Abstract
Photodynamic therapy (PDT), an anticancer treatment modality, has recently been shown to be an effective treatment for several autoimmune disease models including antigen-induced arthritis. PDT was found to induce the expression of IL-10 messenger RNA (mRNA) and protein in the skin, and this expression has similar kinetics to the appearance of PDT-induced suppression of skin-mediated immune responses such as the contract hypersensitivity (CHS) response. Some aspects of the UVB-induced suppression of the immune response have been linked to the induction of IL-10. IL-10 has been shown to inhibit the development and activation of Th1 cells, which are critical for many cell-mediated immune responses, including CHS. We have examined the effect of PDT and UVB irradiation on the activity of the IL-10 gene promoter and on IL-10 mRNA stability using the murine keratinocyte line, PAM 212. In vitro PDT induces IL-10 mRNA and protein expression from PAM 212 cells, which can be correlated with an increase in AP-1 DNA binding activity and activation of the IL-10 gene promoter by PDT. Deletion of an AP-1 response element from the IL-10 gene promoter was shown to abrogate the PDT-induced promoter activity indicating that the AP-1 response element is critical to IL-10 induction by PDT. In addition, PDT results in an increase in IL-10 mRNA stability, which may also contribute to the increased IL-10 expression in PAM 212 cells following PDT. In vitro UVB irradiation also results in activation of the IL-10 promoter. However, in contrast to PDT, UVB-induced activation of the IL-10 promoter is not AP-1 dependent and did not increase IL-10 mRNA stability.
INTRODUCTION
Photodynamic therapy (PDT)† involves the activation of a photosensitizer (PS) by light of a specific wavelength (reviewed in Dougherty et al. [1]). Activated PS undergo oxygen-dependent photochemical reactions that lead to cytotoxicity at the site of PS localization and illumination. Although PDT was initially developed and is approved in the United States as a cancer therapy, recent interest has focused on the use of PDT in the treatment of nonmalignant maladies, specifically in the treatment of autoimmune and vascular diseases. Several preclinical studies have demonstrated that PDT is an effective therapy for antigen-induced arthritis, a model for rheumatoid arthritis (2,3). Additionally transcutaneous PDT, systemic delivery of the PS followed by whole-body irradiation, has been shown to inhibit disease onset and symptoms in murine experimental autoimmune encephalomyelitis (4). Transcutaneous PDT is under investigation for the treatment of psoriasis (5). Transcutaneous PDT has also been shown to be an effective treatment for age-related macular degeneration (AMD) (6).
Several mechanisms have been proposed for the suppression of autoimmunity by PDT. Activated lymphocytes have been shown to be more sensitive to PDT than naive cells (7–9); thus PDT may act to eliminate the autoreactive effector cells. PDT has also been shown to alter the antigen presenting capabilities, and therefore T cell stimulatory function, of dendritic cells by reducing the levels of major histocompatibility antigens (MHC) and costimulatory molecules (CD80, CD86) expressed on the cell surface (10).
We have demonstrated that cutaneous PDT induces IL-10 expression (messenger RNA [mRNA] and protein) in skin (11) and results in an increase in systemic levels of IL-10 (12). IL-10 is the key regulator of many aspects of the inflammatory response, has multiple effects on T cell function and is known to inhibit MHC expression and antigen presenting function of Langerhans cells (13–16). IL-10 has also been shown to play a key role in psoriasis (17); psoriatic individuals exhibit low levels of cutaneous IL-10 and IL-10 therapy has an antipsoriatic activity (18). Recent studies have suggested that IL-10 induction maybe the causative agent in transcutaneous PDT-induced suppression of the contact hypersensitivity (CHS) response (19). Thus it is possible that some aspects of PDT suppression of autoimmune reactions are mediated by IL-10. Increased IL-10 expression following UVB irradiation has also been shown to be involved in UVB-induced immunosuppression (14,20) and UVB is known to induce IL-10 expression in keratinocytes (21).
PDT has also been shown to induce several other cytokines including IL-1β, IL-2, IL-6, tumor necrosis factor-α, interferon-γ, and granulocyte colony-stimulating factor from a wide variety of cells, including leukocytes and tumor cells (11,22–28). However, few studies have investigated the mechanism behind cytokine induction by PDT. PDT is known to induce IL-6 (11,23) and the IL-6 gene promoter contains an AP-1 regulatory element. Kick et al. (26) have shown that PDT induces AP-1 DNA binding activity. In addition to inducing AP-1 activity, PDT induces prolonged expression of c-fos and c-jun, which make up the AP-1 heterodimer (29). Although direct analysis of the role of AP-1 in PDT-induced IL-6 expression was not performed, these studies suggest that induction of AP-1 binding activity by PDT is responsible for the increase in IL-6 levels. Like the IL-6 gene promoter, the murine IL-10 gene promoter also contains an AP-1 regulatory element (30). In this study, we provide functional evidence that the PDT-induced IL-10 mRNA and protein expression in a mouse keratinocyte line, PAM 212, is a result of increased AP-1 DNA binding activity. Deletion of the AP-1 regulatory element from the IL-10 promoter abrogates the effect of PDT on IL-10 induction indicating that this element is critical for PDT-induced transcription of IL-10. We also demonstrate that PDT increases IL-10 mRNA stability in PAM 212 cells. Thus the enhanced expression of IL-10 following PDT is due to both an increase in gene promoter activity and to an increase in mRNA stability.
MATERIALS AND METHODS
In vitro PDT treatment
PAM 212 cells (31) were obtained from Dr. Stuart Yuspa (National Cancer Institute, Bethesda, MD) and maintained in Roswell Park Memorial Institute 1640 supplemented with 10% fetal bovine serum, nonessential amino acids, glutamine, sodium pyruvate, 2-mercaptoethanol and antibiotics (all from GIBCO-BRL, Grand Island, NY) in a humidified atmosphere of 5% CO2 in air. Exponentially growing cells were exposed to 2.5 μg/mL Photofrin® in complete medium for 24 h, followed by exposure to drug-free complete medium for 3 h. Clinical-grade, pyrogen-free Photofrin® (QLT Inc., Vancouver, B.C., Canada) was reconstituted to 2.5 mg/mL in pyrogen-free 5% dextrose (D5W; Baxter Corp., Deerfield, IL) prior to use. At the end of the drug efflux period, cells were exposed to 630 nm light delivered with an argon-dye laser system (Spectra Physics, Mountain View, CA) for a total dose of 0.4 J/cm2 (lethal dose [~LD20]). At selected time points thereafter cells were collected for RNA, enzyme-linked immunosorbent assay (ELISA) or electrophoretic mobility shift assay (EMSA) analysis. In experiments in which the supernatant was collected, culture medium was replaced with CHO serum-free medium (GIBCO-BRL) supplemented as above (with the exception of serum) immediately following treatment. Cell viability was determined by the 3-(4,5-dimethylthiazole-2-yl)-2,5-biphenyl tetrazolium bromide assay (32).
In vitro UVB treatment
PAM 212 cells were washed twice in phosphate buffered saline (PBS) and 0.5 mL of PBS was added to each 10 cm2 tissue culture dish. Cells were irradiated with a pair of FS20 sunlamps, which have an output peak at 310 nm, and received a total dose of 95 J/m2 at 3.8 W/m2 (~LD20). Following treatment, 8 mL of complete culture medium was added and the cells were cultured as described above.
