Abstract
The Drosophila proteases Gastrulation Defective and Snake function in embryonic polarity establishment and bind heparin, a surrogate for anionic species present in the extracellular matrix. Here we demonstrate binding of GD, but not Snake, to anionic species that appear to be tightly associated with a highly purified eggshell matrix.
Keywords: Gastrulation Defective, Snake, Pipe, embryonic polarity, serine protease cascade, Drosophila
INTRODUCTION
The dorsoventral axis of the early Drosophila embryo is established by the ventrally restricted activity of a serine protease cascade within the extracellular space between the embryo plasma membrane and the eggshell (reviewed in [1,2]). Genetic and biochemical experiments have established that Gastrulation Defective (GD) activates Snake, which in turn activates Easter, the protease directly responsible for activating a pro-form of an NGF-related ligand, Spätzle, for the transmembrane receptor Toll. The activation of Easter and Spätzle is strictly dependent on spatial control involving Pipe, a putative sulfotransferase hypothesized to modify a secreted glycan that is fixed in the extracellular matrix on the ventral side of the embryo. GD appears to be activated uniformly around the embryo circumference, while the activation of Snake is still under investigation, so either GD activation of Snake or Snake activation of Easter could be the first reaction that is spatially regulated by an as-yet-unknown target of Pipe-mediated sulfation.
Additional extracellular matrix molecules may play roles in the apparently complex interactions of the proteases in this cascade. GD is localized and normally fixed at the oocyte surface [3], and appears to be quickly bound rather than freely diffusible following secretion [4]. GD and Snake show allele-specific interactions that suggest there is a functional requirement for a GD-Snake complex beyond the simple proteolytic activation of Snake [5]. Studies in insect cell culture systems have further revealed a network of feedback cleavages among the proteases [3,6], as well as differing activities of mutant Snake forms in cell culture compared to in embryos [7]. All of these results indicate a complex regulation of the cascade, rather than a simple linear activation pathway, and together with the search for potential Pipe targets provide motivation to examine interactions of the proteases with fixed components of the extracellular space surrounding the early embryo.
We recently described the isolation and proteomics analysis of an ovary extracellular matrix fraction that is highly enriched in eggshell components and minor oocyte surface proteins [8]. Here we describe the application of biochemical methods to characterize interactions of GD and Snake with this matrix preparation, as well as with anionic components of a less-stringently washed matrix preparation that may retain loosely bound components lost from the originally described preparation. We demonstrate strong interaction of GD, but not Snake, with specific components of both matrix preparations.
MATERIALS AND METHODS
Materials
Chemicals were purchased from Sigma except cell culture media and serum (Invitrogen); acrylamide: bis-acrylamide (37.5:1; Bio-Rad); heparin-Sepharose (GE HealthCare); non-fat dry milk (Carnation); and microBCA assay kit and PicoWest Chemiluminescent Substrate (Pierce). Plastics included six-well tissue culture plates (Falcon BD), 96-well ELISA plates (Nunc Maxisorp), disposable columns (Bio-Rad), and Centricon-PM-10 and syringe filter units (Millipore). Commercial antibodies included rabbit polyclonal anti-HA (Babco), rabbit polyclonal anti-myc (Santa Cruz Biotechnology), 10E4 anti-heparan sulfate (Seikagaku/Associates of Cape Cod) and HRP-conjugated goat anti-rabbit IgG or IgM (Jackson ImmunoResearch). Rabbit polyclonal anti-GD [3] and anti-Snake [7] antibodies have been described.
Cell culture expression of GD and Snake
Transient transfection of Drosophila S2 cells was performed as previously described [3]. The proteins expressed were HA-tagged GD ([3]), myc-tagged Snake ([3]; used only for Fig. 1), and untagged Snake ([7]; used for remaining Snake studies).
Figure 1.
