Abstract
There is still controversy in the literature whether a single episode of mild traumatic brain injury (MTBI) results in short-term functional and/or structural deficits as well as any induced long-term residual effects. With the inability of traditional structural brain imaging techniques to accurately diagnosis MTBI, there is hope that more advanced applications like functional magnetic resonance imaging (fMRI) and diffusion tensor imaging (DTI) will be more specific in diagnosing MTBI. In this study, 15 subjects who have recently suffered from sport-related MTBI and 15 age-matched normal controls underwent both fMRI and DTI to investigate the possibility of traumatic axonal injury associated with functional deficits in recently concussed but asymptomatic individuals. There are several findings of interest. First, MTBI subjects had a more disperse brain activation pattern with additional increases in activity outside of the shared regions of interest (ROIs) as revealed by FMRI blood oxygen level–dependent (BOLD) signals. The MTBI group had additional activation in the left dorsal-lateral prefrontal cortex during encoding phase of spatial navigation working memory task that was not observed in normal controls. Second, neither whole-brain analysis nor ROI analysis showed significant alteration of white matter (WM) integrity in MTBI subjects as evidenced by fractional anisotropy FA (DTI) data. It should be noted, however, there was a larger variability of fractional anisotropy (FA) in the genu, and body of the corpus callosum in MTB subjects. Moreover, we observed decreased diffusivity as evidenced by apparent diffusion coefficient (ADC) at both left and right dorsolateral prefrontal cortex (DL-PFC) in MTBI subjects (P < 0.001). There was also a positive correlation (P < 0.05) between ADC and % change of fMRI BOLD signals at DL-PFC in MTBI subjects, but not in normal controls. Despite these differences we conclude that overall, no consistent findings across advanced brain imaging techniques (fMRI and DTI) were observed. Whether the lack of consistency across research techniques (fMRI & DTI) is due to time frame of scanning, unique nature of MTBI and/or technological issues involved in FA and Apparent Diffusion Coefficient (ADC) quantification is yet to be determined.
Keywords: Mild traumatic brain injury (MTBI), Functional magnetic resonance imaging (FMRI), Diffusion tensor imaging (DTI), Fractional Anisotropy (FA), White matter (WM)
Introduction
Mild traumatic brain injury (MTBI), otherwise known as concussion (Shaw 2002; Cantu 2006) is the most common head injury seen in athletics as well as in other activities of daily life. It can occur in a myriad of different situations including but not limited to athletic events, recreational outings, and transportation accidents where the brain accelerates and/or decelerates differentially in the skull. The consequences of MTBI are not clear, although current findings suggest the possibilities of long-term functional deficits in concussed individuals (van der Naalt et al. 1999; Slobounov et al. 2007, 2008, 2009). For example, 30% of MTBI patients with acute post-traumatic amnesia in van der Naalt et al. (1999) study have persistent symptoms 1 year post-injury. It should be noted that all of these patients appeared to be asymptomatic based upon conventional clinical structural imaging techniques [e.g., magnetic resonance imaging (MRI) and computed tomography (CT)].
It has been challenging to establish clear links between the observable neuropsychological and behavioral symptoms of MTBI along with underlying structural and/or functional deficits based upon current clinical brain imaging techniques (see Schrader et al. 2009 for review). Sporadic evidence suggests, however, a variety of functional deficits in concussed individuals that correlate with brain imaging (fMRI) data (Ptito et al. 2007). McAllister et al. (2001) have shown enhanced and diffused activation primarily in the pre-frontal cortex (PFC) of concussed subjects who successfully performed the cognitive tasks. Similarly, Jantzen et al. (2004) observed increased activation in the parietal frontal and cerebellar regions in concussed individuals when compared with pre-injury fMRI data although there were no changes in the subjects’ cognitive performance. In contrast, Chen et al. (2004) reported the opposite fMRI findings suggesting a reduction in the BOLD signal in the mid-dorsolateral prefrontal cortex (DL-PFC) in symptomatic concussed athletes who also showed poor performance on the working memory tasks. Although the nature of these discrepancies is beyond the scope of this report, it should be noted that one of the possible reasons for these differences is variation in subjects’ inclusion criteria and subject performance.
Presently, there is a growing interest in newer imaging techniques, such as diffusion tensor imaging (DTI), which may be more sensitive to the diffuse white matter damage that often occurs in MTBI (Levin 2003). Since water molecules diffuse more easily parallel to myelinated axons compared to the perpendicular direction, DTI can use this property to map the underlying axonal connections in the brain. One important measure is that of fractional anisotropy (FA) which represents the ratio of water molecules diffusing in parallel as opposed to perpendicular directions and thus reflects organized white matter bundles. Tears in these axon bundles from trauma may result in differential FA ratios. Thus, DTI and the FA ratio appears to be a promising tool to detect diffuse axonal injury (DAI), which is one of the serious consequences of TBI, induced by sudden acceleration/deceleration and/or rotational/vibrational forces causing a shearing of nerve fibers (cf. Singh et al. 2010). The other common measure of DTI is that radial diffusivity (RD) which denotes the extent of diffusion that perpendicular to the direction of maximal diffusivity, which presumably includes diffusion via intracellular and extracellular space perpendicular to the predominant orientation of the axons (Wilde et al. 2008). The apparent diffusion coefficient (ADC) is the overall average measure of diffusion. Diffusion anisotropy, although varies across WM regions, likely reflecting the differences in cellular membrane integrity, fiber myelination, fiber diameter, and directionality (Bigler and Bazarian 2010). Currently, FA and ADC are considered proxies for white matter integrity (Alexander et al. 2007).
That said, it should be noted, however, that DTI alterations may reflect not only white matter integrity but also other aspects and physiological mechanisms not fully understood yet. For example, it has been recently reported that reduced FA values were found in the corticospinal track in professional musicians (Imfeld et al. 2009). This suggests that FA is associated with increased motor proficiency and reflects long-term experience. Similarly, the pattern of FA alteration in the fasciculus arcuatus in absolute pitch musicians does not fit into the simple dichotomy that reduced FA and increased ADC may solely reflect white matter integrity (Oechslin et al. 2010). In addition, it was shown that FA values in CC may reflect primarily fiber density rather than the degree of myelination or axon diameter. Overall, alteration of DTI values may reflect a variety of different, yet poorly understood physiological mechanisms and differentially reflect short and long-term changes.
However, in terms of short-term changes, DTI has evolved in recent years as a valuable complementary technique to investigate traumatic axonal injury (Inglese et al. 2005; Rutgers et al. 2008; Basser 1995). Indeed, DTI tractography, which creates three-dimensional maps of axonal connections, provides a mechanism to localize and quantify pathways affected by the injury (Singh et al. 2010).
There are a number of recent DTI studies indicating the reduction of FA and increase of apparent diffusion coefficient (ADC) in acute TBI (Arfanakis et al. 2002; Inglese et al. 2005; Rutgers et al. 2008). Significant reduction in FA values has been observed in the corpus callosum in subjects suffering from severe TBI, while changes were not significant in subjects suffering from MTBI (Rutgers et al. 2008). In contrast, it has also been reported that FA values may significantly increase in acute MTBI patients within 24 h (Wilde et al. 2008). Overall, it should be noted that most of the DTI studies revealed alterations of FA and ADC in patients suffering from moderate-to-severe TBI. Moreover, the history of previous TBI and detailed information regarding the subjects inclusion criteria have rarely been reported in the aforementioned DTI studies that may account for the discrepant findings.