RNA isolation and reverse-transcriptase polymerase chain reaction
Total RNA was isolated from cells with Ultraspec (Biotecx Laboratories, Inc., Houston, TX). RNA was quantified spectrophotometrically and diluted to a concentration of 2 μg/μL. Reverse-transcriptase polymerase chain reaction (RT-PCR) was done as previously described (11) using IL-10 (35 PCR cycles) or actin (25 PCR cycles) specific primers (Stratagene, La Jolla, CA). The reaction products were separated on a 2% agarose gel, stained with ethidium bromide, examined under UV light and subjected to densiotometry. Control PCR reactions were performed on samples prepared in the absence of reverse transcriptase to test for the presence of contamination by cellular DNA. Reagents were routinely checked for contamination and results were confirmed in three replicate experiments. RT-PCR results are presented as relative IL-10 mRNA. Relative mRNA levels are calculated following normalization to actin levels.
IL-10 ELISA
Supernatants were collected at various times following treatments and were concentrated 10-fold using 3K Microsep centrifugal concentrators (Pall Filtron Corp., Northborough, MA). Protein determinations were made using BioRad protein assay reagent (BioRad, Hercules, CA). ELISA assays were performed as described previously (11).
IL-10 promoter constructs
A DNA fragment containing the murine IL-10 promoter (−1626 to +92 bp) was isolated from an IL-10 genomic clone obtained from Dr. K. Moore, DNAX Research Inst., Palo Alto, CA (33). The fragment was subcloned into the pCAT3basic reporter plasmid (Promega Corp., Madison, WI) to generate pIL10pro(−1626)CAT. The pCAT3basic vector contains the coding region for chloramphenicol acetyltransferase (CAT). The 5′ deletion constructs (i.e. pIL10pro(−1033)CAT) were generated by restriction enzyme digestion of the full length promoter clone as follows: −1033 bp, Kpn I/Xba I; −885 bp, Bgl II/Xba I; −863 bp, Sca I/Xba I; −125 bp, Bgl I/Xba I. The deletion fragments were isolated and subcloned into the pCAT3basic plasmid. Deletion of the AP-1 regulatory element was performed by two-stage PCR as previously described (34), using the following primer pairs for the primary PCR:
| 5′ fragment: | GCGACGCGTCAGTCAGGAGAGAGGG (−1625 bp to −1613 plus a Mlu I site) |
| GTTGGAATGGAAT/AGAACTGCTGCTCC (−1344 to −1357/−1365 to −1378 bp) | |
| 3′ fragment: | GGAGCAGCAGTTCT/AGGCCAGGCCAAC (−1378 to −1365/−1357 to −1344 bp) |
| TATCTCGTGCAGTTATTGTCTTCC (+96 to +74 bp plus a Xba I site). |
Primers were synthesized in the Roswell Park Biopolymer Core Facility. The primary PCR products were isolated and used for the secondary PCR reaction, along with the outermost primers from the primary PCR. The resulting fragment was isolated and subcloned into the Mlu I and Xba I sites of the pCAT3basic plasmid to create the pIL10pro(ΔAP1)CAT plasmid. All plasmid constructs were con-firmed by sequencing and were purified by double cesium chloride centrifugation.
Transient transfection of PAM 212 cells
Transient transfections were done using Superfect (Qiagen Inc., Valencia, CA) at a ratio of 2.5:1 with supercoiled DNA, according to the manufacturer’s protocol. Briefly, cells were plated at 7.5 × 105 cells/10 cm2 tissue culture dish the day before the transfection. The day of the trans-fection cells were washed two times with PBS and 3 mL of culture media containing Superfect and DNA complexes was added to the plates. Cells were incubated for 3 h at 37°C, 5% CO2. Following incubation, cells were washed twice with PBS and 8 mL of fresh culture medium was added. Cells were then incubated for 2 h, followed by addition of Photofrin® as described above. All transfections were done with 10 μg of pIL10pro DNA and included 5 μg of p-SEAP-control vector (Clontech Lab., Palo Alto, CA) as a transfection control.
CAT assays
CAT assays were done as previously described (34) with 100 μg of cell lysate collected 24–48 h after treatment. Following chromatography to separate the acetylated chloramphenicol products, results were analyzed using a Storm Phosphoimager (Molecular Dynamics, Redwood City, CA) and percent conversion of chloramphenicol to acetylated chloramphenicol was determined. To control for variance in transfection efficiencies each transfection contained 5 μg of pSEAPcontrol plasmid and all results were normalized by assaying for secreted alkaline phosphatase activity (SEAP Assay, Clontech). The data are representative of at least three independent transfections for a given recombinant and at least two independent preparations of each plasmid DNA were used.
Nuclear extract preparation and EMSA
Nuclear extracts were prepared as previously described (34) at various times after PDT or UVB treatment. EMSA was performed using 4 μg of nuclear extract incubated with 0.2–1 ng of 32P-labeled oligonucleotides for 20 min at room temperature (34). Oligonucleotides corresponding to the cyclic adenosine monophosphate (cAMP) response element (CRE), NF-κB, Oct-1 and AP-1 consensus sequences were obtained from Promega. One hundred nanograms of each oligonucleotide was end-labeled with T4 DNA kinase and separated from unincorporated radioactivity using Select-D G-25 columns (5-Prime-3-Prime, Inc., Boulder, CO). The specificity of the protein:DNA complexes was examined by incubating the nuclear extracts with a 100 or 500-fold molar excess of unlabeled competitor oligonucleotides for 15 min at room temperature prior to incubation with labeled oligonucleotides. The composition of the protein within the complex was determined by including rabbit polyclonal antibodies specific for either c-fos or c-jun/AP-1 (Santa Cruz Biotechnology, Santa Cruz, CA) or rabbit IgG (Sigma Chemical Co., St. Louis, MO). Antibodies were added to the extract in the presence of the oligonucleotide and incubated at 4°C overnight. A total of 4 μg of antibody was added to each reaction. Protein:DNA complexes were resolved by electrophoresis on 4% nondenaturing polyacrylamide gels, which were subsequently dried and exposed to Phosphoimager screens. Assays were performed on extracts isolated from a minimum of three independent experiments. To ensure equivalent nuclear extract activity, extracts were tested for binding activity to Oct-1, which was unchanged by PDT treatment (data not shown).
mRNA stability studies
To determine whether PDT alters the stability of IL-10 mRNA, PAM 212 cells were treated with UVB or PDT, then incubated with actinomycin D (10 μg/mL) 1 or 4 h after activation, respectively, to stop further transcription. Total RNA was isolated from cells 0, 0.5, 1, 2 or 4 h after actinomycin D addition and subjected to RT-PCR as described above. Densitometry of the ethidium stained gel was performed and the results were normalized to the actin mRNA levels. A total of three independent experiments were performed and the results are presented as the mean ± standard error of mean of the relative IL-10 mRNA expression following normalization to the expression of actin mRNA.
