Abstract
Objective
As the use of atypical antipsychotics in children and adolescents has increased, concerns have been raised about their long-term safety. We aimed to investigate the association between risperidone-induced weight gain, leptin concentration, and the leptin gene (LEP) -2548G/A variants in youths.
Methods
Medically healthy 7-17yo children and adolescents, in extended naturalistic treatment with risperidone, were recruited through pediatric psychiatry clinics. Anthropometric measures and laboratory testing were conducted. Growth and medication history was obtained from the medical record. The effect of the LEP genotypes on leptin concentration and on the slopes of the weight and body mass index (BMI) z score curves before and after the onset of risperidone treatment was investigated.
Results
In 74 subjects, chronically treated with risperidone, the A allele was associated with higher leptin concentration at low weight and BMI z scores. There was no effect of the LEP genotypes on weight or BMI z scores before risperidone was started. Afterwards, however, the A allele carriers showed a steeper rate of increase in weight and BMI z scores. As a result, the GG genotype carriers were 2.5 times less likely to be overweight/obese (i.e. having a BMI above the 85th percentile). This genetic effect on risperidone-associated weight gain did not extend to weight loss related to psychostimulants.
Conclusions
The LEP -2548G/A variants appear to moderate the weight-altering effect of risperidone but not psychostimulants. This may be related to genetic differences in tissue sensitivity to leptin, resulting in differential body composition.
Keywords: Leptin gene, Antipsychotics, Weight Gain, Children, Adolescents, Variants, Predictors
INTRODUCTION
Atypical antipsychotics increase appetite by modulating metabolic homeostatic mechanisms in the hypothalamus, resulting in weight gain (Kim et al., 2007). Consequently, due to the involvement of leptin in appetite regulation and energy balance (Friedman, 1997, Schwartz et al., 2000, Morton et al., 2006), interest in investigating its role in the development of this antipsychotic-associated adverse event has emerged.
In adults, leptin concentrations correlate with body mass index (BMI), total body fat, and percent body fat (Sivitz et al., 2003). Moreover, prospective studies have documented an increase in leptin following weight gain, including that induced by antipsychotic medications (Scarpace and Zhang, 2007, Baptista et al., 2007, Haupt et al., 2005, Unger, 2003). In children, leptin regulation is more complex, involving an interaction of gender, age, stage of sexual development, and adiposity (Blum et al., 1997). Thus, for an equivalent fat mass, leptin serum concentration rises in girls but declines in boys as they grow older and more sexually mature (Blum et al., 1997, Mansoub et al., 2006).
The relationship between leptin and body fat is also possibly regulated by variants of the promoter region of the leptin gene (LEP) (van der Lende et al., 2005). Among the single nucleotide polymorphisms that have been identified in the LEP promoter region, the -2548G/A variants have received the most attention since they appear to alter the transcription rate of the gene (Hoffstedt et al., 2002). In fact, studies in non-psychiatric samples have found the -2548G/A variants to affect leptin concentrations (Hoffstedt et al., 2002, Le Stunff et al., 2000, Mammes et al., 1998, Mammes et al., 2000, Yiannakouris et al., 2001) and, in adults with schizophrenia, these variants have been associated with antipsychotic-related weight gain (Zhang et al., 2003, Ellingrod et al., 2007, Templeman et al., 2005). To our knowledge, however, no such studies have been reported in youths treated with antipsychotics.
MATERIAL & METHODS
Subjects
Children and adolescents, ages 7 to 17, treated with risperidone for six months or more, irrespective of diagnosis and indication, were recruited from pediatric psychiatry outpatient clinics. Patients concomitantly treated with other antipsychotic drugs were excluded as were patients with mental retardation, traumatic brain injury, or other neurological disorders. Pregnant females or those receiving hormonal contraception were not eligible. Patients with metabolic, hormonal, or autoimmune disorders requiring medical treatment were also excluded.
Procedures
This study was approved by the University of Iowa Institutional Review Board. Assent was obtained from children ≤ 11 years old and consent from adolescents and all parents or legal guardians.
Race and ethnicity were based on self-report and the clinical diagnoses on chart review. As reviewed elsewhere (Calarge et al., 2009), all pediatric and psychiatric records were reviewed to extract anthropometric measurements and treatment history. This documentation, which was confirmed by a physician, also reflected deviations from the prescribed treatments. All dosages of psychostimulants were expressed in methylphenidate-equivalent for amphetamines (x 2) (Swanson et al., 2007).
Height was measured while standing erect, to the nearest 0.1 cm, using a stadiometer (Holtain Ltd., UK). Weight was recorded while wearing indoor clothes without shoes, to the nearest 0.1 kg, using a digital scale (Scaletronix, Wheaton, IL). Following standard procedures (Prevention, 2000), triceps and subscapular skinfold thickness was measured with a Lange skinfold caliper to the nearest 0.1 mm by one of two research dieticians (inter-rater agreement ICC > 95%, n = 16). The average of two measurements was used.
Pubertal stage was evaluated by a physician. In addition, patients completed a self-assessment form (Marshall and Tanner, 1969, Marshall and Tanner, 1970). Inter-rater agreement between the physician and self-rating was high (weighted kappa= 0.81, 95% confidence interval = [0.74-0.88], N=74).
