SUMMARY
Background
The mini-mental state exam (MMSE) has been used to address questions such as determination of appropriate cutoff scores for differentiation of individuals with intact cognitive function from patients with dementia and rate of cognitive decline. However, little is known about the relationship of performance in specific cognitive domains to subsequent overall decline.
Objective
To examine the specific and/or combined contribution of four MMSE domains (orientation for time, orientation for place, delayed recall, and attention) to prediction of overall cognitive decline on the MMSE.
Methods
Linear mixed models were applied to 505 elderly nursing home residents (mean age = 85, >12 years education = 27%; 79% F, mean follow-up = 3.20 years) to examine the relationship between baseline scores of these domains and total MMSE scores over time.
Results
Orientation for time was the only domain significantly associated with MMSE decline over time. Combination of poor delayed recall with either attention or orientation for place was associated with significantly increased decline on the MMSE.
Conclusions
The MMSE orientation for time predicts overall decline on MMSE scores over time. A good functioning domain added to good functioning delayed recall was associated with slower rate of decline.
Keywords: MMSE, cognitive domains, cognitive decline, prediction, dementia, Alzheimer’s disease
INTRODUCTION
Cognitive decline or dementia, has become one of the major concerns in the elderly population. Alzheimer’s disease (AD) is one of the most debilitating and prevalent forms of dementia, accounting for approximately 70% of dementia cases. It is characterized by progressive cognitive and functional decline. In the United States alone two to four million people suffer from AD, and the number is expected to increase as the elderly population is growing older (Mendez and Cummings, 2003). Thus, research studies have focused on the investigation of cognitive tests that can be sensitive to change in cognitive functioning. Since its development, the mini-mental state exam (MMSE; Folstein et al., 1975) has become one of the most widely used cognitive screening instruments for dementia. Its items cover various areas of cognitive domains (e.g., orientation, memory, language, and visual construction). The MMSE has become a widely used cognitive screening tool in both clinical and research settings due to factors including its brevity and straightforward administration. Indeed, the MMSE has inspired a myriad of scientific questions ranging from examining appropriate MMSE cutoff scores for differentiation of individuals with intact cognitive function from patients with dementia (Monsch et al., 1995), delineation of specific performance deficits on this instrument found at different stages of AD (Ashford et al., 1989; Galasko et al., 1990; Fillenbaum et al., 1994), examining prediction and rate of cognitive decline in AD (see Small et al., 1997; Han et al., 2000) and relating ante-mortem cognitive performance to post-mortem AD markers (Koepsell et al., 2008).
Much is known about the MMSE in terms of its validity as a screening tool for dementia, cutoff scores, sensitivity of items for differentiating subjects across levels of dementia severity, and rate of cognitive decline. However, little is known about the relationship of decline in specific cognitive domains such as orientation, memory, or attention to the natural course of dementia. What is the additional contribution of the combination of specific domains to overall cognitive decline? The main goal of this study was to predict the total MMSE score over time, from the scores of specific baseline MMSE domains and their combinations.
METHODS
Participants
Participants were 505 elderly nursing home residents from the Jewish Home and Hospital (JHH) in Bronx, NY and Manhattan, NY. The JHH has been an academic affiliate of the Mount Sinai School of Medicine (MSSM) for the last 25 years. Participants were part of a prospective, longitudinal study of cognition in old age, the Clinical and Biological Studies of Early Alzheimer’s Disease project, at the Department of Psychiatry, MSSM. Inclusion criteria in this study were age >60, at least two MMSE assessments, baseline MMSE scores of 10–25 (to avoid ceiling and floor effects), and complete demographic information (age, sex, and education). The study was approved by both the MSSM and JHH institutional review boards.
Administration of MMSE and definition of its subdomains
We followed standard administration protocols for the MMSE (Folstein et al., 1975) except for the administration of the attention domain, which was assessed based on spelling world backward only—serial seven calculations were not assessed. Four MMSE domains were examined: (1) orientation for time (month, date, year, day of week, season); (2) orientation for place (building, floor, city, county, state); (3) delayed recall, which was assessed by asking participants to recall three words (book, telephone, and penny) that they had previously been asked to repeat and memorize; and (4) attention (spelling world backward). Our definition of good performance was indicated by a score equal to or greater than the median score. For example, the possible score for the domain orientation for time ranges from 0 to 5, and good performance on this variable was defined by a score equal to or greater than 3. The domains orientation for place, delayed recall, and attention had median scores of 4, 1, and 3, respectively.
