Abstract
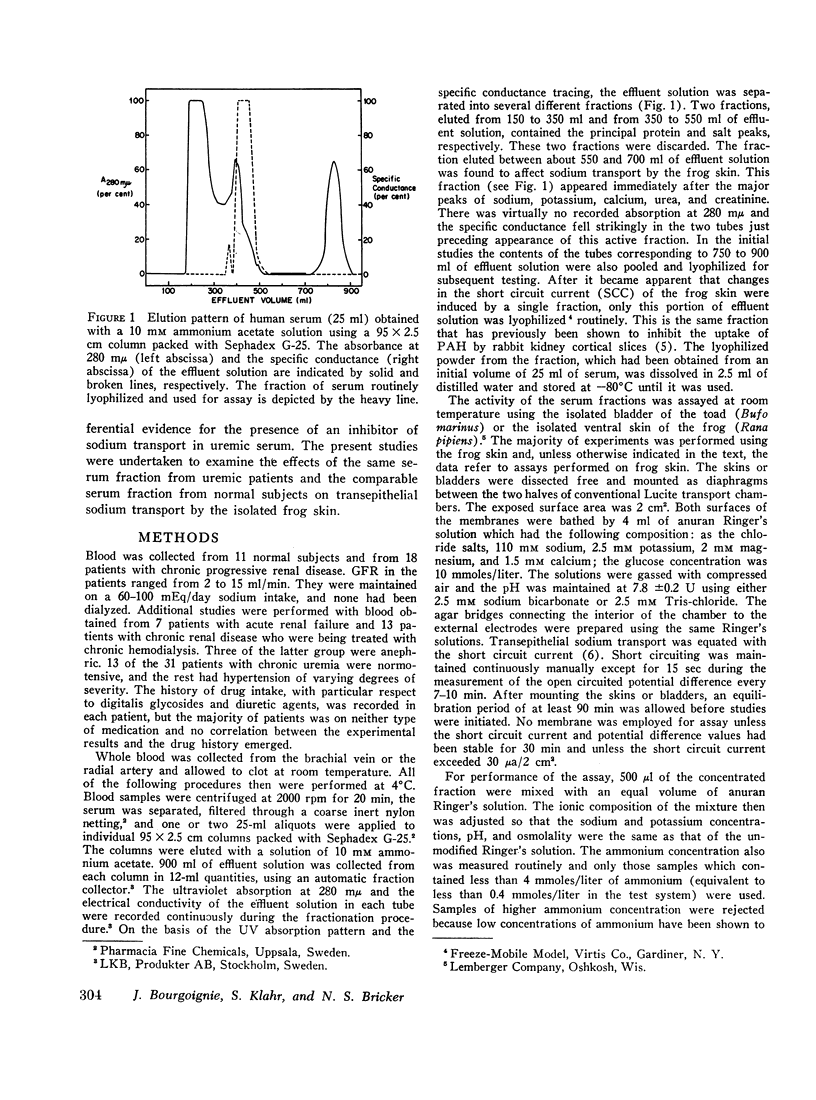
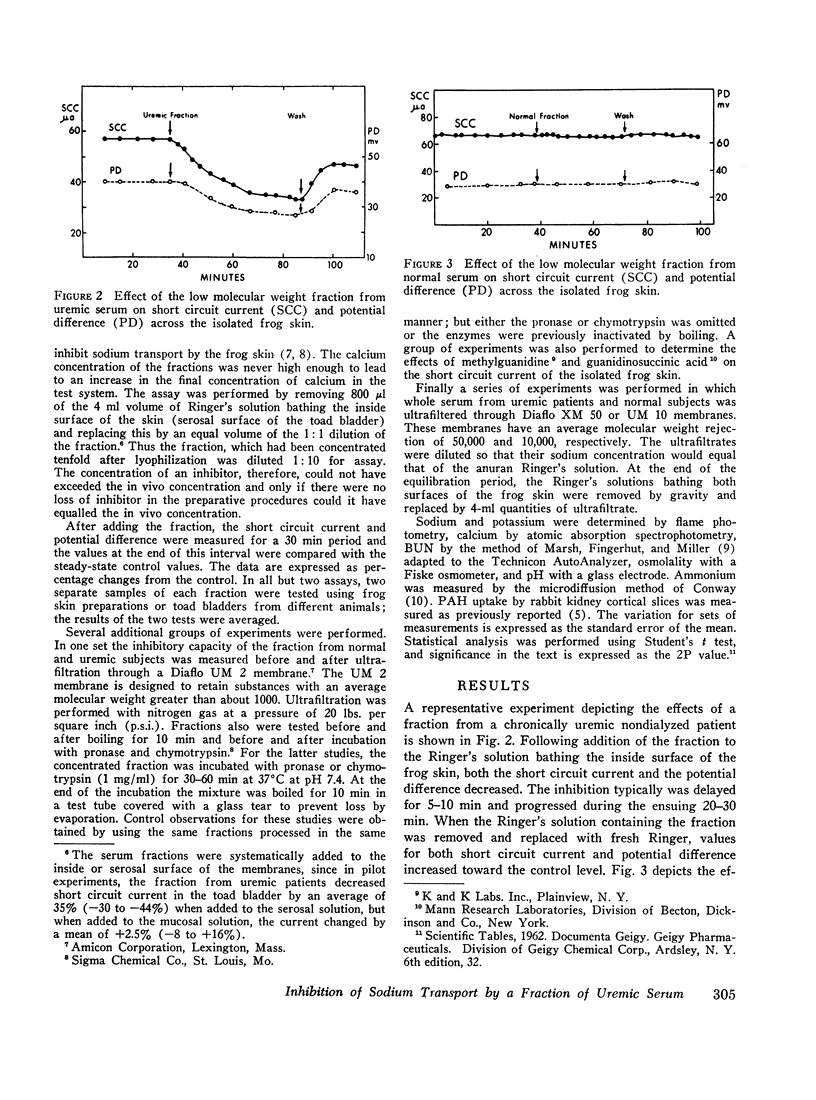
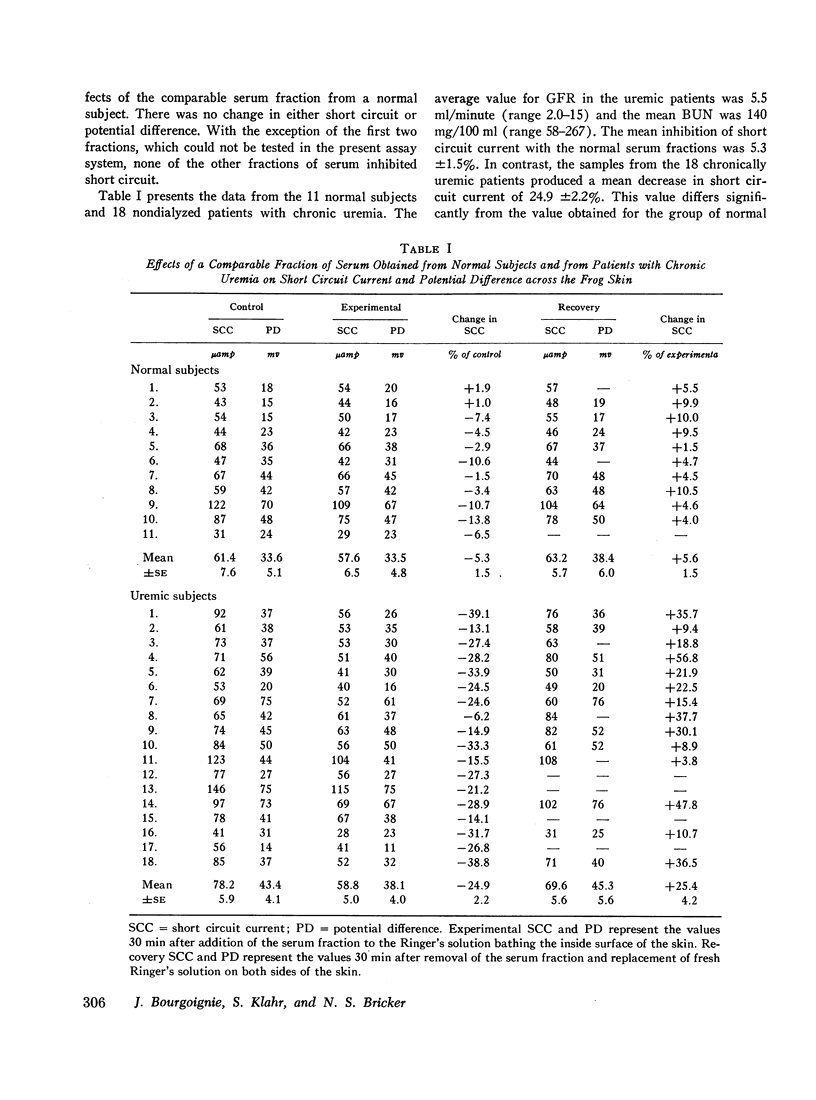
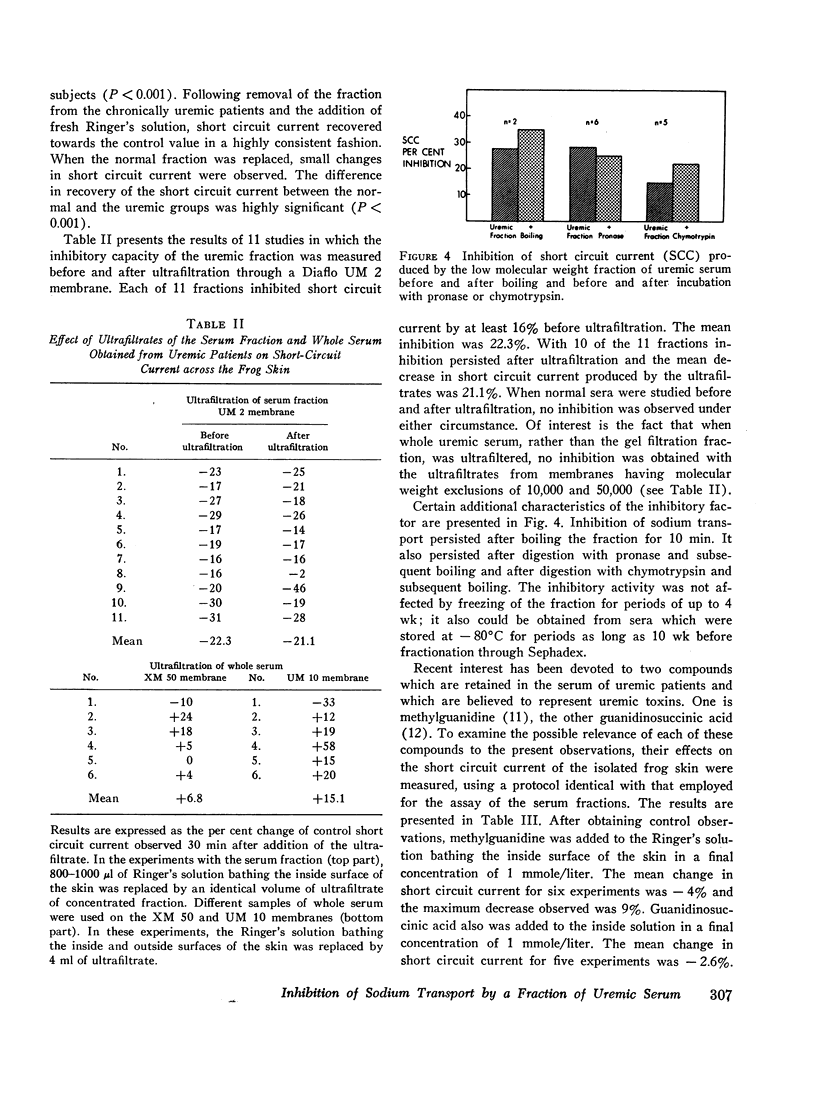
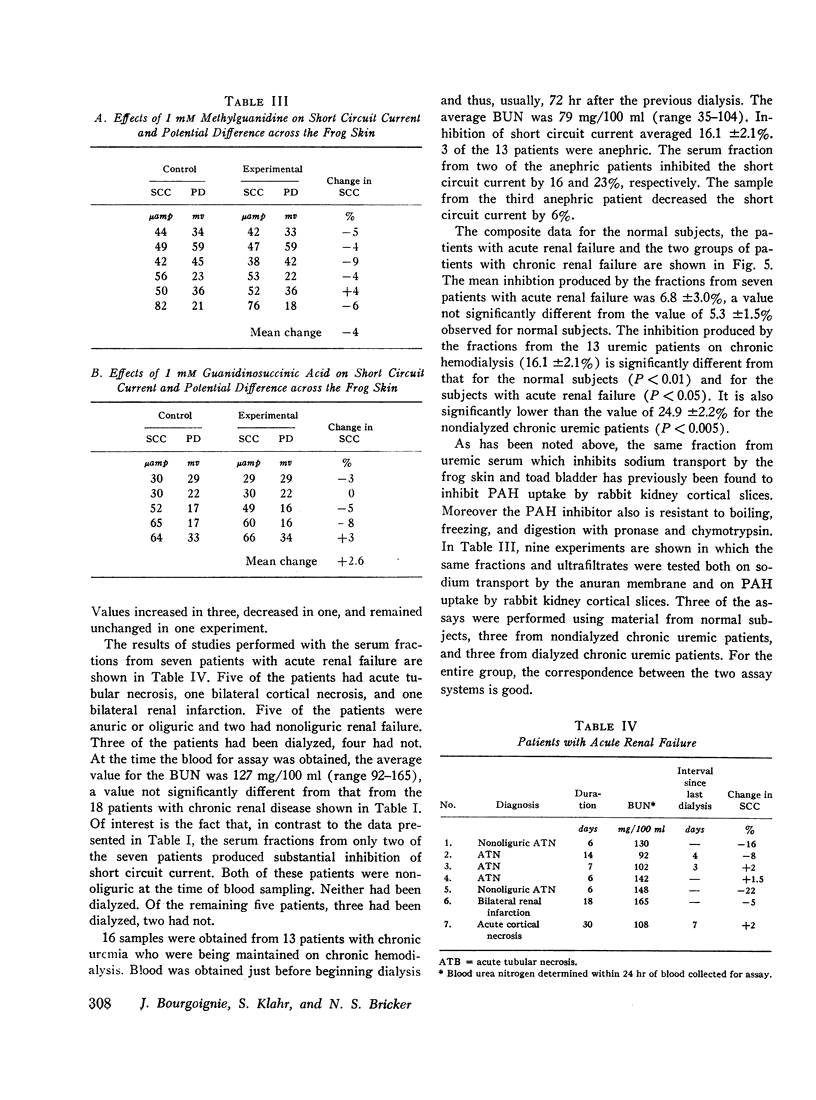
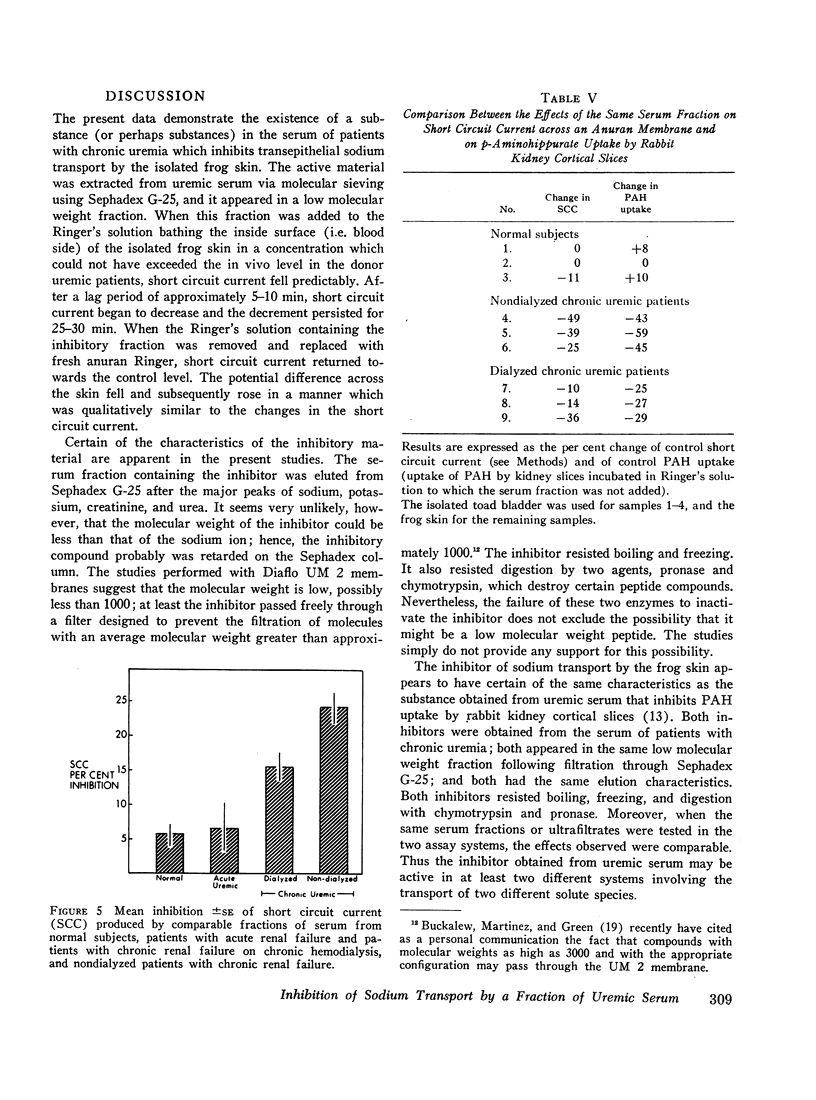
An inhibitor of transepithelial sodium transport was found in a low molecular weight fraction obtained from serum of patients with far advanced chronic renal disease. In 18 nondialyzed patients, the mean inhibition of short circuit current (SCC) was 24.9 ±2.2% (SE). With a comparable fraction from 11 normal subjects. SCC decreased by only 5.3 ±1.5%. There was significantly greater inhibition with the serum fractions of patients with end stage renal disease being maintained on chronic hemodialysis than in the normal control group; but the degree of inhibition in the dialyzed population was significantly less than that observed in the nondialyzed chronically uremic patients. The inhibition of SCC produced by the serum fractions