, Madrigal-Estebas L, McEntee G, Traynor O, Hegarty JE, O’Farrelly C. The human liver contains multiple populations of NK cells, T cells, and CD3+CD56+ natural T cells with distinct cytotoxic activities and Th1, Th2, and Th0 cytokine secretion patterns. J Immunol. 1999;163:2314–2321. [PubMed] [Google Scholar]
- 57.Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ, Dustin ML, Littman DR. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005;3:e113. doi: 10.1371/journal.pbio.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shimaoka T, Seino K, Kume N, Minami M, Nishime C, Suematsu M, Kita T, Taniguchi M, Matsushima K, Yonehara S. Critical role for CXC chemokine ligand 16 (SR-PSOX) in Th1 response mediated by NKT cells. J Immunol. 2007;179:8172–8179. doi: 10.4049/jimmunol.179.12.8172. [DOI] [PubMed] [Google Scholar]
- 59.Salazar-Mather TP, Hokeness KL. Cytokine and chemokine networks: pathways to antiviral defense. Curr Top Microbiol Immunol. 2006;303:29–46. doi: 10.1007/978-3-540-33397-5_2. [DOI] [PubMed] [Google Scholar]
- 60.Su AI, Pezacki JP, Wodicka L, Brideau AD, Supekova L, Thimme R, Wieland S, Bukh J, Purcell RH, Schultz PG, Chisari FV. Genomic analysis of the host response to hepatitis C virus infection. Proc Natl Acad Sci U S A. 2002;99:15669–15674. doi: 10.1073/pnas.202608199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoneyama H, Matsuno K, Zhang Y, Murai M, Itakura M, Ishikawa S, Hasegawa G, Naito M, Asakura H, Matsushima K. Regulation by chemokines of circulating dendritic cell precursors, and the formation of portal tract-associated lymphoid tissue, in a granulomatous liver disease. J Exp Med. 2001;193:35–49. doi: 10.1084/jem.193.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nattermann J, Zimmermann H, Iwan A, von Lilienfeld-Toal M, Leifeld L, Nischalke HD, Langhans B, Sauerbruch T, Spengler U. Hepatitis C virus E2 and CD81 interaction may be associated with altered trafficking of dendritic cells in chronic hepatitis C. Hepatology. 2006;44:945–954. doi: 10.1002/hep.21350. [DOI] [PubMed] [Google Scholar]
- 63.Napoli J, Bishop GA, McGuinness PH, Painter DM, McCaughan GW. Progressive liver injury in chronic hepatitis C infection correlates with increased intrahepatic expression of Th1-associated cytokines. Hepatology. 1996;24:759–765. doi: 10.1002/hep.510240402. [DOI] [PubMed] [Google Scholar]
- 64.Murai M, Yoneyama H, Harada A, Yi Z, Vestergaard C, Guo B, Suzuki K, Asakura H, Matsushima K. Active participation of CCR5(+)CD8(+) T lymphocytes in the pathogenesis of liver injury in graft-versus-host disease. J Clin Invest. 1999;104:49–57. doi: 10.1172/JCI6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ajuebor MN, Hogaboam CM, Le T, Proudfoot AE, Swain MG. CCL3/MIP-1alpha is pro-inflammatory in murine T cell-mediated hepatitis by recruiting CCR1-expressing CD4(+) T cells to the liver. Eur J Immunol. 2004;34:2907–2918. doi: 10.1002/eji.200425071. [DOI] [PubMed] [Google Scholar]
- 66.Curbishley SM, Eksteen B, Gladue RP, Lalor P, Adams DH. CXCR3 Activation Promotes Lymphocyte Transendothelial Migration across Human Hepatic Endothelium under Fluid Flow. Am J Pathol. 2005;167:887–899. doi: 10.1016/S0002-9440(10)62060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hess C, Altfeld M, Thomas SY, Addo MM, Rosenberg ES, Allen TM, Draenert R, Eldrige RL, van Lunzen J, Stellbrink HJ, Walker BD, Luster AD. HIV-1 specific CD8+ T cells with an effector phenotype and control of viral replication. Lancet. 2004;363:863–866. doi: 10.1016/S0140-6736(04)15735-8. [DOI] [PubMed] [Google Scholar]