RESULTS
PDT induces IL-10 expression in PAM 212 cells
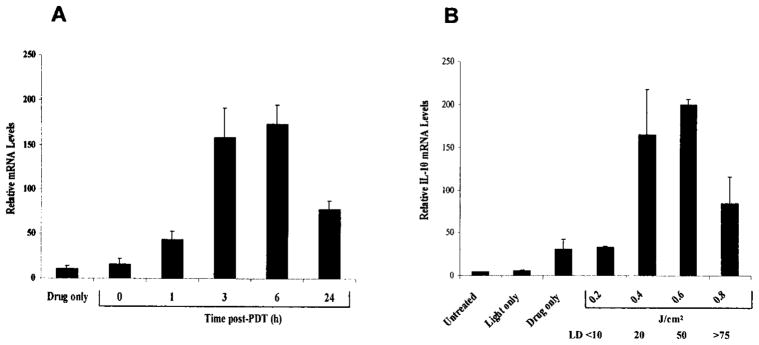
We have previously shown that large cutaneous surface area PDT induces IL-10 mRNA and protein expression in mouse skin (11). UVB also induces IL-10 expression in the skin, which appears to be due in part to increased IL-10 expression from keratinocytes (21). To determine whether PDT also induces IL-10 expression in keratinocytes, PAM 212 cells were treated in vitro and the levels of IL-10 mRNA were measured by RT-PCR. As shown in Fig. 1A, PDT (~LD20) induces IL-10 mRNA within 3 h of treatment. The increased mRNA levels persist for at least 24 h after treatment. To determine whether the IL-10 mRNA induction was dose dependent PAM 212 cells were treated with increasing light dose following 24 h incubation with 2.5 μg/mL Photofrin®. The levels of IL-10 mRNA were determined by RT-PCR 4 h after PDT. The induction of IL-10 mRNA by PDT exhibits a sharp dose dependency with a minimal induction at 0.2 J/cm2 (LD<10) and maximal levels reached following illumination with 0.4 J/cm2 (LD20) (Fig. 1B). Treatment with 0.4 and 0.6 J/cm2 (LD50) were not significantly different in the level of mRNA induced. The decrease in mRNA levels at 0.8 J/cm2 is most likely a result of cell death as this dose represents the LD75 for these cells. The induction of IL-10 mRNA by drug alone could reflect either a minimal exposure to light during sample manipulation or minimal activation of IL-10 expression by Photofrin® alone as has been shown for other genes (35). The kinetics of IL-10 mRNA induction in PAM 212 cells by PDT are similar to those seen in the skin following in vivo PDT treatment (11).
Figure 1.
Induction of IL-10 mRNA expression in PAM 212 cells by PDT. (A) PAM 212 cells were treated in vitro, with 2.5 μg/mL Photofrin® in complete medium for 24 h, followed by 3 h drug efflux in complete medium and illumination at 630 nm with 0.4 J/cm2 (~LD20). Total RNA was isolated from cells harvested immediately or at 1, 3, 6 and 24 h post-PDT, and 2 μg from each sample were subjected to RT-PCR analysis as described in “Materials and Methods.” Drug only controls were exposed to Photofrin® and were harvested after 6 h. Results are presented as in relative IL-10 mRNA levels ± SEM following normalization to the actin mRNA levels and represent the results of three independent experiments. (B) PAM 212 cells were treated in vitro, with 2.5 μg/mL Photofrin® in complete medium for 24 h, followed by 3 h drug efflux in complete medium and illumination at 630 nm with 0.2–0.8 J/cm2. Total RNA was isolated from cells harvested 4 h post-PDT and 2 μg from each sample were subjected to RT-PCR analysis as described in “Materials and Methods.” Drug only and light only controls were exposed to Photofrin® or 0.8 J/cm2 light only, respectively. Results are presented as in relative IL-10 mRNA levels ± SEM as described in “Materials and Methods” and represent the results of three independent experiments. The LD levels corresponding to each light dose are shown below the J/cm2 dose levels.
The induction of IL-10 by PDT in PAM 212 cells was further confirmed by examining the levels of IL-10 secretion following light exposure. PDT induces IL-10 secretion from PAM 212 cells within 6 h of treatment and reaches a maximum at 24 h after treatment (Fig. 2). The levels of IL-10 remain above background levels for up to 72 h after treatment. Photofrin® incubation in the dark is able to slightly increase the IL-10 secretion within 48 h. This suggests that the increase in IL-10 secretion seen with drug alone is due to one of the following: a minimal exposure to light during sample manipulation, inadvertent exposure to light during the longer incubation periods or minimal activation of expression by Photofrin® alone.
Figure 2.
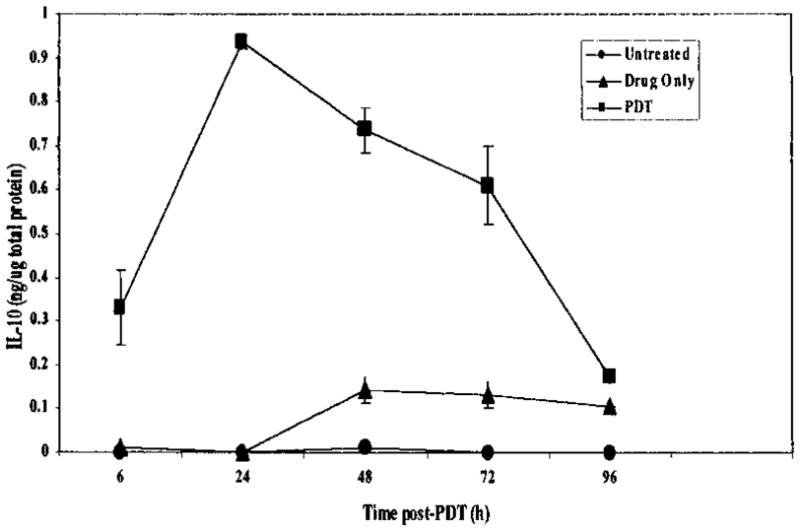
Induction of IL-10 secretion from PAM 212 cells by PDT. PAM 212 cells were treated in vitro, with 2.5 μg/mL Photofrin® in complete medium for 24 h, followed by 3 h drug efflux in complete medium and illumination at 630 nm with 0.4 J/cm2 (~LD20). Immediately following PDT, complete medium was replaced by serum-free medium and the cells were incubated at 37°C in 5%CO2. Supernatants were harvested at 6, 24, 48 and 72 h post-PDT, cells were removed by centrifugation and the supernatants were concentrated 10-fold and the amount of IL-10 present was determined by ELISA as described in “Materials and Methods.” Drug only controls were exposed to Photofrin® and harvested at the same time as the treated cells. Results are reported as the mean ± SEM ng of IL-10 per microgram of total protein.
PDT induces IL-10 gene promoter activity
An increase in protein expression can be a result of an increase in promoter activity, an increase in mRNA stability or an increase in mRNA translation. To determine whether the increase in IL-10 expression following PDT is a result of an increase in promoter activity, PAM 212 cells were transiently transfected with a CAT reporter plasmid containing the IL-10 gene promoter, pIL10pro(−1626)CAT. Transfected cells were treated with Photofrin®-PDT or for comparison, UVB irradiation at ~LD20 levels, and promoter activity was determined. As shown in Fig. 3, PDT significantly induces IL-10 gene promoter activity (P < 0.002 when compared to untreated cells). Minimal promoter activation was seen in untreated transfected cells or in transfected cells treated with light or drug alone. Therefore, the increase in IL-10 expression in PAM 212 cells post-PDT treatment is due, at least in part, to an increase in IL-10 gene promoter activity.