The other MMSE domains were excluded either based on previous research suggesting they tend to be affected later in the course of dementia (e.g., language items; Ashford et al., 1989; Small et al., 1997; Blair et al., 2007), which was one of the exclusion criteria in this study, or due to the narrow range (0–1) of possible scores (repetition, reading, writing, and praxis).
Data analysis
Descriptive analyses were performed to describe the study sample. Linear mixed models were used to examine, separately, the relationships of baseline dichotomies from four MMSE domains, and the interactions of the six pairs of dichotomies, with the total MMSE scores over time. The use of the mixed models enabled study of the MMSE scores across time while accounting for the within-subject correlation, thus providing an efficient way to use all the information from each participant. Another advantage of using the mixed models is that it permits analysis of the unbalanced data—i.e., different numbers of follow-up occasions or different follow-up times. This fits the needs of the present analysis, since the number of follow-up assessments varied from 1 to 14 and the follow-up time ranged from 0.016 to 12.93 years due to participant death and other attrition factors. All of the linear mixed models fitted in the present analysis included random intercept (i.e., fitted baseline) and random slope (in follow-up time), to take into account the fact that participants have different baseline MMSE scores and extents of decline over the follow-up period. For analyses of each domain separately, the model included the baseline domain (above or at median relative to below), time since baseline, the interaction of the baseline domain with time (i.e., the effect of the baseline domain on change over time), baseline age, female sex (relative to male), and education (under 8 years or 9 through 12 years, relative to over 12 years). For analyses of pairs of domains, the two baseline domains and their interaction replaced a single domain in the model. SAS GLIMMIX package was used to fit all linear mixed models.
RESULTS
The mean age of participants was 84.94 (SD = 7.35, range = 60–104). Of the 505 participants 399 (79.01%) were women, reflecting the old age and the residence type (nursing home) of the study sample. The initial mean MMSE score was 19.01 (SD = 4.27, range by definition = 10–25), and the mean follow-up period was 3.20 years (SD = 2.28, range = 0.016–12.93). Of all participants, 137 (27.02%) had an education level equal to or below 8 years, 235 (46.35%) had 9–12 years of education, and 135 (26.63%) had 13–24 years.
Table 1 shows the number of participants scoring below (poor) versus at or above the median (good) in all combinations of the four cognitive domains. As expected, the largest numbers of participants were in the extreme cells in which participants were either performing poorly on all domains (n = 65) or performing well on all domains (n = 66). There were fewer participants in cells in which the pattern of performance across the different domains was mixed (e.g., two good/two poor). The minimum was 13 people who performed well on delayed recall and on orientation for place, yet performed poorly on attention and orientation for time. Table 2 shows baseline demographic characteristics, length of follow-up, and MMSE scores of participants with good and poor performances in different MMSE domains. There were no significant differences, in any of the socio-demographic characteristics, between participants with good and poor performance in any domain. Table 3 presents results of linear mixed models to determine whether specific MMSE domains at baseline can characterize the MMSE total scores over time, after controlling for age (at baseline), sex, and education. In all analyses, except delayed recall when paired with orientation for time (p = 0.04), each domain was strongly positively associated with the MMSE total score (p <0.0001). Orientation for time was the only domain with a significant interaction with time (with less decline over time for participants with good baseline orientation for time) in its analysis. Figure 1 compares fitted models for participants with poor and good orientation for time at baseline, in which the covariates, age, gender, and education, were evaluated at the mean levels obtained from the overall sample.
Table 1.
