Abstract
PURPOSE
To assess the influence of arterial and venous vascular compliances in the neck region on the measurement of the change in intracranial volume during the cardiac cycle.
MATERIALS AND METHODS
Arterial and venous blood flows were imaged by MRI phase contrast at two different locations, one close to the skull base (upper) and one 2-3 cm lower, around C3 level (lower). Maximal intracranial volume change (ICVC) measurements were derived from the momentary differences between the arterial inflow and venous outflow rates at the upper and lower locations separately to assess the influence of the compliances of the vessel segments bounded by the two different imaging locations. Imaging location for the cranio-spinal cerebrospinal fluid flow was a constant variable in this experiment.
RESULTS
The systolic ICVC obtained using the lower location was consistently larger than when using the upper location. Comparison between arterial and venous flow dynamics revealed a much larger changes in flow dynamic and lumen areas in the veins compared with the arteries, which explain the large venous influence on the intracranial volume change measurement.
CONCLUSION
Arterial inflow and venous outflow should be sampled at a level close to the skull base (C1-C2) to minimize the influence of the compliance of arteries and the collapsibility of veins for a reliable measurement of ICVC.
Keywords: Intracranial volume change, cerebral arterial inflow and venous outflow, phase contrast magnetic resonance imaging, vascular compliance
INTRODUCTION
Intracranial volume (ICV) is the sum of the volumes of brain tissue, cerebral blood and cerebral spinal fluid (CSF) in the cranial compartment. The intracranial volume slightly increases during systole and decreases in diastole due to the pulsatility of the blood flow (1-3). These small fluctuations are utilized for noninvasive measurements of intracranial compliance and pressure, which are derived from the ratio of the maximal volume and pressure changes during the cardiac cycle (2-5). The MR measurement of this small volume change is obtained from the momentary difference between transcranial inflow and outflow of blood and CSF to and from the cranium. This is a challenging measurement because it is derived from the difference between large inflow and outflow volumes. Reliable measurement of maximal ICV change (ICVC) requires careful consideration of the scanning protocol and utilization of the cine phase contrast technique. It has been previously shown that a synchronized measurement of the CSF and blood flow considerably reduced measurement variability, from 18% (2) to about 10% (6), because it eliminates erroneous time delays when the CSF and blood flows are sampled at different heart rates. A synchronous measurement of blood and CSF flow rates can be achieved using a single scan with interleaved dual velocity encoding (VENC) phase contrast technique (6), or with two separate scans where the CSF and blood flow images are reconstructed at the same time points of the cardiac cycle and at the same projected heartrate. Large measurement variability in the ICVC, over 30%, has been recently reported with unsynchronized sampling of the blood and CSF flow waveforms (7). In addition, the location of the imaging plane at which the arterial and venous blood flows was measured was lower and further away from the cranium, compared with levels used in earlier reports (1-6).
This note focuses of the influence of the level at which the arterial inflow and venous outflow are measured on the calculation of the ICVC. The influence of the location at which the CSF is measured will be considered in a separate report. From review of the literature, it seems that different groups choose different levels above the carotid bifurcation for the measurement of the total cerebral blood flow. The two most common locations are an upper level that intersects the v2 segment of the vertebral arteries (upper C2 just before the vertebral veins turn to enter the skull base) (1-6) and a lower location, further away from the skull base, typically between C2 and C3 (7,8). Due to the pulsatile nature of the arterial and venous flow, and due to the finite compliance of the vessel segment between these two levels, the shape of the volumetric flow waveform can be different between the two locations with the same total volumetric flow. Therefore, it is expected that the location of the measurement may influence the calculation of the intracranial volume change. The degree by which the pulsatility of the arterial and venous blood flows influences the measurements of the ICV change, respectively, were investigated in order to account for this influence for improved reliability of ICV change, intracranial compliance and pressure measurements by MRI.
