Summary
Regulatory T cells (Tregs) and the PD-1: PD-ligand (PD-L) pathway are both critical to terminating immune responses. Elimination of either can result in the breakdown of tolerance and the development of autoimmunity. The PD-1: PD-L pathway can thwart self-reactive T cells and protect against autoimmunity in many ways. In this review, we highlight how PD-1 and its ligands defend against potentially pathogenic self-reactive effector T cells by simultaneously harnessing two mechanisms of peripheral tolerance: (i) the promotion of Treg development and function and (ii) the direct inhibition of potentially pathogenic self-reactive T cells that have escaped into the periphery. Treg cells induced by the PD-1 pathway may also assist in maintaining immune homeostasis, keeping the threshold for T-cell activation high enough to safeguard against autoimmunity. PD-L1 expression on non-hematopoietic cells as well as hematopoietic cells endows PD-L1 with the capacity to promote Treg development and enhance Treg function in lymphoid organs and tissues that are targets of autoimmune attack. At sites where transforming growth factor-β is present (e.g. sites of immune privilege or inflammation), PD-L1 may promote the de novo generation of Tregs. When considering the consequences of uncontrolled immunity, it would be therapeutically advantageous to manipulate Treg development and sustain Treg function. Thus, this review also discusses how the PD-1 pathway regulates a number of autoimmune diseases and the therapeutic potential of PD-1: PD-L modulation.
Keywords: PD-1, PD-L1, PD-L2, regulatory T cells, Tregs, Foxp3
Introduction
The immune system has the difficult challenge of discerning and defending against a diversity of microbial pathogens, while simultaneously avoiding self-reactivity. While central tolerance mechanisms result in deletion of the majority of self-reactive T lymphocytes, some T cells specific for self-antigens escape into the periphery (1, 2). To further control the development of autoimmunity, multiple mechanisms of peripheral tolerance have evolved, including T-cell anergy, deletion, and suppression by regulatory T cells (Tregs). Failure of any of these tolerance mechanisms can result in autoimmune disease. T-cell costimulatory pathways play critical roles in regulating the delicate balance between protective immunity and tolerance.
The field of T-cell costimulation began with the two-signal model for T-cell activation, which was originally proposed to explain how the encounter of a naive T cell with antigen could lead to activation or anergy (antigen-specific hyporesponsiveness) (3). According to this model, effective activation of a naive T cell requires two signals delivered by APCs. The first signal confers specificity to the immune response and involves antigen recognition, provided by the interaction of antigenic peptide/major histocompatibility complex (MHC) with the T-cell receptor (TCR). The second antigen-independent signal is the ‘costimulatory signal’, delivered by costimulatory molecules expressed on antigen-presenting cells (APCs) to receptors expressed on T cells. According to this model, if a T cell receives only antigen-specific TCR stimulation in the absence of costimulation, it will be rendered unresponsive (anergic) to subsequent antigenic challenge (4). The critical immunoregulatory function of T-cell costimulation has led significant advances in the identification and characterization of T-cell costimulatory pathways (5). We now appreciate that costimulatory pathways can provide positive second signals that promote T-cell activation as well as negative second signals that inhibit T-cell responses, mediate T-cell tolerance, and prevent autoimmunity. In addition, costimulatory pathways not only regulate responses of naive T cells but can also control effector, memory, and regulatory T cells. Thus, the functions of T-cell costimulatory pathways in regulating T-cell activation and tolerance have expanded. Costimulatory pathways can prevent effector T-cell responses and promote the development and function of Treg cells as well as control the fate of naive T cells upon antigen encounter.
The costimulatory pathway consisting of the programmed death-1 (PD-1) receptor (CD279) and its ligands, PD-L1 (B7-H1; CD274) and PD-L2 (B7-DC; CD273), delivers inhibitory signals that regulate the balance among T-cell activation, tolerance, and immune-mediated tissue damage (6–8). This pathway exerts critical inhibitory functions in the setting of persistent antigenic stimulation such as during encounter of self-antigens, chronic viral infections, and tumors (6, 9). This pathway has a central role in regulating the interplay between host defenses aimed at eradicating microbial pathogens and tumors as well as microbial and tumor strategies that evolved to resist immune responses. The PD-1:PD-L pathway contributes directly to T-cell exhaustion and lack of viral control during chronic infections (10–13) as well as the suppressive tumor microenvironment (14, 15). This pathway controls multiple tolerance checkpoints that prevent autoimmunity. This review discusses recent advances in our understanding of PD-1 and its ligands in regulating T-cell tolerance and autoimmunity, focusing on its role in controlling Treg cell development and function. To provide a context for these studies, we first introduce PD-1 and its ligands and discuss the roles of Tregs in peripheral tolerance and autoimmunity.
