Abstract
Caloric restriction (CR), in the absence of malnutrition, delays aging and prevents aging-related diseases through multiple mechanisms. A reduction in chronic inflammation is widely observed in experimental models of caloric restriction. The low inflammation status may contribute to the reduced incidence of osteoporosis, Alzheimer's disease, cardiovascular diseases and cancer in the aging subjects. The association of caloric restriction with low inflammation suggests a role of energy accumulation in the origin of the chronic inflammation. This point is enforced by recent advances in obesity research. Abundant literature on obesity suggests that chronic inflammation is a consequence of energy accumulation in the body. The emerging evidence strongly supports that the inflammatory response induces energy expenditure in a feedback manner to fight against energy surplus in obesity. If this feedback system is deficient (Inflammation Resistance), energy expenditure will be reduced and energy accumulation will lead to obesity. In this perspective, we propose that an increase in inflammation in obesity promotes energy expenditure with a goal to get rid of energy surplus. A decrease in inflammation under caloric restriction contributes to energy saving. Inflammation is a mechanism for energy balance in the body. Inflammation resistance will lead to obesity. We will review the recent literature in support of the viewpoints.
Keywords: Inflammation, caloric restriction, obesity, energy expenditure
Introduction
Caloric restriction (CR) reduces the levels of multiple aspects of inflammation [1-3], suggesting a link between energy status and inflammation. This linkage is enforced by recent progress in obesity research. Chronic inflammation is widely observed in obesity (metabolic syndrome). The obesity-associated inflammation is involved in pathogenesis of type 2 diabetes, hypertension, atherosclerosis, fatty liver, cancer metastasis, and asthma in obesity. Obesity has a higher prevalence in the aging population as a result of reduced energy expenditure with less physical activity. Physical activities consume a major portion of energy in our daily life, which are usually reduced in the aging population. This reduction in energy expenditure may lead to energy accumulation in the body and consequently a gain in adiposity. In obesity, systemic chronic inflammation occurs with elevated proinflammatory cytokines (IL-6, MCP-1, CRP, PAI-1, et al.) in the circulation. The systemic inflammation is due to an inflammatory response in adipose tissues that are under quick expansion. Adipocytes produce these cytokines. In addition, macrophage infiltration into the adipose tissue contributes significantly to the cytokine production. Although we have learned a lot about the signaling pathways that link energy accumulation (adiposity) to chronic inflammation, we know little about the real biological significance of the inflammation. This article addresses this issue, and provides an overview of the interaction of inflammation and energy balance.
1. Chronic inflammation from energy accumulation
In obesity research, the link between chronic inflammation and energy (fat) accumulation is well established. The initial observation of TNF-α elevation in adipose tissue of obese mice provides the first evidence for the chronic inflammation in 1993 by Hotamisligil and colleagues [4]. Thereafter, the concept was enforced by abundant literature identifying increases in many other inflammatory cytokines, such as plasma C-reactive protein (CRP), interleukin 6 (IL-6), plasminogen activator inhibitor-1 (PAI-1), in models of obesity. Activation of inflammatory kinases such as IKKβ (IkBα kinase beta) and JNK1 (c-Jun N-terminal kinase 1) provides additional evidence for activation of intracellular inflammatory pathways in obesity [5-6]. Obesity-associated inflammation is chronic, systemic, low-grade, and not linked to any infection. In contrast to inflammation induced by bacteria or virus infection where neutrophil granulocytes are elevated in the circulation, neutrophil granulocytes are not increased in blood in obesity. The inflammation is systemic since the inflammatory cytokines are increased in the circulation. The inflammation is at a low grade in obesity since there is no fever and malaise, which are often observed for inflammation associated with bacteria/viral infection.
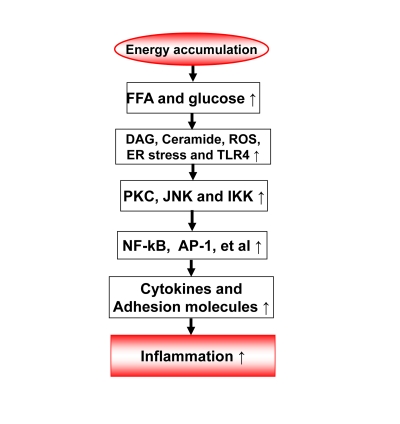
2. Inflammation origin: Energy accumulation may induce inflammation through metabolites of fatty acids and glucose (Figure 1)
Figure 1. Energy accumulation induces inflammation.