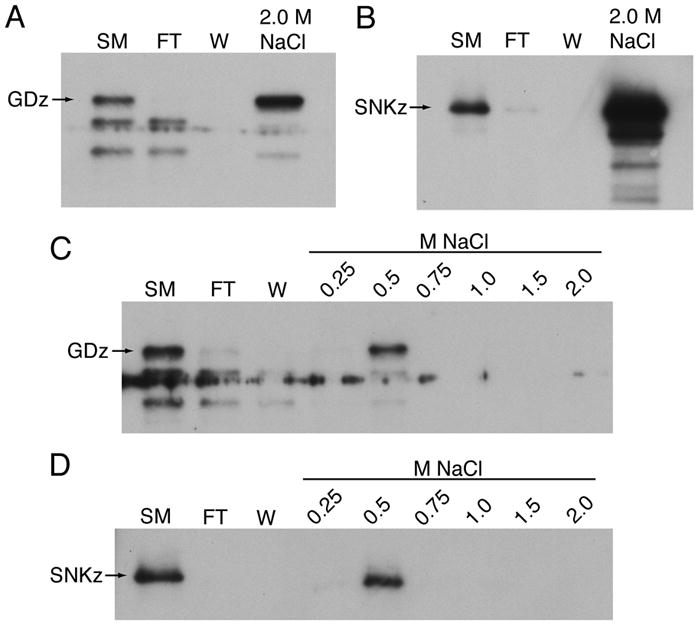
Interaction of GD and Snake with heparin-Sepharose. (A, C) Binding of HA-tagged GD with elution in a single 2.0 M NaCl step (A) or in sequential steps between 0.25 and 2.0 M NaCl (C). Detection was with polyclonal anti-HA antibody. (B, D) Binding and elution of myc-tagged Snake under same conditions. Detection with polyclonal anti-myc antibody. Abbreviations: SM, starting material, FT, flowthrough, W, wash, GDz, full-length GD zymogen, SNKz, full-length Snake zymogen.
Matrix preparations
Fly strains included the wild-type Oregon R (OR) strain, and pipeZH1/Df(3L)pipeA13 physically lacking the box 7 and box 10 pipe isoforms normally expressed in the ovary [9,10]. Young females were mated in groups of 15–20 to 3–4 males in heavily yeasted vials for 2 days to stimulate egg production. For the experiment shown in Fig. 4B, a highly purified eggshell matrix was prepared from 40 wild-type ovary pairs as described for LC-MS/MS analysis [8], including nuclease treatment and extensive washing, then extracted by boiling in 200 μl Laemmli sample buffer containing 100 mM dithiothreitol.
Figure 4.
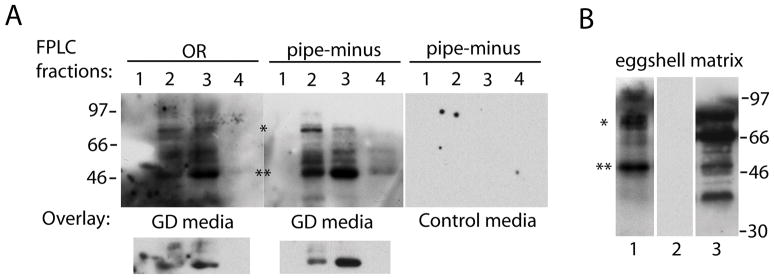
Blot overlay assay to determine GD binding to anionic FPLC fractions or to highly purified eggshell matrix. (A) FPLC fractions 1 – 4 from OR and pipe-minus were incubated with GD or control medium as indicated prior to detection of bound GD using a polyclonal anti-GD antibody. Below the main blots are lighter exposures showing just the relative reactivity with the ~50 kDa anionic species. (B) Purifed eggshell matrix from OR ovaries was blotted and reacted with GD media overlay (lane 1) or control media overlay (lane 2) prior to detection of bound GD, or with 10E4 anti-heparan sulfate antibody (lane 3). Positions of the major ~50 kDa (**) and ~85 kDa (*) bands discussed in the text are indicated.