Regulation of tolerance by PD-1 and its ligands
Structure and expression of PD-1 and its ligands
First identified as a gene upregulated in a T-cell hybridoma undergoing cell death (16), PD-1 is an immunoglobulin (Ig) superfamily member that has an N-terminal IgV-like domain, an approximately 20 amino acid stalk separating the IgV-like domain from the plasma membrane, a transmembrane domain, and a cytoplasmic domain with an immunoreceptor tyrosine-based inhibitory motif (ITIM) and an immunoreceptor tyrosine-based switch motif (ITSM). Mutagenesis studies indicate that the tyrosine within the ITSM motif is essential for PD-1 function in T cells and B cells (17, 18). The protein tyrosine phosphatases SHP-1 and SHP-2 can bind to the ITSM sequence in the PD-1 cytoplasmic tail (18–20), but it is not yet known whether PD-1 recruits SHP-1 and/or SHP-2 under physiologic conditions. PD-1 is monomeric on the cell surface and cannot form covalent homodimers, because it lacks the extracellular cysteine found in CD28, cytotoxic T-lymphocyte antigen-4 (CTLA-4), and inducible costimulator (ICOS), which allows these molecules to homodimerize (21).
PD-1 is expressed during thymic development and is induced in a variety of hematopoietic cells in the periphery by antigen receptor signaling and cytokines. PD-1 is expressed on immature CD4−CD8− (double negative) thymocytes during TCRβ rearrangement (22) (Fig.1A). PD-1 is inducibly expressed on peripheral CD4+ and CD8+ T cells, natural killer T (NKT) cells, B cells, and monocytes, and some dendritic cell (DC) subsets upon their activation (9). In addition, estrogen can stimulate PD-1 expression on T cells and APCs (23). PD-1 is induced by TCR or B-cell receptor (BCR) signaling (24) and remains high in the setting of persistent antigen (either foreign or self) stimulation (Fig.1B). In particular, PD-1 is highly expressed on non-functional, exhausted T cells in the setting of chronic viral infection (25). The common γ chain cytokines interleukin-2 (IL-2), IL-7, IL-15, and IL-21, which have key roles in T-cell expansion and survival, can also induce PD-1 expression on T cells (26). Recent analysis of the PD-1 promoter region has begun to define the transcriptional basis for regulation of PD-1 expression in several cell types. Nuclear factor of activated T cells c1 (NFATc1) is a critical factor for PD-1 induction in T cells (27). The calcineurin inhibitor cyclosporine A and the NFAT-specific inhibitor VIVIT markedly reduce PD-1 expression. Mutation of an NFATc1 consensus binding site leads to complete loss of PD-1 expression in T cells using PD-1 reporter constructs. In macrophages, interferon-sensitive response element (ISRE) is key for interferon-α (IFN-α)-induced PD-1 upregulation (28). PD-1 can be selectively induced on some myeloid DCs by Listeria monocytogenes infection or by ligation of Toll-like receptor 2 (TLR2), TLR3, TLR4, or nucleotide binding oligomerization domain (NOD) but inhibited by IL-4 and TLR9 ligation (29).