Our fMRI portion of this study (Slobounov et al. 2010), which is consistent with the majority of previous reports, indicates increased neural activity and more elaborate neural networks with additional recruitment of dorso-lateral prefrontal cortex (DL-PFC) and cerebellum in clinical sample of subjects performing spatial navigation tasks (see “Results” section for details). We hypothesized that these alterations reflect the functional compensatory mechanisms substituting for either structural/functional disruption of the brain default network or deficits within some local regions in this network resulting from mild TBI. Since brain functions are realized by neural networks, and functional deficits after TBI are believed to indicate traumatic axonal injury (Sugiyama et al. 2009), it is feasible to anticipate the alteration of structural integrity within white matter that parallel the alteration of BOLD signal in concussed individuals. Surprisingly, few studies examining concussion have reported both FMRI and DTI changes in the same population. Given that our population of concussed individuals was able to perform the VR spatial memory task as accurately as the controls, our current report allowed us to compare the increased fMRI BOLD signal seen in the concussed group with DTI. Thus, in this study, we examined if functional deficits observed via fMRI BOLD data are consistent with structural white/gray matter alterations (DTI data) in our MTBI participants along with age and physically active matched controls.
Materials and methods
Participants
Initially 15 neurologically normal student-athletes with no history of MTBI (mean age 21.3 ± 1.5 years) and 15 student-athletes (mean age 20.8 ± 1.7 years) recently suffered from sport-related MTBI (collegiate rugby, ice hockey and soccer players) were recruited for this study. The sample comprised 70% men and 30% women. Academic grade average score for all subjects under study was 3.2 ± 0.5. All injured subjects suffered from grade 1 MTBI (Cantu Data Driven Revised Concussion Grading Guideline, 2006) and were scanned on day 30 (± 2 days) post-injury. Inclusion criteria for this fMRI/DTI study were the commonly accepted clinical symptoms of MTBI such as complaints of loss of concentration, dizziness, fatigue, headache, irritability, visual disturbances, and light sensitivity (Bryant and Harvey 1999). The initial diagnosis of MTBI was made on the field by a certified athletic trainer (AT). As a part of routine protocol of Sport Concussion Program at Penn State University, the research team administered a Post-Concussive Symptoms Checklist prior to the scanning. Several conventional neuropsychological measure were also employed including the Hopkins Verbal Learning Test—Revised (HVLT), Stroop tests, Trailmaking tests A and B, the Symbol Digit Modalities Test (SDMT), and Reported Fatigue Scores. Both traditional pen-and-pencil procedures and computerized testing (ImPact) were used. All MTBI subjects were asymptomatic based upon aforementioned neuropsychological (NS) measures. All MTBI subjects were clinically asymptomatic within 10 days post-injury as well and cleared for sport participation by the medical practitioners at the Penn State Center for Sport Medicine based upon neurological assessments (Co-operative Ataxia Rating Scale, World Federation of Neurology, Trouillas et al. 1997) as well as clinical symptoms resolution. All subjects were right handed according to Edinburgh Handedness Inventory (Oldfield 1971). Specifically, all of the subjects have their handedness score above 90. All subjects signed an informed consent form, and the protocol was approved by the Institutional Review Board of the Pennsylvania State University. It should be noted that fMRI and DTI data were obtained in the same scanning session. There were a few incidental findings detected by neuroradiologist in anatomical MRI in normal controls. These subjects were excluded from further analysis. Thus, there were neither lesions nor hyperintense signals present in the MTBI and normal controls (NC) subjects under study.
Data acquisition
Anatomical and functional images were acquired on a 3.0 Tesla Siemens Trio whole-body scanner (Siemens, Erlangen, Germany) using a 12 channel head coil. fMRI, DTI, T1, and T2 anatomical images were acquired in the axial plane parallel with the Anterior and Posterior Commisure axis covering the entire brain. Two-dimensional 20 direction echo planar DTI (1.8 × 1.8 ×3 mm resolution, TE = 93 ms, TR = 6,500 ms, EPI factor = 128, 47 slices, iPAT = GRAPPA, acceleration factor = 2, NSA = 4). Three-dimensional isotropic T1-weighted magnetization prepared rapid gradient echo (MP-RAGE: 0.9 × 0.9 × 0.9 mm resolution, TE = 3.46 ms, TR = 2,300 ms, TI = 900 ms, flip angle = 9°, 160 slices, iPAT = none, NSA = 1). Two-dimensional T2-weighted fast spin echo (0.7 × 0.7 × 5 mm resolution, TE = 90 ms, TR = 6,000 ms, flip angles = 90°, 120°, ETL = 18, 30 slices, iPAT = GRAPPA, acceleration factor = 2, NSA = 1). Identical two-dimensional blood oxygen level-dependent (BOLD) echo planar fMRI images obtained for each experimental task (3.1 × 3.1 × 5 mm resolution, TE = 25 ms, TR = 2,000 ms, EPI factor = 64, flip angle = 79°, 30 slices, iPAT = none, NSA = 1).
fMRI experiment
The experimental paradigm used in this study consisted of a virtual corridor (see Fig. 1) in which participants (a) were shown the navigation route to encode (E); (b) navigated randomly (RN); and (c) navigated purposefully with the goal of reaching specific target room location (active navigation, (AN) using an MRI-compatible high-resolution joystick (http://www.magconcept.com). The subjects “moved” around using their right thumb to freely navigate in forward, backward, and side-to-side directions. During scanning, the subjects watched the visual scenes in a supine posture within the MRI scanner via a mirror mounted on the head coil.
Fig. 1.
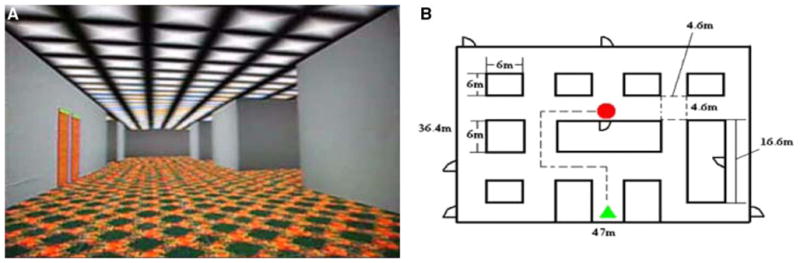
a View of the virtual corridor used for navigation tasks under study and b floor plan, and a sample of the route for one of the runs. The subjects were instructed to reproduce (e.g., retrieve the previously shown route (e.g., encoding) via navigation through virtual corridor by MRI-compatible joystick and to find the target location
Each subject performed 4 runs of the spatial memory tasks in the MRI scanner. In run 1 and run 3, the task was to memorize one route in a virtual reality (VR) corridor (encoding) and repeat it (retrieval) after a distraction period. There were four paths in total for each run. In run 2 and run 4, subjects were asked to memorize all four routes at first and then repeat them randomly. fMRI scans were performed during the tasks in order to examine the brain activation patterns associated with encoding and retrieval in both concussed and normal control groups. Detail of this experiment and the fMRI results were presented in a previous report (Slobounov et al. 2010).
