Abstract
Objective
A cross-sectional study of mixed connective tissue disease (MCTD) was performed to determine if there were identifiable differences in the clinical expression of MCTD associated with race or ethnicity.
Methods
Miami, Florida, and Midwestern US (Missouri) Caucasian MCTD cohorts were studied. Clinical and laboratory features of the 2 MCTD cohorts were compared. A concurrently collected cohort of Sm-positive patients with systemic lupus erythematosus (SLE) was studied as a control. Disease activity and severity and functional status were measured. CD4+CD25high-expressing T-regulatory cells were enumerated and serum soluble L selectin was measured as biomarkers of disease activity.
Results
The Miami and Missouri Caucasian MCTD groups, while differing from the SLE group, were largely similar; however, gastroesophageal reflux, sclerodactyly, and malar rash were significantly more frequent in the Missouri MCTD group and alopecia was more frequent in the Miami MCTD group. Significant clinical and laboratory differences were found between the Miami MCTD and Miami SLE groups despite similar disease duration, activity, severity and functional status. Raynaud's phenomenon (RP), hand swelling, synovitis, myositis, and sclerodactyly were all significantly more common in RNP-positive MCTD versus Sm-positive SLE subjects.
Conclusion
Ethnic differences were observed in the frequency of end-organ involvement in the Miami MCTD versus the Missouri Caucasian MCTD groups. Clinical and laboratory features of all MCTD groups were clearly different from the SLE group, despite similar disease activity, disease severity, and functional status. Disease activity measures appeared to behave similarly as valid measures of disease activity in SLE and MCTD.
Key Indexing Terms: Mixed Connective Tissue Disease, Systemic Lupus Erythematosus, L-Selectin, T-Regulatory Cells, Hispanic
Important differences in clinical manifestations of disease, disease severity, and health outcomes have been described in many diseases among different racial and ethnic groups1,2. Understanding health disparities between such groups is an issue of accelerating importance as the US population becomes increasingly diverse. Miami has been called a microcosm of the demographic future of the US and it currently has one of the most diverse populations of any US city3. No studies on mixed connective tissue disease (MCTD) examining this unique population have previously been published.
We performed a cross-sectional study on the clinical and laboratory manifestations of MCTD in the ethnically and racially diverse population of Miami, and compared these patients to a well characterized Midwestern MCTD cohort (Missouri). In addition, because there is no universally accepted classification criterion for MCTD, a concurrently collected cohort of Sm-positive patients with systemic lupus erythematosus (SLE) was included as a comparator.
Disease activity, severity and functional status were measured in MCTD and SLE using a series of disease activity and damage assessment instruments. MCTD and SLE groups were also compared using clinical, laboratory, and immunologic testing, including 2 novel biomarkers recently reported to be informative in SLE, CD4-positive/CD25-high T-regulatory cells (CD4+CD25high) and serum soluble L selectin (sCD62L) level4-7.
Materials and Methods
Study design and statistical analysis
The investigation was designed to be a cross-sectional, non-blinded, observational study. The target enrollment was 20 patients with MCTD. The rationale for inclusion of the Sm-positive control group was that the RNP and Sm antigens are both contained within the spliceosome complex, and sera reactive with one antigen frequently have additional serologic reactivity with the other8-10. Further, we and others have shown that intermolecular spreading can occur to additional antigenic peptides within the spliceosome complex, and that the onsets of clinical manifestations are closely linked to the development of anti-RNP or anti-Sm antibodies11,12. Finally, in the absence of universally accepted classification criteria for MCTD, it was reasoned that a concurrently collected Sm-positive cohort could serve as an internal control for the RNP-positive cohort.
Concurrently collected anti-Sm positive patients were enrolled as a comparator group to the RNP-positive group. It was anticipated that the Sm-positive group would be equal to or greater in number than the 20-patient MCTD cohort collected during the same period based upon enrollment in previous studies. It was anticipated that, despite being an uncommon disease, at least 20 patients with MCTD could be enrolled within 24 months. Power calculation for sample size was based upon a study group size of 20 and a comparator group size of 25, where using proportion estimates there was a 95% probability of observing clinical differences between groups at the p < 0.05 level. Additionally, the size of the MCTD group was based upon our previous studies, where it was observed using groups of similar size that there were a number of significant differences between MCTD and SLE8,13-16. As appropriate, variables between groups were analyzed using the Student t-test, nonparametric statistics, and correlation using StatView GraphPad software (StatView, SAS Institute, Cary, NC, USA). A p value < 0.05 was considered significant. Continuous variables are shown as mean ± standard error of the mean.
Patients and controls
MCTD and SLE patients were enrolled in a cross-sectional observational study among patients recruited from the Division of Rheumatology and Immunology at the University of Miami Miller School of Medicine, including the University of Miami Hospital and Clinics and Jackson Memorial Hospital, following an institutional review board (IRB) approved protocol. Patients were recruited from those seen during the period February 2005 through February 2007 in both outpatient and inpatient settings. Patients were identified for recruitment based upon the presence of either anti-RNP antibodies or anti-Sm antibodies. Sixty patients agreed to participate, including 21 who were clinically diagnosed as MCTD and 39 clinically diagnosed as SLE during the enrollment period. Among the MCTD patients, 21/21 met the classification criteria of Alarcon-Segovia and Cardel for MCTD and among the SLE patients, 39/39 met 4 or more American College of Rheumatology criteria for classification of SLE17,18. Complete clinical, laboratory, and immunologic data were collected on all patients, as described8,14. All Missouri patients were examined by a single physician (RWH), while Miami patients were examined by one or both of the lead investigators (RWH, MEM). Patients enrolled in Miami were also compared to MCTD patients who were enrolled in a prospective longitudinal observational study at the University of Missouri-Columbia and then de-identified following an IRB approved protocol. These well characterized patients have in part been the subject of previous publications8,14-16. Subset analysis was also done on the Miami Hispanic group compared to the Missouri Caucasian group.