Figure 3.
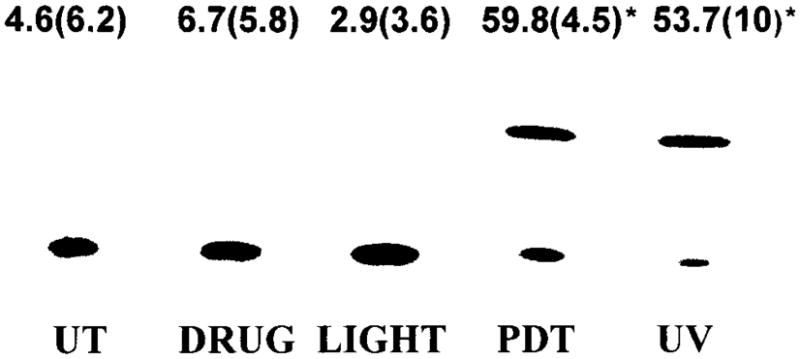
PDT induces IL-10 gene promoter activity. PAM 212 cells were transiently transfected with pIL10pro(−1626)CAT followed by either PDT or UVB treatment as described in “Materials and Methods.” Cells were harvested 48 h after treatment and CAT assays were performed with 100 μg of cell lysates. A representative experiment is shown. The numbers above each lane reflect the results [normalized percent conversion ± (SEM)] of a minimum of three independent experiments. Controls are represented as transfected untreated cells (UT) and transfected cells subjected to either light alone (light) or Photofrin® alone (drug). *Significant compared to controls.
UVB irradiation has been shown to stimulate IL-10 expression in PAM 212 cells (21). As seen in Fig. 3, UVB irradiation also significantly induces IL-10 gene promoter activity (P < 0.001 when compared to untreated cells).
Deletion analysis of IL-10 promoter activation by PDT
The murine IL-10 promoter is known to contain several potential response elements including AP-1, NF-κB, IL-6RE and a CRE (33). To determine if any of these elements are involved in the PDT induction of IL-10 gene promoter activity, 5′-deletion analysis of pIL10pro(−1626)CAT was performed. The schematic diagram of the IL-10 promoter shown in Fig. 4 indicates the sites of response elements that have been shown to be involved in redox reactions (AP-1, NF-κB, IL-6RE) or in IL-10 regulation (CRE). The deletion of the promoter region from −1626 to −1033 bp resulted in a significant ablation of the PDT induction of promoter activity. Promoter activity in cells transiently transfected with the truncated promoter constructs (pIL10pro(−1033)CAT; pIL10pro(−883)CAT; pIL10pro(−863)CAT; pIL10pro(−125)CAT) were not significantly different from each other or from activity in cells transfected with the control vector, pCAT3basic. Analysis of promoter activity in transiently transfected cells, which were either treated with drug alone (Fig. 4) or untreated (data not shown), demonstrated that 5′ deletion of the promoter had no effect on promoter activity in the absence of PDT as promoter activity in these cells remained low (Fig. 4). Therefore, it is unlikely that the deleted region contains a repressive element that suppresses promoter activity. In addition, these data suggest that PDT is not abrogating the activity of a repressive factor. The deletion of the −1626 to −1033 bp fragment did not render the promoter unresponsive to any stimulus; cells transiently transfected with the truncated promoter constructs were treated with UVB irradiation as above. UVB-induced promoter activity in cells transfected with the truncated promoter constructs was not significantly different from activity seen in cells transfected with the pIL10pro(−1626)CAT construct (Fig. 4). These results suggest that UVB and PDT regulate the IL-10 promoter differently and that the deleted promoter constructs are still functional.
Figure 4.
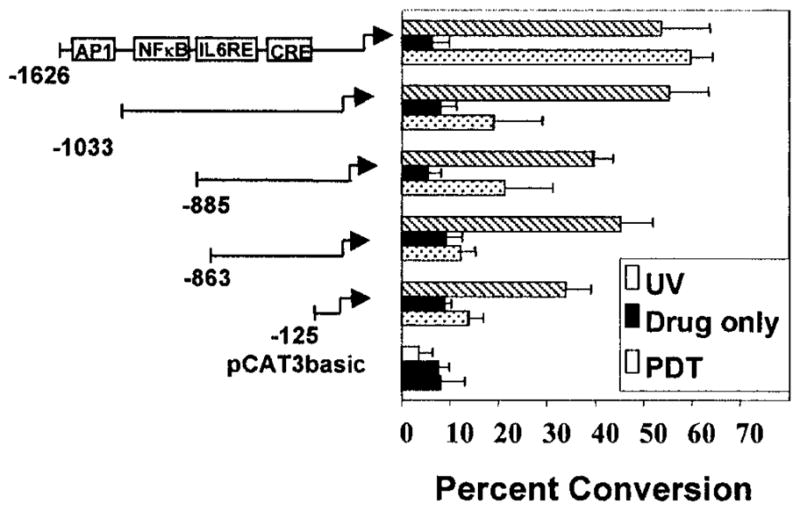
Identification of a 593 bp region of the IL-10 promoter required for PDT induction. (A) PAM 212 cells were transiently transfected with 5′-deletion constructs of the IL-10 promoter (pIL10pro(−1033)CAT, pIL10pro(−885)CAT, pIL10pro(−863)CAT, pIL10pro(−125)CAT), the full length promoter pIL10pro(−1626)CAT construct or the pCAT3basic vector, followed by PDT or UVB irradiation. CAT assays were performed as above. Results are presented as normalized mean percent conversion of chloramphenicol to acetylated chloramphenicol ± SEM.