Data analysis
fMRI data
Images processing and statistical analysis were conducted with Statistical Parameter Mapping (SPM) software package (Friston et al. 1995) version 8 (2008, Wellcome Department of Cognitive Neurology, London UK; http://www.fil.ion.ucl.ac.uk/spm). Pre-processing with SPM8 included realignment, co-registration, and spatial normalization (template of Montreal Neurological Institute, MNI). Then, a Gaussian filter of 8 mm Full Width at Half Maximum was applied to smooth the data spatially. Cerebral activation was rendered onto the standard MNI 152 brain.
DTI data
Whole-brain voxel-wise statistical analysis of the FA data was carried out using TBSS (tract-based spatial statistics, Smith et al. 2006), part of FSL software package (Smith et al. 2004). First, FA images were created by fitting a tensor model to the raw diffusion data using FDT, and then brain-extracted using BET (Smith 2002). All 30 subjects’ FA data were then aligned into a common space using the nonlinear registration tool FNIRT (Andersson et al. 2007a, b), which uses a b-spline representation of the registration warp field (Rueckert et al. 1999). Next, the mean FA image was created and thinned to create a mean FA skeleton which represents the centers of all tracts common to the group. Each subject’s aligned FA data were then projected onto this skeleton and the resulting data fed into voxelwise cross-subject statistics.
The DTI images were eddy current corrected, in order to remove the artifacts from motion and eddy-current-induced distortion (Reese et al. 2003). A white matter tract skeleton was constructed as the template using data from all the subjects. Each subject’s main tracts were registered to that template to form an average white matter atlas. TBSS tool-boxes generated both atlas for the control and patient groups, and performed a statistical analysis for differences. Obtained DTI data were corrected for multiple comparisons. The algorithm was implemented in SPM software package.
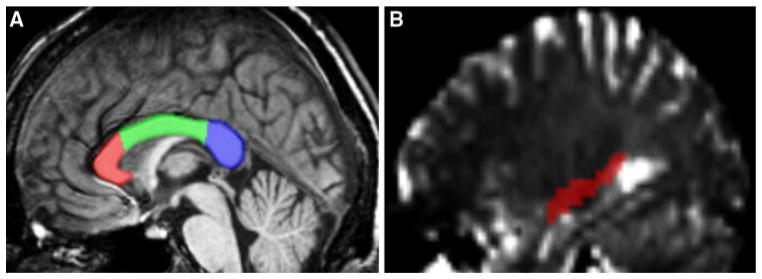
Due to the possibility that the averaging process might wash out significant differences between groups, we opted to perform Region of Interest (ROI) analysis for our next step to ensure a more thorough study. The corpus callosum (CC) which forms the largest and highest density commissural white matter bundle in the brain and connects the left and right hemispheres was chosen as a primary ROI and subdivided into the genu, body, and splenium. In addition to the corpus callosum, we also evaluated the right hippocampus, left and right dorso-lateral prefrontal cortex (DL-PFC), Precuneus and Primary Visual Cortex (V1) as a ROI because of our fMRI findings (see below fMRI results). Subregions of CC and other ROI under study were hand drawn according to Wilson procedure. Figure 2 shows the ROIs within the corpus callosum and the right hippocampus on the sagittal plane.
Fig. 2.
a Sagittal view of corpus callosum subdivided into genu, body, and splenium ROIs. b Sagittal view of the right hippocampus ROI used in the tractography analysis
Eddy-current-corrected DTI images were exported from FSL and imported into SPM. The DTI images and anatomical T1 images were normalized to MNI 152 standard brain template which was built by the Montreal Neurological Institute and adopted by the International Consortium of Brain Mapping (ICBM) as a standard. ROIs were drawn for each subject in MedINRIA software (http://www-sop.inria.fr/asclepios/software/MedINRIA/) to carry out tractography.
FA quantifies the directionality of water diffusion and is a value between zero and one, with values closer to one indicating more restricted radial diffusion indicative of white matter tracts. FA maps were calculated by diagonalizing the diffusion-weighted images information. Each voxel has three eigenvalues of the diffusion tensor matrix, (λ1, λ2, λ3). FA and ADC are defined in the following equations:
| (1) |
where
| (2) |
The FA threshold was set at 0.2, and the minimum length of the fibers was set at 10 voxels. Both voxel-based and tract-based statistics about FA, ADC, and number of fibers were obtained. DTI data were corrected for multiple comparisons. The algorithms were implemented in MedINRIA software package.
Results
Neuropsychological test performance data
Table 1 shows the neuropsychological data for the concussion and healthy control groups. As can be seen from the data presented in Table 1, no significant differences were observed between MTBI subjects and normal controls for all of the variables under NS testing.
Table 1.
Neuropsychological test performance variables including reported fatigue scores prior to MRI scanning for normal controls (NC) and MTBI subjects
| Subjects Groups | NC | MTBI | T-test | P-value |
|---|---|---|---|---|
| M (SD) | M (SD) | |||
| Reported fatigue: Beatty test fatigue rating | ||||
| Cognitive fatigue | 9.5 (2.8) | 11.4 (4.7) | 2.03 | 0.073 |
| Physical fatigue | 10.9 (3.3) | 11.7 (4.8) | 2.02 | 0.096 |
| Total fatigue | 22.6 (6.1) | 23.3 (10.3) | 2.04 | 0.086 |
| Neuropsychologial (NS) test performance: | ||||
| Trailmaking A | 30 (5) | 29 (6) | 1.93 | 0.074 |
| Trailmaking B | 87 (8) | 85 (7) | 1.23 | 0.123 |
| Stroop color-word (CS) & color-word interference (CW-I) | ||||
| CW total time | 50.9 (7.9) | 49.3 (6.4) | 3.15 | 0.072 |
| CW-I total time | 101.4 (25.5) | 100.4 (21.6) | 3.16 | 0.087 |
| CW total errors | 0.0 (0.0) | 0.3 (0.7) | 1.40 | > 0.10 |
| CW-I total errors | 1.5 (0.7) | 1.7 (1.6) | 1.49 | 0.094 |
fMRI data
fMRI activation patterns show that encoding requires more cerebral resources than retrieval during the spatial memory tasks as evidenced by the larger cluster sizes and increased blood oxygen level-dependent (BOLD) signal intensities in the shared ROI’s. The two groups’ (encoding-baseline) contrast patterns showed four common areas, visual cortex, parietal cortex, right dorsal-lateral prefrontal cortex, and right hippocampus. Within those common areas, compared to the normal controls, MTBI patients had significantly larger cluster sizes and increased BOLD signal percent change (P < .001) most notably in the right hippocampus. Furthermore, there were additional regions of activation in MTBI patient group in the left dorsal-lateral prefrontal cortex and cerebellum not observed in normal controls. Figure 3a, b shows the series of the activation maps of the two groups.
Fig. 3.