Clinical, laboratory, and immunologic studies
Complete blood count, urinalysis including microscopic examination, routine blood chemistry, and complete antinuclear antibody (ANA) testing were done on all patients. Serologic testing for reactivity with specific ANA was also done by ELISA and immunoblotting, as described8,11,19. ELISA results were reported as ELISA optical density units (EU)/ml. C3 and C4 were measured by nephelometry. Anti-RNP and anti-Sm reactivity were scored as positive based upon the presence of an EU result at least 4 standard deviations above the mean for apparently healthy blood donors when tested in RNP-specific or Sm-specific ELISA and/or immunoblot reactivity with sera compared to well characterized positive and negative control sera, as described8,11,12.
Disease activity, damage, and functional status measures
Disease activity was measured using the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI), British Isles Lupus Activity Group (BILAG), and Systemic Lupus Activity Measure (SLAM)20-23. Systemic Lupus International Collaborating Clinics (SLICC) damage index scores were measured in all patients24. The Medical Outcomes Study Short-Form 36 Health Survey (SF-36) was used to measure functional status25.
CD4+CD25high T-cell subset
CD4+CD25high T-regulatory cells were enumerated by flow cytometry26,27. In brief, peripheral blood was subjected to Histopaque density gradient separation (Sigma, St. Louis, MO, USA) to isolate mononuclear cells and these were adjusted to concentration of 2 × 106 cells/ml. Cells were stained using anti-CD4 monoclonal antibody conjugated to fluorescence isothiocyanate (FITC; Becton Dickinson, Franklin Lakes, NJ, USA) and anti-CD25 monoclonal antibody conjugated to phycoerythrin (PE; Becton Dickinson). Immunoglobulin G2b (IgG2b)-FITC and IgG2b-PE (Becton Dickinson) were used as isotype controls. The samples were examined on a FACScan Cytometer and the results analyzed using FACSDiva software (Becton Dickinson). After initial gating on the lymphocyte population, cells were enumerated for those positive for CD4 (CD4+) and those expressing a high level of CD25 (CD25high). The absolute numbers of each cell subset were calculated based on the absolute number of CD4+ cells and the total lymphocyte count.
FoxP3 real-time polymerase chain reaction (PCR)
Cells were stained with anti-CD4-FITC and anti-CD25-PE and then sorted using the LSR II (Becton Dickinson). RNA was extracted from the CD4+CD25high subset, the CD4+CD25 cells expressing a low level of CD25 (CD4+CD25low), and the CD4-negative (CD4−) populations using RNeasy (Invitrogen, Carlsbad, CA, USA) and the RNA was further purified from DNA using a Turbo DNA-free kit (Ambion, Austin, TX, USA) following the manufacturer's instructions. cDNA was generated using the SuperScript First-Strand cDNA kit (Invitrogen) and oligo-(dt) primers. Real-time PCR was performed on 1 μg of cDNA in duplicate per sample using an Applied BioSystems 7300 real-time PCR machine. Taqman Universal PCR Master Mix, FoxP3, and GAPDH primers (Applied BioSystems, Foster City, CA, USA) were used in the reaction. Results were expressed as relative amplification units, normalized to glyceraldehyde-3-phosphate dehydrogenase expression28,29.
Circulating L-selectin (sCD62L)
The level of circulating L-selectin (sCD62L) in serum from patients and controls was measured using a quantitative sandwich ELISA kit (R&D Systems, Minneapolis, MN, USA). Sera were diluted 100-fold with sample diluents and loaded in duplicate onto a precoated ELISA plate. This was incubated 1 h at 25°C, followed by addition of anti-sCD62L conjugate. The plate was incubated again for 30 min at 25°C, aspirated, and washed 6 times, and then substrate was added. After a final 30-min incubation, stop solution was added to each well, and the plate was read at 450 nm on a LabSystems MultiSkan Plus plate reader (ThermoScan-Fisher Scientific, St. Louis, MO, USA). Results were calculated using a 4-parameter logistic curve fit expressed in ng/ml.
Results
Demographics
The demographic features of the Miami MCTD and SLE patients are shown in Table 1. The mean age of the Miami MCTD patient group was 39 ± 2.4 years (range 13–57) with mean disease duration of 60 ± 18 months (range 2–276). The mean age of the SLE patient group was 33 ± 1.9 years (range 20–66) with mean disease duration 57 ± 13 months (range 1–453). Among MCTD patients, there were 12/21 (57%) Hispanic, 6/21 (29%) African American, and 3/21 (14%) non-Hispanic Caucasian patients. In the SLE group there was a similar racial and ethnic distribution, with 20/39 (51%) Hispanic, 18/39 (46%) African American, and 1/39 (3%) non-Hispanic Caucasian patients. The differences of proportion between groups were not statistically significant.
Table 1.