In 89% of the sample, a morning fasting blood sample was collected to measure TSH and leptin and for genetic testing. In the other 11%, a non-fasting sample was obtained. Plasma TSH and leptin concentrations were measured by electro-chemiluminescence immunoassay (Roche Diagnostics, Indianapolis, IN) with ruthenium-labeled monoclonal antibody (mouse/human). The assay limit of detection of TSH is 0.005 μIU/ml with intra- and inter-assay coefficients of variation < 4.3%. The leptin assay limit of detection is 0.5 ng/ml with intra- and inter-assay coefficients of variation < 7%.
Polymerase chain reaction (PCR) and sequencing primers for the LEP -2548G/A variant (dbSNP rs7799039) were designed using Pyrosequencing SNP Primer Design Version 1.01 software (http://www.pyrosequencing.com). PCR reactions were done in a 30 μl volume with 1.5 mM Mg2+, 10 pmol of forward primer (5'-TTTCCTGTAATTTTCCCGTGAG), and 10 pmol of reverse primer (5'-AAAGCAAAGACAGGCTAAAAA) according to the following specifications: initial denaturation time of 5 minutes at 95° C followed by 45 cycles of 95° C for 30 s, 43° C for 30 s, 72° C for 30 s, and a final extension of 10 minutes at 72° C. Genotyping was done with Pyrosequencing™ Technology (Ronaghi, 2003). PCR products were visualized by electrophoresis on 1.8% agarose gels stained with ethidium bromide before Pyrosequencing. The sequencing primer used was 5'-TTTGCGACAGGGTTG.
Statistical Analysis
All weight and BMI measurements were converted into z scores using the 2000 CDC growth charts (Ogden et al., 2002). Leptin values were log transformed to normalize the data distribution. The sample was divided based on the presence of the A allele. Differences across the two genotype groups were compared using the Student t-test for continuous variables and Fisher's exact test for categorical variables. The Wilcoxon Rank Sum test was used for continuous variables that were not normally distributed.
Analysis of covariance (ANCOVA) was used to investigate the effect of the leptin genotype groups on leptin concentration. Covariates included BMI z score at study enrollment, age, gender, and stage of sexual development. Because leptin is more closely related to percent body fat (Sivitz et al., 2003), compared to weight or BMI, this was estimated using skinfold thickness measurements following Slaughter et al. (Slaughter et al., 1988) and used as a covariate.
In order to investigate the effect of the genotype groups on risperidone-associated weight gain, a random coefficient regression model was fitted, having weight (or BMI) z score as the dependent variable and genotype group, time, risperidone treatment status, methylphenidate dose (mg/kg/day), and genotype group-time, risperidone treatment status-time, and genotype group-risperidone treatment status-time interactions as the independent variables. The model included a linear and quadratic effect for time as well as baseline weight (or BMI) z score as covariates. This model involved estimating a mean curve for weight (or BMI) z score over time for the genotype groups from the individual curve of each child. Because we expected to find a quadratic effect of duration of risperidone treatment on weight gain, we required a minimum of three weight (or BMI) measurements before or three after risperidone initiation in order for a participant to contribute to this analysis. We also required that a baseline weight, obtained within one month before the initiation of risperidone, be available and excluded participants with prior antipsychotic treatment other than risperidone. The measure of association of the genetic groups with weight (or BMI) z score was derived from the maximum likelihood ratio test statistic of weight gain from models with and without the genetic groups included.
In order to test whether the effect of the LEP -2548G/A variants is specific to risperidone or extends to other psychotropics with weight-altering potential (2004, Swanson et al., 2006), we conducted a similar analysis involving patients treated with psychostimulants before the onset of risperidone treatment (n= 50). In this analysis, the initiation of psychostimulants represented the reference time point before and after which the growth curves of each participant were compared.
All the statistical tests were two-tailed with statistical significance set at α = 0.05, and performed using SAS version 9.1.3 for Windows (SAS Institute Inc, Cary, North Carolina).
RESULTS
In 74 participants (91% males) who have been treated only with risperidone, the LEP -2548G/A genotype frequencies were GG 41% (n=30), GA 42% (n=31), and AA 18% (n=13), and in Hardy-Weinberg equilibrium (χ2 = 1.0, p=0.3). The Table shows the demographic and clinical characteristics of the two genotype groups. Attention deficit hyperactivity disorder was by far the most common psychiatric diagnosis, given to 84% (n=62) of the subjects, though comorbidity was the rule (Calarge et al., 2009). Sixty nine percent (n=51) of the participants received risperidone to reduce irritability and aggression, at an average daily dose consistent with published clinical trials (Aman et al., 2002, Snyder et al., 2002). Besides risperidone, psychostimulants, selective serotonin reuptake inhibitors (SSRIs), and α2-agonists were the most commonly used medications, prescribed in 69% (n=51), 57% (n=42), and 24% (n=18) of the sample, respectively. Except for racial/ethnic composition (Table), there were no significant differences across the two genotype groups in demographic or clinical characteristics.
Table.