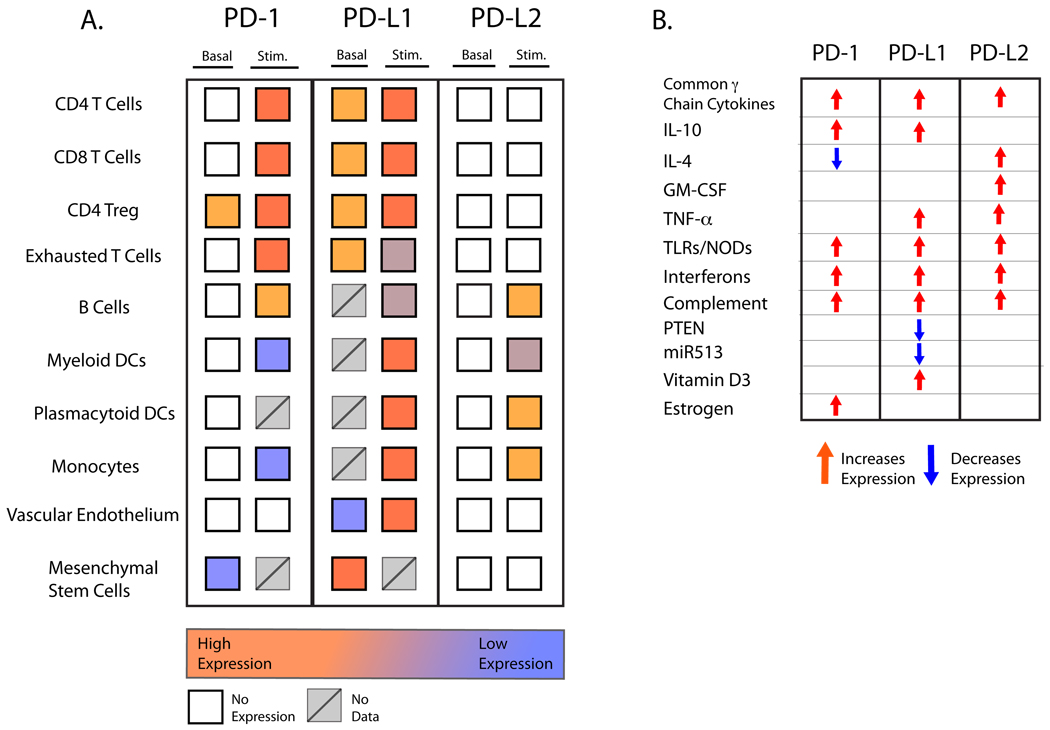
Fig. 1. Relative expression of PD-1 and its ligands.
(A) Comparison of expression of PD-1, PD-L1 and PD-L2 on immune and non-immune cells in naive or activated states. (B) Factors that regulate expression of PD-1, PD-L1, and PD-L2. Regulation of expression on specific cell types is discussed in detail in the text. There are some differences in expression of human and mouse PD-1, PD-L1, and PD-L2 expression. Murine expression is summarized in this figure. Expression of human PD-L1 differs from mouse PD-L1 in that human PD-L1 is primarily an inducible molecule.
The two PD-1 ligands have IgV-like and IgC-like extracellular domains, similar to other B7 family members. PD-L1 has a short cytoplasmic domain (~30 amino acids) that is conserved across species but without any known function (30, 31). The PD-L2 cytoplasmic domain is short in rodents (only 4 amino acids) but is longer (~30 amino acids) and conserved in other mammals without any apparent signaling motifs (32, 33). PD-L1 and PD-L2 differ in their affinities for PD-1; PD-L2 has a three-fold higher affinity for PD-1 as compared to PD-L1. B7-1 is an additional binding partner for PD-L1, but it does not bind to PD-L2 (34).
PD-L2 is expressed in far fewer cell types than PD-L1 (Fig.1A). PD-L2 is inducibly expressed on DCs, macrophages, peritoneal B1 B cells, memory B cells, and cultured bone marrow (BM)-derived mast cells. In contrast, PD-L1 is broadly expressed on hematopoietic and non-hemopoietic cells. PD-L1 is constitutively expressed on B cells, DCs, macrophages, BM-derived mast cells, and T cells, and further upregulated upon their activation. Constitutive expression of PD-L1 is higher in mice than in humans. PD-L1 can also be expressed on a wide variety of non-hematopoietic cell types, including vascular endothelial cells, fibroblastic reticular cells, epithelia, pancreatic islet cells, astrocytes, neurons, and in cells at sites of immune privilege including trophoblasts in the placenta and retinal pigment epithelial cells and neurons in the eye.
The expression of PD-L1 and PD-L2 is regulated by the inflammatory milieu (Fig. 1B). Cytokines are potent stimuli for PD-L1 and PD-L2 expression. Type 1 and type 2 interferons and TNF-α induce PD-L1 expression in T cells, B cells, endothelial cells, and epithelial cells (9). The common γ chain cytokines IL-2, IL-7, and IL-15 increase PD-L1 on human T cells, but IL-21 does not (26). However, IL-21 can stimulate PD-L1 expression on B (CD19+) cells from peripheral blood mononuclear cells (PBMCs). IL-10 also induces PD-L1 on monocytes. Interferons, IL-4, and granulocyte/macrophage colony-stimulating factor (GM-CSF) stimulate expression of PD-L2 on DCs in vitro, and the common γ chain cytokines can induce PD-L1 and to a lesser extent PD-L2 on human monocytes/macrophages (26). Studies of the human PD-L1 promoter show that both constitutive and inducible PD-L1 expression are dependent on two IFN regulatory factor-1 (IRF-1) binding sites (35). These IRF-1 binding sites are also found in mouse, although their importance is not yet clear. Signal transducer and activator of transcription 3 (STAT3) binds to the CD274 promoter, as shown in the gel electromobility shift and chromatin immunoprecipitation assays, and is required for PD-L1 gene expression, as demonstrated by siRNA-mediated STAT3 depletion (36). MicroRNA-513, which is expressed in human biliary epithelial cells, is downregulated by IFN-γ exposure, and targets the 3’ untranslated region (UTR) of PD-L1 and translationally represses it (37). Relief of miR-513-mediated translational repression of PD-L1 may be involved in IFN-γ-induced expression of PD-L1 in biliary epithelial cells and other cell types. Less is known about transcriptional regulation of PD-L2. Its induction by IFN-γ is partially dependent on NF-κB-binding sites upstream of the transcriptional start site (38).