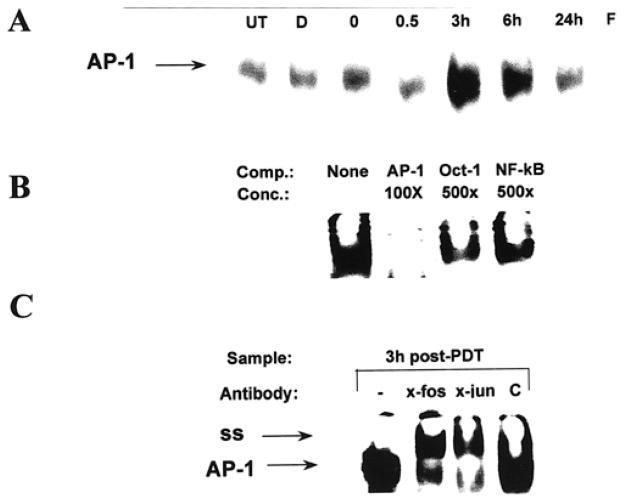
PDT treatment results in activation of AP-1 binding activity
Previous studies have shown that Photofrin®-PDT results in an increase in AP-1 and NF-κB binding activity in L1210 cells (36). Both NF-κB and AP-1 are known to be redox-regulated (37–40). EMSA analysis showed that PDT also increased AP-1 DNA binding activity in PAM 212 cells (Fig. 5A). AP-1 activity was increased by 3 h after treatment and remained high up to 6 h after treatment in three independent experiments. EMSA analysis performed with oligonucleotides corresponding to the CRE and NF-κB elements indicated no change in DNA binding activity following PDT treatment (data not shown). The specificity of the protein: DNA complex was confirmed by competition with excess cold oligonucleotides. Excess cold AP-1 oligonucleotide eliminated binding to radiolabeled oligonucleotide (Fig. 5B). Binding was not eliminated by inclusion of an 100-fold excess cold Oct-1 or NF-κB oligonucleotides; however, induction of NF-κB was seen following UVB treatment (data not shown). Minimal reduction in AP-1 DNA binding activity was observed in reactions performed in the presence of 500-fold excess Oct-1 or NF-κB oligonucleotides (Fig. 5B). To confirm the composition of the shifted complexes, EMSA analysis was performed with antibodies specific for c-fos or c-jun/AP-1. Both antibodies shifted the protein:DNA complex (Fig. 5C). Control antibodies (rabbit IgG) had no effect on the complex mobility.
Figure 5.
PDT induces AP-1 DNA binding activity in PAM 212 cells. (A) Nuclear extracts were isolated from PAM 212 cells at 0, 0.5, 3, 6, 24 h post-PDT and subjected to EMSA with radiolabeled AP-1, oligonucleotide as described in “Materials and Methods.” Nuclear extracts isolated from untreated cells (UT) and cells treated with Photofrin® (D) alone were used as controls. Free probe is also shown (F). Results from a representative experiment are shown. Extracts isolated from three independent experiments showed similar results. An arrow indicates the AP-1 complex. (B) Nuclear extracts were isolated 3 h post-PDT treatment were subjected to EMSA with radiolabeled AP-1 oligonucleotide in the presence or absence of 100 or 500-fold excess cold oligonucleotides to test for protein:DNA complex specificity. (C) Nuclear extracts were isolated 3 h post-PDT treatment and subjected to EMSA with radiolabeled AP-1 oligonucleotide in the presence or absence of 4 μg of anti-c-fos, anti-c-jun/AP-1 or control rabbit IgG, C, to identify proteins within the protein: DNA complex. The AP-1 and supershifted (ss) complexes are indicated with arrows.
Deletion of the AP-1 element abrogates induction of the IL-10 promoter by PDT
To confirm that the AP-1 element was critical to induction of the IL-10 gene promoter by PDT, the AP-1 element was specifically deleted from the full length promoter. Deletion of the 7 bp AP-1 element (−1365 to −1357 bp) inactivated the promoter response to PDT (Fig. 6A; 31 vs 0%; P < 0.002) but had no effect on UVB induction of promoter activity (Fig. 6B; 41.5 vs 37%; P < 0.1602). These results indicate that the AP-1 regulatory element is critical to induction of IL-10 promoter activity by PDT and suggest that activation of AP-1 DNA binding activity by PDT results in an increase in IL-10 promoter activity.
Figure 6.
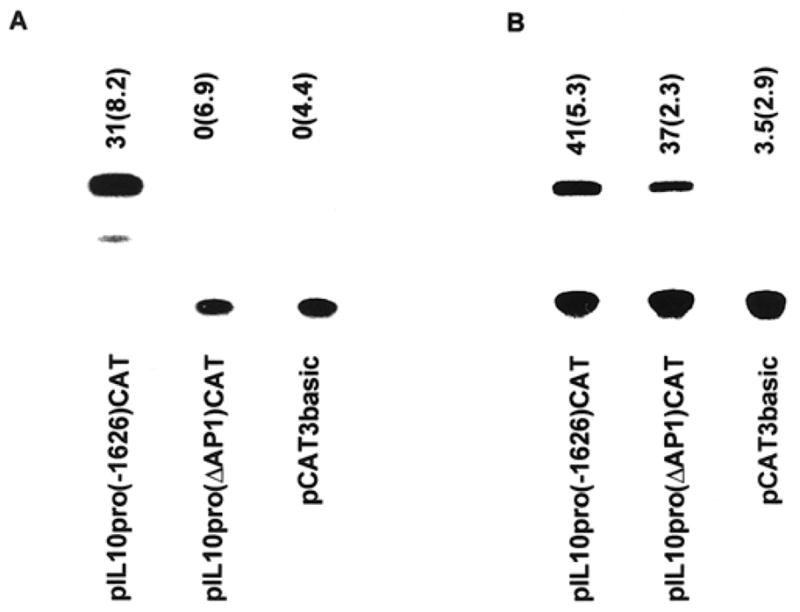
Deletion of the AP-1 response element from the IL-10 promoter abrogates the PDT responsiveness. PAM 212 cells were: (A) transiently transfected with pIL10(−1626)CAT, pIL10(ΔAP1)CAT or pCAT3basic followed by PDT; or (B) UVB irradiation as described above. CAT assays were done as described in “Materials and Methods.” A representative experiment is shown; the numbers above each lane reflect the results [normalized percent conversion (SEM)] of a minimum of three independent experiments.
PDT increases IL-10 mRNA stability
Increased protein and mRNA expression can also be a result of increased mRNA stability. To determine whether PDT also increases the stability of the IL-10 mRNA, PAM 212 cells were treated with actinomycin D (10 μg/mL) 4 h after PDT treatment or 1 h after UVB irradiation. Previous studies have shown that UVB irradiation induces IL-10 mRNA within 1 h of treatment (21). Cells were harvested immediately (0 h) and 0.5, 1, 2 or 4 h after treatment, and the level of IL-10 mRNA was determined by RT-PCR. UVB-induced IL-10 mRNA is detectable up to 1 h after actinomycin D addition. However, PDT-induced IL-10 mRNA is detectable up to 4 h following actinomycin D addition, indicating that PDT prolongs the half-life of the IL-10 mRNA (Fig. 7). Kick et al. (29) have shown that PDT also prolongs the stability of the c-fos and c-jun mRNA.
Figure 7.
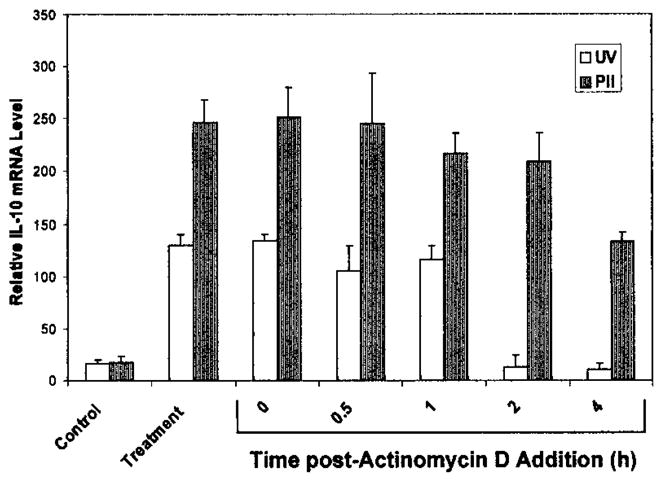
PDT prolongs IL-10 mRNA half-life. Actinomycin D (10 μg/mL) was added to PAM 212 cells 1 or 4 h following UVB irradiation or PDT treatment, respectively. Total RNA was isolated from cells harvested immediately (0) or at 1, 2 or 4 h post-actinomycin D addition and 2 μg from each sample were subjected to RT-PCR analysis as described in “Materials and Methods.” Results are presented as in relative IL-10 mRNA levels ± SEM following normalization to the actin mRNA levels. Control values refer to untreated controls for the UVB sample and Photofrin® treatment only for the PDT sample. Controls and treatment samples were harvested at 1 h for the UVB samples and 4 h for the PDT samples.