Demographic and Clinical Characteristics Of Subjects in the Two Leptin Genetic Groups
| Characteristics | All Subjects (N = 74) | GG Genotypes (N =30) | AA/AG Genotype (N =44) | Statistical Analysis | P Value |
|---|---|---|---|---|---|
| Age, mean ± SD, y | 11.7 ± 2.9 | 11.9 ± 3.0 | 11.7 ± 2.8 | t= 0.35, df=72 | p=0.7 |
| Male, n (%) | 67 (91) | 25 (83) | 42 (95) | Fisher's Exact | p=0.1 |
| Race/Ethnicity, % | |||||
| Non-Hispanic Caucasian / African | |||||
| American / Hispanic / Other | 84/12/3/1 | 73/23/0/3 | 91/5/5/0 | Fisher's Exact | p=0.01 |
| Baseline Weight z score | 0.1 ± 1.1 [n=58] | 0 ± 1.1 [n=23] | 0.1 ± 1.1 [n=35] | t=-0.51, df=56 | p=0.6 |
| Baseline BMI z score | 0.1 ± 1.1 [n=57] | -0.1 ± 1.2 [n=23] | 0.2 ± 1.1 [n=34] | t=-0.99, df=55 | p=0.3 |
| Pubertal Status, % at Tanner stage I, II, III, IV, V | 42/19/4/25/10 | 41/14/7/28/10 | 43/23/2/23/9 | Wilcoxon S = 1110 | p=0.7 |
| Risperidone, median (quartiles), (mg/kg/d) | 0.03 (0.01-0.04) | 0.02 (0.01-0.04) | 0.03 (0.01-0.04) | Wilcoxon S = 1106 | p=0.8 |
| Treatment Duration, median (quartiles), y | 2.6 (1.2-3.7) | 3.0 (1.5-3.7) | 2.3 (1.1-3.8) | Wilcoxon S = 1199 | p=0.4 |
| Elevated TSH, n (%) | 10 (14) | 4 (13) | 6 (14) | Fisher's Exact | p=1.0 |
| Leptin, median (quartiles), ng/ml | 4.5 (2.0-8.5) [n=71] | 2.5 (1.9-7.6) [n=29] | 5.2 (2.3-8.5) [n=42] | Wilcoxon S = 967 | p=0.4 |
| 4.6 (2.2-8.9) | 19.3 (11.8-24.3) | 22.3 (14.4-26.5) | |||
| Percent Fat, median (quartiles), % | [n=69] | [n=27] | [n=42] | Wilcoxon S = 822 | p=0.1 |
Legend for Table: ADHD: attention deficit hyperactivity disorder, DBD: disruptive behavior disorder, PDD: pervasive developmental disorder, SSRI: selective serotonin reuptake inhibitors. In no cases was the TSH > 6.8 μU/ml (normal range: 0.27-4.20).
Percent body fat was computed using the skinfold thickness measurements, following slaughter et al. (Slaughter et al., 1988).
The statistical comparison was between the two genetic groups.
Between brackets is the number of participants included in the specific analysis when different from the total sample, due to missing data.
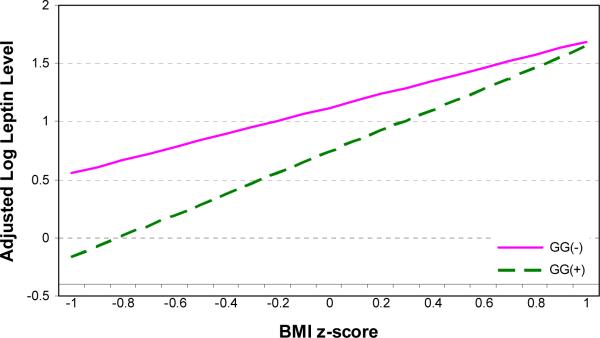
Using ANCOVA, we evaluated whether the leptin genotypes predicted leptin concentration (log transformed) after adjusting for BMI z score, duration of risperidone treatment, age, gender, and stage of sexual development. We found a significant effect of the genotype group (F(1,62) = 4.7, p<0.04) and a significant interaction between BMI z score and the genotype group (F(1,62) = 4.6, p<0.04) predicting leptin concentration: at lower BMI z scores, the A allele carriers had a higher leptin concentration (Figure 1). However, when percent of body fat, as estimated by skinfold thickness (Slaughter et al., 1988), was entered into the model (F(1,66) = 157.7, p<0.0001), the effects of all other predictor variables became non-significant. In fact, log leptin was more strongly correlated with percent body fat (Spearman's r = 0.86, p<0.001) compared to weight z score (Spearman's r = 0.50, p<0.001) or BMI z score (Spearman's r = 0.63, p<0.001).
Figure 1.
After adjusting for BMI z score, age, gender, and stage of sexual development, the LEP -2548G/A genotype interacted with BMI z score to predict leptin concentration (log transformed). Thus, at lower BMI z scores, the A allele carriers had significantly higher leptin concentrations.