Number of participants performing at or below the median and at or above the median in all cognitive domains
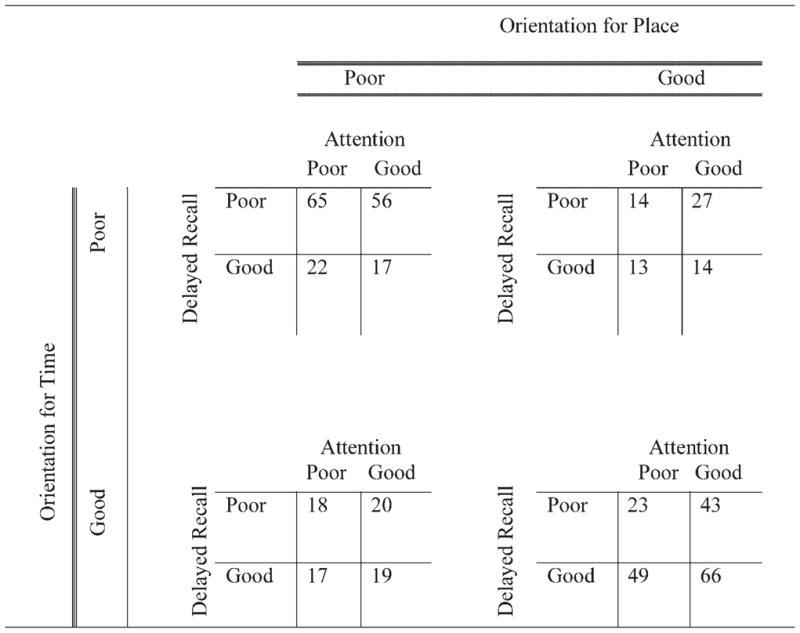 |
Table 2.
Baseline demographic characteristics, length of follow-up, and MMSE raw scores of good and poor performers in MMSE orientation-time, orientation-place, attention, and delayed recall domains
| Variables | Orientation-time |
Orientation-place |
Attention |
Delayed recall |
||||
|---|---|---|---|---|---|---|---|---|
| Good | Poor | Good | Poor | Good | Poor | Good | Poor | |
| Age (years) | ||||||||
| Mean | 84.80 | 85.31 | 85.14 | 84.93 | 85.03 | 85.12 | 84.19 | 85.75 |
| SD | 7.43 | 6.94 | 7.44 | 6.95 | 6.47 | 7.96 | 7.75 | 6.63 |
| Education (years) | ||||||||
| Mean | 11.22 | 10.79 | 11.45 | 10.56 | 10.90 | 11.12 | 11.79 | 9.99 |
| SD | 3.90 | 4.03 | 3.78 | 4.14 | 3.90 | 4.06 | 3.72 | 3.95 |
| Sex (%) | ||||||||
| Male | 59.43 | 40.57 | 55.66 | 44.34 | 50.00 | 50.00 | 50.00 | 50.00 |
| Female | 51.63 | 48.37 | 50.13 | 49.87 | 55.38 | 44.62 | 44.86 | 55.14 |
| Follow-up (years) | ||||||||
| Mean | 3.35 | 3.04 | 3.23 | 3.18 | 3.09 | 3.30 | 3.43 | 3.15 |
| SD | 2.39 | 2.15 | 2.31 | 2.26 | 2.29 | 2.28 | 2.37 | 2.16 |
| MMSE | ||||||||
| Mean | 21.41 | 16.34 | 21.44 | 16.51 | 20.93 | 17.44 | 21.03 | 16.71 |
| SD | 3.04 | 3.80 | 2.99 | 3.90 | 3.52 | 4.16 | 3.33 | 3.91 |
| n | 269 | 236 | 259 | 246 | 232 | 273 | 262 | 221 |
Note: Data represent mean and standard deviations; good = scores at or above the median; poor = scores below the median; educ = education; MMSE = mini-mental state exam; n = sample size.
For delayed recall: due to missing data, gender distribution does not add up to 100%.
Table 3.
Linear mixed models of change in MMSE score over time based on poor performance on single cognitive domains
| Domain | Delayed recall | Attention | Orientation for time | Orientation for place | ||||
|---|---|---|---|---|---|---|---|---|
| Variable | Estimatea | p-value | Estimate | p-value | Estimate | p-value | Estimate | p-value |
| Baseline age | −0.0858 | 0.0142 | −0.0971 | 0.0082 | −0.1044 | 0.0009 | −0.1249 | 0.0002 |
| Female | 0.0083 | 0.9892 | −0.4295 | 0.5035 | 0.2970 | 0.5912 | 0.2577 | 0.6578 |
| Education ≤ 8 yrs | −2.4037 | 0.0003 | −1.1834 | 0.0988 | −2.4323 | <0.0001 | −1.5425 | 0.0146 |
| 9 ≤ education ≤ 12 | −0.6004 | 0.3086 | −0.2954 | 0.6359 | −0.4114 | 0.4396 | −0.6399 | 0.2538 |
| Domain (above median) | 3.5890 | <0.0001 | 3.3335 | <0.0001 | 5.2985 | <0.0001 | 5.0991 | <0.0001 |
| Time | −1.5682 | <0.0001 | −1.4579 | <0.0001 | −1.7799 | <0.0001 | −1.5517 | <0.0001 |
| Domain*time | 0.1776 | 0.1420 | −0.0951 | 0.4399 | 0.4635 | 0.0001 | 0.1054 | 0.3768 |
Estimates represent the extent of annual increase on the MMSE.
