Abstract
Rationale
Epidemiological studies demonstrate a clear association of adverse intrauterine environment with an increased risk of ischemic heart disease in adulthood. Hypoxia is a common stress to the fetus, and results in decreased protein kinase C epsilon (PKCε) expression in the heart and increased cardiac vulnerability to ischemia and reperfusion injury in adult offspring in rats.
Objectives
The present study tested the hypothesis that fetal hypoxia-induced methylation of CpG dinucleotides at the PKCε promoter is repressive and contributes to PKCε gene repression in the heart of adult offspring.
Methods and Results
Hypoxic treatment of pregnant rats from day 15 to 21 of gestation resulted in significant decreases in PKCε protein and mRNA in fetal hearts. Similar results were obtained in ex vivo hypoxic treatment of isolated fetal hearts and rat embryonic ventricular myocyte cell line H9c2. Increased methylation of PKCε promoter at SP1 binding sites, −346 and −268, were demonstrated in both fetal hearts of maternal hypoxia and H9c2 cells treated with 1% O2 for 24 hours. Whereas hypoxia had no significant effect on the binding affinity of SP1 to the unmethylated sites in H9c2 cells, hearts of fetuses and adult offspring, methylation of both SP1 sites reduced SP1 binding. The addition of 5-aza-2’-deoxycytidine blocked the hypoxia-induced increase in methylation of both SP1 binding sites and restored PKCε mRNA and protein to the control levels. In hearts of both fetuses and adult offspring, hypoxia-induced methylation of SP1 sites was significantly greater in males than in females, and decreased PKCε mRNA was seen only in males. In fetal hearts, there was significantly higher abundance of estrogen receptor α (ERα ) and β (ERβ ) isoforms in females than in males. Both ERα and ERβ interacted with the SP1 binding sites in the fetal heart, which may explain the gender differences in SP1 methylation in the fetal heart. Additionally, selective activation of PKCε restored the hypoxia-induced cardiac vulnerability to ischemic injury in offspring.
Conclusion
The findings demonstrate a direct effect of hypoxia on epigenetic modification of DNA methylation and programming of cardiac PKCε gene repression in a sex-dependent manner, linking fetal hypoxia and pathophysiological consequences in the hearts of adult offspring.
Keywords: Fetal heart, PKCε, hypoxia, epigenetics, DNA methylation
Heart disease is the leading cause of death in the United States. In addition to other risk factors, recent epidemiological and animal studies have shown a clear association of adverse intrauterine environment with an increased risk of hypertension and ischemic heart disease in adulthood.1–4 Hypoxia is a common form of intrauterine stress, and the fetus may experience prolonged hypoxic stress under a variety of conditions, including pregnancy at high altitude, pregnancy with anemia, placental insufficiency, cord compression, preeclampsia, heart, lung and kidney disease, or with hemoglobinopathy. Animal studies suggest a possible link between fetal hypoxia and increased risk of cardiovascular disease in offspring.5–13 Studies in rats have demonstrated that maternal hypoxia results in an increase in cardiac vulnerability to ischemia and reperfusion injury in male offspring.10,14,15 In addition, it has been demonstrated that down-regulation of protein kinase C epsilon (PKCε) protein expression in the hearts of adult offspring is a mechanism for the increased heart susceptibility to ischemia and reperfusion injury in the animals exposed to hypoxia before birth.14
Among other mechanisms, PKCε plays a pivotal role of cardioprotection in heart ischemia and reperfusion injury.16–18 The study in a PKCε knock-out mouse model has demonstrated that PKCε expression is not required for cardiac function under normal physiological conditions, but PKCε activation is necessary for acute cardioprotection during cardiac ischemia and reperfusion.19 The finding that fetal hypoxia resulted in a decrease in PKCε protein expression in the heart of adult offspring10,14 suggests that an epigenetic mechanism may explain PKCε gene repression in the heart. Epigenetic mechanisms are essential for development and differentiation, and allow an organism to respond to the environment through changes in gene expression patterns.20–22 DNA methylation is a chief mechanism for epigenetic modification of gene expression patterns, and occurs at cytosines in the CpG dinucleotide sequence.23 Methylation in promoter regions is generally associated with repression of transcription, leading to a long-term shutdown of the associated gene. Methylation of CpG islands in gene promoter regions alters chromatin structure and transcription. Similarly, methylation of CpG dinucleotides within transcription factor binding sites generally represses transcription.20,24,25 The present study tested the hypothesis that fetal hypoxia-induced methylation of CpG dinucleotides at the PKCε promoter contributes to PKCε gene repression in the heart of adult offspring. Herein, we present evidence that hypoxia has a direct effect on PKCε expression in the fetal heart, and demonstrate in a cell model that hypoxia causes an increase in methylation of CpG dinucleotides at the proximal promoter region of PKCε gene, resulting in decreased binding of sequence-specific transcription factors to the promoter and reduced PKCε gene expression.
Methods
An expanded Methods section is available in the online data supplement.
Experimental animals
Pregnant rats were randomly divided into two groups: 1) normoxic control; and 2) hypoxic treatment of 10.5% O2 from day 15 to 21 of gestation.14 Hearts were isolated from near-term (21 day) fetuses and 3 months old offspring. To study the direct effect of hypoxia on the fetal heart, hearts were isolated from day 17 fetal rats and cultured at 37 °C in 95% air/5% CO2 and 1% O2 for 48 hours, as reported previously.24
Cell culture
H9c2 cells were grown and sub-cultured and experiments were performed at 70– 80% confluent. For hypoxic studies, cells were treated with 1%, 3%, or 10.5% O2, respectively, for 24 hours.
Western blot analysis
Protein abundance of PKCε, specificity protein 1 (SP1), alpha (ERα ) and beta (ERβ ) subtypes of estrogen receptors in H9c2 cells and hearts of fetuses and adult offspring were measured with Western blot analysis, and were normalized to beta2-microglobulin (B2M), as described previously.25, 26
Real-time RT-PCR
PKCε mRNA abundance in H9c2 cells and hearts of fetuses and adult offspring was determined by real-time RT-PCR and was normalized to B2M.25, 26
Quantitative methylation-specific PCR
DNA collected from H9c2 cells and hearts of fetuses and adult offspring was treated with sodium bisulfite at 55 °C for 16 hours. Bisulfite-treated DNA was used as a template for real-time fluorogenic methylation-specific PCR (MSP) using primers created to amplify promoter binding sites containing possible methylation sites based on our previous sequencing of rat PKCε promoter.25
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts were collected from H9c2 cells and hearts of fetuses and adult offspring. EMSA was performed using the oligonucleotide probes with either CpG or mCpG in the two SP1 binding sites ( –346 and –268) at rat PKCε promoter region, as described previously.25
Chromatin immunoprecipitation (ChIP)
Chromatin extracts were prepared from H9c2 cells and fetal hearts. ChIP assays were performed for the two SP1 binding sites at the PKCε promoter in DNA sequences pulled-down by SP1, ERα , and ERβ antibodies.25
Hearts subjected to ischemia and reperfusion
Isolated hearts from 3 months old male offspring were subjected to 20 minutes of global ischemia followed by 30 minutes of reperfusion in the absence or presence of a PKCε activator peptide ψ-εRACK (0.5 μM, KAE-1, KAI Pharmaceuticals) in a Langendorff preparation, as previously described.14, 27 Post-ischemic recovery of left ventricular function and lactate dehydrogenase (LDH) release were determined.14
Statistical analysis
Data are expressed as mean ± SEM. Statistical significance (P < 0.05) was determined by analysis of variance (ANOVA) followed by Neuman-Keuls post hoc testing or Student's t test, where appropriate.