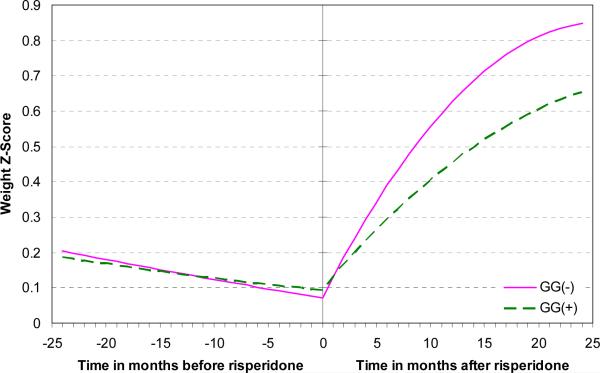
We then investigated the effect of the genetic variants on risperidone-associated weight gain. Following the restrictions described in the statistical analysis section, 50 participants were included in the weight z score analysis and 46 in the BMI z score analysis. After controlling for the effect of baseline weight z score (F(1,47.7) = 810.0, p<0.0001) and the dose of methylphenidate/kg of body weight (F(1,1128) = 77.0, p<0.0001), the leptin genotype groups predicted a differential rate of weight z score gain after, but not before, risperidone was started (three-way interaction of time, risperidone treatment status, and leptin genotype group: F(1,54) = 5.1, p<0.03). In other words, the leptin genotype did not influence the trajectory of weight z score before risperidone was started (Figure 2). However, holding all the significant covariates constant and equal to their respective average values in the sample, after one year of risperidone treatment, weight z score increased by an average 0.36 z score point in the GG genotype and by 0.56 in the A allele carriers. After two years of treatment, it increased by an average 0.56 z score point in the GG genotype and 0.78 in the A allele carriers.
Figure 2.
Before risperidone was started, there was no effect of the LEP -2548G/A genotype on weight z score. Afterwards, however, weight z score increased faster in the A allele carriers than in the GG genotype group. Weight z scores are adjusted for baseline weight z score and the dose of methylphenidate/kg of body weight.
The BMI z score analysis yielded similar results (three-way interaction of time, risperidone treatment status, and leptin genotype group: F(1,101) = 6.83, p=0.01). The effect of genotype on the slope of weight (or BMI) z score change corresponds to a likelihood ratio-based estimate R2 of 0.11 for weight z score and 0.14 for BMI z score. Furthermore, when weight gain was defined categorically, 66% (n=20) of the A allele carriers had gained at least 0.5 weight z score point compared to 44% (n=9) of the participants with the GG genotype. This translated into 50% (n=15) of the A allele carriers being overweight or obese (i.e. BMI ≥ 85th percentile) upon recruitment while only 20% (n=4) of the participants with the GG genotype were (Fisher's Exact p=0.03).
SSRIs and α2 agonists may lead to weight gain during extended treatment (Vanina et al., 2002). Since, along with psychostimulants, these were the most common used psychotropics received by the participants, we repeated the above analyses while controlling for treatment with either of these two groups of psychotropics. Neither was significant (results available upon request).
As shown in the Table, non-Hispanic Caucasians were more common among the A allele carriers. Therefore, we repeated the analyses after excluding minority groups since non-Hispanic Caucasians represented the vast majority of our sample. The three-way interaction of time, risperidone treatment status, and leptin genotype group remained significant for weight z score (F(1,30) = 4.8, p<0.04) and BMI z score (F(1,88.9) = 6.4, p=0.01).
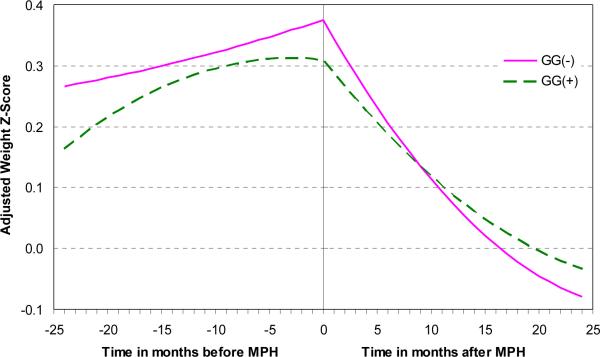
A similar analysis was then applied to treatment with psychostimulants prior to the initiation of risperidone. After controlling for weight z score at the onset of psychostimulant treatment, we found the expected negative impact of psychostimulant treatment, over time, on weight z score (F(1,100) = 20.4, p<0.0001). However, there was no evidence of a genotype group effect (F(1,52.6) = 0.18, p=0.7). In fact, none of the interactions between genotype group, psychostimulant treatment status, and time were significant in the prediction of weight z score change (Figure 3).
Figure 3.
Neither before nor after the onset of psychostimulant treatment did either of the LEP -2548G/A genotype groups have an effect on the change in weight z score. As expected, weight z score gradually decreases after psychostimulants (MPH) are started.
DISCUSSION
To our knowledge, this is the first study to evaluate the potential role of genetic variants in antipsychotic-associated weight gain in a pediatric population. We restricted this investigation to the -2548G/A variants in the promoter region of LEP due to 1) the central role that leptin plays in energy homeostasis, 2) the functional characteristics of these variants and their effect on leptin concentrations, 3) the common prevalence of both alleles in the general population, and 4) initial findings in adults with schizophrenia implicating these variants in antipsychotic-induced weight gain.