Energy accumulation leads to elevation in glucose and fatty acids. These substrates lead to production of diaglycerids (DAG), Ceramide, reactive oxygen species (ROS) and activation of toll-like receptor 4 (TLR4) in cells including macrophages and endothelial cells. All of these events may activate the inflammatory signaling pathways, such as IKK/NF-kB and JNK/AP-1. As a consequence, expression of inflammatory cytokines and adhesion molecules may increase for chronic local inflammation. When inflammatory cytokines are elevated in the circulation, the energy accumulation causes systemic chronic inflammation, which is observed in obesity. This kind of chronic inflammation is limited or prevented by calorie restriction
The metabolites of fatty acids and glucose include diaglyceride (DAG), Ceramide, and reactive oxygen species (Figure 1). They activate inflammatory response through several approaches. They may direct interact with signaling kinases (PKCs, JNKs and IKKs) in cells [7]. They may also act through cell membrane receptors for lipids, such as TLR4, CD36 or GPR [8-11]. The reactive oxygen species (ROS) are generated from fat or glucose oxidation in mitochondria. ROS may induce activation of the inflammatory kinases (JNK and IKK). The lipids also induce endoplasmic reticulum (ER) stress for activation of JNK and IKK [12-13]. In CR, these metabolites of glucose and fatty acids are reduced from less calorie intake. The risk of inflammation is reduced.
In obesity, adipose tissue is a major source of chronic inflammation [14-15]. In adipose tissue, adipocytes and adipose tissue macrophages (ATM) are the major cell types responsible for the production of inflammatory cytokines. The representative cytokines include TNF-α, IL-6, MCP-1 and PAI-1. Adipokines (Leptin and adiponectin) are produced by adipocytes and also involved in the regulation of inflammation. Macro-phages and adipocytes are activated during the process of adipose tissue expansion. Recent studies suggest that the adipose tissue expansion induces a local hypoxia response [16]. The hypoxia response serves as a common root for all of the stress responses in adipose tissue, such as oxidative stress, ER stress, and inflammatory stress [17-19]. Hypoxia directly promotes the chronic inflammation through activation of transcription factors (NF-kB and HIF-1) in adipocytes and macrophages [16]. The hypoxia response is a result of tissue expansion. In CR, adipose tissue expansion is reduced or under controlled. The risk factors for inflammation, such as adipose tissue hypoxia, lipid accumulation, ER stress and oxidative stress are all reduced or absent. These may explain why CR reduces the risk for chronic inflammation in the body.
3. Inflammation feedback to energy accumulation
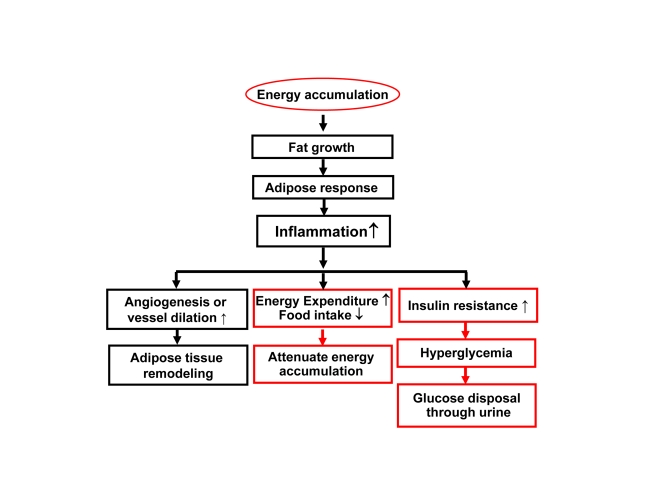
The inflammation observed in adipose tissue likely serves as a feedback signal locally in adipose tissue and systemically for energy expenditure (Figure 2). In adipose tissue, inflammation inhibits adipocyte expansion and adipocyte differentiation, changes adipocyte endocrine and induces extracellular matrix remodeling [20]. The local response is translated into a systemic response through cytokines and free acids released from adipose tissue.
Figure 2. Inflammation in obesity.
Rapid growth of adipose tissue leads to quick expansion of adipose tissue. When angiogenesis or vessel dilation can not meet the demand for blood supply, there will be an adipose tissue hypoxia (ATH) from lack of blood supply. ATH will induce angiogenesis and trigger inflammation. Inflammation will promote angiogenesis and vasodilation locally in the tissue for extracellular remodeling. When inflammatory cytokines and fatty acids are elevated in the circulation, they will promote energy expenditure systemically. The inflammatory response may also induce hyperglycemia and energy disposal through glucose excretion in urine. In this way, inflammation acts through insulin resistance and hyperglycemia.