MATERIALS AND METHODS
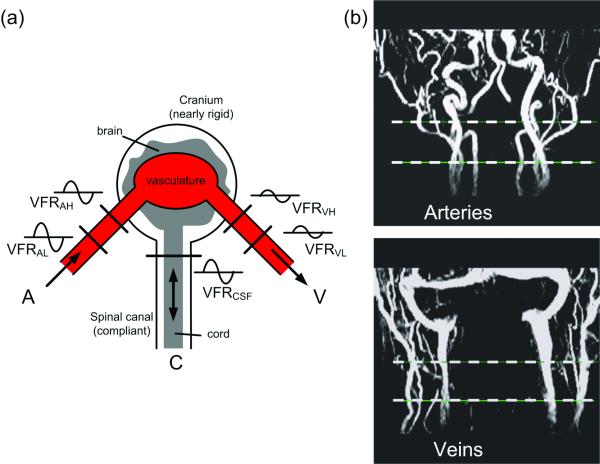
MRI data sets from 9 subjects who underwent measurements of intracranial compliance and pressure (MRICP) were analyzed. All subjects signed an informed consent approved by theinstitutional review board. Each data set included two retrospectively gated high velocity encoded cine phase contrast (PC) scans (70 cm/sec) for measurements of the total cerebral blood flow and a third cine PC scan with low VENC (7 cm/sec) at the upper C2 level for measurement of the cranio-spinal oscillatory CSF flow volume. Other imaging parameters include FOV of 14cm, slice thickness of 6 mm, acquisition matrix of 256×160, minimal TR and TE of 12 and 7ms, respectively, and flip angle of 20 degrees. The two high VENC scans were placed one at an upper location (upper C2 level) and the second at a lower level (lower C2-upper C3). The average distance between these two levels was 21.6±7.3 mm. Images were reconstructed into 32 cardiac phases using the same projected heart rate for both locations. A short 2D TOF MRA scan covering the upper cervical spine was used as a scout for guiding the selection of the upper and lower locations. The imaging planes were selected at orientations that are as perpendicular as possible to the main 4 arteries leading blood to the brain (internal carotid and vertebral arteries), and internal jugular veins (IJV). Schematic model of the location of the inflow and outflow measurements and examples of coronal MIP MRA and MRV with the 2 locations are shown in Figure 1a and 1b, respectively. The time-dependent volumetric flow rate waveforms were obtained by integrating the flow velocities inside the lumen cross-sectional area for each of the 32 time frames. The vessel lumen boundary was obtained using the previously described pulsatility-based lumen segmentation (PUBS) method (9). This method utilizes the temporal velocity information to differentiate between lumen and background pixels. The PUBS method provides an average of fivefold reduction in lumen area and volumetric flow measurements’ variability compared with manual segmentation for these in-vivo flow data (9)
Figure 1.
(a) The compartmental model of the craniospinal system demonstrating the measurement of intracranial volume changes from the differences between volumetric arterial inflow (A), venous outflow (V), and CSF flow (C) rates. The level at which the blood inflow and outflow is imaged can influence the measurement of intracranial volume change. (b) Coronal MIP MRA (top) and MRV (bottom) with the 2 locations used for the blood flows measurements are indicated by the dashed line.
The ICV change waveform is calculated following a previously described method (2). Briefly, a transcranial volumetric flow rate (VFR) is first obtained for each time point of the cardiac cycle by subtracting the venous and CSF outflow rates from the arterial inflow rate. Then the ICV change is derived by integration of the transcranial flow rate waveform with respect to time as shown in Eq. [1].
| [1] |
whereVFRART, VFRVEN, andVFRCSF are the arterial, venous, and CSF volumetric flow rates, respectively, and RR is the period of one cardiac cycle.
The maximal volume change occurring in the arterial and venous vessel segments that are bounded by the upper and lower imaging planes were calculated to assess their influence on the derivation of the ICV changes measurement, respectively. The volume change waveform of the individual segment was calculated by subtracting the outlet flow rates from the inlet flow rates for each time point and then integrating the net volumetric flow rate with respect to time, as described in Eq. [2] and Eq. [3].
| [2] |
| [3] |
whereVFRI andVFRO are measured inlet and outlet volumetric flow rates, respectively. dVFR and ΔVC are the net volumetric flow rate between in-outlet flows and the volume change of vessel segment, respectively. Prior to the calculation of the volume change, a constant value (dc) is either added or subtracted to maintain volume conservation over the cardiac cycle as described in Eq. [4] and Eq. [5].
| [4] |
| [5] |
whereCVFR represents the dc-corrected volumetric flow rate. In addition, the maximal intracranial volume change was calculated using the arterial and venous flow waveforms derived from the upper and lower locations, separately, with the same CSF flow waveform.