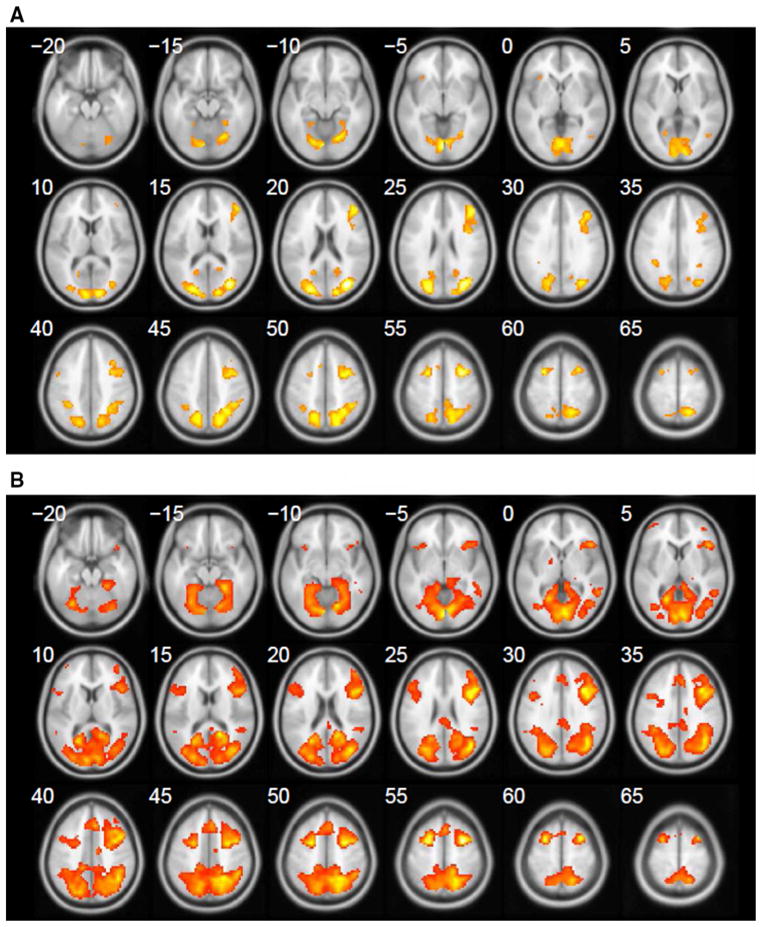
a Series of axial images showing the activation pattern of encoding (E) versus baseline (BL) contrast, normal controls. b Axial activation pattern during (E-BL) contrast, MTBI subjects
Whole-brain-level TBSS analysis showed no significant difference between MTBI and control groups at significance level P < 0.05 (see also Fig. 4).
Fig. 4.
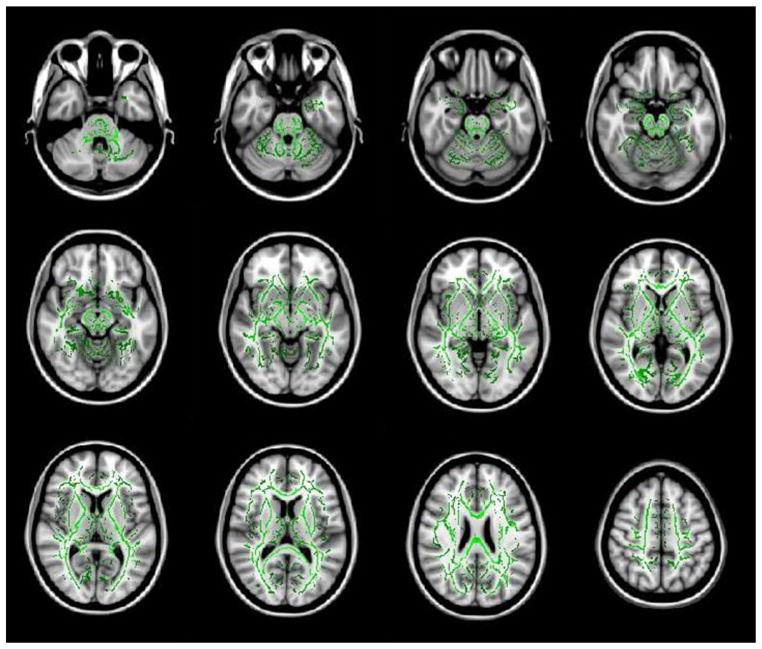
Cascade display of TBSS results showing no statistical significant difference between normal control and MTBI groups. Common white matter fiber tracts and FA values are designated in green. No statistical significance was found which would be designated by the color red
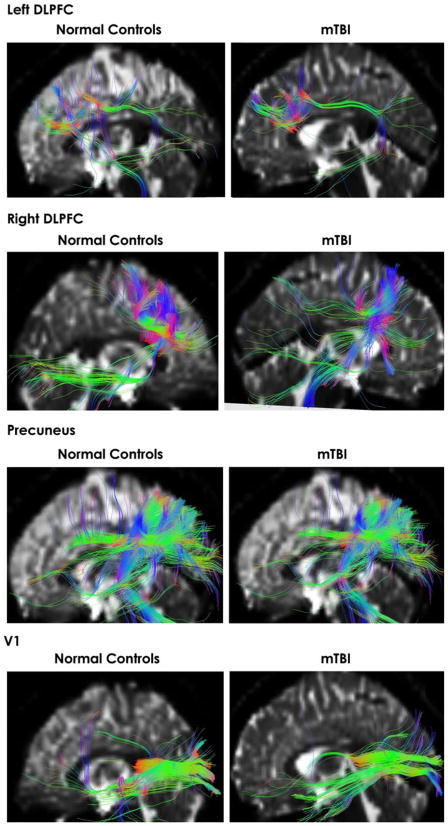
We initially focused on the ROI analysis of the corpus callosum, as the most common region examined in DTI studies and suggesting that CC is the most frequently damaged in TBI (Blumbergs et al. 1995; Benson et al. 2007; Rutgers et al. 2008; Wilde et al. 2008; Levin 2003). We also investigated the right hippocampus with a ROI, considering our fMRI findings. Figure 5 shows representative examples of tractography for those ROI from both groups. As can be seen from this figure, both MTBI and normal controls demonstrate both excellent quality of the tractography results and common white matter tract characteristics fiber pattern that emerges using the quantitative tractography method analysis.
Fig. 5.