DISCUSSION
This study demonstrates that PDT-induced IL-10 mRNA and protein expression in keratinocytes is a result of activation of the IL-10 gene promoter and increased mRNA stability. Kick et al. (26) have shown that PDT enhances AP-1 DNA binding activity in a protein kinase C-independent manner and have suggested that AP-1 mediates the induction of IL-6 by PDT. The current study has expanded these results using functional analysis to demonstrate conclusively that AP-1 has a critical role in the induction of IL-10 by PDT. AP-1 has been shown to be induced by changes in the redox potential (38–40) and hypoxia (41); therefore modulation of AP-1 activity by PDT in vitro might be mediated by changes in the oxidative potential as a result of the generation of singlet oxygen. In addition, PDT-induced hypoxia has the potential to affect AP-1 activity in vivo. AP-1 is composed of homodimers of the products of the c-jun gene family or heterodimeric combinations of c-jun and c-fos gene family members. PDT has been shown to induce prolonged expression of both c-fos and c-jun as a result of oxidative stress (29,42). AP-1 has also been shown to be involved in tumor necrosis factor-α induction of IL-10 in monocytes (43). In contrast T cells appear to regulate IL-10 expression through elements other than AP-1 (44,45).
The IL-10 promoter contains several other regulatory elements including CRE and SP-1 sites. Regulation of the IL-10 promoter in macrophages/monocytes appears to be stimuli dependent. The CRE element has been implicated in the induction of IL-10 in monocytes by cAMP elevating drugs (43,46). Recent studies have shown that SP1 and SP3 play a prominent role in lipopolysaccharide regulation of IL-10 in monocytes (45,47). Our data suggest that UVB and PDT regulate the IL-10 promoter by different mechanisms in keratinocytes, as UVB induction of the IL-10 promoter appears to be AP-1 independent. Thus, regulation of IL-10 appears to be both cell type and stimuli specific. Studies are currently underway to determine the UVB responsive site in the IL-10 gene promoter.
While our studies fail to demonstrate activation of NF-κB in PAM 212 cells by Photofrin®-PDT, several other studies have demonstrated NF-κB activation by PDT in other cell types. For example, Chen et al. (48) have shown that Photofrin®-PDT activates NF-κB in L1210 cells and Granville et al. (49) have demonstrated NF-κB activation by verteporfin-PDT in HL-60 cells. In contrast, similar to our studies Mu et al. (35) were unable to demonstrate NF-κB activation in HeLa cells with Photofrin®-PDT.
In addition to changes in promoter activity, increases in protein expression can be a result of increased mRNA stability and/or increased translational efficiency. Naora and Young (50) have shown that although transcriptional control is the predominant feature in regulation of IL-10 mRNA levels in T cells, mRNA stability also appears to play a role. Our results suggest that increases in mRNA stability may also contribute to alterations of IL-10 expression by PDT, as it does with alterations in expression of c-fos and c-jun (29). In our studies UVB irradiation is unable to stabilize IL-10 mRNA. A recent report by Powell et al. (51) has demonstrated that IL-10 gene expression in macrophages and T cells is regulated through AUUUA motifs in the 3′ untranslated region of the gene. This study suggests that variable expression levels and mRNA are determined by heterogeneity of mRNA-stabilizing signals. It appears as though UVB and PDT regulate IL-10 gene expression through different signals. Thus it is feasible that there are also differences in the mRNA stabilizing signal sent through these different stimuli.
Suppression of the CHS response by PDT has been shown to be a result of increased expression of IL-10 (19), and we have previously demonstrated that PDT induced IL-10 in the skin (11). In the current study we have shown that in vitro PDT-treated keratinocytes express high levels of IL-10. UVB irradiation also induces IL-10 expression in keratinocytes (14,20,21). UVB-induced IL-10 mediates suppression of delayed type hypersensitivity (52). However, keratinocyte-derived IL-10 does not appear to enter the circulation and systemic immunosuppression following UVB irradiation is thought to be the result of an alternative prostaglandin E2 dependent mechanism, involving IL-4 (53). We have recently demonstrated an induction of systemic IL-4 by PDT (Gollnick and Henderson, in preparation). Further studies are underway to determine what, if any, role IL-4 plays in the suppressive effects of cutaneous PDT on the immune system.
Previous work by this laboratory has demonstrated that tumor directed PDT, in contrast to transcutaneous PDT, can cause a decrease in IL-10 mRNA expression in the tumor bed. In these studies, IL-10 mRNA expression was examined in the total tumor bed, rather than in individual cell populations. As mentioned above, IL-10 regulation is cell type specific and thus a more detailed analysis of specific cell subsets is required before conclusions can be drawn regarding the molecular mechanisms of IL-10 regulation in the tumor bed. It is possible that tumor directed PDT is inducing keratinocyte expression of IL-10, however a much smaller area of skin is treated and thus any induction is unlikely to be detected. Additionally tumor directed PDT induces a strong inflammatory response, which is accompanied by an influx of granulocytes (1) and may result in further dilution of the IL-10 signal.
In summary, we have shown that in vitro PDT is able to induce IL-10 mRNA and protein secretion from PAM 212 cells. The induction of IL-10 by PDT in this cell line involves an increase in IL-10 gene promoter activity, which is mediated by activation of the trans-acting transcription factor, AP-1, as well as an increase in IL-10 mRNA stability.
Acknowledgments
We are grateful to Dr. Kevin Moore, DNAX Research Institute, for the gift of the murine IL-10 genomic clone and for his advice. We are also grateful to Dr. Shawn Murphy, Department of Immunology, and Dr. Adrian Black, Deartment of Experimental Therapeutics, for critical review of this manuscript. This work was supported by NIH Grant PO1 CA55791, The Roswell Park Alliance, and partially supported by shared resources of the Roswell Park Cancer Center support grant (P30 CA16056).
Footnotes
Posted on the website on 11 January, 2001.
Abbreviations: cAMP, cyclic adenosine monophosphate; CAT, chloramphenicol acetyltransferase; CHS, contact hypersensitivity; CRE, cAMP response element; ELISA, enzyme-linked immunosorbent assay; EMSA, electrophoretic mobility shift assay; LD, lethal dose; PBS, phosphate buffered saline; PDT, photodynamic therapy; PS, photosensitizer; mRNA, messenger RNA; RT-PCR, reverse transcriptase-polymerase chain reaction.