Demographic data of Miami patients with MCTD and SLE.
| Characteristics | MCTD, n = 21 |
SLE, n = 39 |
|---|---|---|
| Age at enrollment, mean (range) yrs | 39 (13–57) | 33 (20–66) |
| Disease duration, mean (range) mo | 60 (2–276) | 57 (1–453) |
| Hispanic, n (%) | 12 (57) | 20 (51) |
| African American, n (%) | 6 (29) | 18 (46) |
| Caucasian, n (%) | 3 (14) | 1 (3) |
Clinical features of MCTD and SLE patients
Cumulative clinical features in the Miami MCTD and SLE groups are shown in Table 2. The most common clinical features in the MCTD patient group were RP 18/21 (86%), hand swelling 16/21 (76%), alopecia 16/21 (76%), synovitis 16/21 (76%), dry eyes or dry mouth 12/21 (57%), lymphopenia 12/21 (57%), gastroesophageal reflux 11/21 (52%), photosensitivity 8/21 (38%), and myositis 8/21 (38%). There were statistically significant differences between the MCTD and SLE groups in the clinical manifestations of disease. Hand swelling was seen in 16/21 (76%) of the MCTD group versus 16/39 (41%) in the SLE group (p < 0.01); synovitis was seen in 16/21 of the MCTD group versus 19/39 in the SLE group (p < 0.05); myositis was seen in 8/21 (38%) of the MCTD group versus 1/39 (2%) in the SLE group (p < 0.0005); sclerodactyly was seen in 4/21 (19%) of the MCTD group versus 0/39 in the SLE group (p < 0.01).
Table 2.
Clinical manifestations of disease in Miami MCTD and SLE cohorts. Data are number (%).
| Manifestation | MCTD, n = 21 |
SLE, n = 39 |
|---|---|---|
| Raynaud's* | 18 (86) | 18 (46) |
| Hand swelling** | 16 (76) | 16 (41) |
| Alopecia* | 16 (76) | 31 (80) |
| Synovitis** | 16 (76) | 19 (49) |
| Dry eyes and/or dry mouth* | 12 (57) | 15 (38) |
| Lymphopenia* | 12 (57) | 14 (36) |
| Gastroesophageal reflux* | 11 (52) | 12 (30) |
| Pericarditis or pleuritis* | 10 (48) | 23 (59) |
| Photosensitivity* | 8 (38) | 19 (49) |
| Myositis** | 8 (38) | 1 (2) |
| Sclerodactyly** | 4 (19) | 0 (0) |
| Malar rash** | 3 (14) | 18 (46) |
| Renal disease** | 2 (9) | 21 (54) |
No significant differences between the 2 groups (Fisher's test).
p < 0.05 comparing the 2 groups (Fisher's test).
In contrast to MCTD, the most common clinical features in the Miami SLE group were alopecia in 31/39 (80%), pericarditis or pleuritis 23/39 (59%), renal disease 21/39 (54%), and malar rash 18/39 (46%). Malar rash was significantly more common in the SLE group versus MCTD group (p < 0.02) as was renal disease (p < 0.001). Other prevalent clinical manifestations in both groups, including RP, alopecia, sicca, lymphopenia, gastroesophageal reflux, and photosensitivity, did not show statistically significant differences in MCTD versus SLE (Table 2).
Immunologic and laboratory findings among MCTD and SLE
There were differences in serum C3 and C4 complement levels and anti-double-stranded DNA (ds-DNA) between the MCTD and the SLE groups (Figure 1). MCTD patients had, on average, less depletion of C3 compared to the SLE group, with a mean C3 of 114 ± 6.6 mg/dl (range 44–197) versus 87 ± 5.7 mg/dl (range 16–147) (p < 0.005) in SLE. The MCTD group also showed a trend toward a higher mean level of C4 (mean 25 ± 2.2 mg/dl; range 7–140) compared to SLE (mean 16 ± 1.4 mg/dl; range 2–34) [not significant (NS)]. The SLE group had higher mean anti-dsDNA (mean 530 ± 155 EU/ml; range 0–3486) compared to the MCTD group (mean 45 ± 12 EU/ml; range 0–186) (p < 0.05). MCTD patients had a higher mean creatine phosphokinase level (572 ± 271 U/l; range 20–3757) compared to SLE (mean 209 ± 145 U/l; range 4.6–2656), but this difference was not statistically significant. The mean erythrocyte sedimentation rate was 46 ± 8 mm/h (range 4–96) in the MCTD group and 32 ± 5 mm/h (range 1–117) in the SLE group. Both groups had normal white blood cell counts, with a mean total white cell count 5.9 ± 0.5 × 103/μl in MCTD and 6.9 ± 0.9 × 103/μl in SLE, with mean absolute lymphocyte counts of 1100 ± 104 cells/μl (range 300–2200) in the MCTD group and 1320 ± 170 cells/μl (range 1000–3000) in SLE.
Figure 1.
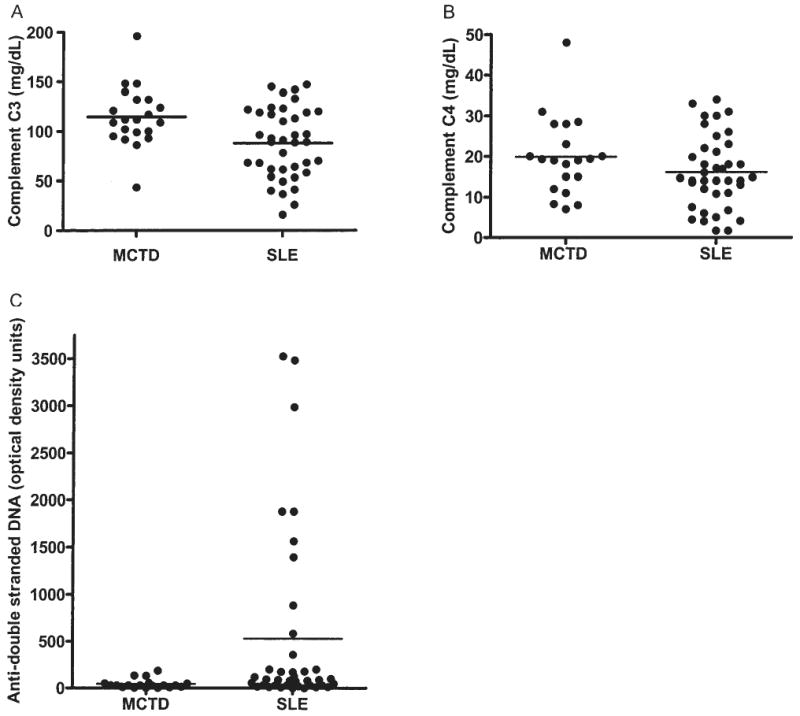
A. Serum C3 complement levels in individual patients with MCTD and SLE. B. C4 complement levels in individual patients with MCTD and SLE. C. Anti-dsDNA levels in the MCTD and SLE patients.