- 68.Butera D, Marukian S, Iwamaye AE, Hembrador E, Chambers TJ, Di Bisceglie AM, Charles ED, Talal AH, Jacobson IM, Rice CM, Dustin LB. Plasma chemokine levels correlate with the outcome of antiviral therapy in patients with hepatitis C. Blood. 2005;106:1175–1182. doi: 10.1182/blood-2005-01-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Larrubia JR, Calvino M, Benito S, Sanz-de-Villalobos E, Perna C, Perez-Hornedo J, Gonzalez-Mateos F, Garcia-Garzon S, Bienvenido A, Parra T. The role of CCR5/CXCR3 expressing CD8+ cells in liver damage and viral control during persistent hepatitis C virus infection. J Hepatol. 2007;47:632–641. doi: 10.1016/j.jhep.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 70.Lagging M, Romero AI, Westin J, Norkrans G, Dhillon AP, Pawlotsky JM, Zeuzem S, von WM, Negro F, Schalm SW, Haagmans BL, Ferrari C, Missale G, Neumann AU, Verheij-Hart E, Hellstrand K. IP-10 predicts viral response and therapeutic outcome in difficult-to-treat patients with HCV genotype 1 infection. Hepatology. 2006;44:1617–1625. doi: 10.1002/hep.21407. [DOI] [PubMed] [Google Scholar]
- 71.Helbig KJ, George J, Beard MR. A novel I-TAC promoter polymorphic variant is functional in the presence of replicating HCV in vitro. J Clin Virol. 2005;32:137–143. doi: 10.1016/j.jcv.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 72.Narumi S, Tominaga Y, Tamaru M, Shimai S, Okumura H, Nishioji K, Itoh Y, Okanoue T. Expression of IFN-inducible protein-10 in chronic hepatitis. J Immunol. 1997;158:5536–5544. [PubMed] [Google Scholar]
- 73.Cruise MW, Lukens JR, Nguyen AP, Lassen MG, Waggoner SN, Hahn YS. Fas ligand is responsible for CXCR3 chemokine induction in CD4+ T cell-dependent liver damage. J Immunol. 2006;176:6235–6244. doi: 10.4049/jimmunol.176.10.6235. [DOI] [PubMed] [Google Scholar]
- 74.Edwards S, Lalor PF, Nash GB, Rainger GE, Adams DH. Lymphocyte traffic through sinusoidal endothelial cells is regulated by hepatocytes. Hepatology. 2005;41:451–459. doi: 10.1002/hep.20585. [DOI] [PubMed] [Google Scholar]
- 75.Eksteen B, Afford SC, Wigmore SJ, Holt AP, Adams DH. Immune-mediated liver injury. Semin Liver Dis. 2007;27:351–366. doi: 10.1055/s-2007-991512. [DOI] [PubMed] [Google Scholar]
- 76.Hokeness KL, Deweerd ES, Munks MW, Lewis CA, Gladue RP, Salazar-Mather TP. CXCR3-dependent recruitment of antigen-specific T lymphocytes to the liver during murine cytomegalovirus infection. J Virol. 2007;81:1241–1250. doi: 10.1128/JVI.01937-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alabraba EB, Lai V, Boon L, Wigmore SJ, Adams DH, Afford SC. Coculture of human liver macrophages and cholangiocytes leads to CD40-dependent apoptosis and cytokine secretion. Hepatology. 2007 doi: 10.1002/hep.22011. [DOI] [PubMed] [Google Scholar]
- 78.Ehl S, Hombach J, Aichele P, Hengartner H, Zinkernagel RM. Bystander activation of cytotoxic T cells: studies on the mechanism and evaluation of in vivo significance in a transgenic mouse model. J Exp Med. 1997;185:1241–1251. doi: 10.1084/jem.185.7.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Spengler U, Nattermann J. Immunopathogenesis in hepatitis C virus cirrhosis. Clin Sci (Lond) 2007;112:141–155. doi: 10.1042/CS20060171. [DOI] [PubMed] [Google Scholar]
- 80.Nattermann J, Sherzada R, Iwan A, Bogen D, Niederle IM, Schulte D, Mertens E, Nischalke HD, Kramer B, Sauerbruch T, Leifeld L, Spengler U. Hepatitis C Virus-Induced Secretion of Inflammatory Chemokines Preferentially Recruits NKG2A(+)CD8(+) T Cells. J Infect Dis. 2008;198:213–217. doi: 10.1086/589309. [DOI] [PubMed] [Google Scholar]
- 81.Goddard S, Williams A, Morland C, Qin S, Gladue R, Hubscher SG, Adams DH. Differential expression of chemokines and chemokine receptors shapes the inflammatory response in rejecting human liver transplants. Transplantation. 2001;72:1957–1967. doi: 10.1097/00007890-200112270-00016. [DOI] [PubMed] [Google Scholar]