Results
Effect of hypoxia on PKC protein and mRNA in fetal cardiomyocytes
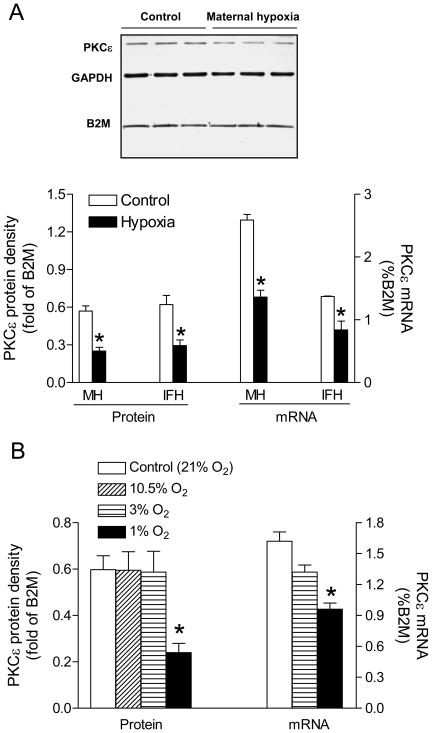
Maternal hypoxia resulted in significant decreases in both protein and mRNA abundance of PKCε in fetal rat hearts (Figure 1A). Similar findings were obtained in isolated fetal hearts treated ex vivo with 1% O2 (Figure 1A), demonstrating a direct effect of hypoxia on PKCε expression in the heart. Further study demonstrated in rat embryonic ventricular myocyte cell line H9c2 that 1% O2, but not 3% or 10.5% O2, significantly decreased PKCε mRNA and protein levels as compared to 21% O2 (Figure 1B).
Figure 1. Effect of hypoxia on PKCε protein and mRNA.
(A) Fetal hearts of maternal hypoxia (MH) and isolated fetal hearts (IFH) treated with 1% (hypoxia) and 21% O2 (control) for 48 hours. (B) H9c2 cells treated with 1%, 3%, 10.5%, and 21% O2 for 24 hours. Data are means ± SEM. * P < 0.05 vs. control (n = 5–15)
Methylation of SP1 binding sites at the PKC promoter
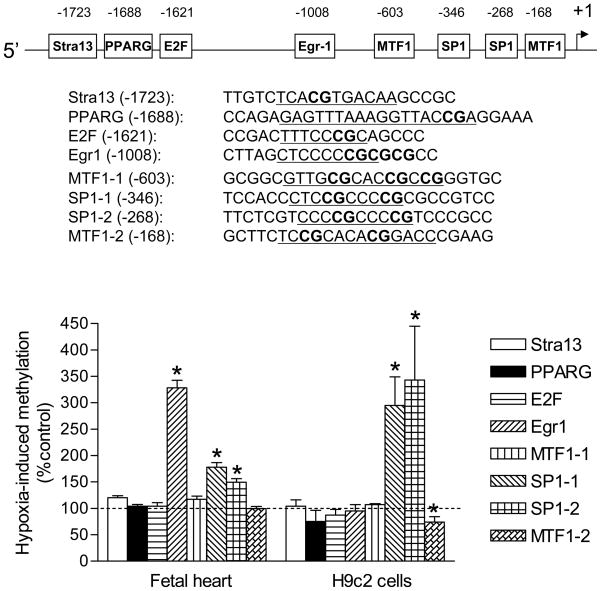
Eight putative transcription binding sites containing CpG dinucleotides at rat PKCε promoter were previously identified.25 Treatment of H9c2 cells with 1% O2 for 24 hours resulted in a significant increase in methylation of SP1 binding sites at −346 and −268, but decreased methylation of metal regulatory transcription factor 1 (MTF1) binding site at −168, as compared to 21% O2 control (Figure 2). Hypoxia did not change significantly the methylation status of class B basic helix-loop-helix protein 2 (Stra13), peroxisome proliferator-activated receptor gamma (PPARG), E2F transcription factor (E2F), early growth response protein 1 (Egr1), and MTF1 at −603. Consistent with the findings in H9c2 cells, maternal hypoxic treatment revealed a similar pattern of increased methylation of the two SP1 binding sites, −346 and −268, at the PKCε promoter in the fetal hearts (Figure 2). In addition, in vivo hypoxia resulted in an increase in methylation of the putative Egr1 binding site in the fetal heart (Figure 2).
Figure 2. Effect of hypoxia on methylation of the PKCε promoter.
Methylation patterns were determined in fetal hearts of maternal hypoxia and H9c2 cells treated with 1% O2 vs. 21% O2 for 24 hours. Data are means ± SEM. * P < 0.05 vs. control (n = 5–10)
Methylation inhibits SP1 binding
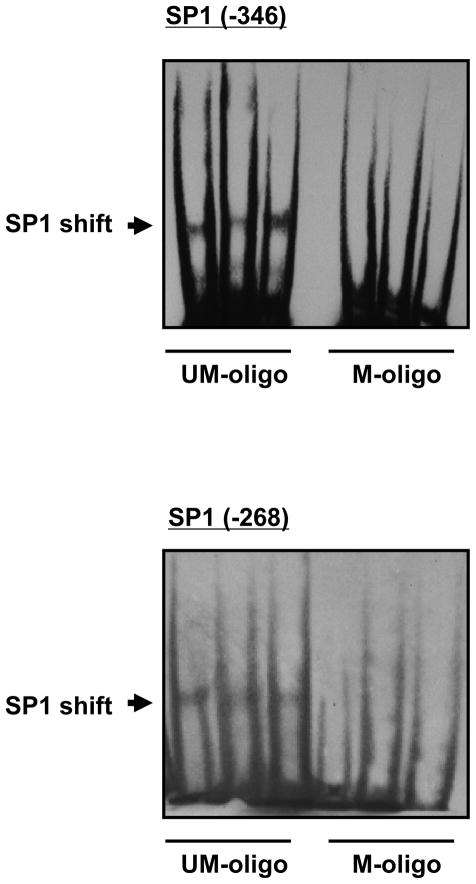
H9c2 cells were used to further determine the role of methylation in PKCε down-regulation. Given the previous finding that deletions of the regions containing the putative Egr1 site and MTF1 site at −168 had no significant effects on the PKCε promoter activity,25 our further investigation focused on the two SP1 binding sites. SP1 binding and the functional significance of the SP1 binding sites in the regulation of PKCε gene activity was demonstrated previously.25 To determine if methylation of the SP1 sites inhibits SP1 binding from nuclear extracts of H9c2 cells, EMSA was performed with methylated and unmethylated oligonucleotide probes containing the SP1 sites at −346 and −268. As shown in Figure 3, nuclear extracts from H9c2 cells bound and shifted the double-stranded unmethylated SP1 oligonucleotides at both sites, but failed to cause a shift of the methylated SP1 oligonucleotides. This is consistent with the previous findings in fetal rat hearts showing a loss of binding of the nuclear extracts to the methylated SP1 oligonucleotides.24
Figure 3. Effect of methylation on SP1 binding.