For preparation of anionic matrix fractions by Mono Q chromatography, a less-stringently washed matrix was used as starting material. Typically, 200 ovary pairs were dissected from each genotype on ice, divided to tubes in groups of 50 ovary pairs, and each group homogenized in 250 μl ice-cold homogenization buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% Triton X-100, protease inhibitor cocktail). Without further washing, RNase A and DNase I were added at 200 μg/ml final concentration and 10 mM MgCl2, and the tubes were nutated at room temperature for 2 hours. Following a 100 g centrifugation, supernatants were discarded, and pellets extracted with 400 μl 6 M urea, 50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% Triton X-100 at 4° C overnight. Extracted proteins were separated from insoluble material by another 100 g spin and combined for each genotype, then concentrated and buffer-exchanged into Mono Q Buffer A (6 M urea, 50 mM Tris-HCl, pH 8.0) using Centricon-PM-10 filters, subjected to microBCA protein assay, and filtered using 0.44 μm syringe filters prior to loading < 1 ml volume onto the Mono Q column.
Column chromatography
For heparin-Sepharose chromatography, GDHA or SM –containing media (about 12 ml total) were dialyzed into 50 mM MOPS, pH 6.8, 150 mM NaCl, 2 mM EDTA. After filtration through a 0.22 μm filter, this starting material was loaded onto 0.5 ml bed volume heparin-Sepharose in a disposable column pre-equilibrated in 50 mM MOPS, pH 6.8, 150 mM NaCl, and flowthrough collected by gravity flow. Following washing in the same buffer, step elutions of 2 column volumes each were performed in MOPS buffer containing 0.25 – 2.0 M NaCl as indicated in the Results and Discussion.
Anion exchange chromatography was performed on an AKTAbasic 10 FPLC system fitted with a Mono Q_5/50_GL column having a 1 ml bed volume (GE HealthCare). Prior to loading the sample, the column was equilibrated in 6 column volumes of Mono Q Buffer A at a flow rate of 1 ml/min; following washout of the flowthrough, the column was further washed with 10 column volumes of Buffer A before the initiation of a linear gradient over 6 column volumes from 0% to 100% Mono Q Buffer B (6 M urea, 50 mM Tris-HCl, pH 8.0, 2 M NaCl). The “unbound” fractions used as a control in the GD ELISA binding assay were prepared by collecting flowthrough, re-concentrating, and running a second time through the Mono Q column and collected as flowthrough, to ensure removal of any anionic species.
ELISA
FPLC fractions were diluted to a standard concentration in TBS (50 mM Tris, pH 8.0, 150 mM NaCl), and coated at 500 ng to 2 μg per well in 96-well Nunc Maxisorp plates for 3 hours at 37° C. Blocking was with 0.5% Tween-20 in TBS for 1 hour at 37° C. Three washes between each subsequent step were in 0.05% Tween-20 in TBS, while protein and antibody dilutions were in 0.5% Tween-20 in TBS. Conditioned S2 cell culture media (GD, Snake, or control, diluted 1:10 except where otherwise indicated) were incubated overnight at 4° C; anti-GD or anti-Snake antibodies (1:2000) were incubated overnight at 4° C; HRP goat anti-rabbit secondary antibody (1:5000) was incubated for 2 hours at room temperature; and 0.25 mg/ml ortho-phenylenediamine in 100 mM citrate-phosphate buffer, pH 5.2 containing 0.03% H2O2 was incubated for 15–30 minutes at room temperature to develop the color reaction. Absorbance at 490 nm was measured in a Biotek Synergy microplate reader.
Protein gels, Western blotting, and blot overlay assays
Proteins separated on 13% SDS-PAGE gels were transferred to nitrocellulose and blotted with 10E4 anti-heparan sulfate antibody (1:1000). For blot overlay assays, control or GD media diluted 1:5 in 5% non-fat dry milk in 50 mM Tris, pH 7.5/150 mM NaCl/0.1% TX-100 (wash buffer) were incubated with pre-blocked membranes overnight at 4° C, washed, incubated with anti-GD (1:1000) overnight at 4° C, washed, and incubated with HRP goat anti-rabbit IgG (1:80,000) 2 hours at room temperature prior to developing with PicoWest Chemiluminescent Substrate.