Figure 1.
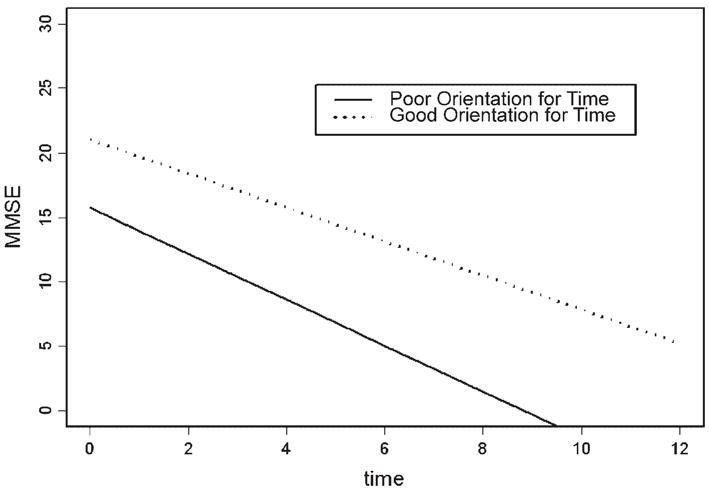
Fitted linear models for MMSE score over time according to baseline performance on orientation for time.
Table 4 presents results of linear mixed models to determine whether pairs of MMSE domains at baseline can characterize the MMSE total scores over time, after controlling for age (at baseline), sex, and education. In contrast to the specific domains, no interaction of pairs of domains was significantly associated with the total score. In the analyses of interactions of pairs of domains with time, there were significant interactions for delayed recall with attention and delayed recall with orientation for place, and a trend level interaction for attention with orientation for time. In each interaction, participants who performed well on both domains declined less than would have been expected from the separate effects of performing well on each domain separately. Thus participants who scored well on both domains had a decline that was less than or similar to the other three combinations of good and poor baseline performance. Figure 2 compares fitted models for participants with combinations of good and poor attention and delayed recall, in which the covariates, age, gender, and education, were evaluated at the overall sample mean values.
Table 4.
Linear mixed models of change in MMSE score over time based on poor performance on pairs of cognitive domains
| Domain combination | Domain 1 |
Delayed recall |
Domain 1 |
Delayed recall |
Domain 1 |
Delayed recall |
Domain 1 |
Attention |
Domain 1 |
Attention |
Domain 1 |
Orientation for time |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Domain 2 | Attention | Domain 2 | Orientation for time | Domain 2 | Orientation for place | Domain 2 | Orientation for time | Domain 2 | Orientation for place | Domain 2 | Orientation for place | |
| Variable | Estimatea | p-value | Estimate | p-value | Estimate | p-value | Estimate | p-value | Estimate | p-value | Estimate | p-value |
| Baseline age | −0.0666 | 0.0545 | −0.0872 | 0.0050 | −0.1045 | 0.0012 | −0.0875 | 0.0055 | −0.1097 | 0.0010 | −0.1101 | 0.0003 |
| Female | −0.2945 | 0.6251 | 0.2825 | 0.6022 | 0.2868 | 0.6103 | −0.0973 | 0.8600 | −0.1209 | 0.8348 | 0.3854 | 0.4672 |
| Education ≤ 8 yrs | −1.3083 | 0.0520 | −2.4971 | <0.0001 | −1.7151 | 0.0051 | −1.6592 | 0.0070 | −0.6000 | 0.3546 | −1.9999 | 0.0005 |
| 9 ≤ Education ≤ 12 | −0.4241 | 0.4690 | −0.4636 | 0.3743 | −0.6769 | 0.2119 | −0.3120 | 0.5591 | −0.4423 | 0.4327 | −0.5106 | 0.3165 |
| Domain 1(above median) | 3.4949 | <0.0001 | 1.6096 | 0.0358 | 3.0971 | <0.0001 | 2.6058 | 0.0003 | 2.7374 | 0.0002 | 4.3719 | <0.0001 |
| Domain 2(above median) | 3.1882 | <0.0001 | 4.3152 | <0.0001 | 4.9403 | <0.0001 | 4.8033 | <0.0001 | 4.8446 | <0.0001 | 3.9984 | <0.0001 |
| Domain 1*domain 2 | 0.3815 | 0.7180 | 0.7939 | 0.4316 | −1.0105 | 0.3191 | 0.5168 | 0.5973 | 0.1517 | 0.8822 | −1.0233 | 0.3179 |