- 82.Nattermann J, Nischalke HD, Feldmann G, Ahlenstiel G, Sauerbruch T, Spengler U. Binding of HCV E2 to CD81 induces RANTES secretion and internalization of CC chemokine receptor 5. J Viral Hepat. 2004;11:519–526. doi: 10.1111/j.1365-2893.2004.00545.x. [DOI] [PubMed] [Google Scholar]
- 83.Volkov Y, Long A, Freeley M, Golden-Mason L, O’Farrelly C, Murphy A, Kelleher D. The hepatitis C envelope 2 protein inhibits LFA-1-transduced protein kinase C signaling for T-lymphocyte migration. Gastroenterology. 2006;130:482–492. doi: 10.1053/j.gastro.2005.10.047. [DOI] [PubMed] [Google Scholar]
- 84.Promrat K, Liang TJ. Chemokine systems and hepatitis C virus infection: is truth in the genes of the beholders? Hepatology. 2003;38:1359–1362. doi: 10.1016/j.hep.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 85.Woitas RP, Ahlenstiel G, Iwan A, Rockstroh JK, Brackmann HH, Kupfer B, Matz B, Offergeld R, Sauerbruch T, Spengler U. Frequency of the HIV-protective CC chemokine receptor 5-Delta32/Delta32 genotype is increased in hepatitis C. Gastroenterology. 2002;122:1721–1728. doi: 10.1053/gast.2002.33660. [DOI] [PubMed] [Google Scholar]
- 86.Promrat K, McDermott DH, Gonzalez CM, Kleiner DE, Koziol DE, Lessie M, Merrell M, Soza A, Heller T, Ghany M, Park Y, Alter HJ, Hoofnagle JH, Murphy PM, Liang TJ. Associations of chemokine system polymorphisms with clinical outcomes and treatment responses of chronic hepatitis C. Gastroenterology. 2003;124:352–360. doi: 10.1053/gast.2003.50061. [DOI] [PubMed] [Google Scholar]
- 87.Hellier S, Frodsham AJ, Hennig BJ, Klenerman P, Knapp S, Ramaley P, Satsangi J, Wright M, Zhang L, Thomas HC, Thursz M, Hill AV. Association of genetic variants of the chemokine receptor CCR5 and its ligands, RANTES and MCP-2, with outcome of HCV infection. Hepatology. 2003;38:1468–1476. doi: 10.1016/j.hep.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 88.Goulding C, McManus R, Murphy A, MacDonald G, Barrett S, Crowe J, Hegarty J, McKiernan S, Kelleher D. The CCR5-delta32 mutation: impact on disease outcome in individuals with hepatitis C infection from a single source. Gut. 2005;54:1157–1161. doi: 10.1136/gut.2004.055699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wasmuth HE, Werth A, Mueller T, Berg T, Dietrich CG, Geier A, Schirin-Sokhan R, Gartung C, Lorenzen J, Matern S, Lammert F. CC chemokine receptor 5 delta32 polymorphism in two independent cohorts of hepatitis C virus infected patients without hemophilia. J Mol Med. 2004;82:64–69. doi: 10.1007/s00109-003-0505-0. [DOI] [PubMed] [Google Scholar]
- 90.Cosmi L, De PR, Santarlasci V, Maggi L, Capone M, Frosali F, Rodolico G, Querci V, Abbate G, Angeli R, Berrino L, Fambrini M, Caproni M, Tonelli F, Lazzeri E, Parronchi P, Liotta F, Maggi E, Romagnani S, Annunziato F. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med. 2008;205:1903–1916. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Northfield JW, Kasprowicz V, Lucas M, Kersting N, Bengsh B, Kim A, Phillips RE, Walker BD, Thimme R, Lauer G, Klenerman P. CD161 expression on hepatitis C virus-specific CD8+ T cells suggests a distinct pathway of T cell differentiation. Hepatology. 2008;47:396–406. doi: 10.1002/hep.22040. [DOI] [PubMed] [Google Scholar]
- 92.Wald O, Pappo O, Safadi R, gan-Berger M, Beider K, Wald H, Franitza S, Weiss I, Avniel S, Boaz P, Hanna J, Zamir G, Eid A, Mandelboim O, Spengler U, Galun E, Peled A. Involvement of the CXCL12/CXCR4 pathway in the advanced liver disease that is associated with hepatitis C virus or hepatitis B virus. Eur J Immunol. 2004;34:1164–1174. doi: 10.1002/eji.200324441. [DOI] [PubMed] [Google Scholar]