(a) Adipocyte inhibition. A major function of adipocytes is to store fat. In addition, the adipocytes secrete many cytokines/hormones in its endocrine activity. Inflammatory cytokines inhibit adipocyte function in multiple aspects. These include inhibition of preadipocyte differentiation, induction of lipolysis and suppression of adiponectin expression in mature adipocytes. These inhibitory activities are well documented for TNF-α and IL-1 [21-23]. At the molecular level, inflammation inhibits insulin signaling pathway [24-26] and PPARγ activities in adipocytes [27]. These effects contribute to suppression of tissue expansion, and alteration in cytokine profile. The disorders in lipid metabolism and cytokine balance contribute to the whole body insulin resistance, a result of impaired insulin signaling in multiple organs (skeletal muscle, liver, and adipose tissue) [28-30]. Insulin resistance may induce hyperglycemia, which in turn leads to glucose excretion through urine (type 2 diabetes). The type 2 diabetes is an extreme condition in the body to get ride of energy surplus in an effort to prevent energy accumulation in the body.
(b) Adipose tissue remodeling: Macrophage infiltration is a major marker of local inflammation in the adipose tissue in obesity. Adipose tissue macrophages (ATM) have been under active investigation since 2004 when macrophage infiltration was initially identified in obese mice [31-34]. The discovery provides a source for TNF-α in adipose tissue since mature adipocytes produces very little TNF-α [31-34]. The biological significance of macrophage infiltration remains to be elucidated. However, more and more evidence suggests that macrophages are required for adipose tissue remodeling and adipogenesis of preadipocytes. Macrophages may serve as a signal amplifier in the adipose tissue for stimulation of angiogenesis [35]. Macrophages produce many angiogenic factors, such as PDGF, TGF-β and HGF, which are increased in adipose tissue in obese individuals [36-37]. Interestingly, this activity of macrophages is required for adipose tissue growth in lean mice [38-39] and obese mice [35]. Macrophages may also regulate blood flow through production of vasodilators (such as NO). Macrophages may clean the cell debris of dead adipocytes within the adipose tissue [40]. An increase in adipocyte death was reported in the adipose tissue of obese mice, and the dead cells were surrounded by ATMs to form the "Crown" like structure [40-41]. The cell death in adipose tissue may be a result of the hypoxia response [42]. In CR, the adipose tissue expansion is under control, there are not such risk factors for macrophage activation in adipose tissue.
(c) Fuel mobilization. Inflammation regulates fuel mobilization. Fuel (fatty acids) mobilization from adipose tissue to other tissues is controlled by the nervous system and hormones/cytokines. The role of inflammatory cytokines has drawn a lot of attention in the fuel mobilization. Cytokines such as TNF-α, IL-1, IL-6, et al., activate fuel efflux in adipocytes through lipolysis, in which free fatty acids (FFAs) are generated from triglycerides under hydrolysis and released into blood stream. FFAs are normally oxidized in mitochondria for ATP production. An increase in FFA supply may lead to acceleration of energy expenditure. However, when FFA supply overrides the consumption, they deposit in non-adipocytes in the form of ectopic fat deposition. The ectopic fat contributes to pathogenesis of fatty liver disease and atherosclosis (deposit on the blood vessel wall). In the physiological conditions, IL-6 secreted by contracting muscle is involved in coordination of fuel mobilization between adipose tissue and skeletal muscle during exercise [43-44]. In CR, the fatty acid supply is limited as a result of reduced calorie intake, the risk for ectopic fat deposition will be reduced. This may help in prevention of fatty liver and atherosclosis.
(d) Energy intake. Inflammatory cytokines are involved in the regulation of energy intake and expenditure. IL-1 and IL-6 reduces food intake and prevent hyperphagia [45-46]. Cytokines (IL-1, IL-6 and TNF-α) also induce energy expenditure [46-50]. These activities of cytokines are dependent on their actions in the central nervous system [46-47,51-52]. Therefore, inflammatory cytokines may serve as an anti-obesity signal by modifying both energy intake and energy expenditure. Additionally, these data indicate that the inflammatory cytokines may serve as a link between peripheral tissues and central nervous system in the control of energy balance.
4. Energy expenditure by inflammation
The activities of inflammatory cytokines on adipocytes and neurons suggest that inflammation may inhibit energy accumulation. They induce energy expenditure and inhibits food intake. These possibilities are strongly supported by phenotypes of transgenic mice with chronic inflammation and by cytokine infusion studies. Transgenic mice of IKK2/NF-kB have provided new evidence.
The IKK2/NF-kB pathway is a dominant inflammation signaling pathway. The pathway has been under active investigation in the obesity field after IKKβ was found to induce insulin resistance in obese mice [5]. The serine kinase IKK has three major isoforms including IKKα (IKK1), IKKβ (IKK2) and IKKγ, in which IKKβ is required for NF-kB activation [53]. In obesity, IKKβ is activated by several intracellular signals, such as ROS, ER stress, DAG, and Ceramide. IKKβ is also activated by the extracellular stimuli including TNF-α, IL-1, and fatty acids [8], and hypoxia [54]. IKKβ induces NF-kB activation by phosphorylation of the Inhibitor Kappa B alpha (IkBα) [55].