References
- 1.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ratkay LG, Chowdhary RK, Iamaroon A, Richter AM, Neyndorff HC, Keystone EC, Waterfield JD, Levy JG. Amelioration of antigen-induced arthritis in rabbits by induction of apoptosis of inflammatory cells with local application of transdermal photodynamic therapy. Arthritis Rheum. 1998;41:525–534. doi: 10.1002/1529-0131(199803)41:3<525::AID-ART19>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 3.Trauner K, Gandouredwards R, Bamberf M, Nishioka NS, Flotte T, Autry S, Hasan T. Influence of light delivery on photodynamic synovectomy in an antigen-induced arthritis model for rheumatoid arthritis. Lasers Surg Med. 1998;22:147–156. doi: 10.1002/(sici)1096-9101(1998)22:3<147::aid-lsm1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 4.Leong S, Chan AH, Levy JG, Hunt DWC. Transcutaneous photodynamic therapy alters the development of an adoptively transferred form of murine experimentall autoimmune encephalomyelitis. Photochem Photobiol. 1996;64:751–757. doi: 10.1111/j.1751-1097.1996.tb01830.x. [DOI] [PubMed] [Google Scholar]
- 5.Boehncke WH, Elshorst-Schmidt T, Kaufmann R. Systemic photodynamic therapy is a safe and effective treatment for psoriasis. Arch Dermatol. 2000;136:271–272. doi: 10.1001/archderm.136.2.271. [DOI] [PubMed] [Google Scholar]
- 6.Fine SL. Photodynamic therapy with verteporfin is effective for selected patients with neovascular age-related macular degeneration. Arch Ophthalmol. 1999;117:1400–1402. doi: 10.1001/archopht.117.10.1400. [DOI] [PubMed] [Google Scholar]
- 7.Hryhorenko EA, Oseroff AR, Morgan J, Rittenhouse-Diakun K. Deletion of alloantigen-activated cells by aminolevulinic acid-based photodynamic therapy. Photochem Photobiol. 1999;69:560–565. [PubMed] [Google Scholar]
- 8.Chowdhary RK, Ratkay LG, Neyndorff HC, Richter AM, Obochi M, Waterfield JD, Levy JG. The use of transcutaneous photodynamic therapy in the prevention of adjuvant-enhanced arthritis in mlr/lpr mice. Clin Immunol Immunopathol. 1994;72:255–263. doi: 10.1006/clin.1994.1139. [DOI] [PubMed] [Google Scholar]
- 9.Hunt DWC, Jiang H, Granville DJ, Chan AH, Leong S, Levy JG. Consequences of the photodynamic treatment of resting and activated peripheral T lymphocytes. Immunopharmacology. 1999;41:31–44. doi: 10.1016/s0162-3109(98)00051-4. [DOI] [PubMed] [Google Scholar]
- 10.King DE, Jiang H, Simkin G, Obochi M, Levy JG, Hunt DWC. Photodynamic alteration of the surface receptor expression pattern of murine splenic dendritic cells. Scand J Immunol. 1999;49:184–192. doi: 10.1046/j.1365-3083.1999.00498.x. [DOI] [PubMed] [Google Scholar]
- 11.Gollnick SO, Liu X, Owczarczak B, Musser DA, Henderson BW. Altered expression of interleukin 6 and interleukin 10 as a result of photodynamic therapy in vivo. Cancer Res. 1997;57:3904–3909. [PubMed] [Google Scholar]
- 12.Musser DA, Gollnick SO, Oseroff AR, Henderson BW. Photodynamic therapy (PDT) induces long term suppression of CHS which is correlated with systemic and localized expression of IL-10. Photochem Photobiol. 1998;67S:102S. [Google Scholar]
- 13.Moore KW, O’Garra A, deWaal Malefyt R, Vieira P, Mosmann TR. Interleukin-10. Ann Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 14.Ullrich SE. Mechanism involved in the systemic suppression of antigen-presenting cell function by UV irradiation. Keratinocyte-derived IL-10 modulates antigen-presenting cell function of splenic adherent cells. J Immunol. 1994;152:3410–3416. [PubMed] [Google Scholar]
- 15.Fiorentino DF, Zlotnik A, Vieira P, Howard M, Moore KW, O’Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–3451. [PubMed] [Google Scholar]
- 16.Beissert S, Hosoi J, Grabbe S, Asahina A, Granstein RD. IL-10 inhibits tumor antigen presentation by epidermal antigen-presenting cells. J Immunol. 1995;154:1280–1286. [PubMed] [Google Scholar]
- 17.Asadullah K, Sterry W, Stephanek K, Jasulaitis D, Leupold M, Audring H, Volk HD, Döcke WD. IL-10 is a key cytokine in psoriasis. J Clin Invest. 1998;101:783–794. doi: 10.1172/JCI1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asadullah K, Döcke W-D, Ebeling M, Friedrich M, Belbe G, Audring H, Volk H-D, Sterry W. Interleukin 10 treatment of psoriasis—clinical results of a phase 2 trial [Review] Arch Dermatol. 1999;135:187–192. doi: 10.1001/archderm.135.2.187. [DOI] [PubMed] [Google Scholar]
- 19.Simkin G, Tao JS, Levy JG, Hunt DWC. IL-10 contributes to the inhibition of contact hypersensitivity in mice treated with photodynamic therapy. J Immunol. 2000;164:2457–2462. doi: 10.4049/jimmunol.164.5.2457. [DOI] [PubMed] [Google Scholar]
- 20.Ullrich SE, McIntyre BW, Rivas JM. Suppression of the immune response to alloantigen by factors released from ultraviolet-irradiated keratinocytes. J Immunol. 1990;145:489–498. [PubMed] [Google Scholar]
- 21.Rivas JM, Ullrich SE. Systemic suppression of DTH by supernatants from UV-irradiated keratinocytes: an essential role for interleukin 10. J Immunol. 1992;149:3865–3871. [PubMed] [Google Scholar]
- 22.de Vree WJ, Essers MC, De Bruijn HS, Star WM, Koster JF, Sluiter W. Evidence for an important role of neutrophils in the efficacy of photodynamic therapy in vivo. Cancer Res. 1996;56:2908–2911. [PubMed] [Google Scholar]
- 23.Nseyo UO, Whalen RK, Duncan MR, Berman B, Lundahl SL. Urinary cytokines following photodynamic therapy for bladder cancer. Urology. 1990;36:167–171. doi: 10.1016/0090-4295(90)80220-h. [DOI] [PubMed] [Google Scholar]
- 24.Evans S, Matthews W, Perry R, Fraker D, Norton J, Pass HI. Effect of photodynamic therapy on tumor necrosis factor production by murine macrophages. J Natl Cancer Inst. 1990;82:34–39. doi: 10.1093/jnci/82.1.34. [DOI] [PubMed] [Google Scholar]
- 25.Canti G, Franco P, Marelli O, Ricci L, Nicolin A. Hematoporphyrin derivative rescue from toxicity caused by chemotherapy or radiation in a murine leukemia model (L1210) Cancer Res. 1984;44:1551–1556. [PubMed] [Google Scholar]
- 26.Kick G, Messer G, Goetz A, Plewig G, Kind P. Photodynamic therapy induces expression of interleukin 6 by activation of AP-1 but not NF-kB DNA binding. Cancer Res. 1995;55:2373–2379. [PubMed] [Google Scholar]