The Janus kinase (JAK)/STAT, mitogen-activated protein kinase (MAPK), and PI3K/AKT pathways mediate IFN signaling, and recent studies indicate that the JAK/STAT and MAPK signaling pathways are involved in IFN-induced PD-L1 expression. Studies using pharmacological inhibitors show that PD-L1 expression in cell lines is decreased when myeloid differentiation factor 88 (MyD88), tumor necrosis factor receptor-associated factor 6 (TRAF6), and MEK are inhibited (39). JAK2 has also been implicated in PD-L1 induction. Loss or inhibition of phosphatase and tensin homolog (PTEN), a cellular phosphatase that modifies phosphatidylinositol 3-kinase (PI3K) and Akt signaling, increases post-transcriptional PD-L1 expression in cancers (40). Inhibition of PI3K or Akt decreased PD-L1 in tumor cells. Complement c5a also promotes expression of PD-L1 and PD-L2 (41).
Signaling through PD-1
There are many potential bidirectional interactions between PD-1 and PD-L1 due to the expression of PD-1 on T cells, B cells, macrophages, and some DCs, and the broad expression of PD-L1 on hematopoietic and non-hematopoietic cells. PD-1 function is best characterized in T cells, but several studies demonstrate that PD-1 can also function in other cell types (29, 42). PD-1 can transmit an inhibitory signal when engaged along with either the TCR or BCR (17, 24). In addition, PD-1 can inhibit macrophage and DC responses to TLR agonists and microbes (29, 42).
Although PD-1 is inducibly expressed on T cells upon their activation, the effects of PD-1 ligation on T cells can be seen as early as two hours after activation (17). Engagement of PD-1 by either of its ligands during TCR signaling can block T-cell proliferation, cytokine production and cytolytic function, and impair T-cell survival (19). The extent of PD-1 mediated inhibition depends on the strength of the TCR signal, with more inhibition occurring at lower levels of TCR stimulation. PD-1 often inhibits cytokine production to a greater extent than cell proliferation. CD28 costimulation (31) or IL-2 (43) can override PD-1-mediated inhibition. It is not yet clear how the level of PD-1 expression relates to PD-1 function. PD-1 may inhibit T-cell function and survival directly, by blocking early activation signals that are promoted by CD28, or indirectly through IL-2. CD28 and IL-2 promote T-cell expansion and survival by stimulating anti-apoptotic, cell cycle, and cytokine genes. PD-1 engagement prevents the induction of the cell survival factor Bcl-xL as well as expression of transcription factors associated with effector cell function, including GATA-3, T-bet, and Eomes (17, 44).
PD-1 alters membrane-proximal signaling events in T cells (Fig. 2). PD-1 inhibits the induction of phosphatidylinositol-3-kinase (PI3K) activity and downstream activation of Akt (45). PI3K and Akt activity are key for glucose transport and glycolysis, so PD-1-mediated inhibition of these signaling molecules can hamper cell bioenergetics. The effects of PD-1 on Akt activation also provide an explanation for why IL-2 can rescue T cells from PD-1 inhibition. IL-2 can trigger Akt activation through STAT5 and circumvent PD-1 mediated inhibition of Akt activation. Although CTLA-4 also blocks Akt activation, it does not interfere with PI3K activity but instead blocks Akt phosphorylation by activation of protein phosphatase 2 (PP2A) (19, 45). Thus, the PD-1 and CTLA-4 immunoinhibitory receptors target different signaling molecules. PD-1 engagement can reduce phosphorylation of CD3ζ, ζ-associated protein of 70 kDa (ZAP70), and protein kinase Cθ (PKCθ) (45). PD-1 ligation also inhibits Erk activation, but this effect can be overcome through cytokine receptor signaling, particularly cytokines that activate STAT5, such as IL-2, IL-7, and IL-15 (46).