NF-kB (nuclear factor kappa B) is a ubiquitous transcription factor that is formed by two subunits of Rel family, which include seven members, p65 (RelA), p50 (NF-kB1), c-Rel, RelB, p100, p105, p52 [56]. These members form a homodimer or heterodimer in the regulation of gene transcription. In most case, NF-kB is a heterodimer of p65 and p50. P65 contains the transactivation domain and mediates the transcriptional activity of NF-kB. P50 usually inhibits the transcriptional activity of p65 [57], and the inhibition disappears in the NF-kB p50 knockout mice [58]. In the classical pathway, NF-kB activation is mediated by IKKβ-induced phosphorylation, proteasome-mediated degradation of IkBα [53]. In response to stress responses, NF-kB promotes lipid mobilization through suppression of PPARγ activity in the nucleus [59]. It also induces transcription of inflammatory cytokines (TNF-α, IL-1, IL-6, MCP-1, et al.). In the alternative pathway, NF-kB is activated by hypoxia in the absence of IkBα degradation. This type of NF-kB activation in adipocytes and macrophages contributes to chronic inflammation in the adipose tissue of obese individuals [16].
NF-kB activity may promote energy expenditure. This activity of NF-kB is supported by documents on energy expenditure in cachexia [60-61] and infection. However, the role of NF-kB in energy expenditure was not tested in transgenic models. To this point, we investigated energy metabolism in transgenic mice with elevated NF-kB activities. The transcriptional activity of NF-kB is enhanced either by over-expression of NF-kB p65 (RelA) in the fat tissue, or inactivation of NF-kB p50 (NF-kB1) by global gene knockout [65]. In these two models, inflammatory cytokines (TNF-α and IL-6) were elevated in blood and energy expenditure was increased in day and night [65]. The oxygen consumption and CO2 production were both increased in the mice. Locomotion was not altered, but food intake was increased in the mice. Expression of inflammatory cytokines (TNF-α and IL-6) was elevated in adipose tissue and macrophages. On a high fat diet (HFD), both lines of transgenic mice were protected from obesity and insulin resistance [65-66]. The data suggests that the transcription factor NF-kB promotes energy expenditure and inhibits energy accumulation. The inflammatory cytokines may mediate the NF-kB activity in energy expenditure. In the mice, lipid accumulation is prevented by the enhanced energy expenditure. The studies suggest that inflammation may prevent insulin resistance by eliminating lipid accumulation. IKKβ was investigated in the control of insulin sensitivity [5,62-63] and food intake in transgenic mice [64]. However, IKKβ was not investigated in the control of energy expenditure in these studies.
NF-kB may promote energy expenditure through the inflammatory cytokines. In the two transgenic models, systemic inflammation was observed with elevated proteins for TNF-α and IL-6 in the serum [65-66]. Expression of TNF-α and IL-1 mRNA was increased in adipose tissue and macrophages. These cytokines are positively associated with energy expenditure in the body [61]. In transgenic mice with deficiency in these cytokines or their receptors, energy accumulation is enhanced, suggesting a reduction in energy expenditure. This positive energy balance was reported in transgenic mice with deficiency in TNF-α [50], IL-1 [45] or IL-6 [46]. On the other side, when these cytokine activities are enhanced in transgenic mice, energy accumulation is decreased leading to a lean phenotype [48-49,67-68]. The cytokines may act in the hypothalamus of central nervous system to regulate the energy balance [46-47,51-52]. In addition to the central mechanism, activation of mitochondria by the cytokines in the peripheral tissues may also contribute to the energy expenditure. TNF-α and IL-1 enhances mitochondrial function through phosphorylation-mediated activation of PGC-1α [69]. This activity of inflammatory cytokines may contribute to energy consumption in mitochondria-enriched tissues/organs such as liver, skeletal muscle and brown fat. Inflammation may be a drug target in the management of energy metabolism [70-71].
5. CR and chronic inflammation
Studies have demonstrated that CR decreases the circulating levels of inflammatory cytokines and inflammatory signaling activities in a wide variety of tissues [1-3]. CR is able to decrease global levels of inflammatory responses in the body. Interestingly, the beneficial effects of CR may be related to a decrease in visceral fat and adipose reactivity [3,72]. It has been documented that adiposity during aging contributes to a number of morbidity factors including insulin resistance, dyslipidemia, atherosclerosis, hypercoagula-bility and hypertension [73-74]. However, it is important to remember that the most inflammation data are derived from the visceral fat and ectopic fat [72-74]. For example, subcutaneous fat has been observed to have beneficial effects on lipid and energy homeostasis, and even counteract the negative effects of visceral adipose tissue [75]. It is important to note that CR has beneficial effects in non-obese humans as well as non-obese rodents [76-77], indicating that decreased adiposity may not be the only mediator of beneficial effects of CR. This fact suggests that a decrease in energy accumulation is more important in the control of inflammation since this may apply to both obese and non-obese conditions.