Before discussing the results, we review the study limitations. First, our sample includes youths treated with only risperidone. Since antipsychotics vary with regard to their receptor binding profiles (Richelson and Souder, 2000) and their mechanism to induce weight gain (Kim et al., 2007), findings from this study might not apply to other antipsychotic agents. However, identifying the genetic moderators of weight gain with other antipsychotics is crucial, especially that some may lead to more dramatic weight gain compared to risperidone (Allison et al., 1999, Correll and Carlson, 2006). Second, acute and chronic weight gain secondary to antipsychotics might be associated with different genetic predictors (Templeman et al., 2005). Using the same analytical method, we have found that the T variant at position -759 in the promoter region of the 5HT2C receptor gene also increases the risk of risperidone-induced weight gain (Cole et al., 2008). Thus, it is likely that multiple genetic and environmental factors are involved in the emergence of this side effect. Another shortcoming of this study is its naturalistic design. Several factors, including excessive weight gain, could lead to the discontinuation of risperidone. Though the impact of attrition on the role of variants of 5HT2C receptor gene is not known, it does not appear to have selectively affected a particular genotype since the allele frequencies remained in Hardy-Weinberg equilibrium. In addition, we aimed to investigate the safety of long-term, as opposed to acute, treatment with risperidone. Since clinical trials in psychiatry are characterized by high attrition (Anderson et al., 2007, Correll et al., 2007), we designed the study to optimize retention by recruiting subjects already stable on their medications. However, if the -2548G/A variants differentially predict weight gain not only following chronic risperidone treatment, as our data suggest, but during acute treatment as well, then, the discontinuation of risperidone is likely to underestimate the effect of the A allele on this adverse drug event. In our analysis, we control for the effect of risperidone and psychostimulants, but some patients were receiving other psychotropics. However, when all the potential weight-inducing agents were combined (Vanina et al., 2002), the average exposure period did not surpass 10% of the total period of observation in either genotype group, which was not different across the two groups. The same was true for non-psychostimulant weight-suppressing drugs (Vanina et al., 2002). In addition, we could not find an independent effect of either SSRIs or α2 agonists on weight gain in our sample. Finally, our findings require replication in a larger sample which would provide more statistical power to control for other potentially significant moderators of weight gain.
Notwithstanding the shortcomings reviewed above, our findings remain significant in several respects. First, leptin concentration varied based on the patients' genotype and fat mass as reflected by BMI. Second, as has been reported elsewhere (Ellingrod et al., 2007, Templeman et al., 2005, van der Lende et al., 2005, Zhang et al., 2007, Zhang et al., 2003), we found no effect of the -2548G/A variants on baseline weight or BMI z scores. Finally, after accounting for baseline weight and psychostimulant treatment, we found that the growth curves in the two genotype groups were different, with the AA and AG genotypes showing steeper increase in weight (or BMI) z score during extended risperidone treatment. As a result, GG genotype carriers were 2.5 times less likely to be overweight or obese (i.e., having a BMI above the 85th percentile).
Our results are in agreement with one of two studies conducted in Chinese Han adults with schizophrenia and contrast findings from other studies, including two that involved Caucasians (Ellingrod et al., 2007, Gregoor et al., 2009, Kang et al., 2008, Ruano et al., 2007, Templeman et al., 2005, Zhang et al., 2007, Zhang et al., 2003). This apparent inconsistency might be due to developmental factors or to differences in study design and sample characteristics. For example, ours is the first study to involve antipsychotic-naïve children and to compare changes in growth curves over time rather than weight measurements obtained at two time points. In contrast, more than one study recruited adults with chronic psychosis, evaluated cross-sectionally (Gregoor et al., 2009, Ruano et al., 2007, Zhang et al., 2007). In addition, our analysis included children treated with only one antipsychotic medication, unlike other studies where adults with schizophrenia treated with a variety of antipsychotics were pooled into a single analysis (Gregoor et al., 2009, Ruano et al., 2007, Templeman et al., 2005, Zhang et al., 2007). Furthermore, the gender distribution in our sample was strongly skewed toward males, consistent with the gender distribution of those pediatric psychiatric disorders that often require antipsychotic treatment (Association, 2000, Olfson et al., 2006). This is potentially significant in light of recent evidence of a moderating effect of gender on the risk of obesity associated with the LEP -2548G/A variants in adults treated with antipsychotics (Gregoor et al., 2009). Finally, 16% of our sample consisted of non-Caucasians, possibly introducing racial/ethnic heterogeneity. However, when we restricted the analysis to non-Hispanic Caucasians, the results remained significant.
As anticipated, we found a negative effect of psychostimulant use on the change in weight z score (2004, Swanson et al., 2006, Poulton, 2005). This, however, was not predicted by the LEP -2548G/A variants suggesting that psychostimulants interfere with a different pathway of appetite and energy homeostasis than risperidone (Morton et al., 2006). This is consistent with evidence suggesting that psychostimulants do not attenuate risperidone-associated weight gain (Aman et al., 2004, Calarge et al., 2009).