| Time | −1.3418 | <0.0001 | −1.8720 | <0.0001 | −1.4743 | <0.0001 | −1.5731 | <0.0001 | −1.5988 | <0.0001 | −1.7532 | <0.0001 |
| Domain 1*time | −0.2542 | 0.1852 | 0.3430 | 0.0911 | −0.1841 | 0.3350 | −0.3966 | 0.0338 | 0.0851 | 0.6299 | 0.4988 | 0.0045 |
| Domain 2*time | −0.3893 | 0.0148 | 0.6008 | 0.0001 | −0.1731 | 0.2724 | 0.2313 | 0.2128 | 0.3217 | 0.0858 | −0.0681 | 0.7335 |
| Domain 1*domain 2*time | 0.7002 | 0.0046 | −0.4012 | 0.1135 | 0.5717 | 0.0225 | 0.4247 | 0.0838 | −0.3557 | 0.1450 | −0.0008 | 0.9976 |
Estimates represent the extent of annual increase on the MMSE.
Figure 2.
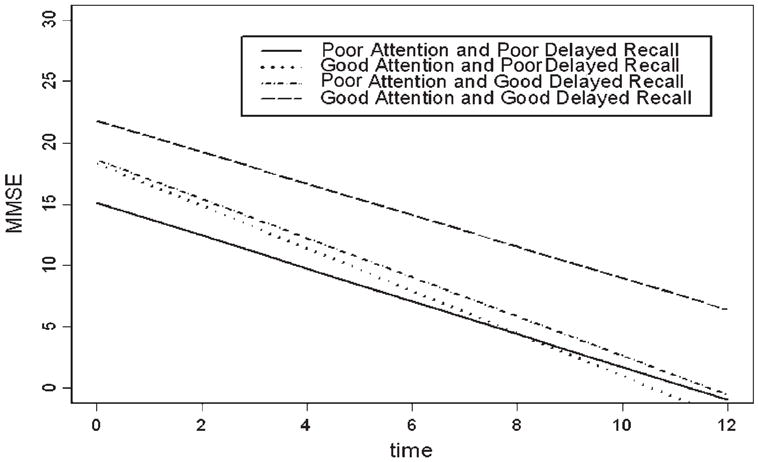
Fitted linear models for MMSE score over time according to baseline performance on attention and delayed recall.
For all analyses of both domains and pairs, total MMSE declined over time (p <0.0001). With the exception of the analysis for delayed recall and attention (p = 0.055), the MMSE total score decreased with increasing age. There was no association of total MMSE with sex or education of 9 through 12 years. Education less than nine years was significantly associated with lower total MMSE in all analyses that did not include attention.
DISCUSSION
The MMSE is one of the most widely used cognitive screening tools in dementia research; however, research examining whether specific or combinations of MMSE domains can predict cognitive decline has been limited. Our analysis revealed that orientation for time was the only MMSE domain for which poor baseline performance was significantly associated with faster rate of decline in the total MMSE score after controlling for age, sex, and education. Poor orientation for time doubled the rate of decline in the MMSE. Additionally, although delayed recall by itself did not predict the rate of decline in the MMSE, when considered in combination with attention or orientation for place, those performing well on both delayed recall and the other domain had slower or similar rate of decline to those with poor or mixed baseline performance on the pair of domains.
These results are similar to those of Ashford et al. (1989), who found that MMSE-orientation (e.g., date) and delayed recall were among the earliest items to be lost in AD. Orientation for time and place were the MMSE domains with the largest extent of change over time in patients with AD (Small et al., 1997). The longitudinal nature, length of follow-up, and sample size of this study extend those findings. Other studies aimed at differentiating normal controls from early dementia and in identifying the different stages of dementia, have found that MMSE-orientation is impaired early in the disease process (e.g., Galasko et al., 1990; Fillenbaum et al., 1994). Impairment in orientation as measured by the MMSE constitutes a clear specific deficit, and is not due to diffused cognitive dysfunction.