RESULTS AND DISCUSSION
Dissing et al. [6] reported the binding of untagged GD and Snake expressed in the baculovirus system to heparin-Sepharose beads, which could be eluted by the addition of 2.0 M NaCl to disrupt ionic interactions. As a first step to looking at GD and Snake interactions using proteases expressed in Drosophila S2 cells [3], we reproduced their experiment using HA-tagged GD and myc-tagged Snake (Fig. 1A, B). We extended their findings by substitution of a sequential stepped elution (Fig. 1C, D), demonstrating that both GD and Snake were completely eluted from the column by a single step from 0.25 to 0.5 M NaCl.
Heparin-protease interactions disrupted by as little as 0.1 M NaCl have been correlated with significant effects of heparin on protease interactions with substrates or inhibitors, although more typically 0.3 – 0.5 M NaCl is required [11,12], so the heparin binding affinities seen for GD and Snake could be biologically important. However, recent genetic studies have cast serious doubt on the possibility that heparan sulfate or other glycosaminoglycans could be part of the Pipe target [13,14], so it is possible that binding to heparin-Sepharose reflects an affinity of GD and Snake towards binding to different sulfated or other negatively charged species. Such binding might be relevant to spatial control of the protease cascade or to interactions of GD and Snake with other cofactor molecules.
To further address the significance of the observed heparin-Sepharose binding, we chose to examine whether GD and Snake bind to anionic species isolated from Drosophila ovary extracellular matrix. In the course of preparing matrix from wild-type (OR) and pipe-minus (pipeZH1/Df(pipe)A13) ovaries, we observed that the pipe-minus ovaries were usually larger than the OR ovaries (Fig. 2), due to larger numbers of egg chambers. Matrix protein yields from pipe-minus ovaries were consequently about 20–40% higher than yields from the same number of OR ovaries. We have seen similar differences in ovary size and eggshell matrix yields from a different pipe mutant combination (pipe1/pipe2) when compared to a different wild-type strain (w1118; EKL, unpublished). Although we have not explored the underlying mechanism of the increased ovary size, we suggest the possibility that a target of Pipe modification may have a role in the regulation of cell proliferation in the ovary.
Figure 2.
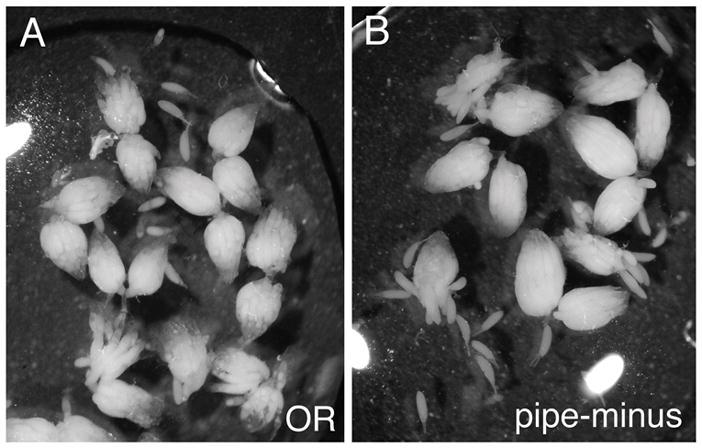
pipe-mutant ovaries tend to be larger than wild-type ovaries. (A) OR. (B) pipeZH1/Df(pipe)A13.