- 93.Terada R, Yamamoto K, Hakoda T, Shimada N, Okano N, Baba N, Ninomiya Y, Gershwin ME, Shiratori Y. Stromal cell-derived factor-1 from biliary epithelial cells recruits CXCR4-positive cells: implications for inflammatory liver diseases. Lab Invest. 2003;83:665–672. doi: 10.1097/01.lab.0000067498.89585.06. [DOI] [PubMed] [Google Scholar]
- 94.Efsen E, Grappone C, Defranco RM, Milani S, Romanelli RG, Bonacchi A, Caligiuri A, Failli P, Annunziato F, Pagliai G, Pinzani M, Laffi G, Gentilini P, Marra F. Up-regulated expression of fractalkine and its receptor CX3CR1 during liver injury in humans. J Hepatol. 2002;37:39–47. doi: 10.1016/s0168-8278(02)00065-x. [DOI] [PubMed] [Google Scholar]
- 95.Burton JR, Jr, Klarquist J, Im K, Smyk-Pearson S, Golden-Mason L, Castelblanco N, Terrault N, Rosen HR. Prospective analysis of effector and regulatory CD4(+) T cells in chronic HCV patients undergoing combination antiviral therapy. J Hepatol. 2008 doi: 10.1016/j.jhep.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 96.Hjelmstrom P, Fjell J, Nakagawa T, Sacca R, Cuff CA, Ruddle NH. Lymphoid tissue homing chemokines are expressed in chronic inflammation. Am J Pathol. 2000 Apr;156(4):1133–8156. 1133–1138. doi: 10.1016/S0002-9440(10)64981-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bonacchi A, Petrai I, Defranco RM, Lazzeri E, Annunziato F, Efsen E, Cosmi L, Romagnani P, Milani S, Failli P, Batignani G, Liotta F, Laffi G, Pinzani M, Gentilini P, Marra F. The chemokine CCL21 modulates lymphocyte recruitment and fibrosis in chronic hepatitis C. Gastroenterology. 2003;125:1060–1076. doi: 10.1016/s0016-5085(03)01194-6. [DOI] [PubMed] [Google Scholar]
- 98.Saadoun D, Bieche I, Maisonobe T, Asselah T, Laurendeau I, Piette JC, Vidaud M, Cacoub P. Involvement of chemokines and type 1 cytokines in the pathogenesis of hepatitis C virus-associated mixed cryoglobulinemia vasculitis neuropathy. Arthritis Rheum. 2005;52:2917–2925. doi: 10.1002/art.21270. [DOI] [PubMed] [Google Scholar]
- 99.Grant AJ, Goddard S, Ahmed-Choudhury J, Reynolds G, Jackson DG, Briskin M, Wu L, Hubscher SG, Adams DH. Hepatic expression of secondary lymphoid chemokine (CCL21) promotes the development of portal-associated lymphoid tissue in chronic inflammatory liver disease. Am J Pathol. 2002;160:1445–1455. doi: 10.1016/S0002-9440(10)62570-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Marra F. Chemokines in liver inflammation and fibrosis. Front Biosci. 2002;7:d1899–d1914. doi: 10.2741/A887. [DOI] [PubMed] [Google Scholar]
- 101.Marra F, Romanelli RG, Giannini C, Failli P, Pastacaldi S, Arrighi MC, Pinzani M, Laffi G, Montalto P, Gentilini P. Monocyte chemotactic protein-1 as a chemoattractant for human hepatic stellate cells. Hepatology. 1999;29:140–148. doi: 10.1002/hep.510290107. [DOI] [PubMed] [Google Scholar]
- 102.Schwabe RF, Schnabl B, Kweon YO, Brenner DA. CD40 activates NF-kappa B and c-Jun N-terminal kinase and enhances chemokine secretion on activated human hepatic stellate cells. J Immunol. 2001;166:6812–6819. doi: 10.4049/jimmunol.166.11.6812. [DOI] [PubMed] [Google Scholar]
- 103.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bonacchi A, Romagnani P, Romanelli RG, Efsen E, Annunziato F, Lasagni L, Francalanci M, Serio M, Laffi G, Pinzani M, Gentilini P, Marra F. Signal transduction by the chemokine receptor CXCR3: activation of Ras/ERK, Src, and phosphatidylinositol 3-kinase/Akt controls cell migration and proliferation in human vascular pericytes. J Biol Chem. 2001;276:9945–9954. doi: 10.1074/jbc.M010303200. [DOI] [PubMed] [Google Scholar]