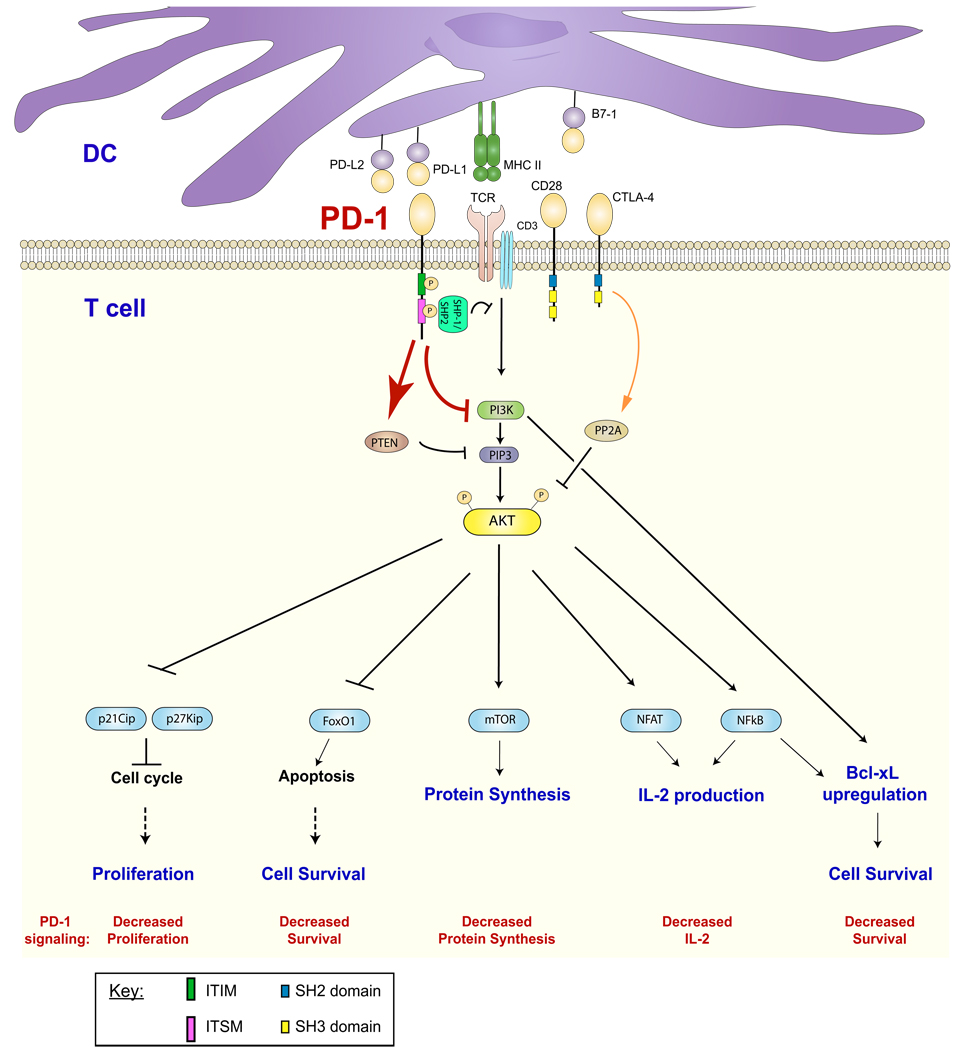
Fig. 2. PD-1 signaling.
Ligation of TCR and PD-1 leads to tyrosine phosphorylation (P) of the ITIM and ITSM of PD-1. Binding of the ITSM by SHP-1 or SHP-2 results in the dephosphorylation of proximal signaling molecules and augmentation of PTEN expression. This effectively attenuates the activation of the PI3K and Akt pathways. PD-1 signaling may result in decreased T-cell proliferation, survival, protein synthesis, and IL-2 production. (Red arrows and text indicate consequence of PD-1-mediated signaling)
Comparison of the consequences of PD-1 and CTLA-4 ligation on CD4+ T-cell activation by gene expression profiling has revealed that PD-1 inhibits T-cell activation to a greater extent than CTLA-4 (45). Ligation of CTLA-4 reduced the number of transcripts whose expression levels were altered substantially (at least a five-fold increase or decrease) by CD3 and CD28 costimulation by ~67%. Ligation of PD-1 reduced the number of these CD3/CD28 regulated transcripts by ~90%. Importantly, the cell survival gene Bcl-xL was upregulated following ligation of CTLA-4 but not PD-1, suggesting a means by which PD-1 engagement could make T cells more susceptible to apoptosis. In contrast to what is known about how PD-1 impacts signaling pathways during the initial activation of naive T cells, it is not yet clear if there are differences in PD-1 signaling in distinct types of T cells. We have investigated signaling by the PD-1 pathway during the development of adaptive regulatory T cells, as discussed later in this review.