of a group of seven patients with acute renal failure was not significantly different from the control group despite the presence of high grade uremia in the former. The inhibitory fraction has characteristics identical with the uremic serum fraction which previously has been shown to inhibit p-aminohippurate (PAH) uptake by rabbit kidney cortical slices. With gel filtration through Sephadex G-25, the active fraction appears after the major peaks of substances as small as urea and sodium; hence it may have been retarded on the column. But its ultrafiltration characteristics suggest that its molecular weight may be less than 1000. The inhibitory capability was not destroyed by boiling, freezing, or digestion with chymotrypsin or pronase. Neither methylguanidine nor guanidinosuccinic acid in concentrations well above those present in the serum of uremic patients inhibited sodium transport in the frog skin. The data suggest that there is an inhibitor of sodium transport in the serum of patients with chronic uremia. The role of this material in the regulation of sodium excretion in uremia as well as its possible role as a uremic toxin are subjects of both theoretical and practical interest.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bricker N. S., Klahr S., Purkerson M., Schultze R. G., Avioli L. V., Birge S. J. In vitro assay for a humoral substance present during volume expansion and uraemia. Nature. 1968 Sep 7;219(5158):1058–1059. doi: 10.1038/2191058a0. [DOI] [PubMed] [Google Scholar]
- Bricker N. S. The control of sodium excretion with normal and reduced nephron populations. The pre-eminence of third factor. Am J Med. 1967 Sep;43(3):313–321. doi: 10.1016/0002-9343(67)90188-x. [DOI] [PubMed] [Google Scholar]
- Buckalew V. M., Jr, Martinez F. J., Green W. E. The effect of dialysates and ultrafiltrates of plasma of saline-loaded dogs on toad bladder sodium transport. J Clin Invest. 