Nuclear extracts of H9c2 cells were incubated with unmethylated (UM-oligo) or methylated (M-oligo) oligonucleotides containing SP1 consensus sequence at sites −346 and −268.
Effect of hypoxia on SP1 abundance and binding affinity
Western blots revealed that hypoxia caused a significant increase in nuclear SP1 abundance in H9c2 cells (Online Figure I). In contrast, there was no significant difference in SP1 abundance either in fetal hearts between control and maternal hypoxic treatments, or in the hearts of both male and female offspring between the control and prenatally hypoxic animals (Online Figure I). The binding affinity of SP1 to the unmethylated SP1 binding sites was determined in competition studies performed in pooled nuclear extracts with the increasing ratio of unlabeled/labeled oligonucleotides encompassing the SP1 sites at −346 and −268, respectively. Hypoxia had no significant effect on the binding of nuclear extracts to either SP1 sites at −346 or −268 in H9c2 cells, fetal hearts, or the hearts of both male and female offspring (Online Figure II).
Inhibition of DNA methylation restored PKCε expression
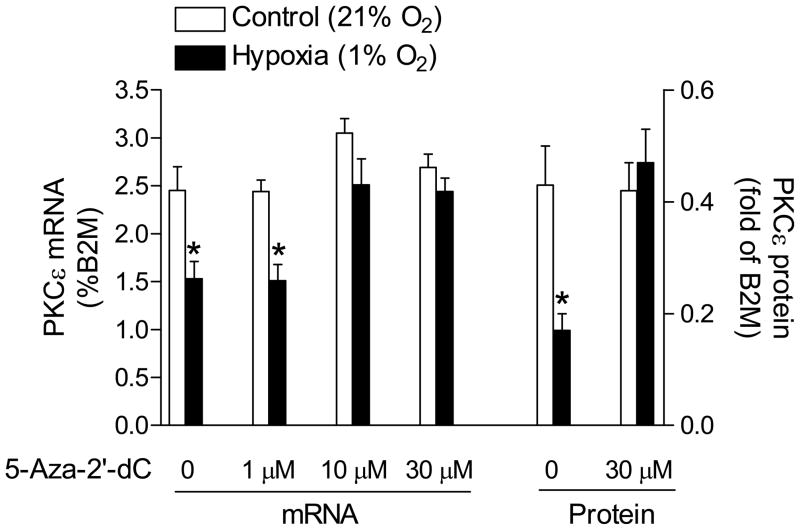
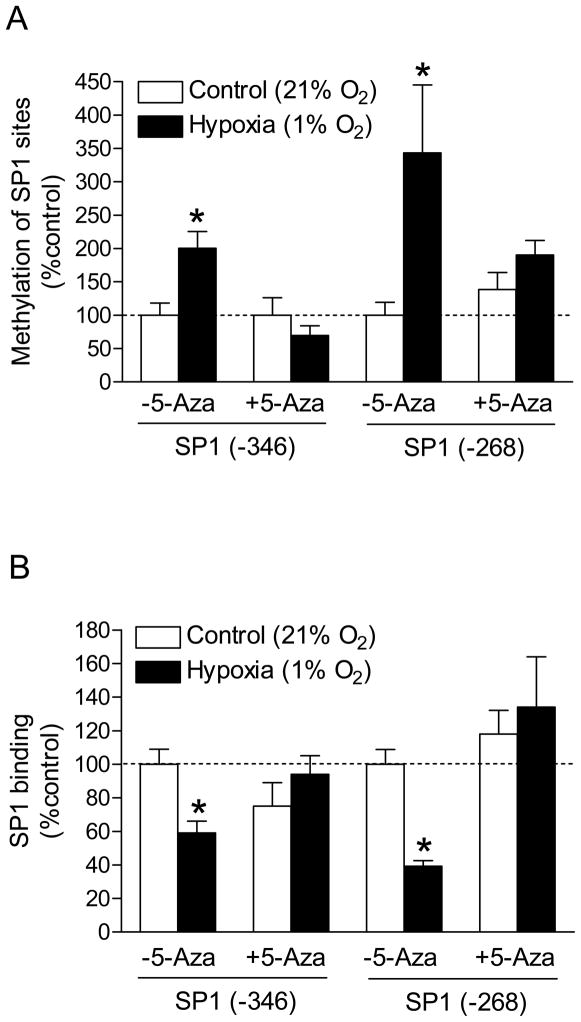
To determine the causal role of CpG dinucleotide methylation in the down-regulation of PKCε expression, we exposed H9c2 cells to 1% O2 in the absence or presence of increasing concentrations of the DNA methylation inhibitor 5-aza-2’-deoxycytodine. As shown in Figure 4, 5-aza-2’-deoxycytodine produced a concentration-dependent inhibition of hypoxia-induced decrease in PKCε mRNA and 30 μM 5-aza-2’-deoxycytodine restored the mRNA to normoxic levels. This was accompanied by restoration of PKCε protein (Figure 4). The inhibition of hypoxia-induced methylation of the two SP1 binding sites by 5-aza-2’-deoxycytodine was demonstrated (Figure 5A). To confirm that hypoxia-induced methylation alters SP1 binding to the PKCε promoter in the context of intact chromatin, ChIP assays were performed using a SP1 antibody. Figure 5B shows that hypoxia caused significant decreases in the SP1 binding to both SP1 sites at −346 and −268, respectively, in H9c2 cells. However, in the presence of 5-aza-2-deoxycytine, there were no significant differences in SP1 binding to the either sites between hypoxic and normoxic samples (Figure 5B).
Figure 4. Effect of 5-aza-2’-deoxycytidine on PKCε mRNA and protein.
H9c2 cells were treated with 1% O2 and 21% O2 for 24 hours in the absence or presence of increasing concentration of 5-aza-2’-deoxycytidine (5-Aza-2’-dC). Data are means ± SEM. * P < 0.05 vs. control (n = 4–17).
Figure 5. Effect of 5-aza-2’-deoxycytidine on methylation of SP1 sites and SP1 binding.
H9c2 cells were treated with 1% O2 and 21% O2 for 24 hours in the absence or presence of 30 μM 5-aza-2’-deoxycytidine (5-Aza). (A) Methylation of SP1 binding sites was determined by methylation-specific PCR. (B) SP1 binding was determined by ChIP assays. Data are means ± SEM. * P < 0.05 vs. control (n = 4–9).