This study does not address the mechanism by which the A allele increases the risk for antipsychotic-related weight gain. Nevertheless, our findings and those of others suggest that the A allele is associated with a higher concentration of circulating leptin (Hoffstedt et al., 2002, Mammes et al., 1998, van der Lende et al., 2005). In fact, after controlling for significant confounders, the A allele carriers in our sample appear to produce more leptin at a low weight compared to participants with the GG genotype. Whether this reflects a state of relative leptin resistance in youths of normal weight treated with risperidone is not known. It is of note, however, that the association between serum leptin and the A allele disappeared when percent body fat, as opposed to BMI which reflects total fat mass, was introduced into the regression model. This raises the possibility that the LEP -2548G/A variants are associated with differential body composition. In fact, after controlling for BMI z score, gender, age, and sexual development, we found that percent body fat was 2.9% lower in the GG genotype (F(1,63) = 4.3, p=0.04). This preliminary finding suggests that, compared to the GG genotype, the A allele carrier state is not only associated with more weight gain induced by risperidone but also with a higher percent body fat, for the same BMI z score, thus potentially compounding the cardiovascular risk.
In summary, as evidence accumulates for the efficacy of antipsychotic medications in a variety of pediatric psychiatric disorders, their use will continue to grow as will the number of patients affected by their side effects. Weight gain and metabolic abnormalities are some of the most common and potentially serious adverse events that follow antipsychotic treatment (ADA, 2004, Newcomer and Haupt, 2006). We present, here, the first evidence implicating the A allele of the leptin promoter region at position -2548 with excessive risperidone-associated weight gain in youths. The ultimate aim of such efforts to identify genetic predictors of serious adverse events induced by psychotropics is to develop treatments based on every individual's strengths and vulnerabilities.
ACKNOWLEDGMENTS
The authors would like to thank the patients and their families for their commitment to this research, the child psychiatry staff, the research assistants, and the staff in the General Clinical Research Center. Dr. Raymond Crowe and Dr. Patricia Donohoue offered critical feedback. Dr. Jennifer McWilliams assisted in data collection.
Funding This study was funded by a 2005 Young Investigator Award to Chadi Calarge and by the National Institute of Health General Clinical Research Centers Mechanism (RR00059).
Footnotes
FINANCIAL DISCLOSURE: Conflict of Interest; Drs. Calarge, Zimmerman, Sivitz, and Schlechte and Ms. Acion report no biomedical financial interests or potential conflicts of interest.
Dr. Ellingrod is on an advisory board for Lilly and receives royalties for authorship and consultation from Lexi Comp.
References
- National Institute of Mental Health Multimodal Treatment Study of ADHD follow-up: changes in effectiveness and growth after the end of treatment. Pediatrics. 2004;113:762–9. doi: 10.1542/peds.113.4.762. [DOI] [PubMed] [Google Scholar]
- ADA Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27:596–601. doi: 10.2337/diacare.27.2.596. [DOI] [PubMed] [Google Scholar]
- Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, Weiden PJ. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156:1686–96. doi: 10.1176/ajp.156.11.1686. [DOI] [PubMed] [Google Scholar]
- Aman MG, Binder C, Turgay A. Risperidone effects in the presence/absence of psychostimulant medicine in children with ADHD, other disruptive behavior disorders, and subaverage IQ. J Child Adolesc Psychopharmacol. 2004;14:243–54. doi: 10.1089/1044546041649020. [DOI] [PubMed] [Google Scholar]
- Aman MG, De Smedt G, Derivan A, Lyons B, Findling RL. Double-blind, placebo-controlled study of risperidone for the treatment of disruptive behaviors in children with subaverage intelligence. Am J Psychiatry. 2002;159:1337–46. doi: 10.1176/appi.ajp.159.8.1337. [DOI] [PubMed] [Google Scholar]
- Anderson GM, Scahill L, McCracken JT, McDougle CJ, Aman MG, Tierney E, Arnold LE, Martin A, Katsovich L, Posey DJ, Shah B, Vitiello B. Effects of short- and long-term risperidone treatment on prolactin levels in children with autism. Biol Psychiatry. 2007;61:545–50. doi: 10.1016/j.biopsych.2006.02.032. [DOI] [PubMed] [Google Scholar]
- Association AP. Diagnostic and Statistical Manual of Mental Disorders, Text Revision. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Baptista T, Davila A, El Fakih Y, Uzcategui E, Rangel NN, Olivares Y, Galeazzi T, Vargas D, Pena R, Marquina D, Villarroel V, Teneud L, Beaulieu S. Similar frequency of abnormal correlation between serum leptin levels and BMI before and after olanzapine treatment in schizophrenia. Int Clin Psychopharmacol. 2007;22:205–11. doi: 10.1097/YIC.0b013e328080ca44. [DOI] [PubMed] [Google Scholar]