1970 May;49(5):926–935. doi: 10.1172/JCI106312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cort J. H., Dousa T., Pliska V., Lichardus B., Safárová J., Vranesić M., Rudinger J. Saluretic activity of blood during carotid occlusion in the cat. Am J Physiol. 1968 Oct;215(4):921–927. doi: 10.1152/ajplegacy.1968.215.4.921. [DOI] [PubMed] [Google Scholar]
- Friedman R. T., Aiyawar R. M., Hughes W. D., Huf E. G. Effects of NH4+-ions on acid-base properties and ion movements in isolated frog skin. Comp Biochem Physiol. 1967 Dec;23(3):847–869. doi: 10.1016/0010-406x(67)90346-5. [DOI] [PubMed] [Google Scholar]
- Giovannetti S., Biagini M., Balestri P. L., Navalesi R., Giagnoni P., De Matteis A., Ferro-Milone P., Perfetti C. Uraemia-like syndrome in dogs chronically intoxicated with methylguanidine and creatinine. Clin Sci. 1969 Jun;36(3):445–452. [PubMed] [Google Scholar]
- HUF E. G., WILLS J. Influence of some inorganic cations on active salt and water uptake by isolated frog skin. Am J Physiol. 1951 Oct;167(1):255–260. doi: 10.1152/ajplegacy.1951.167.1.255. [DOI] [PubMed] [Google Scholar]