Disease activity and severity
SLEDAI, BILAG, and SLAM were used to measure disease activity in the 2 groups of Miami patients (Figure 2). The MCTD group had a mean SLEDAI score of 8.5 ± 1.0 (range 0–20) and the SLE group had a mean SLEDAI 12.0 ± 1.4 (range 0–33) (NS). The MCTD group had a mean BILAG score of 16.4 ± 1.6 (range 2–35) and the SLE group had a mean BILAG 14.4 ± 1.9 (range 2–52) (p < 0.05).
Figure 2.
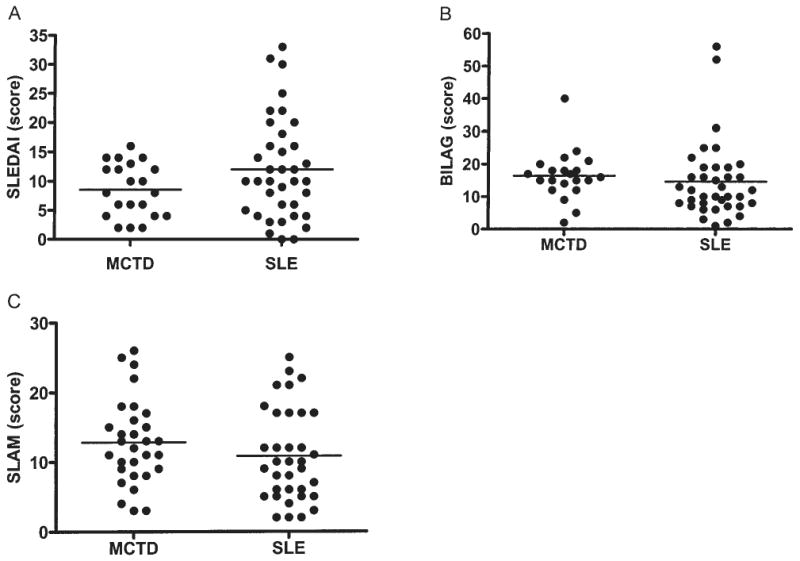
A. SLE Disease Activity Index (SLEDAI) scores for individual patients with MCTD) and SLE. B. British Isles Lupus Activity Group (BILAG) scores for MCTD and SLE patients. C. Systemic Lupus Activity Measure (SLAM) scores for MCTD and SLE patients.
The MCTD group had a mean SLAM score of 12.7 ± 1.3 (range 3–26) and the SLE group had a mean SLAM of 10.5 ± 1.1 (range 2–25) (NS). The mean SLICC scores for the 2 groups were similar; the MCTD group had a mean score of 1.9 ± 0.4 (range 0–6) and the SLE group had a mean score of 1.7 ± 0.3 (range 0–7) (NS).
The SF-36 Physical Component Summary and Mental Component Summary and each of the 8 SF-36 domains were examined. While differing from controls, there were no statistically significant differences in the Miami MCTD or SLE groups in either of the 2 component summaries or the 8 domains, consistent with well matched functional status between the groups as measured by the SF-36 (data not shown).
T-regulatory cells in MCTD and SLE patient groups
CD4+CD25high T-cells were measured in the Miami MCTD group and SLE group. Real-time PCR using FoxP3-specific primers was performed on cells sorted into CD4+CD25high and CD4+CD25low subsets. As expected, the FoxP3-expressing cells were highly enriched within the CD4+CD25high subset (Figures 3A, 3B), confirming that this population was phenotypically characteristic of the CD4+CD25high T-regulatory cell subset28,29. The MCTD cohort had a mean of 1.4% ± 0.3% (range 0.2%–6.5%) CD4+CD25high cells detected in peripheral blood, compared to a mean of 1.3% ± 0.2% (range 0.2%–3.3%) in the SLE group.As shown in Figure 3C, in the MCTD group the number of CD4+CD25high cells decreased with increasing levels of disease activity measured using the SLEDAI (r2 = 0.11; NS); the trend was similar comparing CD4+CD25high cells to activity scores using the BILAG and SLAM (data not shown).
Figure 3.