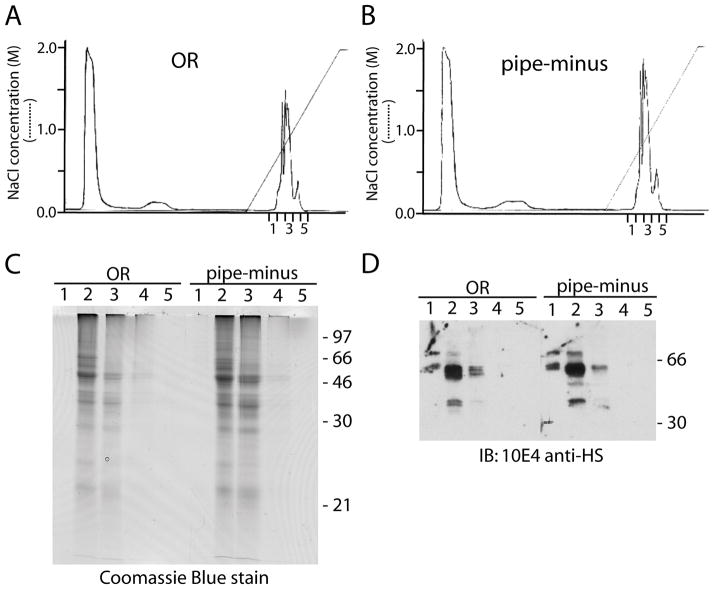
Urea-extracted matrix proteins were separated using Mono Q anion-exchange chromatography, yielding a characteristic set of peaks between 0.5 and 1.5 M NaCl that were captured in five 0.5 ml column fractions (Fig. 3A, B). Coomassie blue staining showed a complex array of bands in fractions 2 and 3 with high molecular weight smearing that might represent glycosaminoglycans expected in such anionic fractions (Fig. 3C). Immunoblotting with 10E4 monoclonal anti-heparan sulfate antibody confirmed the presence of heparan sulfate proteoglycans, with highest levels in Fraction 2 (Fig. 3D). There were no major differences seen between OR and pipe-minus preparations.
Figure 3.
Generation and basic characterization of anionic matrix fractions. (A, B) Plots of absorbance at 280 nm over the course of Mono Q anion exchange chromatography runs for OR (A) and pipe-minus (B) matrix preparations. For each, five anionic fractions were collected and used in subsequent experiments. (C) Coomassie blue stained gel of the indicated fractions. (D) A blot of the anionic fractions was probed with 10E4, a monoclonal anti-heparan sulfate antibody.
The binding of GD and Snake to the anionic fractions was tested by incubating GD, Snake, or control conditioned media in wells previously coated with 2.0 μg protein prepared from column fractions, then detecting bound GD or Snake by ELISA assay with the relevant antibodies. In initial trials, GD media gave a robust signal in the subsequent GD ELISA compared to control media (Table 1), while Snake media gave no significantly increased signal in the subsequent Snake ELISA over control (Table 2). Dilution series in which the GD and Snake medias were coated directly onto plastic and detected by ELISA with their respective antibodies (Tables 1 and 2, righthand two columns) did illustrate that there is about 4-fold decreased signal in the ability to detect bound Snake compared to bound GD; however, it is still likely that a significant interaction of Snake with the anionic matrix fractions would have been detectable over the background signal seen with control media (Table 2). These results suggested that GD, but not Snake, has significant interactions with anionic matrix species detectable in this assay, and Snake interactions were not pursued further in this study.
Table 1.
GD binding to anionic fractions measured by ELISA, with dilution series of direct GD plating on plastic for determining ELISA parameters
| Ctrl Media | GD Media | OD | GD Media Dilution | |
|---|---|---|---|---|
| OR fxn 2 | 0.175 | 1.773 | 3.308 | 1:3 |
| OR fxn 3 | 0.269 | 2.421 | 3.212 | 1:9 |
| Pipe fxn 2 | 0.186 | 2.402 | 2.834 | 1:27 |
| Pipe fxn 3 | 0.191 | 2.997 | 2.267 | 1:81 |
| 1.777 | 1:243 | |||
| 1.057 | 1:729 | |||
| 0.592 | 1:2187 | |||
| 0.133 | 1:6561 | |||
Table 2.