Sex differences in hypoxia-induced changes in methylation and PKCε expression
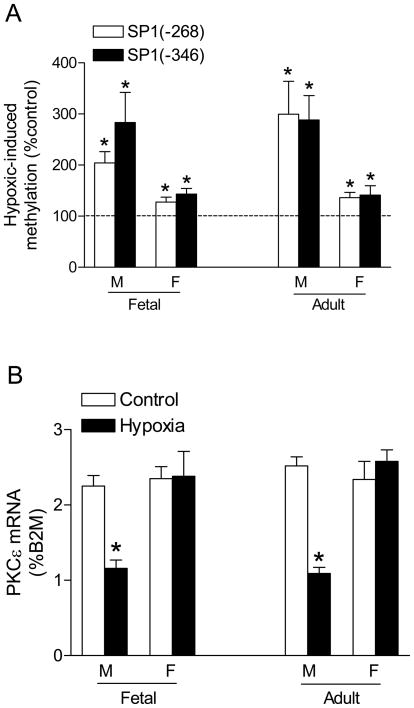
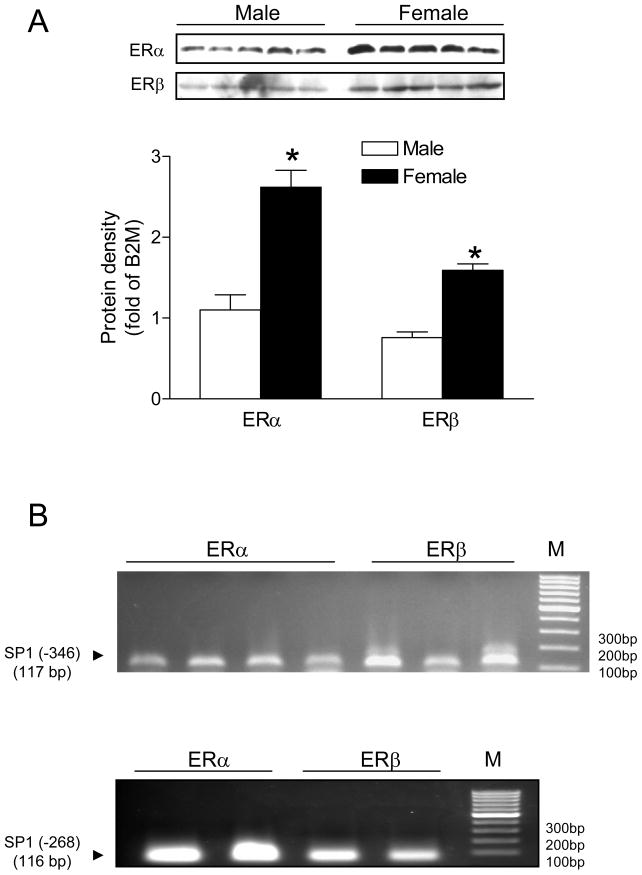
We further investigated the potential sex differences in hypoxia-induced methylation of the SP1 binding sites and PKCε transcription. Maternal hypoxia caused a minimal but significant increase in methylation at both SP1 binding sites in the hearts of female fetuses, but induced significantly greater methylation in male fetal hearts (Figure 6A). PKCε mRNA was significantly decreased in the hearts of male but not female fetuses (Figure 6B). The similar pattern of sex differences in the hypoxia-induced SP1 methylation and PKCε transcription was demonstrated in the hearts of male and female offspring (Figure 6A, B). The sex difference observed in the fetal hearts was intriguing given that male and female fetuses were likely exposed to the similar concentrations of steroid hormones in utero. However, both ERα and ERβ abundance was significantly higher in the hearts of female, as compared with male fetuses (Figure 7A). Chromatin immunoprecipitation assay demonstrated the PCR products of the two SP1 binding sites in the DNA sequences pulled-down by both ERα and ERβ antibodies in the fetal hearts (Figure 7B), suggesting an interaction between estrogen receptors and the SP1 binding sites at the PKCε promoter.
Figure 6. Effect of hypoxia on SP1 methylation and PKCε mRNA.
Pregnant rats were treated with hypoxia, and hearts were isolated from near-term fetuses or 3 months old offspring. M, male; F, female. (A) Methylation of SP1 binding sites at the PKCε promoter. (B) PKCε mRNA. Data are means ± SEM. * P < 0.05 vs. control (n = 5)
Figure 7. Estrogen receptors and SP1 binding sites in the fetal heart.
Hearts were isolated from near-term fetuses. (A) Protein abundance of estrogen receptor alpha (ERα ) and beta (ERβ ) subtypes. Data are mean ± SEM. * P < 0.05 vs. male (n = 5). (B) PCR products of the SP1 binding sites (−346 and −268) following chromatin immunoprecipitation (ChIP) with ERα and ERβ antibodies, respectively, in the fetal heart. M, markers.
PKCε activation restored hypoxia-induced cardiac vulnerability to ischemic injury
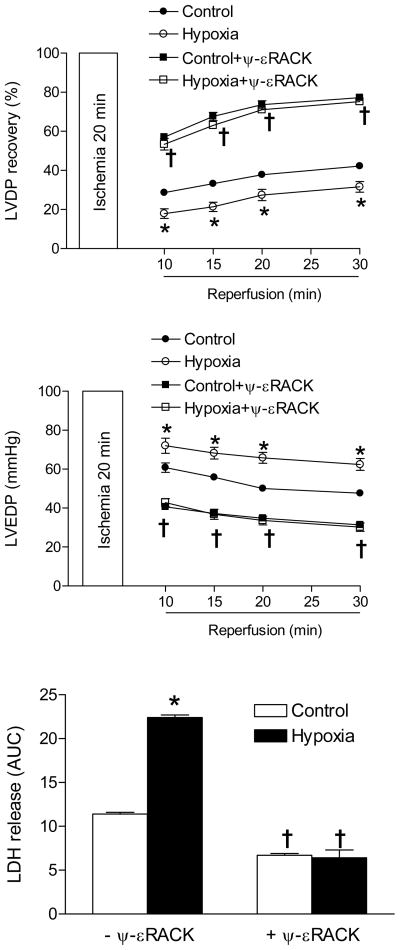
The cause-and-effect evidence of the functional importance of PKCε in hypoxia-mediated, sex-dependent programming of increased heart vulnerability to ischemia and reperfusion injury in adult male offspring has been previously demonstrated by selective inhibition of PKCε with a PKCε translocation inhibitor peptide.14 To further demonstrate that decreased PKCε is an important factor in the hypoxia-induced increase in cardiac ischemic susceptibility in male offspring, additional studies were performed in the hearts in a Langendorff preparation using a selective PKCε activator peptide ψ-εRACK obtained from KAI Pharmaceuticals.16, 27–29 There were no significant differences in left ventricle developed pressure (LVDP), heart rate (HR), dP/dtmax, dP/dtmin and coronary flow rate at the baseline among all groups (Online Table I). In the absence of ψ-εRACK, fetal hypoxia caused a significant decrease in post-ischemic recovery of LVDP and increases in left ventricle end diastolic pressure (LVEDP) and lactate dehydrogenase (LDH) release (Figure 8), as well as decreases in the recovery of dP/dtmax and dP/dtmin (Online Figure III), as previously reported.14ψ-εRACK increased post-ischemic recovery of left ventricular function as shown in the previous studies27, 29 and abolished the hypoxic effects (Figure 8 and Online Figure III).