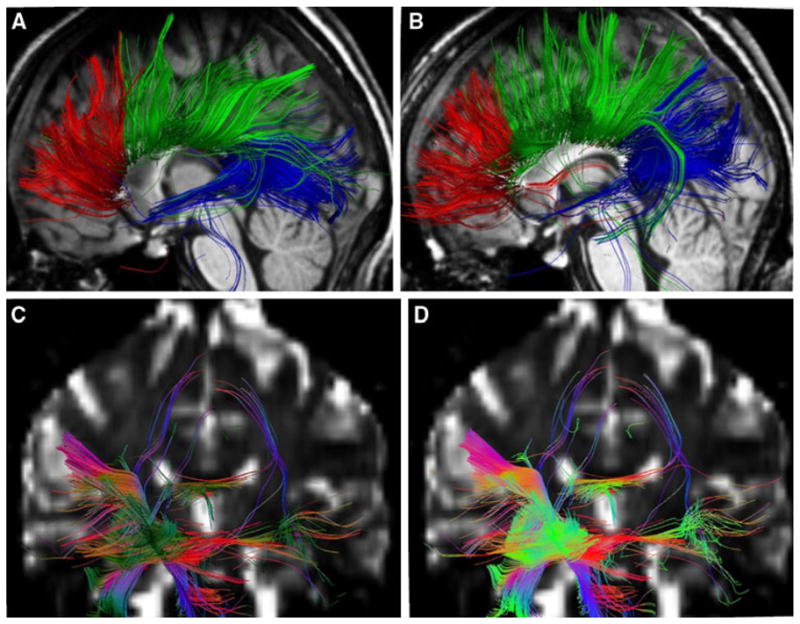
Fiber tracts going through the corpus callosum ROIs of a representative normal control subject (a) and a representative MTBI patient (b). Fiber tracts connected to the right hippocampus ROI of a representative normal control subject (c) and a representative MTBI patient (d)
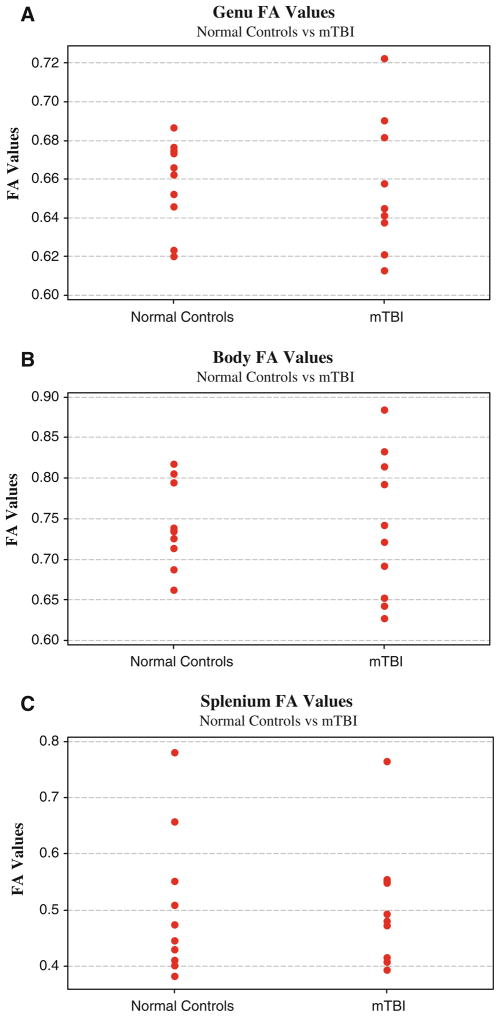
Using the MedINRIA software (http://www-sop.inria.fr/asclepios/software/MedINRIA/), the statistics of the fiber bundles were obtained for all corpus callosum subdivisions and the right hippocampus. Two-sample t-tests were applied to all of the above data. There were no significant differences between normal controls and MTBI subjects (P > .05). Even with no statistical significance, it is of interest to mention that the MTBI showed more variability (Standard Deviation) of FA and ADC values in the genu and body of the corpus callosum (see also Fig. 6). MTBI subjects showed increased FA variability in the genu and body. MTBI subjects also demonstrated a larger number of fibers in the genu and body, but a decrease in the numbers of fibers in the right hippocampus, although statistically significant (P > .05).
Fig. 6.
The FA values scatter plots of the control and patient group. It should be noted that the FA variations of patients are larger than those of controls except the splenium ROI
Considering the results of the fMRI portion of our study, particularly the data showing that four common areas (visual cortex, parietal cortex, right dorsal-lateral prefrontal cortex, and right hippocampus) were differentially sensitive to encoding of spatial information in MTBI subjects, we performed ROI analysis focused on these regions. Figure 7 shows representative examples of tractography for these additional ROI for both groups of subjects. Again, both MTBI and normal controls showed similar track characteristics fiber patterns supporting the results of quantitative tractography method analysis shown in Table 2.
Fig. 7.
Fiber tracts going through additional ROI including left and right DL-PFC, Precuneus (both sagittal and axial views) and Visual Cortex (V1) for both normal controls and MTBI subjects
Table 2.
The mean values of the FA, ADC, and number of fibers from the 8 ROIs
| ROI analysis |
|||
|---|---|---|---|
| Normal controls (n = 15) | mTBI (n = 15) | P-value | |
| Genu | |||
| FA | 0.658204 (0.022498) | 0.65312 (0.035125) | 0.705 |
| ADC (mm2/s) | 2.32832 (0.172617) | 2.408549 (0.101279) | 0.226 |
| Number of fibers | 1578.9 (277.4633) | 1615.4 (322.8664) | 0.790 |
| Body | |||
| FA | 0.741879 (0.050517) | 0.740227 (0.088266) | 0.960 |
| ADC (mm2/s) | 2.326389 (0.142296) | 2.392431 (0.261316) | 0.495 |
| Number of fibers | 1693.8 (363.0481) | 1932.1 (768.4694) | 0.393 |
| Splenium | |||
| FA | 0.504772 (0.127285) | 0.508863 (0.108362) | |
| ADC (mm2/s) | 2.817709 (0.550361) | 2.775635 (0.23698) | 0.828 |
| Number of fibers | 1896.5 (463.9134) | 1713.1 (301.1472) | 0.311 |
| Right hippocampus | |||
| FA | 0.3351906 (0.02) | 0.341731 (0.015226) | 0.378 |
| ADC (mm2/s) | 2.697601 (0.17) | 2.656498 (0.044002) | 0.463 |
| Number of fibers | 1769 (338.78148) | 1487.5 (417.8023) | 0.115 |
| Left DL-PFC | |||
| FA | 0.368213 (0.016038) | 0.372879 (0.011063) | 0.429 |
| ADC (mm2/s) | 2.355871 (0.049943) | 2.298619 (0.059015) | 0.002 |
| Number of fibers | 477 (326.676) | 480 (239.1445) | 0.977 |
| Right DL-PFC | |||
| FA | 0.355987 (0.009358) | 0.354294 (0.010019) | 0.666 |
| ADC (mm2/s) | 2.354563 (0.031236) | 2.301095 (0.057717) | 0.001 |
| Number of fibers | 1700 (684.5941) | 1585 (385.0126) | 0.633 |
| Precuneus | |||
| FA | 0.372842 (0.016318) | 0.37781 (0.01557) | 0.450 |
| ADC (mm2/s) | 2.434849 (0.060357) | 2.41295 (0.064295) | 0.265 |
| Number of fibers | 1874 (598.0219) | 1735 (445.3598) | 0.535 |
| Primary visual cortex | |||
| FA | 0.381986 (0.023074) | 0.370301 (0.025177) | 0.237 |
| ADC (mm2/s) | 2.561129 (0.222456) | 2.526746 (0.178923) | 0.585 |
| Number of fibers | 338 (157.4606) | 312 (119.5462) | 0.661 |
The numbers in the parenthesis are the standard deviations. FA and ADC values come from the ROI-based analysis. Note significant differences in ADC at right and left DL-PFC between groups
Table 1 shows the mean FA, ADC, and number of fibers of the ROI’s for each groups. As can be seen from this Table, no significant changes in FA and in number of fibers between groups were observed at all ROI under study. However, there was slight but significant differences in ADC at both left DL-PFC (P = .002) and right (P = .001) DL-PFC between groups, indicating decreased diffusivity in MTBI subjects.