- Blum WF, Englaro P, Hanitsch S, Juul A, Hertel NT, Muller J, Skakkebaek NE, Heiman ML, Birkett M, Attanasio AM, Kiess W, Rascher W. Plasma leptin levels in healthy children and adolescents: dependence on body mass index, body fat mass, gender, pubertal stage, and testosterone. J Clin Endocrinol Metab. 1997;82:2904–10. doi: 10.1210/jcem.82.9.4251. [DOI] [PubMed] [Google Scholar]
- Calarge CA, Acion L, Kuperman S, Tansey MJ, Schlechte JA. Metabolic Abnormalities During Extended Risperidone Treatment in Children and Adolescents. J Child Adolesc Psychopharmacol. 2009;19 doi: 10.1089/cap.2008.007. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole D, Calarge CA, Ellingrod VL, D. M, Schlechte JA. American Academy of Child and Adolescent Psychiatry. Chicago, IL: 2008. Effect of the -759C/T variants of the serotonin (5-HT2C) receptor gene on risperidone-associated weight gain in children and adolescents in extended treatment. [Google Scholar]
- Correll CU, Carlson HE. Endocrine and metabolic adverse effects of psychotropic medications in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2006;45:771–91. doi: 10.1097/01.chi.0000220851.94392.30. [DOI] [PubMed] [Google Scholar]
- Correll CU, Nyilas M, Aurang C, Johnson B, Jin N, Marcus R, Pikalov A, McQuade RD, Iwamoto T, Weller W. American Academy of Child and Adolescent Psychiatry. Boston, MA: 2007. Long-Term Safety and Tolerability of Aripiprazole in Children (10-17) with Mania. [Google Scholar]
- Ellingrod VL, Bishop JR, Moline J, Lin YC, Miller del D. Leptin and leptin receptor gene polymorphisms and increases in body mass index (BMI) from olanzapine treatment in persons with schizophrenia. Psychopharmacol Bull. 2007;40:57–62. [PubMed] [Google Scholar]
- Friedman JM. The alphabet of weight control. Nature. 1997;385:119–20. doi: 10.1038/385119a0. [DOI] [PubMed] [Google Scholar]
- Gregoor JG, van der Weide J, Mulder H, Cohen D, van Megen HJ, Egberts AC, Heerdink ER. Polymorphisms of the LEP- and LEPR gene and obesity in patients using antipsychotic medication. J Clin Psychopharmacol. 2009;29:21–5. doi: 10.1097/JCP.0b013e31819359be. [DOI] [PubMed] [Google Scholar]
- Haupt DW, Luber A, Maeda J, Melson AK, Schweiger JA, Newcomer JW. Plasma leptin and adiposity during antipsychotic treatment of schizophrenia. Neuropsychopharmacology. 2005;30:184–91. doi: 10.1038/sj.npp.1300563. [DOI] [PubMed] [Google Scholar]
- Hoffstedt J, Eriksson P, Mottagui-Tabar S, Arner P. A polymorphism in the leptin promoter region (-2548 G/A) influences gene expression and adipose tissue secretion of leptin. Horm Metab Res. 2002;34:355–9. doi: 10.1055/s-2002-33466. [DOI] [PubMed] [Google Scholar]
- Kang SG, Lee HJ, Park YM, Choi JE, Han C, Kim YK, Kim SH, Lee MS, Joe SH, Jung IK, Kim L. Possible association between the -2548A/G polymorphism of the leptin gene and olanzapine-induced weight gain. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:160–3. doi: 10.1016/j.pnpbp.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Kim SF, Huang AS, Snowman AM, Teuscher C, Snyder SH. From the Cover: Antipsychotic drug-induced weight gain mediated by histamine H1 receptor-linked activation of hypothalamic AMP-kinase. Proc Natl Acad Sci U S A. 2007;104:3456–9. doi: 10.1073/pnas.0611417104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Stunff C, Le Bihan C, Schork NJ, Bougneres P. A common promoter variant of the leptin gene is associated with changes in the relationship between serum leptin and fat mass in obese girls. Diabetes. 2000;49:2196–200. doi: 10.2337/diabetes.49.12.2196. [DOI] [PubMed] [Google Scholar]
- Mammes O, Betoulle D, Aubert R, Giraud V, Tuzet S, Petiet A, Colas-Linhart N, Fumeron F. Novel polymorphisms in the 5' region of the LEP gene: association with leptin levels and response to low-calorie diet in human obesity. Diabetes. 1998;47:487–9. doi: 10.2337/diabetes.47.3.487. [DOI] [PubMed] [Google Scholar]
- Mammes O, Betoulle D, Aubert R, Herbeth B, Siest G, Fumeron F. Association of the G-2548A polymorphism in the 5' region of the LEP gene with overweight. Ann Hum Genet. 2000;64:391–4. doi: 10.1017/s0003480000008277. [DOI] [PubMed] [Google Scholar]
- Mansoub S, Chan MK, Adeli K. Gap analysis of pediatric reference intervals for risk biomarkers of cardiovascular disease and the metabolic syndrome. Clin Biochem. 2006;39:569–87. doi: 10.1016/j.clinbiochem.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–95. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Haupt DW. The metabolic effects of antipsychotic medications. Can J Psychiatry. 2006;51:480–91. doi: 10.1177/070674370605100803. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- Olfson M, Blanco C, Liu L, Moreno C, Laje G. National trends in the outpatient treatment of children and adolescents with antipsychotic drugs. Arch Gen Psychiatry. 2006;63:679–85. doi: 10.1001/archpsyc.63.6.679. [DOI] [PubMed] [Google Scholar]