A paired t-test was applied to determine the significance of differences between the two ICVC measurements. The influence of the degree of the pulsatility of the arterial and venous flows on the ICVC measurement was assessed from the relationship between the peak-to-peak amplitude of arterial and venous flows and the ratio of the upper and lower measurements of ICVC. In addition, for each individual arterial and venous vessel segments, the peak-to-peak amplitude of the net volumetric flow was calculated and compared with the maximal volume change of that segment. Linear regression was then used to identify correlations between pulsation levels and volume changes.
RESULTS
In all nine data sets, the ICVC measurement obtained using the upper location was smaller than the one obtained with the lower location
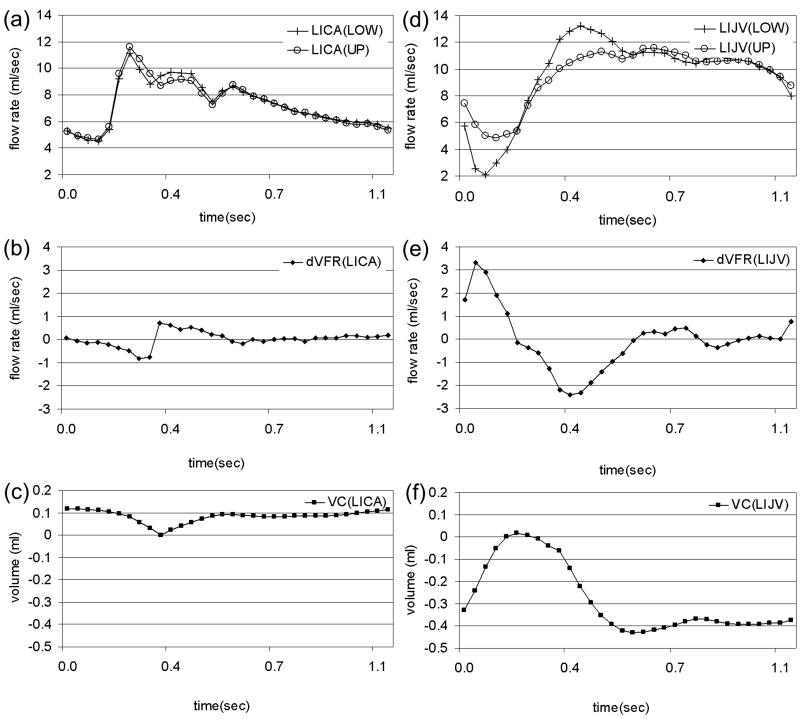
The respective mean ICVC were 0.51 vs. 0.71 mL. The difference was statistically significant with p<0.05. The mean ratio of the lower vs. upper measurements was 1.39±0.32 (mean±SD). Examples of volumetric blood flow and segmental volume change waveforms in an internal carotid artery and internal jugular vein obtained at the upper and lower imaging planes are shown in Figure 2. The measured volumetric flow waveforms are shown in Figure 2a and d, where the waveforms from the upper (○) and lower (+) locations are shown in the same graph. The net volumetric flow rates for these vessel segments are shown in Figure 2b and e, respectively and the volume change for the vessel segments bounded by the upper and lower imaging planes are shown in Figure 2c and f, respectively. As can be seen, the arterial waveforms at the upper and lower locations are very similar and therefore the volume change in the arterial vessel segments are small as shown in Figure 2c. On the other hand, the venous flow is much less similar between the upper and lower location resulting with a larger volume changes in the venous vessel segments as can be seen in Figure 2f.
Figure 2.
An example of the blood flow dynamics in a left carotid arteries (left column) and left internal jugular vein (right column) measured at upper and lower location from one subject. The measured flow waveforms are shown in (a) and (d) respectively, (b) and (e) are the flow differences derived from (a) and (d), respectively. The volume change waveform in (c) and (f) are derived from the integration of the waveforms in (b) and (e) with respect to time, over the cardiac cycle.
The average pulse amplitude (peak-to-peak value) of the arterial and venous volumetric flows is summarized in Table 1. In general, the arterial pulse amplitude is similar at the two levels (mean ratio of 1.04), while the venous pulsatility is larger at the lower location (mean ratio of 1.3). The individual segments and the combined arterial and venous segments maximal volume change are listed in Table 1. The maximal volume change of the venous side is almost twice that of the arterial side, 0.39±0.25 mL vs. 0.21±0.1 mL. Thus these findings suggest that the venous side influence on the ICVC measurements is considerably larger than the arterial side.