Summary
Energy accumulation induces chronic inflammation. This view is supported by data from many model systems of CR and obesity. Inflammation may promote energy expenditure in a regulatory-feedback manner to fight against energy surplus (Figure 2). This concept extends our understanding of biological significance of inflammation in obesity. It also helps us to understand why CR reduces inflammation. The inflammation may act in the peripheral organs/tissues as well as in the central nervous system to regulate energy balance. In the peripheral, inflammation induces fat mobilization and oxidation to promote energy expenditure. Inflammation may induce energy disposal through glucose excretion in urine as a result of insulin resistance and hyperglycemia. In the central, inflammation may inhibit food intake and activate neurons for energy expenditure. If this feedback system is deficient, energy expenditure will be interrupted and fat will be accumulated in the body for adiposity. We call this condition of "Inflammation Resistance". In CR, the energy accumulation is prevented. In turn, the risk factors for the chronic inflammation are limited. In our view, the low inflammation serves as a mechanism for energy saving in CR.
Acknowledgments
This study is supported by NIH fund (R56DK068036-6) and ADA research award (7-07-RA-189) to Ye J.
Footnotes
The authors of this manuscript have no conflict of interests to declare.
References
- 1.Fontana L. Neuroendocrine factors in the regulation of inflammation: excessive adiposity and calorie restriction. Exp Gerontol. 2009;44:41–45. doi: 10.1016/j.exger.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morgan TE, Wong AM, Finch CE. Anti-inflammatory mechanisms of dietary restriction in slowing aging processes. Interdiscip Top Gerontol. 2007;35:83–97. doi: 10.1159/000096557. [DOI] [PubMed] [Google Scholar]
- 3.Dixit VD. Adipose-immune interactions during obesity and caloric restriction: reciprocal mechanisms regulating immunity and health span. J Leukoc Biol. 2008;84:882–892. doi: 10.1189/jlb.0108028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 5.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of Obesity- and Diet-Induced Insulin Resistance with Salicylates or Targeted Disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 6.Hirosumi J, Tuncman G, Chang L, Gorgun C, Uysal K, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 7.Brose N, Rosenmund C. Move over protein kinase C, you've got company: alternative cellular effectors of diacylglycerol and phorbol esters. J Cell Sci. 2002;115:4399–4411. doi: 10.1242/jcs.00122. [DOI] [PubMed] [Google Scholar]
- 8.Lee JY, Ye J, Gao Z, Youn HS, Lee WH, Zhao L, Sizemore N, Hwang DH. Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J Biol Chem. 2003;278:37041–37051. doi: 10.1074/jbc.M305213200. [DOI] [PubMed] [Google Scholar]
- 9.Weigert C, Brodbeck K, Staiger H, Kausch C, Machicao F, Haring HU, Schleicher ED. Palmitate, but not unsaturated fatty acids, induces the expression of interleukin-6 in human myotubes through proteasome-dependent activation of nuclear factor kappa B. J Biol Chem. 2004;279:23942–23952. doi: 10.1074/jbc.M312692200. [DOI] [PubMed] [Google Scholar]
- 10.Gao Z, Zhang X, Zuberi A, Hwang D, Quon MJ, Lefevre M, Ye J. Inhibition of Insulin Sensitivity by Free Fatty Acids Requires Activation of Multiple Serine Kinases in 3T3-L1 Adipocytes. Mol Endocrinol. 2004;18:2024–2034. doi: 10.1210/me.2003-0383. [DOI] [PubMed] [Google Scholar]
- 11.Costanzi S, Neumann S, Gershengorn MC. Seven Transmembrane-spanning Receptors for Free Fatty Acids as Therapeutic Targets for Diabetes Mellitus: Pharmacological, Phylogenetic, and Drug Discovery Aspects. J Biol Chem. 2008;283:16269–16273. doi: 10.1074/jbc.R800014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozcan U, Cao Q, Yilmaz E, Lee A-H, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic Reticulum Stress Links Obesity, Insulin Action, and Type 2 Diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura T, Furuhashi M, Li P, Cao H, Tuncman G, Sonenberg N, Gorgun CZ, Hotamisligil GS. Double-Stranded RNA-Dependent Protein Kinase Links Pathogen Sensing with Stress and Metabolic Homeostasis. Cell. 2010;140:338–348. doi: 10.1016/j.cell.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 15.Halberg N, Wernstedt-Asterholm I, Scherer PE. The adipocyte as an endocrine cell. Endocrinol Metab Clin North Am. 2008;37:753–768, x-xi. doi: 10.1016/j.ecl.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye J, Gao Z, Yin J, He H. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab. 2007;293:E1118–E1128. doi: 10.1152/ajpendo.00435.2007. [DOI] [PubMed] [Google Scholar]