- Lichardus B., Plika V., Uhrín V., Barth T. The cow as a model for investigating natriuretic activity. Lancet. 1968 Jan 20;1(7534):127–129. doi: 10.1016/s0140-6736(68)92728-1. [DOI] [PubMed] [Google Scholar]
- MARSH W. H., FINGERHUT B., MILLER H. AUTOMATED AND MANUAL DIRECT METHODS FOR THE DETERMINATION OF BLOOD UREA. Clin Chem. 1965 Jun;11:624–627. [PubMed] [Google Scholar]
- Schultze R. G., Shapiro H. S., Bricker N. S. Studies on the control of sodium excretion in experimental uremia. J Clin Invest. 1969 May;48(5):869–877. doi: 10.1172/JCI106045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealey J. E., Kirshman J. D., Laragh J. H. Natriuretic activity in plasma and urine of salt-loaded man and sheep. J Clin Invest. 1969 Dec;48(12):2210–2224. doi: 10.1172/JCI106187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedláková E., Lichardus B., Cort J. H. Plasma saluretic activity: its nature and relation to oxytocin analogs. Science. 1969 May 2;164(3879):580–582. doi: 10.1126/science.164.3879.580. [DOI] [PubMed] [Google Scholar]
- Slatopolsky E., Elkan I. O., Weerts C., Bricker N. S. Studies on the characteristics of the control system governing sodium excretion in uremic man. J Clin Invest. 1968 Mar;47(3):521–530. doi: 10.1172/JCI105748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein I. M., Cohen B. D., Kornhauser R. S. Guanidinosuccinic acid in renal failure, experimental azotemia and inborn errors of the urea cycle. N Engl J Med. 1969 Apr 24;280(17):926–930. doi: 10.1056/NEJM196904242801704. [DOI] [PubMed] [Google Scholar]
- USSING H. H., ZERAHN K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand. 1951 Aug 25;23(2-3):110–127. doi: 10.1111/j.1748-1716.1951.tb00800.x. [DOI] [PubMed] [Google Scholar]
- de Wardener H. E. Control of sodium reabsorption. Br Med J. 1969 Sep 20;3(5672):676–683. doi: 10.1136/bmj.3.5672.676. [DOI] [PMC free article] [PubMed] [Google Scholar]