While many studies have shown that PD-Ls can inhibit T-cell proliferation and cytokine production, others have found that PD-Ls enhance T-cell activation. The reasons for these contradictory results are not yet clear and remain controversial. Some studies have shown that PD-L1 can increase T-cell proliferation by inhibiting IFN-γ-induced nitric oxide production (47). When macrophages were used as APCs, anti-PD-L1 and anti-PD-1 increased IFN-γ and IL-2 production by T cells but paradoxically inhibited their proliferation. This effect was found to be due to IFN-γ-dependent induction of nitric oxide production by macrophages, which leads to inhibition of T-cell proliferation. These findings suggest that some of the positive effects of PD-L1 and PD-L2 may be explained by inhibition of negative signaling. In addition, there are data indicating that PD-L1 and/or PD-L2 may signal bidirectionally.
Roles of PD-1 and its ligands in T-cell tolerance
A role for PD-1 in regulating T-cell tolerance and autoimmunity was first suggested by the phenotype of PD-1−/− mice (48). PD-1 deficiency results in the spontaneous development of some features of a late onset lupus-like disease characterized by autoantibodies and mild glomerulonephritis in C57BL/6 mice. Autoimmunity is accelerated by PD-1 deficiency on autoimmune-prone backgrounds (49), providing further evidence for a role for PD-1 in the induction and/or maintenance of tolerance. Analysis of the functions of PD-1 and its ligands in tolerance and autoimmunity is now an active area of investigation. Here we provide an overview of the mechanisms by which PD-1 and its ligands control self-reactive T cells, laying the groundwork for our discussion of the functions of PD-1 and its ligands in autoimmune diseases.
The PD-1:PD-L pathway delivers inhibitory signals that regulate both central and peripheral T-cell tolerance. In the thymus, PD-L1 is expressed broadly on the thymic cortex and on thymocytes, whereas PD-L2 is expressed in the thymic medulla. CD4−CD8− (DN) thymocytes begin to express PD-1 at the stage of TCRβ rearrangement. PD-1:PD-L1 interactions restrict positive selection during the DN to CD4+CD8+ (DP) stage of thymic development (50). Signals through PD-1 regulate signaling thresholds during positive selection, and lack of either PD-1 or PD-L1 increases DP thymocyte cell numbers (51). PD-1 also participates in negative selection (52). PD-1 and PD-L1 have been further implicated in tolerance by gene expression profiling studies of central tolerance in nonobese diabetic (NOD) mice (53).
The PD-1:PD-L pathway controls peripheral T-cell tolerance in several ways. This pathway limits the initial phase of activation and expansion of self-reactive T cells, and restricts self-reactive T-cell effector function and target organ injury. PD-1:PD-L interactions can inhibit expansion of naive self-reactive T cells and/or their differentiation into effector T cells. PD-L1 and PD-L2 are expressed on tolerogenic DCs, providing a means by which these PD-1 ligands may control the decision between T-cell activation and tolerance (54). PD-1 deficiency on antigen-specific T cells increases CD8+ T-cell responses to antigen-bearing resting DCs. The PD-1: PD-L pathway can also negatively regulate reactivation, expansion, and/or functions of effector T cells (55, 56). Bone marrow chimera studies indicate that PD-L1 on non-hematopoietic cells can restrain self-reactive T-cell responses in target organs. The expression of PD-L1 on endothelial cells as well as other nonhematopoietic cells may be important for maintaining tissue tolerance (57). PD-L1 on endothelial cells has the potential to restrict extravasation of T cells into target organs (58). PD-L1 on non-hematopoietic cells may also limit immune-mediated tissue damage by controlling functions of pathogenic effector cells (56, 59). The relative contributions of PD-L1 on lymphoid, endothelial, and other cells in regulating effector T-cell responses are not yet clear. Signals through the PD-1:PD-L pathway also regulate the dynamic interplay between Treg and T effector cells. PD-1 and PD-L1 are both expressed on Tregs (60). As detailed below, this pathway controls the development, maintenance, and function of induced Treg cells.