- 17.Ye J. Emerging Role of Adipose Tissue Hypoxia in Obesity and Insulin Resistance. Int J Obes. 2009;33:54–66. doi: 10.1038/ijo.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–911. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 19.Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJ, Brekken RA, Scherer PE. HIF 1 alpha Induces Fibrosis and Insulin Resistance in White Adipose Tissue. Mol Cell Biol. 2009;29:4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S, Scherer PE. Metabolic Dysregulation and Adipose Tissue Fibrosis: Role of Collagen VI. Mol Cell Biol. 2009;29:1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xing H, Northrop JP, Grove JR, Kilpatrick KE, Su JL, Ringold GM. TNF alpha-mediated inhibition and reversal of adipocyte differentiation is accompanied by suppressed expression of PPARgamma without effects on Pref-1 expression. Endocrinology. 1997;138:2776–2783. doi: 10.1210/endo.138.7.5242. [DOI] [PubMed] [Google Scholar]
- 22.Ruan H, Hacohen N, Golub TR, Van Parijs L, Lodish HF. Tumor necrosis factor-alpha suppresses adipocyte-specific genes and activates expression of preadipocyte genes in 3T3-L1 adipocytes: nuclear factor-kappaB activation by TNF-alpha is obligatory. Diabetes. 2002;51:1319–1336. doi: 10.2337/diabetes.51.5.1319. [DOI] [PubMed] [Google Scholar]
- 23.Suzawa M, Takada I, Yanagisawa J, Ohtake F, Ogawa S, Yamauchi T, Kadowaki T, Takeuchi Y, Shibuya H, Gotoh Y, Matsumoto K, Kato S. Cytokines suppress adipogenesis and PPAR-gamma function through the TAK1/TAB1/NIK cascade. Nat Cell Biol. 2003;5:224–230. doi: 10.1038/ncb942. [DOI] [PubMed] [Google Scholar]
- 24.Guo D, Donner DB. Tumor necrosis factor promotes phosphorylation and binding of insulin receptor substrate 1 to phosphatidylinositol 3-kinase in 3T3-L1 adipocytes. J Biol Chem. 1996;271:615–618. doi: 10.1074/jbc.271.2.615. [DOI] [PubMed] [Google Scholar]
- 25.Feinstein R, Kanety H, Papa MZ, Lunenfeld B, Karasik A. Tumor necrosis factor-alpha suppresses insulin-induced tyrosine phosphorylation of insulin receptor and its substrates. J Biol Chem. 1993;268:26055–26058. [PubMed] [Google Scholar]
- 26.Zhang J, Gao Z, Yin J, Quon MJ, Ye J. S6K Directly Phosphorylates IRS-1 on Ser270 to Promote Insulin Resistance in Response to TNF-α Signaling Through IKK2. J Biol Chem. 2008;283:35375–35382. doi: 10.1074/jbc.M806480200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye J. Regulation of PPARg function by TNF-a. Biochem Biophys Res Commun. 2008;374:405–408. doi: 10.1016/j.bbrc.2008.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hotamisligil GS. Mechanisms of TNF-alpha-induced insulin resistance. Exp Clin Endocrinol Diabetes. 1999;107:119–125. doi: 10.1055/s-0029-1212086. [DOI] [PubMed] [Google Scholar]
- 29.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 30.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 31.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Gregorio GB, Yao-Borengasser A, Rasouli N, Varma V, Lu T, Miles LM, Ranganathan G, Peterson CA, McGehee RE, Kern PA. Expression of CD68 and Macrophage Chemoattractant Protein-1 Genes in Human Adipose and Muscle Tissues: Association With Cytokine Expression, Insulin Resistance, and Reduction by Pioglitazone. Diabetes. 2005;54:2305–2313. doi: 10.2337/diabetes.54.8.2305. [DOI] [PubMed] [Google Scholar]
- 34.Fain JN. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam Horm. 2006;74:443–477. doi: 10.1016/S0083-6729(06)74018-3. [DOI] [PubMed] [Google Scholar]
- 35.Pang C, Gao Z, Yin J, Zhang J, Jia W, Ye J. Macrophage Infiltration into Adipose Tissue May Promote Angiogenesis for Adipose Tissue Remodeling in Obesity. Am J Physiol Endocrinol Metab. 2008;295:E313–E322. doi: 10.1152/ajpendo.90296.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lijnen HR. Angiogenesis and obesity. Cardiovasc Res. 2008;78:286–293. doi: 10.1093/cvr/cvm007. [DOI] [PubMed] [Google Scholar]