Figure 8. Effect of PKCε activation on cardiac ischemia and reperfusion injury.
Hearts were isolated from 3-month-old male offspring that had been exposed to normoxia (control) or hypoxia before birth, and were treated in the absence or presence of the PKCε activator ψ-εRACK (0.5 μM) for 10 minutes before subjecting to 20 minutes of ischemia and 30 minutes of reperfusion in a Langendorff preparation. Post-ischemic recovery of left ventricular developed pressure (LVDP) and end diastolic pressure (LVEDP) were determined. Lactate dehydrogenase (LDH) release over 30 minutes of reperfusion was measured as area under curve (AUC). Data are mean ± SEM. * P < 0.05, hypoxia vs. control; † P < 0.05, + ψ-εRACK vs. -ψ-εRACK. n = 5.
Discussion
The present study demonstrates that hypoxia has a direct effect on fetal cardiomyocytes causing a reduction in PKCε protein and mRNA. This is correlated with increased methylation of CpG dinucleotides in the two SP1 binding sites at the proximal promoter region of PKCε gene, resulting in decreased binding of SP1 to the promoter. The causal effect of methylation in the hypoxia-induced PKCε gene repression is demonstrated through the use of a DNA methylation inhibitor, which blocks the methylation and restores PKCε mRNA and protein expression to normal.
Previous studies demonstrated the long-term adverse effect of maternal hypoxia on cardiac vulnerability to ischemia and reperfusion injury in adult male offspring in a sex-dependent manner.10,14,15 Particularly, the decreased expression of cardioprotective genes, namely PKCε in hearts of adult male offspring are of significant interest.14 Acute hypoxia and reactive oxygen species increase the activity of PKCε in the heart, which plays an important role in cardioprotection during ischemia and reperfusion injury.30 The present study demonstrated that chronic hypoxia during gestation down-regulated PKCε expression in the developing heart through an epigenetic modification. This suggests differential regulations of acute hypoxia and chronic hypoxia on PKCε activity and gene expression in the heart. The causal role of reduced PKCε in sex-dependent programming of increased heart vulnerability to ischemia and reperfusion injury in adult male offspring has been demonstrated by selective inhibition of PKCε with a PKCε translocation inhibitor peptide.14, 31 The present study demonstrated that a selective PKCε activator peptide16, 27–29 restored the hypoxia-induced cardiac vulnerability to ischemic injury, providing further evidence of cause-and-effect role of decreased PKCε in the hypoxia-mediated, sex-dependent programming of increased heart ischemic vulnerability in male offspring. This is consistent with other studies showing that PKCε expression is necessary for acute cardioprotection during cardiac ischemia and reperfusion.19 The present finding of decreased PKCε protein and mRNA in fetal hearts caused by maternal hypoxia suggests that the reduction in PKCε expression observed in adult rats10,14 originates in utero. Previous studies in the same animal model demonstrated that maternal hypoxia increased hypoxia-inducible factor 1α (HIF-1α ) protein in fetal hearts, suggesting tissue hypoxia in the fetal heart.32 The similar results of isolated hearts treated ex vivo with hypoxia indicate that hypoxia is necessary and sufficient to impact the PKCε expression in fetal hearts. Whether this decreased PKCε expression in the heart is a protective mechanism that increases fetal survival is not clear at present. However, the finding that the down-regulation of PKCε resulted in increased heart ischemic vulnerability in offspring would suggest that it is a maladaptive response.
The present study used the embryonic rat ventricular myocyte cell line H9c233 to investigate the underlying mechanisms. H9c2 cell line has been widely used in a variety of myocardiocyte studies, including those investigating apoptosis, differentiation and ischemia and reperfusion injury.34–38 H9c2 cells retain many electrophysical properties found in freshly isolated cardiomyocytes,33 albeit they do not spontaneously contract and are capable of continuous growth.39 In the present study, the hypoxia-induced decrease in PKCε expression observed in fetal hearts in vivo and ex vivo mirrored that found in H9c2 cells, suggesting a congruent underlying mechanism for each model and providing a comparable model of H9c2 cells in the study of PKCε gene regulation. Similar to the present finding, it has been reported that PKCε protein expression is decreased in H9c2 cells exposed to 2 and 8 hours of hypoxia in the presence of low and high glucose.40, 41
Another question pertained to normal physiological PKCε expression in fetal heart cells. 21% O2 (147 torr) is generally considered normal for adult cells. Because fetal partial oxygen pressures are closer to 20 torr,42 21% O2 is hyperoxic for fetal cardiomyocytes. This may alter underlying gene expression patterns since normal oxygen levels in fetal hearts are significantly lower. Interestingly, we found that exposure to 21%, 10.5% and 3% O2 had no significantly different effects on PKCε expression in H9c2 cells. While the potential effect of ‘hyperoxia’ on fetal cardiomyocytes remains to be further investigated, the finding that 1% O2 down-regulated PKCε expression suggests modification in gene expression patterns as a mode of adaptation to oxygen insufficiency in cardiomyocytes. This is in agreement with many previous studies in which hypoxic effects were investigated in H9c2 cells under 1% O2.37,38,43,44
The finding of similar pattern of increased methylation of the two SP1 binding sites at the PKCε promoter between H9c2 cells and the fetal hearts of maternal hypoxic treatment provides further evidence supporting the model of H9c2 cells in the present study. Whereas the differences in methylation patterns in the putative Egr1 and MTF1 (−168) binding sites observed in fetal hearts and H9c2 cells are intriguing, the functional significance of this finding is not clear given that deletions of the regions containing the Egr1 and MTF1 (−168) sites had no significant effects on the PKCε promoter activity.25 In contrast, deletion of the confirmed binding sites for both SP1 (−346) and SP1 (−268) significantly decreased the PKCε promoter activity in H9c2 cells.25 In the present study, we demonstrated by EMSA that methylation of CpG dinucleotides at the core of both SP1 binding sites abolished SP1 binding in H9c2 cells. Previous studies with site-directed methylation of PKCε promoter-luciferase constructs selectively at SP1 binding sites at −268 and −346, demonstrated that mutation of CmG at either SP1 site −268 or −346 alone had no significant effect on the promoter activity, but mutation of CmG at the both SP1 binding sites significantly reduced the promoter activity in H9c2 cells.24 Although increases in nuclear SP1 protein abundance in hypoxic H9c2 cells may serve as a compensatory mechanism, this increase is relatively ineffective because increased methylation of SP1 binding sites caused an even more significant decrease in SP1 binding to the PKCε promoter in the intact chromatin as demonstrated by ChIP assays. The finding that SP1 abundance was not significantly affected by hypoxia in fetal hearts, as well as in the hearts of either male or female adult offspring, together with the finding that hypoxia had no significant effect on SP1 binding affinity in H9c2 cells, fetal and adult hearts, reinforces a primary role of methylation in programming of PKCε gene repression.