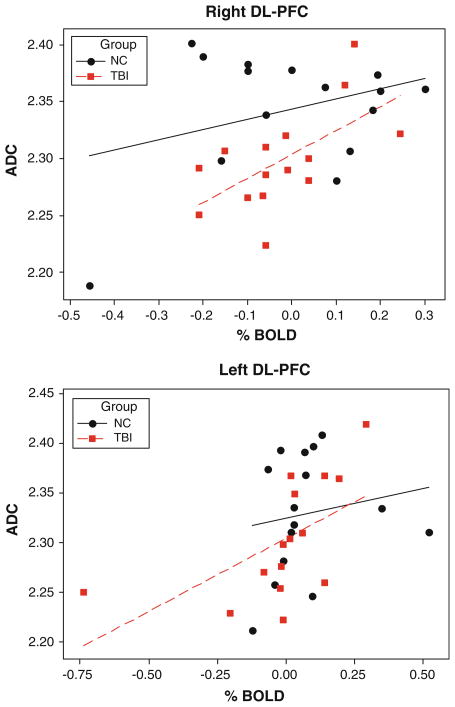
Due to observed reduced diffusivity accompanied by significantly larger cluster sizes and increased BOLD signal percent change (P < .001), we performed Pearson correlation analysis which are depicted in Fig. 8. Interestingly, there was significant positive correlations between ADC values and FMRI % change at both left (r = 0.580, P = 0.024) and right (r = 0.612, P = 0.015) DL-PFC in MTBI subjects, but not in normal controls (r = 0.157, P = 0.575; and r = 0.335, P = 0.222 accordingly).
Fig. 8.
Scatterplots of ADC from left and right DL-PFC seed voxels relative to % change FMRI signal at these ROI for normal controls and MTBI subjects. Best-fit lines are indicated in solid black (NC) and dashed red (MTBI)
Discussion
It is important to better articulate the functional alterations and possibly structural abnormities in white matter in MTBI since concussion is still one of the most puzzling neurological disorders and often happens in high risk sporting events and in everyday life. There is still a debate in the literature whether concussion is just a short-term functional abnormality or may reflect structural deficits in the brain that can be detected by advanced brain imaging methodologies. Accordingly, in the present report, we combined fMRI incorporated with VR and DTI studies in normal controls and subjects suffering from a single episode of mild TBI. The major question under study was whether the functional deficits that may be observed via the BOLD signal associated with cognitive tasks parallel those that may be observed via DTI measures.
In our study, we used a VR procedure to study spatial memory following concussion. Although a variety of studies have examined deficits associated with MTBI, there is little research that has been directed toward spatial memory processing within concussed individuals. Being the most common athletic head injury and the essential role spatial processing plays in athletics, it is an area that deserves attention. During fMRI acquisition, subjects with and without concussions were required to navigate to a target destination in order to study the neural underpinning of encoding and retrieval of spatial information. Similar activation patterns were acquired from both control and patient groups. However, the activation map of the MTBI subjects showed larger activated areas and some additional activation. Therefore, whether concussion patients’ DTI results would also reflect any structural deviation from controls is an important question. To answer this question, we compared the DTI data of the 2 groups (MTBI vs. normal controls).
In this DTI portion of the study, whole-brain TBSS and basic normalized tractography methods were used, but no significant differences between controls and MTBI patients were detected. The only exception is that we observed decreased diffusivity as evidenced by apparent diffusion coefficient (ADC) at both left and right dorsolateral pre-frontal cortex (DL-PFC) in MTBI subjects. There was also a positive correlation (P < 0.05) between ADC and % change of fMRI BOLD signals at DL-PFC in MTBI subjects, but not in normal controls. This is an intriguing finding which needs to be replicated. Given that DTI differences have been found using different tasks (e.g., verbal memory) and with different populations (e.g., schizophrenia and parkinsonism), future research may want to explore such areas as the uncinate fasciculus, anterior corona radiata, forcepts minor, and superior longitudinal fasciculus to better articulate the meaning of DTI differences.
This general lack of DTI differences is difficult to definitively interpret at this time since there are inconsistent findings in the literature in relation to DTI. Some researchers suggest that this results from a lack of sensitivity of DTI measures with MTBI individuals (Rutgers et al. 2008). Further, significant FA reductions but less significant changes in mean diffusivity (MD) have been reported in the internal capsule and corpus callosum during the first 24 h of injury (Arfanakis et al. 2002). Changes in FA, however, were significantly less at 1 month post-injury, suggesting the dynamic nature of MTBI. One possibility for initial measures following MTBI is that the initial axonal swelling which results in the release of Ca2+ may reduce the free space of water which in turn would reduce diffusivity (Chu et al. 2010). Quite contrasting findings have been reported recently by Bazarian et al. (2007), suggesting a significant increase in FA in the posterior corpus callosum and a significant decrease in MD in the left anterior internal capsule within 72 h of injury. These authors also claimed that their DTI results were highly correlated with postconcussive symptoms (PCSs) and neurobehavioral tests. Our subjects were scanned within 30 days post-injury and may be a factor determining lack of FA alterations observed in this study. In other words, the study time frame (e.g., acute vs. advanced stage of injury) and rate of MTBI symptom resolution along with obvious functional deficits (i.e., fMRI portion of this study) may suggest the possibility of long-term functional deficits with delayed white matter structural alterations, as might be evidenced by the lack of the reduction and/or increase in FA values of DTI in our study.
In fact, the presence of DTI alteration in MTBI patients is controversial. In most previous reports, reduced FA and increased ADC in moderate-to-severe TBI patients were observed but not in MTBI subjects. Specifically, the FA in moderate-to-severe TBI was reduced in the posterior corona radiata, cortico-spinal tracts, cingulum, external capsule, forceps minor and major, genu, body and splenium of the corpus callosum, inferior fronto-occipital (IFO) fasciculus, superior longitudinal fasciculus (SLF), and sagittal stratum. In contrast, FA reduction in mild TBI, however, was detected only in the cortico-spinal tract, sagittal stratum, and the SLF (Kraus et al. 2007).
Also, compared with controls, a significant reduction of FA was observed in patients’ corpus callosum, internal capsule, and centrum semiovale in Inglese et al. (2005) study. These authors have also reported significant increases of MD in the corpus callosum and internal capsule. They concluded that although MD and FA abnormalities in patients with TBI were too subtle to be detected with the whole-brain histogram analysis, they are present in brain areas that are frequent sites of DAI. In fact, they also reported that diffusion tensor imaging changes in MTBI patients may be present at both early and late time frame of injury. No differences in FA and ADC using whole-brain TBSS as well as corpus callosum and hippocampus ROI analysis in our study may be attributed to the fact that all our subjects have had suffered from a single episode of MTBI, were asymptomatic within 14 days post-injury, and were cleared by medical personnel for sport participation by the time when scanning was done.
Another feasible reason for the inability to detect differences in MTBI patients can be attributed to current MRI technology constraints. This is highlighted mostly by the inability to obtain sub-millimeter resolution and number of diffusion directions with satisfactory signal to noise ratio in a reasonable scan time. Specifically, in our study, we used a high field 3 Tesla scanner with DTI pulse sequence parameters of 20 directions with 4 number of signal averages. In other studies, Rutgers et al. (2008) a 1.5-Tesla scanner and 25 direction DTI sequence were used, unlike Wilde et al. (2008) where 3.0-Tesla and 30 directions were used. Those DTI parameters, to some extent, could affect the calculation of FA and also the process of tractography. It should be stressed again that DTI alterations may reflect not only white matter integrity but also other aspects and physiological mechanisms not fully understood yet (Imfeld et al. 2009).