Such a deficit is likely to be associated with specific neural substrates. Animal studies have shown the involvement of the hippocampus in the processing of spatial and temporal information (Eichenbaum et al., 1999). A clinicopathological study of AD patients found that poor performance on tasks of orientation was associated with neurofibrillary tangle densities in the CA1 field of the hippocampus, superior parietal, and posterior cingulate cortex of the right hemisphere (Giannakopoulos et al., 2000). Similarly, a more recent study found that impairment in an actual navigation task among patients with AD and mild cognitive impairment (MCI) was associated with right posterior hippocampus and parietal volumes (Delpolyi et al., 2007). Although inconsistent with the findings of Giannakopoulos et al. (2000) who found that both spatial and temporal orientations were associated with neuropathology in the CA1 field of the hippocampus, superior parietal, and posterior cingulate cortex, the fact that in the current study orientation for place, by itself, was not associated with faster decline suggests that specific neural pathways may underlie different aspects of the more general orientation domain. Overall, the unique contribution of orientation as a separate predictor of cognitive decline indicates that cognitive domains other than memory may predict as strongly the progression of AD dementia as memory. Several longitudinal studies of individuals with MCI as well as persons with intact cognition found that memory and non-memory domains can accurately predict decline to dementia (Mickes et al., 2007; Silveri et al., 2007). The present results suggest that assessment of the orientation domain is important in investigations aimed at identifying individuals at high risk of cognitive decline, and in which memory impairment has been the main focus. The current findings may also have implications for clinical trials, which could benefit from enriching their sample of at-risk elderly showing deficits in orientation tasks.
Delayed recall, by itself, at baseline did not predict faster rate of decline in the MMSE. This finding is inconsistent with the myriad of neuropsychological studies reporting an association between poor performance on tests of delayed recall and cognitive decline (Kluger et al., 1999; Lange et al., 2002; Mortimer et al., 2004). It is noteworthy that, unlike neuropsychological tests, the brevity of the MMSE-memory domain (recall of three objects) may affect its sensitivity in predicting cognitive decline (this same rationale can explain the lack of sensitivity of the MMSE-attention domain, which was assessed by spelling world backward—not serial seven calculations, which is a more challenging task). Thus, differences in study design and sample characteristics (i.e., length of follow-up, number of assessments, and severity of dementia) could explain this discrepancy. However, the lack of longitudinal change in delayed recall has been noted (Brooks et al., 1993; Small et al., 1997). Given the broad clinical utility and use of the MMSE, the insensitivity of the MMSE’s delayed recall component to the prediction of cognitive decline or dementia progression is noteworthy.
To the extent that cognitive reserve (Scarmeas et al., 2003) is affecting the synergistic effect between delayed recall and other cognitive domains—e.g., orientation and attention (indeed, we found that elderly with higher levels of education were less likely to decline), these findings may indicate that delayed recall, in addition to impairment of other cognitive domains, passes the threshold of cognitive reserve and the individual is no longer protected against cognitive decline. Moreover, the fact that these interactions included delayed recall (memory) and orientation may imply that these two domains are acutely sensitive to disease progression and/or extensive brain pathology. Indeed, studies have shown that deficits in multiple domains (e.g., memory and attention) are evident in the preclinical stage of dementia (Kluger et al., 1997; Nordlund et al., 2005). Thus it is possible that when performance on MMSE memory in addition to another domain is impaired, there may be enough evidence to recommend a comprehensive dementia evaluation.
A specific contribution of attention functioning in the prediction of cognitive decline was absent. Nonetheless, its interactions with delayed recall and orientation for time show that there were contributions of attention that depended on the level of performance of the other domain. It is important to note that the evaluation of attention functioning is a complex one given the various subdivisions that comprise the attentional processing system, which appear to be distinctively affected at different levels of dementia severity (see Foldi et al., 2002; Silveri et al., 2007).
Although attention can and does affect information encoding, the present findings suggest that memory and attention domains are not fully dependent on each other. For example, 146/505 (28.9%) participants had good scores on attention and poor scores on delayed recall. Moreover, based on these results, it appears that people who score well on a given combination of domains tend to be slower decliners than those whose performance is either generally poor or more varied across domains (e.g., poor orientation for place and good delayed recall).
This study has several strengths. The follow-up period was on average 3 years, with a maximum follow-up time of 13 years. Similarly, the number of assessments was high, comprising a maximum of 15 visits. Moreover, we analyzed longitudinal data from elderly with a wide, yet more or less normally distributed, range of educational attainment and a wide range of cognitive functioning without the confounds of floor and ceiling effects.