The causal effect of increased methylation in hypoxia-induced PKCε gene repression was further demonstrated with a DNA methylation inhibitor 5-aza-2’-deoxycytodine in the present study. 5-Aza-2’-deoxycytodine binds to DNA methyltransferase 1 causing its depletion and preventing DNA methylation,45 and has been widely used to inhibit DNA methylation.46–49 In the present study, we demonstrated that 5-aza-2’-deoxycytodine concentration-dependently inhibited hypoxia-induced down-regulation of PKCε mRNA expression. This is caused by inhibition of the hypoxia-induced methylation of both SP1 binding sites resulting in recovered SP1 binding to the PKCε promoter in the intact chromatin. Whereas the study of 5-aza-2’-deoxycytodine in H9c2 cells is limited, relatively high concentrations of 5-aza-cytidine (a close derivative of 5-aza-2-deoxycytidine) have been shown to rescue luciferase activity in transfected H9c2 cells in excess of 250 μM,50 which may reflect cell-type specific response to 5-aza-2-deoxycytidine and derivatives. The ability of 5-aza-2-deoxycytidine to restore PKCε mRNA and protein expression in cardiomyocytes in the presence of a stressor is consistent with earlier studies that showed that 5-aza-2-deoxycytidine blocked cocaine-mediated repression of PKCε expression in fetal rat hearts.24
Previous studies demonstrated that prenatal hypoxia caused an increase in heart susceptibility to ischemia and reperfusion injury in a sex-dependent manner, which was due to fetal programming of PKCε gene repression resulting in downregulation of PKCε function in the heart of adult male offspring.14 In the present study, no significant differences were found in SP1 methylation and PKCε mRNA abundance between male and female control groups in both fetuses and offspring. This is consistent with the previous findings of no significant difference in PKCε protein abundance in the heart between control males and females.14, 51 However, the sex differences in hypoxia-induced changes in methylation of the SP1 binding sites and PKCε transcription were demonstrated in both fetal and offspring hearts. The finding that the hypoxia-induced methylation was significantly greater in the hearts of male fetuses is intriguing given that male and female fetuses are likely exposed to the similar concentrations of steroid hormones in utero. Whereas the mechanisms are not clear at present, the sex difference observed may be caused in part by the greater expression of ERα and ERβ in the hearts of female fetuses. The finding that both ERα and ERβ interacted with the SP1 binding sites at the PKCε promoter in intact chromatin in the fetal heart suggests a possible mechanism for the increased protection of SP1 binding sites and PKCε transcription in the female hearts in response to hypoxic stress. It has been demonstrated that both ERα and ERβ can activate transcription of the retinoic acid receptor α 1 (RAR-1) gene by the formation of an ER-SP1 complex on the SP1 sites in the RAR-1 promoter.52 It is not clear at present whether the presence of phytoestrogens in the diet of soy-based chows used in the present study might contribute to the gender difference, though the pregnancy is a stage of high estrogen concentrations. This remains as an intriguing area for the future investigation using a casein-based diet. The similar pattern of sex differences in the hypoxia-induced SP1 methylation and PKCε transcription demonstrated in the hearts of male and female offspring supports the notion that fetal hypoxia-induced methylation of CpG dinucleotides at the PKCε promoter contributes to PKCε gene repression in the heart of adult offspring.
The present investigation provides evidence of a novel mechanism of methylation in non-CpG island, sequence-specific transcription factor binding sites in subtle epigenetic modifications of gene expression pattern in fetal programming of cardiac function in response to adverse intrauterine environment. Although it may be difficult to translate the present findings directly into the humans, the possibility that fetal hypoxia may result in programming of a specific gene in the offspring with a consequence of increased cardiac vulnerability provides a mechanistic understanding worthy of investigation in humans. This is because hypoxia is one of the most important and clinically relevant stresses to the fetus, and because large epidemiological studies indicate a link between in utero adverse stimuli during gestation and an increased risk of ischemic heart disease in the adulthood.
Novelty and Significance.
What Is Known?
Adverse intrauterine environment increases the risk of hypertension and ischemic heart disease in adulthood.
Protein kinase C epsilon (PKCε) plays a pivotal role of cardioprotection against ischemia and reperfusion injury.
Fetal hypoxia decreases PKCε gene expression in the heart and increases cardiac vulnerability to ischemic injury in adult male offspring in rats.
What New Information Does This Article Contribute?
Fetal hypoxia causes an epigenetic modification of increased DNA methylation patterns within the PKCε gene promoter, resulting in a repression of PKCε gene expression in the heart of male rats.
Decreased PKCε protein expression results in increased cardiac vulnerability to ischemic injury in male offspring.
The sex-dependent difference in epigenetic modification of PKCε gene expression patterns occurs during fetal development, as a result of the different expression patterns of estrogen receptors in male and female fetal hearts.
Intrauterine stress is associated with an increasde risk of ischemic heart disease in later life. Previous studies in rats showed that fetal hypoxia results in decreased PKCε expression and increased cardiac ischemic injury in male offspring. In this study, we found that hypoxia caused increased DNA methylation within the PKCε gene promoter, resulting in a repression of PKCε gene expression in the hearts of male fetal rats. This epigenetic modification pattern persisted into adulthood. We further demonstrated a causal role of decreased PKCε expression in increased heart ischemic vulnerability. Higher levels of estrogen receptors were found in the female fetal hearts, which may protect females from epigenetic modifications caused by intrauterine stress. This study is the first to show that hypoxia alters the epigenetic signature of a cardioprotective gene in utero, and that this modification can persist throughout life, leading to increased susceptibility to ischemia and reperfusion injury in the heart. Together, these findings provide a novel mechanism for hypoxia-induced programming of cardiovascular disease. Because hypoxia is a common stress to the fetus, this study may lead to further inquiries into the molecular adaptations in other tissues that promote pathophysiological conditions in adults.
Supplementary Material
Acknowledgments
The authors thank KAI Pharmaceuticals for the gift of PKCε activator peptide.
Sources of Funding
This study was supported by the National Institutes of Health grants HL82779 (LZ), HD31226 (LZ), and HL83966 (LZ).
Non-standard Abbreviations and Acronyms
- PKCε
protein kinase C epsilon
- CpG
cytosine-phosphate-guanine
- ERα
β, estrogen receptor alpha, beta
- SP1
specificity protein 1
- MTF1
metal regulatory transcription factor 1
- Stra13
class B basic helix-loop-helix protein 2
- PPARG
peroxisome proliferator-activated receptor gamma
- E2F
E2F transcription factor
- Egr1
early growth response protein 1
- EMSA
electrophoretic mobility shift assay
- ChIP
chromatin immunoprecipitation
- LVDP
left ventricle developed pressure
- LVEDP
left ventricle end diastolic pressure
- LDH
lactose dehydrogenase
Footnotes
Disclosures
None.