Snake binding to anionic fractions measured by ELISA, with dilution series of direct Snake plating on plastic for determining ELISA parameters
| Ctrl Media | Snake Media | OD | Snake Media Dilution | |
|---|---|---|---|---|
| OR fxn 2 | 0.160 | 0.187 | 0.751 | 1:3 |
| OR fxn 3 | 0.137 | 0.161 | 0.591 | 1:9 |
| Pipe fxn 2 | 0.263 | 0.202 | 0.497 | 1:27 |
| Pipe fxn 3 | 0.168 | 0.179 | 0.328 | 1:81 |
| 0.257 | 1:243 | |||
| 0.189 | 1:729 | |||
| 0.143 | 1:2187 | |||
| 0.117 | 1:6561 | |||
Although GD is uniformly distributed and activated [3], in subsequent experiments we compared its interactions with both wild-type and pipe-minus matrix because the proteoglycan or glycoprotein modified by Pipe is likely to be uniformly distributed [9] and could have Pipe-independent as well as Pipe-dependent interactions with GD or other proteases, e.g., through its core protein or sulfated glycans. In further ELISA experiments (where one of three trials is shown in Table 3), we compared GD versus control binding to 0.5 μg protein from each of the OR and pipe-minus anionic fractions, as well as to a Mono Q unbound fraction from each genotype prepared by passing flowthrough through the column for a second time. For both genotypes, GD bound most strongly to fraction 3, and showed significantly greater binding to anionic fractions 2 – 5 than to the unbound (non-anionic) material in the urea-extracted eggshell matrix. These results indicate that GD has binding affinity for one or more anionic species enriched in fraction 3 and present in both wild-type and pipe-mutant matrix.
Table 3.
GD binding to anionic and unbound (non-anionic) fractions measured by ELISA
| Ctrl Media | GD Media | Ctrl Media | GD Media | ||
|---|---|---|---|---|---|
| OR fxn 1 | 0.135 | 0.356 | Pipe fxn 1 | 0.140 | 0.473 |
| OR fxn 2 | 0.165 | 0.684 | Pipe fxn 2 | 0.187 | 0.629 |
| OR fxn 3 | 0.146 | 0.795 | Pipe fxn 3 | 0.131 | 0.681 |
| OR fxn 4 | 0.126 | 0.681 | Pipe fxn 4 | 0.127 | 0.616 |
| OR fxn 5 | 0.123 | 0.635 | Pipe fxn 5 | 0.114 | 0.522 |
| OR unbound | 0.138 | 0.320 | Pipe unbound | 0.156 | 0.328 |
To test whether GD binds to one or several anionic species, we performed blot overlay assays by incubating GD or control conditioned media with anionic fractions separated by 13% SDS-PAGE and transferred to nitrocellulose, followed by Western blotting with anti-GD antibody. While several bands were found to bind GD (Fig. 4A), the most intense interactions were with an ~50 kDa band enriched in fraction 3 (also present in fraction 2 and 4) and an ~85 kDa band enriched in fraction 2 (also present in fraction 3). The ~50 kDa species is unlikely to correspond to the most prominent band seen by Coomassie staining (Fig. 2C), because the latter band is strongly enriched in fraction 2 compared to fraction 3.
Finally, we were interested in determining whether the GD-binding species were also present in the highly purified eggshell matrix from which loosely bound molecules would likely be stripped by stringent washing conditions [8]. A similar array of binding proteins was observed (Fig. 4B, lane 1), including strong interactors at ~50 kDa, ~85 kDa, and a cluster at ~97 kDa that may correspond to a similar cluster observed in anionic fractions 2 and 3 (Fig. 4A). Despite the similar patterns, because of the limited number of trials to date and the use of eggshell matrix and anionic FPLC fractions run on separate gels it is not yet possible to make a solid conclusion that the GD-binding species in the two preparations are identical. The pattern of GD-interacting bands in the eggshell matrix did not correspond to the pattern of heparan sulfate proteoglycans observed by 10E4 immunoblotting (Fig. 4B, lane 3), although the strong ~50 kDa GD interactor appeared to co-migrate with a relatively low-abundance heparan sulfate proteoglycan.
These studies demonstrate that GD has robust interactions with a small number of anionic components that are likely to be tightly associated with the extracellular matrix, and illustrate the utility of ELISA and blot overlay assays in characterizing such interactions. Although Snake showed approximately equivalent binding to heparin-Sepharose as seen for GD, it did not appear to interact with anionic components in the ELISA assay. In the future, it may be possible to identify interactors by mass spectrometry analysis of gel slices or spots, and to combine two-dimensional gels followed by GD blot overlay and 10E4 blotting to determine if the ~50 kDa GD interactor is a heparan sulfate proteoglycan.