- 37.Christiaens V, Lijnen HR. Angiogenesis and development of adipose tissue. Mol Cell Endocrinol. 2010;318:2–9. doi: 10.1016/j.mce.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H, Ohsugi M, Tobe K, Kadowaki T, Nagai R, Sugiura S. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes. 2007;56:1517–1526. doi: 10.2337/db06-1749. [DOI] [PubMed] [Google Scholar]
- 39.Cho C-H, Jun Koh Y, Han J, Sung H-K, Jong Lee H, Morisada T, Schwendener RA, Brekken RA, Kang G, Oike Y, Choi T-S, Suda T, Yoo O-J, Koh GY. Angiogenic Role of LYVE-1-Positive Macrophages in Adipose Tissue. Circ Res. 2007;100:e47–57. doi: 10.1161/01.RES.0000259564.92792.93. [DOI] [PubMed] [Google Scholar]
- 40.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW 2nd, DeFuria J, Jick Z, Greenberg AS, Obin MS. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 42.Yin J, Gao Z, He Q, Ye J. Role of hypoxia in obesity-induced disorders of glucose and lipid metabolism in adipose tissue. Am J Physiol Endocrinol Metab. 2009;296:E333–E342. doi: 10.1152/ajpendo.90760.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pedersen BK. IL-6 signalling in exercise and disease. Biochem Soc Trans. 2007;35:1295–1297. doi: 10.1042/BST0351295. [DOI] [PubMed] [Google Scholar]
- 44.Hoene M, Weigert C. The role of interleukin-6 in insulin resistance, body fat distribution and energy balance. Obesity Reviews. 2008;9:20–29. doi: 10.1111/j.1467-789X.2007.00410.x. [DOI] [PubMed] [Google Scholar]
- 45.Chida D, Osaka T, Hashimoto O, Iwakura Y. Combined interleukin-6 and interleukin-1 deficiency causes obesity in young mice. Diabetes. 2006;55:971–977. doi: 10.2337/diabetes.55.04.06.db05-1250. [DOI] [PubMed] [Google Scholar]
- 46.Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- 47.Anforth HR, Bluthe RM, Bristow A, Hopkins S, Lenczowski MJ, Luheshi G, Lundkvist J, Michaud B, Mistry Y, Van Dam AM, Zhen C, Dantzer R, Poole S, Rothwell NJ, Tilders FJ, Wollman EE. Biological activity and brain actions of recombinant rat interleukin-1alpha and interleukin-1beta. Eur Cytokine Netw. 1998;9:279–288. [PubMed] [Google Scholar]
- 48.Garcia MC, Wernstedt I, Berndtsson A, Enge M, Bell M, Hultgren O, Horn M, Ahren B, Enerback S, Ohlsson C, Wallenius V, Jansson JO. Mature-onset obesity in interleukin-1 receptor I knockout mice. Diabetes. 2006;55:1205–1213. doi: 10.2337/db05-1304. [DOI] [PubMed] [Google Scholar]
- 49.Xu H, Hirosumi J, Uysal KT, Guler AD, Hotamisligil GS. Exclusive action of transmembrane TNF alpha in adipose tissue leads to reduced adipose mass and local but not systemic insulin resistance. Endocrinology. 2002;143:1502–1511. doi: 10.1210/endo.143.4.8715. [DOI] [PubMed] [Google Scholar]
- 50.Pamir N, McMillen TS, Kaiyala KJ, Schwartz MW, LeBoeuf RC. Receptors for Tumor Necrosis Factor-{alpha} Play a Protective Role against Obesity and Alter Adipose Tissue Macrophage Status. Endocrinology. 2009;150:4124–4134. doi: 10.1210/en.2009-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luheshi GN. Cytokines and fever. Mechanisms and sites of action. Ann N Y Acad Sci. 1998;856:83–89. doi: 10.1111/j.1749-6632.1998.tb08316.x. [DOI] [PubMed] [Google Scholar]
- 52.Klir JJ, Roth J, Szelenyi Z, McClellan JL, Kluger MJ. Role of hypothalamic interleukin-6 and tumor necrosis factor-alpha in LPS fever in rat. Am J Physiol. 1993;265:R512–517. doi: 10.1152/ajpregu.1993.265.3.R512. [DOI] [PubMed] [Google Scholar]
- 53.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 54.Cummins EP, Berra E, Comerford KM, Ginouves A, Fitzgerald KT, Seeballuck F, Godson C, Nielsen JE, Moynagh P, Pouyssegur J, Taylor CT. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc Natl Acad Sci U S A. 2006;103:18154–18159. doi: 10.1073/pnas.0602235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hacker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 2006;2006:re13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- 56.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 57.Schmitz ML, Baeuerle PA. The p65 subunit is responsible for the strong transcription activating potential of NF-kappa B. EMBO J. 1991;10:3805–3817. doi: 10.1002/j.1460-2075.1991.tb04950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bohuslav J, Kravchenko VV, Parry GCN, Erlich JH, Gerondakis S, Mackman N, Ulevitch RJ. Regulation of an Essential Innate Immune Response by the p50 Subunit of NF-kappa B. J Clin Invest. 