References
- 1.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 2.Bateson P, Barker D, Clutton-Brock T, Deb D, D'Udine B, Foley RA, Gluckman P, Godfrey K, Kirkwood T, Lahr MM, McNamara J, Metcalfe NB, Monaghan P, Spencer HG, Sultan SE. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- 3.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 5.Heydeck DRJ, Roigas C, Papies B, Lun A. The catecholamine sensitivity of adult rats is enhanced after prenatal hypoxia. Biol Neonate. 1994;66:106–111. doi: 10.1159/000244097. [DOI] [PubMed] [Google Scholar]
- 6.Roigas J, Roigas C, Heydeck D, Papies B. Prenatal hypoxia alters the postnatal development of beta-adrenoceptors in the rat myocardium. Biol Neonate. 1996;69:383–388. doi: 10.1159/000244335. [DOI] [PubMed] [Google Scholar]
- 7.Butler TG, Schwartz J, McMillen IC. Differential effects of the early and late intrauterine environment on corticotrophic cell development. J Clin Invest. 2002;110:783–791. doi: 10.1172/JCI15563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peyronnet J, Dalmaz Y, Ehrstrom M, Mamet J, Roux JC, Pequignot JM, Thoren HP, Lagercrantz H. Long-lasting adverse effects of prenatal hypoxia on developing autonomic nervous system and cardiovascular parameters in rats. Pflugers Arch. 2002;443:858–865. doi: 10.1007/s00424-001-0766-9. [DOI] [PubMed] [Google Scholar]
- 9.Davis LRJ, Thornburg KL, Shokry M, Hohimer AR, Giraud GD. Augmentation of coronary conductance in adult sheep made anaemic during fetal life. J Physiol. 2003;547:53–59. doi: 10.1113/jphysiol.2002.023283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li GXY, Estrella JL, Ducsay CA, Gilbert RD, Zhang L. Effect of fetal hypoxia on heart susceptibility to ischemia and reperfusion injury in the adult rat. J Soc Gynecol Investig. 2003;10:265–274. doi: 10.1016/s1071-5576(03)00074-1. [DOI] [PubMed] [Google Scholar]
- 11.Jones RDMA, Emery CJ. Effects of perinatal exposure to hypoxia upon the pulmonary circulation of the adult rat. Physiol Res. 2004;53:11–17. [PubMed] [Google Scholar]
- 12.Mone SMGM, Miller TL, Herman EH, Lipshultz SE. Effects of environmental exposures on the cardiovascular system: prenatal period through adolescence. Pediatrics. 2004;113:1058–1069. [PubMed] [Google Scholar]
- 13.Zhang L. Prenatal hypoxia and cardiac programming. J Soc Gynecol Investig. 2005;12:2–13. doi: 10.1016/j.jsgi.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Xue Q, Zhang L. Prenatal hypoxia causes a sex-dependent increase in heart susceptibility to ischemia and reperfusion injury in adult male offspring: Role of PKCε. J Pharmacol Exp Ther. 2009;330:624–632. doi: 10.1124/jpet.109.153239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Y, Williams SJ, O'Brien D, Davidge ST. Hypoxia or nutrient restriction during pregnancy in rats leads to progressive cardiac remodeling and impairs postischemic recovery in adult male offspring. FASEB J. 2006;20:1251–1253. doi: 10.1096/fj.05-4917fje. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Hahn H, Wu G, Chen CH, Liron T, Schechtman D, Cavallaro G, Banci L, Guo Y, Bolli R, Dorn GW, 2nd, Mochly-Rosen D. Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc Natl Acad Sci U S A. 2001;98:11114–11119. doi: 10.1073/pnas.191369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ping P, Zhang J, Pierce WM, Jr, Bolli R. Functional proteomic analysis of protein kinase C epsilon signaling complexes in the normal heart and during cardioprotection. Circ Res. 2001;88:59–62. doi: 10.1161/01.res.88.1.59. [DOI] [PubMed] [Google Scholar]
- 18.Murriel CL, Mochly-Rosen D. Opposing roles of delta and epsilonPKC in cardiac ischemia and reperfusion: targeting the apoptotic machinery. Arch Biochem Biophys. 2003;420:246–254. doi: 10.1016/j.abb.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 19.Gray MO, Zhou HZ, Schafhalter-Zoppoth I, Zhu P, Mochly-Rosen D, Messing RO. Preservation of base-line hemodynamic function and loss of inducible cardioprotection in adult mice lacking protein kinase C epsilon. J Biol Chem. 2004;279:3596–3604. doi: 10.1074/jbc.M311459200. [DOI] [PubMed] [Google Scholar]
- 20.Jaenisch RBA. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33:245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 21.Bogdarina I, Welham S, King PJ, Burns SP, Clark AJ. Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ Res. 2007;100:520–526. doi: 10.1161/01.RES.0000258855.60637.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gluckman PDHM, Buklijas T, Low FM, Beedle AS. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat Rev Endocrinol. 2009;5:401–408. doi: 10.1038/nrendo.2009.102. [DOI] [PubMed] [Google Scholar]
- 23.Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science. 2001;293:1068–1070. doi: 10.1126/science.1063852. [DOI] [PubMed] [Google Scholar]
- 24.Meyer K, Zhang H, Zhang L. Direct effect of cocaine on epigenetic regulation of PKCε gene repression in the fetal rat heart. J Mol Cell Cardiol. 2009;47:504–511. doi: 10.1016/j.yjmcc.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H, Meyer KD, Zhang L. Fetal exposure to cocaine causes programming of Prkce gene repression in the left ventricle of adult rat offspring. Biol Reprod. 2009;80:440–448. doi: 10.1095/biolreprod.108.072983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foldager CB, Munir S, Ulrik-Vinther M, Soballe K, Bunger C, Lind M. Validation of suitable house keeping genes for hypoxia-cultured human chondrocytes. BMC Mol Biol. 2009;10:94. doi: 10.1186/1471-2199-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inagaki K, Hahn HS, Dorn GW, 2nd, Mochly-Rosen D. Additive protection of the ischemic heart ex vivo by combined treatment with delta-protein kinase C inhibitor and epsilon-protein kinase C activator. Circulation. 2003;108:869–875. doi: 10.1161/01.CIR.0000081943.93653.73. [DOI] [PubMed] [Google Scholar]
- 28.Inagaki K, Begley R, Ikeno F, Mochly-Rosen D. Cardioprotection by epsilon-protein kinase C activation from ischemia: continuous delivery and antiarrhythmic effect of an epsilon-protein kinase C-activating peptide. Circulation. 2005;111:44–50. doi: 10.1161/01.CIR.0000151614.22282.F1. [DOI] [PubMed] [Google Scholar]