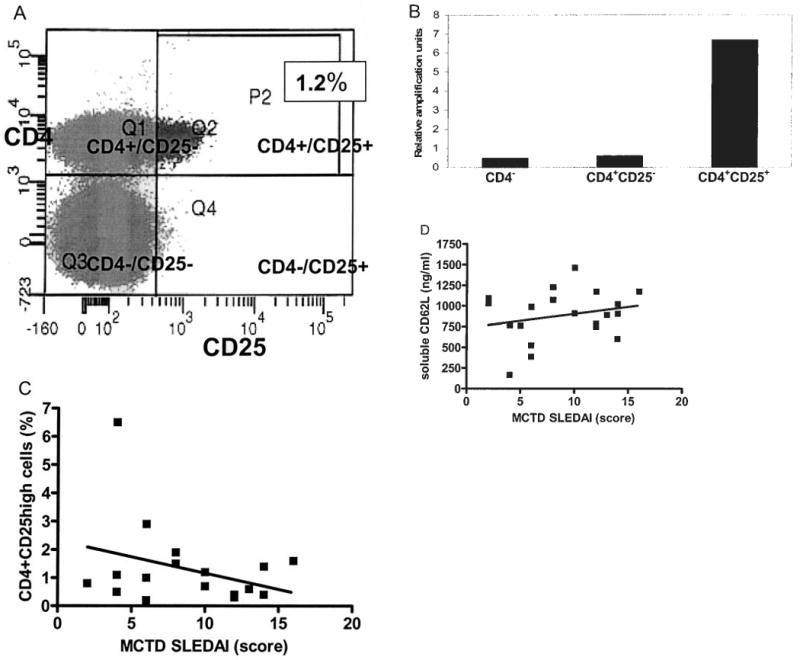
A. Representative flow cytometry result enumerating CD25 monoclonal antibody staining of cells (x-axis) and CD4 staining of cells (y-axis). Quadrant 2 illustrates the gating of cells positive for CD4 and expressing a high level of CD25 (CD4+/CD25high), constituting 1.2% of the total cells. Quadrant 1 shows the CD4+/CD25− and CD4+/CD25low subset. Quadrant 3 shows the CD4−/CD25− subset. Quadrant 4 shows the CD4−/CD25+ subset. B. Representative results examining FoxP3 expression by real-time reverse transcriptase PCR on cells sorted by flow cytometry for those that were CD4−, CD4+CD25low, or CD4+CD25high. C. A plot of individual MCTD patients with SLEDAI scores (x-axis) and CD4+CD25high cells (y-axis) (r2 = 0.05, NS). D. A plot of individual MCTD patients with SLEDAI scores (x-axis) and serum soluble L selectin (sCD62L) levels (y-axis) (r2 = 0.08, NS).
sCD62L serum levels among MCTD and SLE patients
sCD62L was measured by ELISA in the serum of MCTD and SLE patients. These results were compared to disease activity measures in MCTD and SLE. As shown in Figure 3D, there was a trend for the level of sCD62 to increase in parallel with increasing level of disease activity in MCTD, measured by SLEDAI and the other disease activity measures. This correlation did not reach a statistically significant level (r2 = 0.05; NS), however. Levels of sCD62L were similar in the MCTD and SLE groups, with a mean of 887 ± 68 ng/dl (range 167–1462) in the MCTD group versus 783 ± 39 ng/dl (range 312–1251) in the SLE group (NS).
Comparison of Miami and Hispanic MCTD with Missouri MCTD group
Clinical and laboratory features of the Miami MCTD patient group and a subset limited to the Miami Hispanic group were compared to the Missouri MCTD group consisting of 51 well characterized Caucasian patients who were predominantly of Northern European ancestry seen at the University of Missouri-Columbia8. A comparison of clinical features in the 2 MCTD groups is shown in Table 3. The most common clinical disease features in the Missouri MCTD group were RP 46/51 (90%), hand swelling 43/51 (84%), synovitis 41/51 (80%), gastroesophageal reflux 41/51 (80%), lymphopenia 34/51 (67%), and dry eyes or dry mouth 30/51 (59%). These were similar to findings in the overall Miami MCTD group as well as the Miami Hispanic MCTD subset (Table 3). Notably, alopecia was observed more commonly in the Miami MCTD cohort (16/21; 76%) versus the Missouri MCTD cohort (14/51; 28%) (p < 0.001). As shown in Table 3, gastroesophageal reflux, sclerodactyly, and malar rash were more common in the Missouri MCTD cohort than in the Miami MCTD cohort (p < 0.03).
Table 3.
Miami MCTD cohort compared to the Missouri MCTD cohort. Data are number (%).
| Characteristic | All Miami MCTD, n = 21 |
Miami Hispanic MCTD, n = 12 |
Missouri MCTD, n = 51 |
|---|---|---|---|
| Raynaud's††** | 18 (86) | 11 (92) | 45 (90) |
| Hand swelling††** | 16 (76) | 10 (83) | 41 (85) |
| Alopecia†* | 16 (76) | 8 (73) | 14 (29) |
| Synovitis††** | 16 (76) | 12 (100) | 40 (81) |
| Dry eyes and/or dry mouth††** | 12 (57) | 8 (73) | 22 (59) |
| Lymphopenia††** | 12 (57) | 9 (75) | 30 (67) |
| Gastroesophageal reflux†** | 11 (52) | 6 (50) | 40 (80) |
| Pericarditis or pleuritis††** | 10 (48) | 2 (18) | 19 (37) |
| Photosensitivity††** | 8 (38) | 6 (55) | 22 (45) |
| Myositis††** | 8 (38) | 5 (50) | 14 (28) |
| Sclerodactyly†** | 4 (19) | 4 (33) | 25 (50) |
| Malar rash†** | 3 (14) | 2 (18) | 20 (42) |
| Renal disease††** | 2 (9) | 1 (9) | 6 (12) |
p < 0.05 comparing Miami MCTD cohort and Missouri MCTD cohort (Fisher's exact test).
No significant differences between the Miami MCTD cohort and the Missouri MCTD (Fisher's exact test).
p < 0.05 comparing Hispanic MCTD cohort and Missouri MCTD cohort (Fisher's exact test).
No significant differences between the Miami Hispanic MCTD cohort and the Missouri MCTD cohort (Fisher's exact test).
Discussion
Clinically important differences in disease manifestations, severity of disease, and disease outcomes between different racial and ethnic groups have been well established in many diseases; however, previous studies have not examined whether such differences occur in MCTD among different ethnic or racial groups in the US1,2,27-38.
Miami represents a unique ethnically and racially diverse population3. The Miami Hispanic population differs from other US Hispanic groups, which have populations dominated by Mexican immigrants. In contrast, the Miami Hispanic population consists of 60% Caribbean origin (Cuba, Puerto Rico, Dominican Republic) with substantial contributions from Central and South America including Colombia, Nicaragua, Honduras, Peru, and Venezuela, but only a 3% contribution from Mexico.