Another issue which may be accounted as a technical bias is the analyzing software algorithms used in different studies. Among the popular DTI processing software, the tracking algorithms are close in their basic principles, but not exactly the same. Meanwhile, there are many angles of view for DTI results: voxel-based, tract-based, whole-brain, ROI, etc. In our study, we implemented well-accepted TBSS whole-brain tract-based analysis in order to perform a careful group analysis. Voxel-based ROI analysis and tractography were done by the MedINRIA software package which is widely used in DTI studies across the Europe and United States. Indeed, every bit of difference in the algorithm and threshold used could induce biased results. Therefore, it is not surprising that DTI studies of MTBI have shown some inconsistency toward FA and ADC. These methodology issues still remain to be solved.
When compared to moderate-to-severe TBI, MTBI does not induce the overall DAI that have been documented in studies implied by FA and ADC changes. Our current data are overall in agreement with the notion that damage to myelin in MTBI is less common, whereas both the axons and myelin are likely to be damaged in the subject population who has had suffered from moderate-to-severe TBI (Kraus et al. 2007). The exception to this appears to be when the individual is younger (e.g., Wozniak et al. 2007) and thus, the trauma not only compromises the brain but potentially also infers with brain development.
Generally, it is believed that a decrease in FA which associated with an increase in MD and ADC will be observed immediately after TBI; however, one study reported significant increased FA and decreased MD in acute MTBI cases (Bazarian et al. 2007). Longitudinal studies of FA and ADC changes in severe TBI patients were reported. FA as a measure of the white matter integrity is intensely focused on in all DTI studies. In a study of moderate-to-severe TBI, it is observed that FA values decreased significantly in frontal and temporal tracts from 4.5 to 29 months post injuries (Bendlin et al. 2008). There is an evidence of the white matter injury progress over time (Greenberg et al. 2008). To our knowledge, there are no longitudinal studies of FA and ADC evolution in MTBI, which is the most recent focus of our research group. Multiple follow-ups starting with acute phase of injury (within 3 days post-injury), 2 weeks post-injury, and 30 day post-injury etc. investigation of subjects suffering from MTBI is underway in our laboratory to further address this issue.
Besides the MRI scanner limitations, another possible reason why there were no significant difference observed may be due to population characteristics and study inclusion criteria. Participants in our study had an age range from 18 to 21 years old. This population is novel when compared to previous studies that consistently demonstrated a higher mean age in combination to a larger population standard deviation. Along with a younger subject pool all our participants were healthy and engaged in a regular exercise regimen. It has been shown that exercise can help athletes recover quicker and protect them from recurrent injuries (Cotman et al. 2007; Griesbach et al. 2004). That said, it is important to stress that premature return to sport activities may put the injured athletes at higher risk for recurrent injuries with more severe consequences (Cantu 2006).
In conclusion, we found that fMRI and DTI measures were differentially sensitive to cortical changes resulting from MTBI. This adds an initial piece of information as to when fMRI, DTI, and other measures (e.g., MEG, EEG) are most likely to reflect changes following traumatic brain injury. Specifically, the presence of functional abnormalities observed via fMRI BOLDs signal associated with successful performance of the spatial navigation task was not matched with FA and ADC values that may rule out DAI in our MTBI subjects. Whether the lack of consistency across research modalities (fMRI & DTI) are due to time frame of scanning, unique nature of MTBI and/or technological issues involved in FA and ADC quantification is yet to be determined. Further studies are needed to add the overall parametric solution as to which measures are most sensitive to MTBI. Such studies should at least include severity of trauma, rate of symptoms resolution, age of participant, time since injury as well as present and previous health history including exercise history.
Acknowledgments
This study was supported by NIH Grant RO1 NS056227-01A2 “Identification of Athletes at Risk for Traumatic Brain Injury” awarded to Dr. Slobounov, PI.
Contributor Information
K. Zhang, Department of Kinesiology, The Pennsylvania State University, 19 Recreation Building, University Park, PA 16802, USA
B. Johnson, Department of Kinesiology, The Pennsylvania State University, 19 Recreation Building, University Park, PA 16802, USA
D. Pennell, Social, Life & Engineering Sciences Imaging Center, Chandlee Laboratory, The Pennsylvania State University, University Park, PA 16802, USA
W. Ray, Department of Psychology, The Pennsylvania State University, 612 Moore Building, University Park, PA 16802, USA
W. Sebastianelli, Center for Sport Medicine & Hershey Medical School, The Pennsylvania State University, University Park, PA, USA
S. Slobounov, Email: sms18@psu.edu, slobounovsm@mail.nih.gov, Department of Kinesiology, The Pennsylvania State University, 19 Recreation Building, University Park, PA 16802, USA. Center for Sport Medicine & Hershey Medical School, The Pennsylvania State University, University Park, PA, USA. National Institute of Health, NINDS, MSC10 Center Dr., Bethesda, MD 20892, USA
References
- Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J, Jenkinson M, Smith S. Non-linear optimisation. FMRIB technical report TR07JA1 from. 2007a www.fmrib.ox.ac.uk/analysis/techrep.
- Andersson J, Jenkinson M, Smith S. Non-linear registration, aka Spatial normalisation FMRIB technical report TR07JA2 from. 2007b www.fmrib.ox.ac.uk/analysis/techrep.
- Arfanakis K, Haughton VM, Carew JD, et al. Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am J Neuroradiol. 2002;23:794–802. [PMC free article] [PubMed] [Google Scholar]
- Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8:333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- Bazarian JJ, Zhong J, Blythe B, Zhu T, Kavcic V, Peterson D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J Neurotrauma. 2007;24:1447–1459. doi: 10.1089/neu.2007.0241. [DOI] [PubMed] [Google Scholar]
- Bendlin BB, Ries ML, Lazar M, Alexander AL, Dempsey RJ, Rowley HA, Sherman JE, Johnson SC. Longitudinal changes in patients with traumatic brain injury assessed with diffusion-tensor and volumetric imaging. NeuroImage. 2008;42:503–514. doi: 10.1016/j.neuroimage.2008.04.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson RR, Meda SA, Vasudevan S, Kou Z, Govindarajan KA, Hanks RA, Millis SR, Makki M, Latif Z, Coplin W, Meythaler J, Haacke EM. Global white matter analysis of diffusion tensor images is predictive of injury severity in traumatic brain injury. J Neurotrauma. 2007;24(3):446–459. doi: 10.1089/neu.2006.0153. [DOI] [PubMed] [Google Scholar]
- Bigler E, Bazarian J. Diffusion tensor imaging: a biomarker for mild traumatic brain injury? Neurology. 2010 doi: 10.1212/WNL.0b013e3181d3e43a. e-Pub of print on January 27, 2010. www.neurology.org. [DOI] [PubMed]
- Blumbergs PC, Scott G, Manavis J, Wainwright H, Simpson DA, McLean AJ. Topography of axonal injury as defined by amyloid precursor protein and the sector scoring method in mild and severe closed head injury. J Neurotrauma. 1995;12(4):565–572. doi: 10.1089/neu.1995.12.565. [DOI] [PubMed] [Google Scholar]