1998;102:1645–1652. doi: 10.1172/JCI3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao Z, He Q, Peng B, Chiao PJ, Ye J. Regulation of Nuclear Translocation of HDAC3 by I{kappa}B{alpha} Is Required for Tumor Necrosis Factor Inhibition of Peroxisome Proliferator-activated Receptor {gamma} Function. J Biol Chem. 2006;281:4540–4547. doi: 10.1074/jbc.M507784200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strasser F. Appraisal of current and experimental approaches to the treatment of cachexia. Curr Opin Support Palliat Care. 2007;1:312–316. doi: 10.1097/SPC.0b013e3282f3474c. [DOI] [PubMed] [Google Scholar]
- 61.Tisdale MJ. Biology of cachexia. J Natl Cancer Inst. 1997;89:1763–1773. doi: 10.1093/jnci/89.23.1763. [DOI] [PubMed] [Google Scholar]
- 62.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 64.Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKK[beta]/NF-[kappa]B and ER Stress Link Overnutrition to Energy Imbalance and Obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tang T, Zhang J, Yin J, Staszkiewicz J, Gawronska-Kozak B, Mynatt R, Martin RJ, Keenan M, Gao Z, Ye J. Uncoupling of Inflammation and Insulin Resistance by NF-kB in Transgenic Mice through Induction of Energy Expenditure. J Biol Chem. 2010;285:4637–4644. doi: 10.1074/jbc.M109.068007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gao Z, Yin J, Zhang J, He Q, McGuinness OP, Ye J. Inactivation of NF-kB P50 Leads to Insulin Sensitization in liver through Post-Translational Inhibition of p70S6K. J Biol Chem. 2009;284:18368–18376. doi: 10.1074/jbc.M109.007260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matsuki T, Horai R, Sudo K, Iwakura Y. IL-1 Plays an Important Role in Lipid Metabolism by Regulating Insulin Levels under Physiological Conditions. J Exp Med. 2003;198:877–888. doi: 10.1084/jem.20030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Somm E, Henrichot E, Pernin A, Juge-Aubry CE, Muzzin P, Dayer JM, Nicklin MJ, Meier CA. Decreased fat mass in interleukin-1 receptor antagonist-deficient mice: impact on adipogenesis, food intake, and energy expenditure. Diabetes. 2005;54:3503–3509. doi: 10.2337/diabetes.54.12.3503. [DOI] [PubMed] [Google Scholar]
- 69.Puigserver P, Rhee J, Lin J, Wu Z, Yoon JC, Zhang CY, Krauss S, Mootha VK, Lowell BB, Spiegelman BM. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARgamma coactivator-1. Mol Cell. 2001;8:971–982. doi: 10.1016/s1097-2765(01)00390-2. [DOI] [PubMed] [Google Scholar]
- 70.von Haehling S, Genth-Zotz S, Anker SD, Volk HD. Cachexia: a therapeutic approach beyond cytokine antagonism. Int J Cardiol. 2002;85:173–183. doi: 10.1016/s0167-5273(02)00245-0. [DOI] [PubMed] [Google Scholar]
- 71.Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS Jr. NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science. 2000;289:2363–2366. doi: 10.1126/science.289.5488.2363. [DOI] [PubMed] [Google Scholar]
- 72.Muzumdar R, Allison DB, Huffman DM, Ma X, Atzmon G, Einstein FH, Fishman S, Poduval AD, McVei T, Keith SW, Barzilai N. Visceral adipose tissue modulates mammalian longevity. Aging Cell. 2008;7:438–440. doi: 10.1111/j.1474-9726.2008.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barzilai N, Banerjee S, Hawkins M, Chen W, Rossetti L. Caloric restriction reverses hepatic insulin resistance in aging rats by decreasing visceral fat. J Clin Invest. 1998;101:1353–1361. doi: 10.1172/JCI485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gabriely I, Ma XH, Yang XM, Atzmon G, Rajala MW, Berg AH, Scherer P, Rossetti L, Barzilai N. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process. Diabetes. 2002;51:2951–2958. doi: 10.2337/diabetes.51.10.2951. [DOI] [PubMed] [Google Scholar]
- 75.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7:410–420. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith JV, Heilbronn LK, Ravussin E. Energy restriction and aging. Curr Opin Clin Nutr Metab Care. 2004;7:615–622. doi: 10.1097/00075197-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 77.Redman LM, Martin CK, Williamson DA, Ravussin E. Effect of caloric restriction in non-obese humans on physiological, psychological and behavioral outcomes. Physiol Behav. 2008;94:643–648. doi: 10.1016/j.physbeh.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]