The design of our study was distinctive from previous studies on MCTD in that the entry criterion was the presence of anti-RNP or anti-Sm antibodies. This allowed collection of comparable connective tissue disease groups that differed in respect to the fine specificity of their anti-spliceosomal autoantibodies; i.e., all patients had autoantibodies reactive with RNP or Sm antigens. The distribution of clinical features in the Miami MCTD group was similar to that in studies from the US, Mexico, Japan, and Europe33-38. It was particularly notable that end-organ involvement in the Miami MCTD group was substantially different from the Miami SLE group (Table 2). Compared to the matched Miami SLE group there were statistically significant differences in hand swelling (p < 0.01), synovitis (p < 0.05), myositis (p < 0.0005), and sclerodactyly (p < 0.01). The data support the hypothesis that the RNP-positive subset is uniquely different from the Sm-positive subset32-38.
When the Miami MCTD group was compared to a well defined group of Midwestern Caucasians of Northern European ancestry with MCTD (Table 3), substantial similarities and some differences were found between groups. The most significant difference was the increased frequency of alopecia, which was observed more commonly in the Miami MCTD cohort than in the Missouri MCTD cohort (p < 0.001). Also, gastroesophageal reflux, sclerodactyly, and malar rash were more common in the Missouri MCTD cohort than the Miami MCTD cohort (p < 0.03). Thus, overall, the Hispanic and non-Hispanic MCTD groups were closely similar to each other but exhibited select differences in end-organ involvement from the Midwestern MCTD group. All MCTD groups exhibited very different end-organ involvement from the SLE patients studied.
To address the possibility that the Miami MCTD and Miami SLE groups differed in level of disease activity, the SLEDAI, BILAG, and SLAM were used to measure disease activity in the 2 groups of patients (Figure 2). While one group utilized the SLEDAI as a validated disease activity measure in a cohort that included MCTD patients, use of the other tools for measuring disease activity in MCTD has not previously been reported39. In addition, to address the possibility that the groups differed in organ damage or functional status, the SLICC was used to measure damage and the SF-36 was used to measure functional status. Perhaps surprisingly, the disease activity, damage, and functional status measurements were comparable between the 2 groups, and also consistent with results for the CD4+CD25high T-regulatory cell number and serum levels of sCD62L in the MCTD and SLE groups. Again, no previous study used these survey tools or examined these biomarkers in MCTD. While larger confirmatory studies are necessary, our findings suggest that these tools have behavior characteristics similar to those reported in SLE, and may be useful to quantify disease activity in future clinical trials in MCTD.
Circulating T-regulatory cell numbers have recently been reported to be decreased in the small number of studies examining this important T cell subset in SLE4,40,41. No previous studies examined T-regulatory cells in MCTD despite recent recognition of the central role T-regulatory cells play in immune tolerance. We identified a trend toward a reciprocal relationship between disease activity and the percentage of detectable CD4+CD25high T-regulatory cells in peripheral blood of the MCTD patient group (Figure 3C); however, this did not achieve statistical significance with the small number of patients.
With activation, white blood cells shed cell-surface L-selectin (also known as CD62L), and measurement of this sCD62L in inflammatory diseases, including SLE, has been suggested to be a useful biomarker of disease activity6,7,42,43. To examine the possible role of sCD62L as a biomarker in MCTD, it was measured in serum, and the findings were compared to other disease activity measures (Figure 3D). While there was a trend for the level of sCD62L to increase with increasing disease activity, as measured by SLEDAI, this did not achieve statistical significance.
Limitations of our study include the relatively small number of patients who were identified and studied. While precise epidemiologic data are not available, MCTD does appear to be very uncommon or rare. That was reflected by the fact that during active enrollment extending over a 2-year period only 21 patients with MCTD were enrolled, at a major urban tertiary referral center with over 1500 inpatient beds10,33. In addition, the majority of patients studied did have established disease, which might influence the manifestations observed. It can be argued, however, that with established disease it is less likely that the patients studied had disease in evolution. Previous studies of undifferentiated CTD and early MCTD reported that the majority of patients have stable classifiable disease within 5 years of diagnosis8,44,45.
We found significant clinical and laboratory differences between the Miami MCTD and SLE groups despite similar disease duration, disease activity, disease severity, and functional status. Consistent with previous studies of MCTD from the US, Europe, Mexico, and Japan, RP, hand swelling, synovitis, myositis, and sclerodactyly were all significantly more common in patients with MCTD compared to those with SLE. Ethnic differences were observed in the frequency of end-organ involvement in the Miami MCTD compared to the Midwestern MCTD group, with gastroesophageal reflux, sclerodactyly, and malar rash more frequent in the Midwestern cohort and alopecia more frequent in the Miami cohort. The widely used SLEDAI, BILAG, SLAM, SLICC, and SF-36 measures appeared to behave similarly in SLE and MCTD.
Acknowledgments
We gratefully acknowledge the helpful comments on the manuscript and the clinical support of Carlos Lozada, MD, the technical assistance of Kim Jaimes, BS, and Yun Zang, MD, and the clinical support of Patricia Muller, MD.
Supported by the University of Miami NIH General Clinical Research Center. Dr. Hoffman was supported by the National Institutes of Health (AR43308) the Department of Veterans Affairs, and the Lupus Foundation of America. Dr. Greidinger was supported by the Department of Veterans Affairs and the Lupus Research Institute.