TABLE 1.
Blood flow parameters obtained from the upper and lower imaging planes.
| Peak to peak volume change (ml) |
Peak to peak VFR (ml/sec) |
Mean velocity (cm/sec) |
Lumen area (cm2) |
|||||
|---|---|---|---|---|---|---|---|---|
| Intracranial compartment |
Arteries | Veins | RIJV | LIJV | RIJV | LIJV | ||
| MRI | Lower | 0.71 | 11.99 | 7.43 | 14.0 | 12.1 | 0.47 | 0.32 |
| plane location |
Upper | 0.59 | 11.64 | 5.64 | 20.2 | 18.5 | 0.32 | 0.20 |
|
| ||||||||
| Ratio | Mean | 1.39 | 1.04 | 1.30 | 0.71 | 0.69 | 1.49 | 1.72 |
| (L/U) | SD | 0.32 | 0.100 | 0.45 | 0.20 | 0.34 | 0.36 | 0.65 |
Note: RIJV, LIJV=Right, left internal jugular vein.
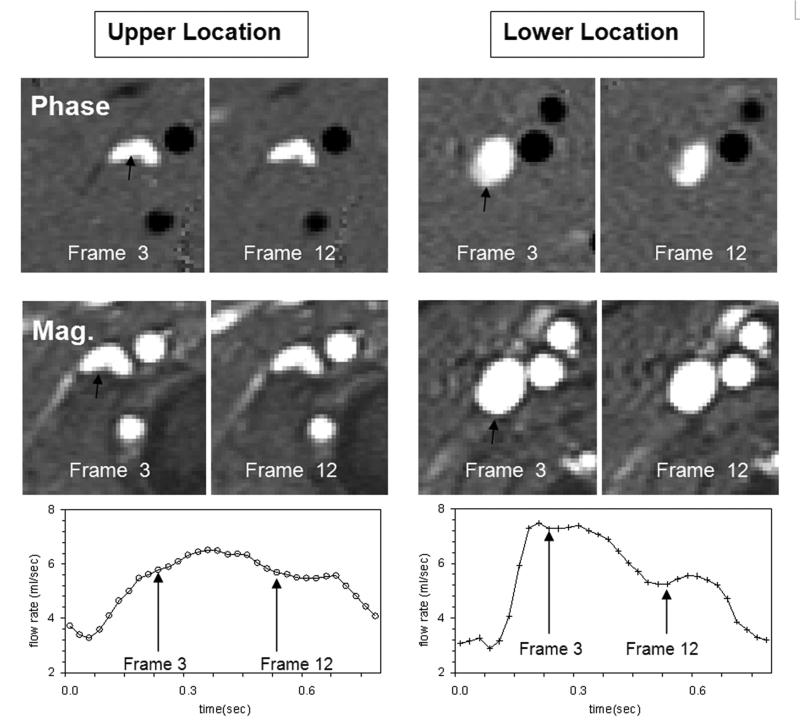
Large differences in the venous lumen area were found between the upper and lower location of the jugular veins. These differences are considerably larger than a measurement error of approximately 3-5% associated with the PUBS method for similar lumen sizes (9). On average, at the lower location, the lumen area is larger and the mean velocity is slower compared with the upper location, by 60% and 30%, respectively. Mean and SD of lumen areas and velocities at the two levels are listed in Table 1. An example of velocity and magnitude images from upper and lower locations is shown in Figure 3. These images also demonstrate that the flow velocities during the cardiac cycle are more uniform in the upper location compared with the lower location (Figure 3 top).
Figure 3.
An example of phase (top) and magnitude (middle) images from the upper (left) and lower (right) locations showing the velocity and the lumen of the right internal jugular vein (black arrow). Two cardiac frames are shown to demonstrate changes in velocities across the lumen. The flow velocities are more uniform during the cardiac cycle in the upper location. The corresponding volumetric flow rate waveforms at both locations are shown at the bottom. As can be seen, the flow at the lower location is more pulsatile.