- Poulton A. Growth on stimulant medication; clarifying the confusion: a review. Arch Dis Child. 2005;90:801–6. doi: 10.1136/adc.2004.056952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevention CfDCa National Health and Nutrition Examination Survey: Anthropometry Procedures Manual. 2000 (Revised December 2000) [Google Scholar]
- Richelson E, Souder T. Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci. 2000;68:29–39. doi: 10.1016/s0024-3205(00)00911-5. [DOI] [PubMed] [Google Scholar]
- Ronaghi M. Pyrosequencing for SNP genotyping. Methods Mol Biol. 2003;212:189–95. doi: 10.1385/1-59259-327-5:189. [DOI] [PubMed] [Google Scholar]
- Ruano G, Goethe JW, Caley C, Woolley S, Holford TR, Kocherla M, Windemuth A, de Leon J. Physiogenomic comparison of weight profiles of olanzapine- and risperidone-treated patients. Mol Psychiatry. 2007;12:474–82. doi: 10.1038/sj.mp.4001944. [DOI] [PubMed] [Google Scholar]
- Scarpace PJ, Zhang Y. Elevated leptin: consequence or cause of obesity? Front Biosci. 2007;12:3531–44. doi: 10.2741/2332. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr., Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–71. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Sivitz WI, Wayson SM, Bayless ML, Larson LF, Sinkey C, Bar RS, Haynes WG. Leptin and body fat in type 2 diabetes and monodrug therapy. J Clin Endocrinol Metab. 2003;88:1543–53. doi: 10.1210/jc.2002-021193. [DOI] [PubMed] [Google Scholar]
- Slaughter MH, Lohman TG, Boileau RA, Horswill CA, Stillman RJ, Van Loan MD, Bemben DA. Skinfold equations for estimation of body fatness in children and youth. Hum Biol. 1988;60:709–23. [PubMed] [Google Scholar]
- Snyder R, Turgay A, Aman M, Binder C, Fisman S, Carroll A. Effects of risperidone on conduct and disruptive behavior disorders in children with subaverage IQs. J Am Acad Child Adolesc Psychiatry. 2002;41:1026–36. doi: 10.1097/00004583-200209000-00002. [DOI] [PubMed] [Google Scholar]
- Swanson J, Greenhill L, Wigal T, Kollins S, Stehli A, Davies M, Chuang S, Vitiello B, Skrobala A, Posner K, Abikoff H, Oatis M, McCracken J, McGough J, Riddle M, Ghuman J, Cunningham C, Wigal S. Stimulant-related reductions of growth rates in the PATS. J Am Acad Child Adolesc Psychiatry. 2006;45:1304–13. doi: 10.1097/01.chi.0000235075.25038.5a. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Elliott GR, Greenhill LL, Wigal T, Arnold LE, Vitiello B, Hechtman L, Epstein JN, Pelham WE, Abikoff HB, Newcorn JH, Molina BS, Hinshaw SP, Wells KC, Hoza B, Jensen PS, Gibbons RD, Hur K, Stehli A, Davies M, March JS, Conners CK, Caron M, Volkow ND. Effects of stimulant medication on growth rates across 3 years in the MTA follow-up. J Am Acad Child Adolesc Psychiatry. 2007;46:1015–27. doi: 10.1097/chi.0b013e3180686d7e. [DOI] [PubMed] [Google Scholar]
- Templeman LA, Reynolds GP, Arranz B, San L. Polymorphisms of the 5-HT2C receptor and leptin genes are associated with antipsychotic drug-induced weight gain in Caucasian subjects with a first-episode psychosis. Pharmacogenet Genomics. 2005;15:195–200. doi: 10.1097/01213011-200504000-00002. [DOI] [PubMed] [Google Scholar]
- Unger RH. The physiology of cellular liporegulation. Annu Rev Physiol. 2003;65:333–47. doi: 10.1146/annurev.physiol.65.092101.142622. [DOI] [PubMed] [Google Scholar]
- van der Lende T, Te Pas MF, Veerkamp RF, Liefers SC. Leptin gene polymorphisms and their phenotypic associations. Vitam Horm. 2005;71:373–404. doi: 10.1016/S0083-6729(05)71013-X. [DOI] [PubMed] [Google Scholar]
- Vanina Y, Podolskaya A, Sedky K, Shahab H, Siddiqui A, Munshi F, Lippmann S. Body weight changes associated with psychopharmacology. Psychiatr Serv. 2002;53:842–7. doi: 10.1176/appi.ps.53.7.842. [DOI] [PubMed] [Google Scholar]
- Yiannakouris N, Yannakoulia M, Melistas L, Chan JL, Klimis-Zacas D, Mantzoros CS. The Q223R polymorphism of the leptin receptor gene is significantly associated with obesity and predicts a small percentage of body weight and body composition variability. J Clin Endocrinol Metab. 2001;86:4434–9. doi: 10.1210/jcem.86.9.7842. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Tan YL, Zhou DF, Haile CN, Cao LY, Xu Q, Shen Y, Kosten TA, Kosten TR. Association of clozapine-induced weight gain with a polymorphism in the leptin promoter region in patients with chronic schizophrenia in a Chinese population. J Clin Psychopharmacol. 2007;27:246–51. doi: 10.1097/jcp.0b013e3180582412. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Yao ZJ, Mou XD, Chen JF, Zhu RX, Liu W, Zhang XR, Sun J, Hou G. Association of -2548G/A functional polymorphism in the promoter region of leptin gene with antipsychotic agent-induced weight gain. Zhonghua Yi Xue Za Zhi. 2003;83:2119–23. [PubMed] [Google Scholar]