References
- 1.Health disparities experienced by black or African Americans — United States. MMWR Morb Mortal Wkly Rep. 2005;54:1–3. [PubMed] [Google Scholar]
- 2.Health disparities experienced by Hispanics — United States. MMWR Morb Mortal Wkly Rep. 2004;53:935–7. [PubMed] [Google Scholar]
- 3.U.S. Census Bureau State and County QuickFacts. Miami-Dade County, Florida: [November 28, 2007]. Available from: http://quickfacts.census.gov/qfd/states/12/12086.html. [Google Scholar]
- 4.Miyara M, Amoura Z, Parizot C, et al. Global natural regulatory T cell depletion in active systemic lupus erythematosus. J Immunol. 2005;175:8392–400. doi: 10.4049/jimmunol.175.12.8392. [DOI] [PubMed] [Google Scholar]
- 5.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25 high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 6.Baraczka K, Pozsonyi T, Nekam K, et al. Soluble L-selectin levels in serum and cerebrospinal fluid in patients with multiple sclerosis and systemic lupus erythematosus. Acta Neurol Scand. 2000;102:114–7. doi: 10.1034/j.1600-0404.2000.102002114.x. [DOI] [PubMed] [Google Scholar]
- 7.Ates A, Kinikli G, Turgay M, Duman M. Serum-soluble selectin levels in patients with rheumatoid arthritis and systemic sclerosis. Scand J Immunol. 2004;59:315–20. doi: 10.1111/j.0300-9475.2004.01389.x. [DOI] [PubMed] [Google Scholar]
- 8.Burdt MA, Hoffman RW, Deutscher SL, Wang GS, Johnson JC, Sharp GC. Long-term outcome in mixed connective tissue disease. Longitudinal clinical and serologic findings. Arthritis Rheum. 1999;42:899–909. doi: 10.1002/1529-0131(199905)42:5<899::AID-ANR8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 9.Pettersson I, Hinterberger M, Mimori T, Gottlieb E, Steitz JA. The structure of mammalian small nuclear ribonucleoproteins. Identification of multiple protein components reactive with anti-(U1)ribonucleoprotein and anti-Sm autoantibodies. J Biol Chem. 1984;259:5907–14. [PubMed] [Google Scholar]
- 10.Pettersson I, Wang G, Smith E, et al. The use of immunoblotting and immunoprecipitation of (U) small nuclear ribonucleoproteins in the analysis of sera of patients with mixed connective tissue disease and systemic lupus erythematosus: a cross-sectional, longitudinal study. Arthritis Rheum. 1986;29:986–96. doi: 10.1002/art.1780290807. [DOI] [PubMed] [Google Scholar]
- 11.Greidinger EL, Hoffman RW. The appearance of U1 RNP antibody specificities in sequential autoimmune human antisera follows a characteristic order that implicates the U1-70 kd and B'/B proteins as predominant U1 RNP immunogens. Arthritis Rheum. 2001;44:368–75. doi: 10.1002/1529-0131(200102)44:2<368::AID-ANR55>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 12.Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–33. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman RW, Rettenmaier LJ, Takeda Y, et al. Human autoantibodies against the 70-kd polypeptide of U1 small nuclear RNP are associated with HLA-DR4 among connective tissue disease patients. Arthritis Rheum. 1990;33:666–73. doi: 10.1002/art.1780330509. [DOI] [PubMed] [Google Scholar]
- 14.Kaneoka H, Hsu KC, Takeda Y, Sharp GC, Hoffman RW. Molecular genetic analysis of HLA-DR and HLA-DQ genes among anti-U1-70-kd autoantibody positive connective tissue disease patients. Arthritis Rheum. 1992;35:83–94. doi: 10.1002/art.1780350113. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman RW, Cassidy JT, Takeda Y, Smith-Jones EI, Wang GS, Sharp GC. U1-70-kd autoantibody-positive mixed connective tissue disease in children. A longitudinal clinical and serologic analysis. Arthritis Rheum. 1993;36:1599–602. doi: 10.1002/art.1780361115. [DOI] [PubMed] [Google Scholar]
- 16.Komatireddy GR, Wang GS, Sharp GC, Hoffman RW. Antiphospholipid antibodies among anti-U1-70 kDa autoantibody-positive patients with mixed connective tissue disease. J Rheumatol. 1997;24:319–22. [PubMed] [Google Scholar]
- 17.Alarcon-Segovia D, Cardiel MH. Comparison between 3 diagnostic criteria for mixed connective tissue disease. Study of 593 patients. J Rheumatol. 1989;16:328–34. [PubMed] [Google Scholar]
- 18.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 19.Greidinger EL, Foecking MF, Magee J, et al. A major B cell epitope present on the apoptotic but not the intact form of the U1-70-kDa ribonucleoprotein autoantigen. J Immunol. 2004;172:709–16. doi: 10.4049/jimmunol.172.1.709. [DOI] [PubMed] [Google Scholar]
- 20.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH, Committee on Prognosis Studies in SLE Derivation of the SLEDAI. A disease activity index for lupus patients. Arthritis Rheum. 1992;35:630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 21.Hay EM, Bacon PA, Gordon C, et al. The BILAG index: A reliable and valid instrument for measuring clinical disease activity in systemic lupus erythematosus. Q J Med. 1993;86:447–58. [PubMed] [Google Scholar]
- 22.Isenberg DA, Rahman A, Allen E, et al. BILAG 2004. Development and initial validation of an updated version of the British Isles Lupus Assessment Group's disease activity index for patients with systemic lupus erythematosus. Rheumatology Oxford. 2005;44:902–6. doi: 10.1093/rheumatology/keh624. [DOI] [PubMed] [Google Scholar]