Regulatory T cells
Biology and function
Although a number of fairly novel CD4+ T-cell subtypes have garnered the attention of the Immunology community, of late, no single cell has burst on the scene quite as dramatically as the CD4+ Treg. Tregs mediate peripheral tolerance by actively suppressing effector T cells and inhibiting immune-mediated tissue damage. Initially revived in the last decade by Sakaguchi, Powrie, Mason and colleagues (61–64), Tregs were identified by high expression of the IL-2 receptor α chain, CD25, and confirmed by subsequent suppressor function. Tregs are hyporesponsive to TCR stimulation but share some surface markers with activated effector T cells (e.g. CTLA-4, GITR, PD-1, PD-L1). It was not until molecular identification of the Treg-restricted transcription factor forkhead box protein 3 (Foxp3) (65–68) that immunologists fully respected Tregs as a lineage-specific subtype of CD4+ T cells. Multiple subsets of Foxp3+ Tregs can be divided into two subsets: naturally occurring (nTregs) and induced Tregs (iTregs). Foxp3+ nTregs are generated in the thymus and are initially detected within the CD4+CD8+ double positive stage to CD4+ single positive stage (69). nTregs express Foxp3, high levels of CD25, and have a TCR repertoire biased towards self-antigens. In contrast, iTregs (or adaptive Tregs) develop in the periphery from CD4+Foxp3− naive T cells. In vitro, the ‘conversion’ of naive T cells toward an iTreg fate is dependent on TGF-β and IL-2 (70–73). Similar to nTregs, iTregs require prior stimulation for Treg activity, potently suppress effector T cells, and express high levels of CD25, CTLA-4, GITR, PD-L1 and PD-1 (74). While other subsets of Tregs (e.g. Foxp3−IL-10+ Tr1 cells or Foxp3−TGF-β+ Th3 cells) may restrain immune responses, we focus this review on the collaboration of natural and induced Foxp3+ Tregs in the maintenance of immune tolerance and homeostasis.
Our understanding of the immunoregulatory nature of Tregs has emerged from the convergence of in vivo and in vitro studies demonstrating the suppressive function of Tregs. Evidence from numerous groups has shown that either depletion of CD25+CD4+ Tregs or ablation of Foxp3+ Tregs results in the development of autoimmune disease (61, 66, 75). That the depletion of one subpopulation of CD4+ T cells can cause severe immunopathology underscored the essential role of Tregs in maintaining peripheral tolerance. Further in vitro evidence convincingly demonstrated that Tregs are able to suppress the proliferation of antigen-stimulated naive T cells (76, 77) and genetic evidence in both mice and humans supported the critical inhibitory role of Tregs in vivo. Scurfy mice, which succumb to lymphoproliferative and wasting disease, result from the disruption of a forkhead/ winged-helix protein, called scurfin (78–81). Scurfin was later found to be the transcription factor, Foxp3 (67, 82). Careful examination of scurfy mice identified a naturally occurring mutation within the Foxp3 gene. Further demonstrating the suppressive power of Tregs, addition of Tregs to Scurfy mice can prevent autoimmune-mediated death (83). Moreover, naive T cells may be converted to functionally suppressive Tregs by induced or forced expression of the Foxp3 gene (66). In humans, a rare X-linked disorder, immune dysregulation, polyendocrinopathy, enteropathy X-linked (IPEX) syndrome, results from mutations in the Foxp3 gene (65, 68). That Treg deficiency or dysfunction results in multiorgan autoimmunity, including severe enteritis, type 1 diabetes mellitus, eczema and hypothyroidism, conclusively demonstrated that a single sub-population of CD4+ T cells was critical for thwarting autoimmune attack.
Mechanisms of Treg suppression
Vindicated, Tregs have become the subject of intense interest and studies of Tregs flood the literature, with studies investigating the development and function of Tregs (76, 84–86) as well as their in vivo roles during autoimmune diseases (87–93). While our understanding of the cellular and molecular basis for Treg-mediated suppression is currently evolving, studies in humans and mice have revealed multiple mechanisms of suppression utilized by Foxp3+ Tregs. Foxp3 negatively regulates IL-2 transcription and upregulates IL-2R expression, rendering exogenous IL-2 a necessity for Treg survival (84, 94, 95). The constitutively high expression of CD25 allows efficient absorption of IL-2 by Tregs, and a concomitant depletion of IL-2 from nearby conventional T cells. Pandiyan et al. reported that consumption of IL-2 by Tregs can result in cytokine deprivation-induced apoptosis of effector T cells (96, 97). Alternatively, work by Shevach and others (76, 77) suggest that Tregs inhibit IL-2 mRNA induction from Foxp3− effector T cells.