There were weaknesses in this study. It is important to note that cognitive measures are sensitive to the effects of culture and education (see Ponton and Ardila, 1999). Performance on the MMSE is affected by age, educational attainment, and cultural factors (Crum et al., 1993; Monsch et al., 1995; Black et al., 1999). We were able to control for age and education, and still the results remained strongly significant. However, the sample was relatively homogeneous ethnically (70.50% Caucasians). Future studies addressing these findings to other elderly populations of different ethnic/cultural backgrounds are warranted (i.e., minority elderly). Another important issue is the possibility that baseline performance in orientation for time may differentially predict cognitive decline across the different types of dementia. Thus, patterns of decline using the MMSE domains across the different types of dementia should also be examined. Our study is unique in that it represents one of the few attempts to directly characterize rate of cognitive decline over a period as long as 13 years using four MMSE domains as predictors. Future research can also be aimed at examining whether MMSE domains, which are relatively simple, can predict cognitive decline using more sophisticated neuropsychological tests and/or clinical rating scales.
KEYPOINTS.
Poor performance on the MMSE-orientation for time domain is associated with faster rate of decline on total MMSE scores over time.
Good performance on the MMSE-delayed recall domain, in addition to good performance on another domain, is associated with slower rate of decline on total MMSE scores over time.
Acknowledgments
This study was supported by NIA grants K01 AG023515–01A2 (Dr. Beeri), AG02219 (Dr. Haroutunian), and AG05138 (Dr. Sano); the Dextra Baldwin McGonagle Foundation; and the Joseph E. and Norma G. Saul Foundation.
Footnotes
CONFLICT OF INTEREST
None declared.
References
- Ashford JW, Kolm P, Colliver JA, Bekian C. Alzheimer patient evaluation and the mini-mental state: item characteristic curve analysis. J Gerontol. 1989;44(5):139–146. doi: 10.1093/geronj/44.5.p139. [DOI] [PubMed] [Google Scholar]
- Black SA, Espino DV, Mahurin R, et al. The influence of noncognitive factors on the mini-mental state examination in older Mexican–Americans: findings from the Hispanic EPESE. J Clin Epidemiol. 1999;52(11):1095–1102. doi: 10.1016/s0895-4356(99)00100-6. [DOI] [PubMed] [Google Scholar]
- Blair M, Marczinski CA, Davis-Faroque N, Kertesz A. A longitudinal study of language decline in Alzheimer’s disease and frontotemporal dementia. J Int Neuropsychol Soc. 2007;13:237–245. doi: 10.1017/S1355617707070269. [DOI] [PubMed] [Google Scholar]
- Brooks JO, III, Yesavage JA, Taylor J, et al. Cognitive decline in Alzheimer’s disease: elaborating on the nature of the longitudinal factor structure of the mini-mental state examination. Int Psychoger. 1993;5(2):135–146. doi: 10.1017/s1041610293001474. [DOI] [PubMed] [Google Scholar]
- Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the mini-mental state examination by age and educational level. J Am Med Assoc. 1993;269(18):2386–2391. [PubMed] [Google Scholar]
- Delpolyi AR, Rankin KP, Mucke L, Miller BL. Spatial cognition and the human navigation network in AD and MCI. Neurology. 2007;69:986–997. doi: 10.1212/01.wnl.0000271376.19515.c6. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Dudchenko P, Wood E, Shapiro M. The hippocampus, memory, and place cells: is it spatial memory or a memory space? Neuron. 1999;23:209–226. doi: 10.1016/s0896-6273(00)80773-4. [DOI] [PubMed] [Google Scholar]
- Fillenbaum GG, Wilkinson WE, Welsh KA, Mohs RC. Discrimination between stages of Alzheimer’s disease with subsets of mini-mental state examination items. Arch Neurol. 1994;51:916–921. doi: 10.1001/archneur.1994.00540210088017. [DOI] [PubMed] [Google Scholar]