- 27.Zioikowski P, Symonowicz K, Milach J, Szkudlarek T. In vivo tumor necrosis factor-alpha induction following chlorin e6-photodynamic therapy in Buffalo rats. Neoplasma. 1996;44:192–196. [PubMed] [Google Scholar]
- 28.Herman S, Kalechman Y, Gafter U, Sredni B, Malik Z. Photofrin II induces cytokine secretion by mouse spleen cells and human peripheral mononuclear cells. Immunopharmacology. 1996;31:195–204. doi: 10.1016/0162-3109(95)00047-x. [DOI] [PubMed] [Google Scholar]
- 29.Kick G, Messer G, Plewig G, Kind P, Goetz AE. Strong and prolonged induction of c-jun and c-fos proto-oncogenes by photodynamic therapy. Br J Cancer. 1996;74:30–36. doi: 10.1038/bjc.1996.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korbelik M, Krosl G, Krosl J, Dougherty GJ. The role of host lymphoid populations in the response of mouse EMT6 tumor to photodynamic therapy. Cancer Res. 1996;56:5647–5652. [PubMed] [Google Scholar]
- 31.Yuspa SH, Hawley-Nelson P, Koechler B, Stanley JR. A survey of transformation markers in differentiating epidermal cell lines. Cancer Res. 1993;40:4694–4697. [PubMed] [Google Scholar]
- 32.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 33.Kim JM, Brannan CI, Copeland NG, Jenkins NA, Khan TA, Moore KW. Structure of the mouse IL-10 gene and chromosomal localization of the mouse and human genes. J Immunol. 1992;148:3618–3623. [PubMed] [Google Scholar]
- 34.Murphy SP, Gollnick SO, Paznarry T, Tomasi TB. Repressional MHC class II gene transcription in trophoblast cells by novel single stranded DNA binding proteins. Mol Reprod Differ. 1997;47:1–14. doi: 10.1002/(SICI)1098-2795(199708)47:4<390::AID-MRD5>3.0.CO;2-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mu J, Yamamoto N, Tsutsui T, Tai X, Kobayashi M, Fujiwara H, Hamaoka T. Administration of recombinant interleukin 12 prevents outgrowth of tumor cells metastasizing spontaneously to lung and lymph nodes. Cancer Res. 1995;55:4404–4408. [PubMed] [Google Scholar]
- 36.Ryter SW, Gomer CJ. Nuclear factor κB binding activity in mouse L1210 cells following Photofrin II-mediated photosensitization. Photochem Photobiol. 1993;58:753–756. doi: 10.1111/j.1751-1097.1993.tb04964.x. [DOI] [PubMed] [Google Scholar]
- 37.Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system. Ann Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 38.Abate C, Patel L, Rauscher FJ, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990;249:1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- 39.Xanthoudakis S, Miao G, Wang F, Pan YC, Curran T. Redox activation of Fos-Jun DNA binding activity is mediated by a DNA repair enzyme. EMBO J. 1992;11:3323–3335. doi: 10.1002/j.1460-2075.1992.tb05411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer M, Schreck R, Baeuerle PA. H2O2 and antioxidants have opposite effects on activation of NF-κB and AP-1 in intact cells: AP-1 as secondary antioxidant-responsive factor. EMBO J. 1993;12:2005–2015. doi: 10.1002/j.1460-2075.1993.tb05850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao KS, Zanthoudakis S, Curran T, O’Dwyer PJ. Activation of AP-1 and of nuclear redox factor, Ref1, in the response of HT29 colon cancer cells to hypoxia. Mol Cell Biol. 1994;14:5997–6003. doi: 10.1128/mcb.14.9.5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luna MC, Wong S, Gomer CJ. Photodynamic therapy mediated induction of early response genes. Cancer Res. 1994;54:1374–1380. [PubMed] [Google Scholar]
- 43.Platzer C, Meisel C, Vogt K, Platzer M, Volk HD. Upregulation and monocytic IL-10 by tumor necrosis factor-α and cAMP elevating drugs. Int Immunol. 1995;7:517–523. doi: 10.1093/intimm/7.4.517. [DOI] [PubMed] [Google Scholar]
- 44.Rooney JW, Hodge MR, McCaffrey PG, Rao A, Glimcher LH. A common factor regulates both Th1- and Th2-specific cytokine gene expresion. EMBO J. 1994;13:625–633. doi: 10.1002/j.1460-2075.1994.tb06300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tone M, Powell MJ, Tone Y, Thompson SA, Waldmann H. IL-10 gene expression is controlled by the transcription factors Sp1 and Sp3. J Immunol. 2000;165:286–291. doi: 10.4049/jimmunol.165.1.286. [DOI] [PubMed] [Google Scholar]
- 46.Platzer C, Fritsch E, Elsner T, Lehmann MH, Volk HD, Prosch S. Cyclic adenosine monophosphate-responsive elements are involved in the transcriptional activation of the human IL-10 gene in monocytic cells. Eur J Immunol. 1999;29:3098–3104. doi: 10.1002/(SICI)1521-4141(199910)29:10<3098::AID-IMMU3098>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 47.Brightbill HD, Plevy SE, Modlin RL, Smale ST. A prominent role for Sp1 during lipopolysaccharide-mediated induction of the IL-10 promoter in macrophages. J Immunol. 2000;164:1940–1951. doi: 10.4049/jimmunol.164.4.1940. [DOI] [PubMed] [Google Scholar]
- 48.Chen Y, Inobe J, Weiner HL. Inductive events in oral tolerance in the TCR transgenic adoptive transfer model. Cell Immunol. 1997;178:62–68. doi: 10.1006/cimm.1997.1119. [DOI] [PubMed] [Google Scholar]
- 49.Granville DJ, Carthy CM, Jiang H, Levy JG, McManus BM, Matroule JY, Piette J, Hunt DWC. Nuclear factor-κB activation by the photochemotherapeutic agent verteporfin. Blood. 2000;95:256–262. [PubMed] [Google Scholar]
- 50.Naora H, I, Young G. Comparison of the mechanisms regulating IL-5, IL-4, and three other lymphokine genes in the Th2 clone D10.G4.1. Exp Hematol. 1995;23:597–602. [PubMed] [Google Scholar]
- 51.Powell MJ, Thompson SA, Tone Y, Waldmann H, Tone M. Posttranscriptional regulation of IL-10 gene expression through sequences in the 3′-untranslated region. J Immunol. 2000;165:292–296. doi: 10.4049/jimmunol.165.1.292. [DOI] [PubMed] [Google Scholar]
- 52.Rivas JM, Ullrich SE. The role of IL-4, IL-10, and TNF-α in the immune suppression induced by ultraviolet radiation. J Leukoc Biol. 1994;56:769–775. doi: 10.1002/jlb.56.6.769. [DOI] [PubMed] [Google Scholar]
- 53.Shreeder V, Giese T, Sung VW, Ullrich SE. A cytokine cascade including prostaglandin E2, IL-4, and IL-10 is responsible for UV-induced systemic immune suppression. J Immunol. 1998;160:3783–3789. [PubMed] [Google Scholar]