- 29.Shinmura K, Nagai M, Tamaki K, Bolli R. Loss of ischaemic preconditioning in ovariectomized rat hearts: possible involvement of impaired protein kinase C epsilon phosphorylation. Cardiovasc Res. 2008;79:387–394. doi: 10.1093/cvr/cvn086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kabir AM, Clark JE, Tanno M, Cao X, Hothersall JS, Dashnyam S, Gorog DA, Bellahcene M, Shattock MJ, Marber MS. Cardioprotection initiated by reactive oxygen species is dependent on activation of PKCepsilon. Am J Physiol Heart Circ Physiol. 2006;291:H1893–H1899. doi: 10.1152/ajpheart.00798.2005. [DOI] [PubMed] [Google Scholar]
- 31.Meyer K, Zhang H, Zhang L. Prenatal cocaine exposure abolished ischemic preconditioning-induced protection in adult male rat hearts: role of PKCε. Am J Physiol Heart Circ Physiol. 2009;296:H1566–H1576. doi: 10.1152/ajpheart.00898.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bae S, Xiao YH, Li G, Casiano C, Zhang L. Effect of maternal chronic hypoxic exposure during gestation on apoptosis in fetal rat heart. Am J Physiol Heart Circ Physiol. 2003;285:H983–H990. doi: 10.1152/ajpheart.00005.2003. [DOI] [PubMed] [Google Scholar]
- 33.Hescheler J, Meyer R, Plant S, Krautwurst D, Rosenthal W, Schultz G. Morphological, biochemical, and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circ Res. 1991;69:1476–1486. doi: 10.1161/01.res.69.6.1476. [DOI] [PubMed] [Google Scholar]
- 34.Hamid T, Gu Y, Ortines RV, Bhattacharya C, Wang G, Xuan YT, Prabhu SD. Divergent tumor necrosis factor receptor-related remodeling responses in heart failure: role of nuclear factor-kappaB and inflammatory activation. Circulation. 2009;119:1386–1397. doi: 10.1161/CIRCULATIONAHA.108.802918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pchejetski D, Kunduzova O, Dayon A, Calise D, Seguelas MH, Leducq N, Seif I, Parini A, Cuvillier O. Oxidative stress-dependent sphingosine kinase-1 inhibition mediates monoamine oxidase A-associated cardiac cell apoptosis. Circ Res. 2007;100:41–49. doi: 10.1161/01.RES.0000253900.66640.34. [DOI] [PubMed] [Google Scholar]
- 36.Kaneda R, Ueno S, Yamashita Y, Choi YL, Koinuma K, Takada S, Wada T, Shimada K, Mano H. Genome-wide screening for target regions of histone deacetylases in cardiomyocytes. Circ Res. 2005;97:210–218. doi: 10.1161/01.RES.0000176028.18423.07. [DOI] [PubMed] [Google Scholar]
- 37.Tang Y, Jackson M, Qian K, Phillips MI. Hypoxia inducible double plasmid system for myocardial ischemia gene therapy. Hypertension. 2002;39:695–698. doi: 10.1161/hy0202.103784. [DOI] [PubMed] [Google Scholar]
- 38.Mizukami Y, Ono K, Du CK, Aki T, Hatano N, Okamoto Y, Ikeda Y, Ito H, Hamano K, Morimoto S. Identification and physiological activity of survival factor released from cardiomyocytes during ischaemia and reperfusion. Cardiovasc Res. 2008;79:589–599. doi: 10.1093/cvr/cvn148. [DOI] [PubMed] [Google Scholar]
- 39.Kimes BW, Brandt BL. Properties of a clonal muscle cell line from rat heart. Exp Cell Res. 1976;98:367–381. doi: 10.1016/0014-4827(76)90447-x. [DOI] [PubMed] [Google Scholar]
- 40.Kim MH, Jung YS, Moon CH, Jeong EM, Lee SH, Baik EJ, Moon CK. Isoform-specific induction of PKC-epsilon by high glucose protects heart-derived H9c2 cells against hypoxic injury. Biochem Biophys Res Commun. 2003;309:1–6. doi: 10.1016/s0006-291x(03)01525-0. [DOI] [PubMed] [Google Scholar]
- 41.Kim MJ, Moon CH, Kim MY, Kim MH, Lee SH, Baik EJ, Jung YS. Role of PKC-delta during hypoxia in heart-derived H9c2 cells. Jpn J Physiol. 2004;54:405–414. doi: 10.2170/jjphysiol.54.405. [DOI] [PubMed] [Google Scholar]
- 42.Bishai JM, Blood AB, Hunter CJ, Longo LD, Power GG. Fetal lamb cerebral blood flow (CBF) and oxygen tensions during hypoxia: a comparison of laser Doppler and microsphere measurements of CBF. J Physiol. 2003;546:869–878. doi: 10.1113/jphysiol.2002.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uetani T, Nakayama H, Okayama H, Okura T, Higaki J, Inoue H, Higashiyama S. Insufficiency of pro-heparin-binding epidermal growth factor-like growth factor shedding enhances hypoxic cell death in H9c2 cardiomyoblasts via the activation of caspase-3 and c-Jun N-terminal kinase. J Biol Chem. 2009;284:12399–12409. doi: 10.1074/jbc.M900463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shin EJ, Schram K, Zheng XL, Sweeney G. Leptin attenuates hypoxia/reoxygenation-induced activation of the intrinsic pathway of apoptosis in rat H9c2 cells. J Cell Physiol. 2009;221:490–497. doi: 10.1002/jcp.21883. [DOI] [PubMed] [Google Scholar]
- 45.Compere SJ, Palmiter RD. DNA methylation controls the inducibility of the mouse metallothionein-I gene in lymphoid cells. Cancer Cell. 1980;25:233–240. doi: 10.1016/0092-8674(81)90248-8. [DOI] [PubMed] [Google Scholar]
- 46.Chuang JC, Yoo CB, Kwan JM, Li TW, Liang G, Yang AS, Jones PA. Comparison of biological effects of non-nucleoside DNA methylation inhibitors versus 5-aza-2'-deoxycytidine. Mol Cancer Ther. 2005;4:1515–1520. doi: 10.1158/1535-7163.MCT-05-0172. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen CTWD, Velicescu M, Gonzales FA, Lin JC, Liang G, Jones PA. Histone H3-lysine 9 methylation is associated with aberrant gene silencing in cancer cells and is rapidly reversed by 5-aza-2'-deoxycytidine. Cancer Res. 2002;62:6456–6461. [PubMed] [Google Scholar]
- 48.Bender CM, Pao MM, Jones PA. Inhibition of DNA methylation by 5-aza-2'-deoxycytidine suppresses the growth of human tumor cell lines. Cancer Res. 1998;58:95–101. [PubMed] [Google Scholar]
- 49.Lee BHYS, Lin X, Nelson WG. Procainamide is a specific inhibitor of DNA methyltransferase 1. J Biol Chem. 2005;280:40749–40756. doi: 10.1074/jbc.M505593200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krishnan M, Park JM, Cao F, Wang D, Paulmurugan R, Tseng JR, Gonzalgo ML, Gambhir SS, Wu JC. Effects of epigenetic modulation on reporter gene expression: implications for stem cell imaging. FASEB J. 2006;20:106–108. doi: 10.1096/fj.05-4551fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bae S, Zhang L. Gender differences in cardioprotection against ischemia/reperfusion injury in adult rat hearts: focus on Akt and protein kinase C signaling. J Pharmacol Exp Ther. 2005;315:1125–1135. doi: 10.1124/jpet.105.090803. [DOI] [PubMed] [Google Scholar]
- 52.Nilsson S, Mäkelä S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. Mechanisms of estrogen action. Physiol Rev. 2001;81:1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.