- Foldi NS, Lobosco J, Schaefer LA. The effect of attentional dysfunction in Alzheimer’s disease: theoretical and practical implications. Semin Speech Lang. 2002;23(2):139–150. doi: 10.1055/s-2002-24990. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Galasko D, Klauber MR, Hofstetter R, Salmon DP. The mini-mental state examination in the early diagnosis of Alzheimer’s disease. Arch Neurol. 1990;47:49–52. doi: 10.1001/archneur.1990.00530010061020. [DOI] [PubMed] [Google Scholar]
- Giannakopoulos P, Gold G, Duc M, Michel J-P. Neural substrates of spatial and temporal disorientation in Alzheimer’s disease. Acta Neuropathol. 2000;100:189–195. doi: 10.1007/s004019900166. [DOI] [PubMed] [Google Scholar]
- Han L, Cole M, Bellavance F, McCusker J. Tracking cognitive decline in Alzheimer’s disease using the mini-mental state examination: a meta-analysis. Int Psychoger. 2000;12(2):231–247. doi: 10.1017/s1041610200006359. [DOI] [PubMed] [Google Scholar]
- Kluger A, Ferris SH, Golomb J, Mittelman MS. Neuropsychological prediction of decline to dementia in nondemented elderly. J Geriatr Psychiatry Neurol. 1999;12:168–179. doi: 10.1177/089198879901200402. [DOI] [PubMed] [Google Scholar]
- Kluger A, Gianutsos JG, Golomb J, et al. Patterns of motor impairment in normal aging, mild cognitive decline, and early Alzheimer’s disease. J Gerontol Psychological Sciences. 1997;52(1):28–39. doi: 10.1093/geronb/52b.1.p28. [DOI] [PubMed] [Google Scholar]
- Koepsell TD, Kurland BF, Harel O, Johnson EA. Education, cognitive function, and severity of neuropathology in Alzheimer’s disease. Neurology. 2008;70:1732–1739. doi: 10.1212/01.wnl.0000284603.85621.aa. [DOI] [PubMed] [Google Scholar]
- Lange KL, Bondi MW, Salmon DP, et al. Decline in verbal memory during preclinical Alzheimer’s disease: examination of the effect of APOE genotype. J Int Neuropsychol Soc. 2002;8:943–955. doi: 10.1017/s1355617702870096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MF, Cummings JL. Dementia: A Clinical Approach. 3. Elsevier: Philadelphia, PA; 2003. [Google Scholar]
- Mickes L, Wixted JT, Fennema-Notestine C, et al. Progressive impairment on neuropsychological tasks in a longitudinal study of preclinical Alzheimer’s disease. Neuropsychology. 2007;21(6):696–705. doi: 10.1037/0894-4105.21.6.696. [DOI] [PubMed] [Google Scholar]
- Monsch AU, Foldi NS, Ermini-Funfschilling DE, Berres M. Improving the diagnostic accuracy of the mini-mental state examination. Acta Neurol Scand. 1995;92:145–150. doi: 10.1111/j.1600-0404.1995.tb01029.x. [DOI] [PubMed] [Google Scholar]
- Mortimer JA, Gosche KM, Riley KP, Markesbery WR. Delayed recall, hippocampal volume and Alzheimer neuropathology: findings from the Nun study. Neurology. 2004;62:428–432. doi: 10.1212/01.wnl.0000106463.66966.65. [DOI] [PubMed] [Google Scholar]
- Nordlund A, Rolstad S, Hellström P, Sjögren M. The Goteborg MCI study: mild cognitive impairment is a heterogeneous condition. J Neurol Neurosurg Psychiatr. 2005;76:1485–1490. doi: 10.1136/jnnp.2004.050385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponton MO, Ardila A. The future of neuropsychology with Hispanic populations in the United States. Arch Clin Neuropsychol. 1999;14(7):565–580. [PubMed] [Google Scholar]
- Scarmeas N, Zarahn E, Anderson KE, et al. Cognitive reserve modulates functional brain responses during memory tasks: a PET study in healthy young and elderly subjects. Neuroimage. 2003;19(3):1215–1227. doi: 10.1016/s1053-8119(03)00074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MC, Reali G, Jenner C, Puopolo M. Attention and memory in the preclinical stage of dementia. J Geriatr Psychiatry Neurol. 2007;20:67–75. doi: 10.1177/0891988706297469. [DOI] [PubMed] [Google Scholar]
- Small BJ, Viitanen M, Backman L. Mini-mental state examination item scores as predictors of Alzheimer’s disease: incidence data from the Kungsholmen project, Stockholm. J Gerontol Medical Sciences. 1997;52(5):M299–M304. doi: 10.1093/gerona/52a.5.m299. [DOI] [PubMed] [Google Scholar]