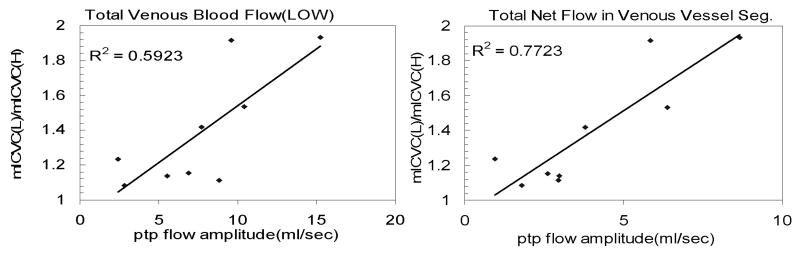
Finally, the relationship between the ratio of the lower vs. upper maximal ICV change and arterial and venous pulsatility were assessed by linear regression. The most significant correlations were found with the pulsatility of the net venous flow (R2 = 0.77, p<0.05) and the flow at the lower level (R2 = 0.59, p<0.05). These relationships are shown in Figure 4. As expected, the higher the venous pulsatility, the larger the expected difference in the ICV change measurements between the two different locations.
Figure 4.
The relation between the lower/upper ratio of maximal ICV change and the peak-to-peak (ptp) flow amplitude of venous flow measured at the lower location (left), and the peak-to-peak amplitude of the net venous flow (right).
DISCUSSION
This study demonstrates that the location along the vessel at which the arterial inflow and venous outflow are imaged influences the measurement of intracranial volume change during the cardiac cycle is a predictive way. Consistently, a larger ICV change is obtained when the lower location is used as compared when the upper location is used. This is expected because the additional volume of the included vessels segments is “added” to the intracranial volume. The mean ratio of the lower vs. upper measurements of the nine data sets was 1.39. Therefore, intracranial compliances measured using a location that is on average lower by 2 cm from the more optimal location (just below the skull base) are expected to be overestimated by an average of about 40%.
Changes in venous pulsatility along the cerebral drainage pathway have been previously reported by Kim et al (10). They proposed a lumped parameter model to predict the extra-cranial venous outflow dynamics based on measured arterial inflow, CSF flow and venous flow in the sagittal and straight sinuses, and validated their prediction that intracranial venous pulsatility is lower than the extra-cranial, i.e., pulsatility in the jugular veins. The increased pulsatility in the upper jugular veins may be in part due to additional drainage from the petrosal sinus (11). The current report points to variation in extra-cranial venous pulsatility between two locations along the same vein with no major branch draining in between these locations. Therefore, it explains the importance of measuring the cerebral venous outflow at a location close to the skull base for a reliable derivation of the ICVC. In addition, this study demonstrates that the measurement of ICVC is sensitive to the blood flow dynamics (timing of onset and magnitude of the pulse amplitude), especially of the venous outflow, which contributed the most to the larger ICVC when the measurement is performed at locations further away from the skull base.
Two causes potentially explain the larger effect of the venous influence of the ICVC measurement than the arterial influence. The arterial pulse wave velocity measured at the carotid arteries is on the order of 5-10 m/sec (12). Therefore, the time delay between the systolic onsets at the inlet and outlet that are 2 cm apart would be on the order of about 4 msec or less. This short delay is below the temporal resolution of the MR measurement, as can be seen in Figure 2a. The systolic onsets of the upper and lower waveforms in the internal carotid artery occur at approximately the same time and therefore, the net arterial flow waveform is relatively flat as seen in Figure 2b. The larger influence of the venous outflow is related to the more complex venous flow due to changes in flow pattern between the upper and lower location of the internal jugular veins. The venous flow in the upper location is more uniform across the lumen during the cardiac cycle compared with the lower location. Furthermore, since the internal jugular vein lumen area is smaller at the higher location, the flow velocity is faster. Therefore, due to the Venturi effect, the fluid pressure is lower at the upper portion of the vein, which explains the partial collapse of the vein at this level compared with the lower level as can be seem in Figure 3. This figure also demonstrates a larger change in the cross sectional area between the cardiac phases in the lower location, possibly due to larger pressure fluctuation during the cardiac cycle.