Acknowledgments
We thank Lora LeMosy for assistance in preparing the tables. This work was supported by NIH grant GM067738.
ABBREVIATIONS
- GD
Gastrulation Defective
- HA
Hemaglutinin
- HRP
Horseradish peroxidase
- ELISA
Enzyme-linked immunosorbent assay
References
- 1.Moussian B, Roth S. Dorsoventral axis formation in the Drosophila embryo -- shaping and transducing a morphogen gradient. Curr Biol. 2005;15(21):R887–R899. doi: 10.1016/j.cub.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 2.LeMosy EK. Proteolytic regulatory mechanisms in the formation of extracellular morphogen gradients. Birth Defects Res C. 2006;78(3):243–255. doi: 10.1002/bdrc.20074. [DOI] [PubMed] [Google Scholar]
- 3.LeMosy EK, Tan YQ, Hashimoto C. Activation of a protease cascade involved in patterning the Drosophila embryo. Proc Natl Acad Sci USA. 2001;98(9):5055–5060. doi: 10.1073/pnas.081026598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeLotto R. Gastrulation defective, a complement factor C2/B-like protease, interprets a ventral prepattern in Drosophila. EMBO Rep. 2001;2(8):721–726. doi: 10.1093/embo-reports/kve153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ponomareff G, Giordano H, DeLotto Y, DeLotto R. Interallelic complementation at the Drosophila melanogaster gastrulation defective locus defines discrete functional domains of the protein. Genetics. 2001;159(2):635–645. doi: 10.1093/genetics/159.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dissing M, Giordano H, DeLotto R. Autoproteolysis and feedback in a protease cascade directing Drosophila dorsal-ventral cell fate. EMBO J. 2001;20(10):2387–2393. doi: 10.1093/emboj/20.10.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian S, LeMosy EK. Mutagenesis of the cysteine-rich clip domain in the Drosophila patterning protease, Snake. Arch Biochem Biophys. 2008;475(2):169–174. doi: 10.1016/j.abb.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fakhouri M, Elalayli M, Sherling D, Hall JD, Miller E, Sun X, Wells L, LeMosy EK. Minor proteins and enzymes of the Drosophila eggshell matrix. Dev Biol. 2006;293(1):127–141. doi: 10.1016/j.ydbio.2006.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sen J, Goltz JS, Stevens L, Stein D. Spatially restricted expression of pipe in the Drosophila egg chamber defines embryonic dorsal-ventral polarity. Cell. 1998;95(4):471–481. doi: 10.1016/s0092-8674(00)81615-3. [DOI] [PubMed] [Google Scholar]
- 10.Sergeev P, Streit A, Heller A, Steinmann-Zwicky M. The Drosophila dorsoventral determinant PIPE contains ten copies of a variable domain homologous to mammalian heparan sulfate 2-sulfotransferase. Dev Dyn. 2001;220(2):122–132. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1094>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 11.Yang L, Manithody C, Rezaie A. Localization of the heparin binding exosite of Factor IXa. J Biol Chem. 2002;277(52):50756–50760. doi: 10.1074/jbc.M208485200. [DOI] [PubMed] [Google Scholar]
- 12.Yang L, Manithody C, Rezaie A. Contribution of basic residues of the 70–80 loop to heparin binding and anticoagulant function of activated Protein C. Biochemistry. 2002;41(19):6149–6157. doi: 10.1021/bi015899r. [DOI] [PubMed] [Google Scholar]
- 13.Zhu X, Sen J, Stevens L, Goltz JS, Stein D. Drosophila Pipe protein activity in the ovary and the embryonic salivary gland does not require heparan sulfate glycosaminoglycans. Development. 2005;132(17):3813–3822. doi: 10.1242/dev.01962. [DOI] [PubMed] [Google Scholar]
- 14.Zhu X, Stevens L, Stein D. Synthesis of the sulfate donor PAPS in either the Drosophila germline or somatic follicle cells can support embryonic dorsal-ventral axis formation. Development. 2007;134(8):1465–1469. doi: 10.1242/dev.003426. [DOI] [PubMed] [Google Scholar]