- 23.Liang MH, Socher SA, Larson MG, Schur PH. Reliability and validity of six systems for the clinical assessment of disease activity in systemic lupus erythematosus. Arthritis Rheum. 1989;32:1107–18. doi: 10.1002/anr.1780320909. [DOI] [PubMed] [Google Scholar]
- 24.Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39:363–9. doi: 10.1002/art.1780390303. [DOI] [PubMed] [Google Scholar]
- 25.Stoll T, Gordon C, Seifert B, et al. Consistency and validity of patient administered assessment of quality of life by the MOS SF-36; its association with disease activity and damage in patients with systemic lupus erythematosus. J Rheumatol. 1997;24:1608–14. [PubMed] [Google Scholar]
- 26.Hoffman P, Eder R, Boeld TJ, et al. Only the CD45RA+subpopulation of CD4+CD25high T cells gives rise to homogeneous regulatory T-cell lines upon in vitro expansion. Blood. 2006;108:4260–7. doi: 10.1182/blood-2006-06-027409. [DOI] [PubMed] [Google Scholar]
- 27.Earle KE, Tang Q, Zhou X, et al. In vitro expanded human CD4+CD25+ regulatory T cells suppress effector T cell proliferation. Clin Immunol. 2005;115:3–9. doi: 10.1016/j.clim.2005.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campbell DJ, Ziegler SF. FOXP3 modifies the phenotypic and functional properties of regulatory T cells. Nat Rev Immunol. 2007;7:305–10. doi: 10.1038/nri2061. [DOI] [PubMed] [Google Scholar]
- 29.Lim HM, Broxmeyer HE, Kim CM. Regulation of trafficking receptor expression in human forkhead box P3+ regulatory T cells. J Immunol. 2006;177:840–51. doi: 10.4049/jimmunol.177.2.840. [DOI] [PubMed] [Google Scholar]
- 30.Hoffman RW. Mixed connective-tissue disease and overlap syndrome. In: Wallace DJ, Hahn BH, editors. Dubois' lupus erythematosus. 7th. New York: Lippincott Williams and Wilkins; 2007. pp. 975–91. [Google Scholar]
- 31.Hoffman RW. Overlap syndromes: mixed connective tissue disease and Sjogren's syndrome. In: Lahita RG, editor. Systemic lupus erythematosus. 4th. New York: Elsevier; 2004. pp. 717–44. [Google Scholar]
- 32.Frandsen PB, Kriegbaum NJ, Ullman S, Hoier-Madsen M, Wiik A, Halberg P. Follow-up of 151 patients with high-titer U1RNP antibodies. Clin Rheumatol. 1996;15:254–60. doi: 10.1007/BF02229703. [DOI] [PubMed] [Google Scholar]
- 33.Amigues JM, Cantagrel A, Abbal M, Mazieres B. Comparative study of 4 diagnosis criteria sets for mixed connective tissue disease in patients with anti-RNP antibodies. J Rheumatol. 1996;23:2055–62. [PubMed] [Google Scholar]
- 34.Kotajima L, Aotsuka S, Sumiya M, Yokohari R, Tojo T, Kasukawa R. Clinical features of patients with juvenile onset mixed connective tissue disease: analysis of data collected in a nationwide collaborative study in Japan. J Rheumatol. 1996;23:1088–94. [PubMed] [Google Scholar]
- 35.Lundberg I, Hedfors E. Clinical course of patients with anti-RNP antibodies. A prospective study of 32 patients. J Rheumatol. 1991;18:1511–9. [PubMed] [Google Scholar]
- 36.Vila LM, Alarcon GS, McGwin G, et al. Early clinical manifestations, disease activity and damage of systemic lupus erythematosus among two distinct US Hispanic subpopulations. Rheumatology Oxford. 2004;43:358–63. doi: 10.1093/rheumatology/keh048. [DOI] [PubMed] [Google Scholar]
- 37.Hoffman IE, Peene I, Meheus L, et al. Specific antinuclear antibodies are associated with clinical features in systemic lupus erythematosus. Ann Rheum Dis. 2004;63:1155–8. doi: 10.1136/ard.2003.013417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swaak AJ, van der Brink HG, Smeenk RJ, et al. Systemic lupus erythematosus: clinical features in patients with disease duration of over 10 years, first evaluation. Rheumatology Oxford. 1999;38:953–8. doi: 10.1093/rheumatology/38.10.953. [DOI] [PubMed] [Google Scholar]
- 39.Hoet RM, Koornneef I, de Rooij DJ, van de Putte LB, van Venrooij WJ. Changes in anti-U1 RNA antibody levels correlate with disease activity in patients with systemic lupus erythematosus overlap syndrome. Arthritis Rheum. 1992;35:1202–10. doi: 10.1002/art.1780351013. [DOI] [PubMed] [Google Scholar]
- 40.Crispin JC, Martinez A, Alcocer-Varela J. Quantification of regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun. 2003;21:273–6. doi: 10.1016/s0896-8411(03)00121-5. [DOI] [PubMed] [Google Scholar]
- 41.Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178:2579–88. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 42.Font J, Pizcueta P, Ramos-Casals M, et al. Increased serum levels of soluble L-selectin (CD62L) in patients with active systemic lupus erythematosus. Clin Exp Immunol. 2000;119:169–74. doi: 10.1046/j.1365-2249.2000.01082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sfikakis PP, Charalambopoulos D, Vaiopoulos G, Mavrikakis M. Circulating P- and L-selectin and T-lymphocyte activation and patients with autoimmune rheumatic diseases. Clin Rheumatol. 1999;18:28–32. doi: 10.1007/s100670050047. [DOI] [PubMed] [Google Scholar]
- 44.Bodolay E, Csiki Z, Szekanecz Z, et al. Five year follow up of 665 Hungarian patients with undifferentiated connective tissue disease. Clin Exp Rheumatol. 2003;21:313–20. [PubMed] [Google Scholar]
- 45.Danieli MG, Fraticelli P, Franceschini F, et al. Five-year follow-up of 165 Italian patients with undifferentiated connective tissue disease. Clin Exp Rheumatol. 1999;17:585–91. [PubMed] [Google Scholar]