Finally, the link between pulsatility and the magnitude of the ICVC obtained using imaging plane at difference locations along the vessel segment is demonstrated from the relationship between the ratio of the lower to upper ICVC as a function the peak-to-peak amplitude of the net (R2 of 0.77, p<0.05) and lower location (R2 of 0.59, p<0.05) venous outflow shown in Figure 4. This clearly implies that the measurement of the venous outflow should be obtained at the level reported in the initial publications of the ICVC method (2-4), i.e., close to the skull base to minimize the influences of the compliance and collapsibility of the arteries and veins, respectively. These findings explain the larger values of ICVC and larger measurement variability reported in a recent investigation of factors affecting the reproducibility of the intracranial volume change measurement by MRI (7).
Acknowledgments
Grant Support: This work is supported by NIH grant RO1NS052122-02.
REFERENCES
- 1.Alperin N, Vikingstad EM, Gomez-Anson B, Levin DN. Hemodynamically independent analysis of cerebrospinal fluid and brain motion observed with dynamic phase contrast MRI. Magn Reson Med. 1996;35:741–754. doi: 10.1002/mrm.1910350516. [DOI] [PubMed] [Google Scholar]
- 2.Alperin NJ, Lee SH, Loth F, Raksin PB, Lichtor T. MR-Intracranial pressure (ICP): a method to measure intracranial elastance and pressure noninvasively by means of MR imaging: baboon and human study. Radiology. 2000;217:877–885. doi: 10.1148/radiology.217.3.r00dc42877. [DOI] [PubMed] [Google Scholar]
- 3.Alperin N, Mazda M, Lichtor T, Lee SH. From cerebrospinal fluid pulsation to noninvasive intracranial compliance and pressure measured by MRI flow studies. Current Medical Imaging Reviews. 2006;2:117–129. [Google Scholar]
- 4.Glick RP, Niebruegge J, Lee SH, Egibor O, Lichtor T, Alperin N. Early experience from the application of a noninvasive magnetic resonance imaging-based measurement of intracranial pressure in hydrocephalus. Neurosurgery. 2006;59:1052–60. doi: 10.1227/01.NEU.0000245586.23710.EF. discussion 1060-1. [DOI] [PubMed] [Google Scholar]
- 5.Miyati T, Mase M, Kasai H, et al. Noninvasive MRI assessment of intracranial compliance in idiopathic normal pressure hydrocephalus. J Magn Reson Imaging. 2007;26:274–278. doi: 10.1002/jmri.20999. [DOI] [PubMed] [Google Scholar]
- 6.Dhoondia H, Alperin N. Improved MR-Intracranial Pressure (MR-ICP) Measurement using a new Data Acquisition Technique. Proceedings of the 11th Annual Meeting of ISMRM; Toronto. 2003; abstract 793. [Google Scholar]
- 7.Marshall I, MacCormick I, Sellar R, Whittle I. Assessment of factors affecting MRI measurement of intracranial volume changes and elastance index. Br J Neurosurg. 2008;22:389–397. doi: 10.1080/02688690801911598. [DOI] [PubMed] [Google Scholar]
- 8.Baledent O, Gondry-Jouet C, Meyer ME, et al. Relationship between cerebrospinal fluid and blood dynamics in healthy volunteers and patients with communicating hydrocephalus. Invest Radiol. 2004;39:45–55. doi: 10.1097/01.rli.0000100892.87214.49. [DOI] [PubMed] [Google Scholar]
- 9.Alperin N, Lee SH. PUBS: Pulsatility-based segmentation of lumens conducting non-steady flow. Magn Reson Med. 2003;49:934–944. doi: 10.1002/mrm.10455. [DOI] [PubMed] [Google Scholar]
- 10.Kim J, Thacker NA, Bromiley PA, Jackson A. Prediction of the jugular venous waveform using a model of CSF dynamics. AJNR Am J Neuroradiol. 2007;28:983–989. [PMC free article] [PubMed] [Google Scholar]
- 11.Beards SC, Yule S, Kassner A, Jackson A. Anatomical variation of cerebral venous drainage: the theoretical effect on jugular bulb blood samples. Anaesthesia. 1998;53:627–633. doi: 10.1046/j.1365-2044.1998.409-az0513.x. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi K, Akishita M, Yu W, Hashimoto M, Ohni M, Toba K. Interrelationship between non-invasive measurements of atherosclerosis: flow-mediated dilation of brachial artery, carotid intima-media thickness and pulse wave velocity. Atherosclerosis. 2004;173:13–18. doi: 10.1016/j.atherosclerosis.2003.10.013. [DOI] [PubMed] [Google Scholar]