- Bryant R, Harvey A. Postconcussive symptoms and posttraumatic stress disorder after mind traumatic brain injury. J Nerv Ment Dis. 1999;187:302–305. doi: 10.1097/00005053-199905000-00006. [DOI] [PubMed] [Google Scholar]
- Cantu R. Concussion classification: ongoing controversy. In: Slobounov S, Sebastianelli W, editors. Foundations of sport-related brain injuries. Springer, NY: 2006. pp. 87–111. [Google Scholar]
- Chen JK, Johnston KM, Frey S, Petrides M, Worsley K, Ptito A. Functional abnormalities in symptomatic concussed athletes: an fMRI study. Neuroimage. 2004;22:68–82. doi: 10.1016/j.neuroimage.2003.12.032. [DOI] [PubMed] [Google Scholar]
- Chu Z, Wilde E, Hunter J, McCauley S, Bigler E, Troyanskaya M, Yallampalli R, Chia J, Levin H. Voxel-based analysis of diffusion tensor imaging in mild traumatic brain injury in adolescents. Am J Neuroradiol. 2010;31:340–346. doi: 10.3174/ajnr.A1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie L-A. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frithh CD, Frackowiak RSJ. Statistical parametric maps in functional neuroimaging. A general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Greenberg G, Mikulis DJ, Ng K, DeSouza D, Green RE. Use of diffusion tensor imaging to examine subacute white matter injury progression in moderate to severe traumatic brain injury. Arch Phys Med Rehabil. 2008;89(Suppl 2) doi: 10.1016/j.apmr.2008.08.211. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Hovda DA, Molteni R, Wuand A, Gomez-Pinilla F. Voluntary exercise following traumatic brain injury: Brain-derived neurotrophic factor pregulation and recovery of function. Neurosci. 2004;125:129–139. doi: 10.1016/j.neuroscience.2004.01.030. [DOI] [PubMed] [Google Scholar]
- Imfeld A, Oechsin M, Meyer M, Loenneker T, Jancke L. White matter plasticity in the corticospinal tract of musicians: a diffusion tensor imaging study. Neuroimage. 2009;45:600–607. doi: 10.1016/j.neuroimage.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Inglese M, Makani S, Johnson G, et al. Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. J Neurosurg. 2005;103:298–303. doi: 10.3171/jns.2005.103.2.0298. [DOI] [PubMed] [Google Scholar]
- Jantzen KL, Anderson B, Steinberg FL, et al. A prospective functional MR imaging study of mild traumatic brain injury in collegiate football players. Am J Neuroradiol. 2004;25:738–745. [PMC free article] [PubMed] [Google Scholar]
- Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. 2007;130(10):2508–2519. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- Levin HS. Neuroplasticity following non-penetrating traumatic brain injury. Brain Inj. 2003;17(8):665–674. doi: 10.1080/0269905031000107151. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Sparling MB, Flashman LA, Guerin SJ, Mamourian AC, Saykin AJ. Differential working memory load effects after mild traumatic brain injury. Neuroimage. 2001;14(5):1004–1012. doi: 10.1006/nimg.2001.0899. [DOI] [PubMed] [Google Scholar]
- Oechslin M, Imfread A, Loenneker T, Meyer M, Jancke L. The plasticity of the superior longitudinal fasciculus as a function of musical expertise: a diffusion tensor imaging study. Frontier Hum Neurosci. 2010;3:1–12. doi: 10.3389/neuro.09.076.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ptito A, Chen J-K, Johnston K. Contribution of functional magnetic resonance imaging (fMRI) to sport concussion evaluation. Neurorehab. 2007;22:217–227. [PubMed] [Google Scholar]
- Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med. 2003;49:177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill DLG, Leach MO, Hawkes DJ. Non-rigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. 1999;18(8):712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- Rutgers DR, Toulgoat F, Cazejust J, Fillard P, Lasjaunias P, Ducreux D. White matter abnormalities in mild traumatic brain injury: a diffusion tensor imaging study. AJNR Am J Neuroradiol. 2008;29:514–519. doi: 10.3174/ajnr.A0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader H, Mickrevičiene D, Gleizniene R, Jakstiene S, Surkiene D, Stovner L, Obelieniene D. Magnetic resonance imaging after most common form of concussion. BMC Med Imaging. 2009;9:11. doi: 10.1186/1471-2342-9-11. from: http://www.biomedcentral.com/1471-2342/9/11. [DOI] [PMC free article] [PubMed]
- Shaw N. The neurophysiology of concussion. Prog Neurobiol. 2002;67:281–344. doi: 10.1016/s0301-0082(02)00018-7. [DOI] [PubMed] [Google Scholar]
- Singh M, Jeongwon J, Hwanga D, Sungkarata W, Gruen P. Novel diffusion tensor imaging methodology to detect and quantify injured regions and affected brain pathways in traumatic brain injury. Magn Reson Imaging. 2010;28:22–40. doi: 10.1016/j.mri.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobounov S, Sebastianelli W, Cao C, Slobounov E, Newell K. Differential rate of recovery in athletes after first versus and second concussion episodes. J Neurosurgery. 2007;61(2):238–244. doi: 10.1227/01.NEU.0000280001.03578.FF. [DOI] [PubMed] [Google Scholar]
- Slobounov S, Cao C, Sebastianelli W, Slobounov E, Newell K. Residual deficits from concussion as revealed by virtual time-to-contact measures of postural stability. Clin Neurophysiol. 2008;119(2):281–289. doi: 10.1016/j.clinph.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Slobounov S, Cao C, Sebastianelli W. Differential effect of single versus recurrent mild traumatic brain injuries on wavelet entropy measures of EEG. Clin Neurophysiol. 2009;120(5):862–867. doi: 10.1016/j.clinph.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobounov S, Zhang K, Pennell D, Ray W, Johnson B, Sebastianelli W. Functional abnormalities in normally appearing athletes following mild traumatic brain injury: a functional MRI study. Exp Brain Res. 2010;202:341–354. doi: 10.1007/s00221-009-2141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(S1):208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TEJ. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Sugiyama K, Kondo T, Oouchida Y, Suzukamo Y, Higano S, Endo M, Watanabe H, Shindo K, Izumi S-I. Clinical utility of diffusion tensor imaging for evaluating patients with diffuse axonal injury and cognitive disorders in the chronic stage. J Neurotrauma. 2009;26(11):1879–1890. doi: 10.1089/neu.2008.0839. [DOI] [PubMed] [Google Scholar]
- Trouillas P, Tkayanagi T, Hallett M, Currier D, Subramony S, Wessel K, Bryer A, Diener H, Massaquoi S, Gomez C, et al. International cooperative ataxia rating scale for pharmacological assessment of the cerebellar syndrome. J Neurolog Sci. 1997;145:205–211. doi: 10.1016/s0022-510x(96)00231-6. [DOI] [PubMed] [Google Scholar]
- van der Naalt J, Hew JM, van Zomeren AH, et al. Computed tomography and magnetic resonance imaging in mild to moderate head injury: early and late imaging related to outcome. Ann Neurol. 1999;46:70–78. doi: 10.1002/1531-8249(199907)46:1<70::aid-ana11>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Wilde EA, McCauley SR, Hunter JV, Bigler ED, Chu Z, Wang ZJ, Hanten GR, Troyanskaya M, Yallampalli R, Li X, Chia J, Levin HS. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008;70(12):948–955. doi: 10.1212/01.wnl.0000305961.68029.54. [DOI] [PubMed] [Google Scholar]
- Wozniak JR, Krach L, Ward E, Mueller BA, Muetzel R, Schnoebelen S, Kiragu A, Lim KO. Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: a diffusion tensor imaging (DTI) study. Arch Clin Neurophysiol. 2007;22:555–568. doi: 10.1016/j.acn.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]