Cytokine mediators of Treg suppression
Further studies have revealed three other cytokines, IL-10, TGF-β and IL-35, as important mediators of Treg function. IL-10-producing Foxp3+ T cells are abundantly found in the lamina propria of the intestine and are thought to play a critical role in mucosal tolerance. In vitro, IL-10-deficient Foxp3+ Tregs are fully suppressive, yet in vivo, they are unable to suppress the development of colitis (98–100). Furthermore, IL-10-deficient Tregs compromise mucosal immunity but not systemic tolerance, suggesting that Treg suppressive mechanisms may be environmentally determined to enable appropriate responses within the required biological context.
TGF-β is critical to the induction and maintenance of Foxp3+ Tregs. However, it is still unclear whether TGF-β functions as a suppressor molecule utilized by Tregs (70, 101, 102). Initial reports using TGF-β-deficient Tregs or anti-TGF-β neutralizing antibodies suggested that TGF-β was not required for nTreg function, but further in vitro and in vivo studies dispute these findings and suggest that membrane-bound TGF-β is critical for nTreg function (76, 103, 104). Surprisingly, neither active nor latent TGF-β can be visualized with antibodies. Following post-translational processing, TGF-β binds latency-associated peptide (LAP) to maintain TGF-β in its inactive form (105). Disrupting interactions between LAP and TGF-β are central to TGF-β activation. Further studies of activated murine and human Tregs revealed that latent TGF-β was likely the suppressor molecule expressed by Tregs, since LAP, but not TGF-β, can be detected by antibody staining upon TCR activation (106, 107). Andersson et al. demonstrated that latent TGF-β on the surface of Tregs converts Foxp3− cells to Foxp3+ cells upon TCR stimulation, thereby mediating ‘infectious tolerance’ or inducing the conversion of T-effector cells towards a Treg subtype (hereby referred to as ‘contra-conversion’).
IL-35 is a newly described inhibitory cytokine that may directly mediate the suppression of T effectors by Tregs (108). IL-35 is member of the IL-12 cytokine family. It consists of a heterodimer of the Epstein-Barr virus-induced gene (Ebi3) and IL-12α (p35). The Ebi3 gene is a Foxp3 target and both Ebi3 and IL-12α are expressed by murine Tregs. Ebi3-deficient or IL-12α-deficient Tregs have significantly reduced suppressive capacity when cultured with effector/responder T cells plus bead-bound anti-CD3 and anti-CD28. In vivo, Ebi3-deficient and IL-12α-deficient Tregs are unable to regulate the onset and extent of inflammatory bowel disease. While IL-35 may be critical for Treg suppressive activity in mice, human studies have failed to identify IL-35 in activated or resting Tregs (109). However, there are data to suggest that human DCs may produce IL-35 (109), but further experiments are required to confirm a role for IL-35 as a suppressive cytokine that may function within a DC-Treg-T effector triad.
Regulation of DC function by Tregs
Interactions between dendritic cells and Treg cells have been identified as another mechanism by which Tregs influence the ensuing immune response. Tang et al.(110) and Tadokoro et al. (111) used two-photon laser scanning microscopy to demonstrate that antigen-experienced T-effector cells arrested with DCs in the absence of Tregs. Upon addition of Tregs, the interaction time between DCs and T effectors was diminished. Tregs also disrupted stable clusters of diabetogenic T cells in the pancreatic lymph nodes (110, 111), thus limiting T-cell priming by DCs.
DCs may also be intrinsically affected by Treg interactions. Misra et al. (112) and Serra et al. (113) demonstrated that Tregs could downregulate costimulatory molecule expression on human and murine DCs. CTLA-4 is expressed constitutively on CD4+Foxp3+CD25+ Tregs (114–116), and recent work by Wing et al. (117) demonstrated that selective ablation of CTLA-4 in Tregs could cause systemic autoimmunity and fatal disease but did not alter Treg development or convert Tregs toward pathogenicity. CTLA-4-deficient Tregs are less suppressive than WT Tregs when cultured with DCs and T effectors. Some studies suggest that Tregs expressing CTLA-4 can induce the downregulation of CD80 and CD86 on DCs, thereby educating the DC to become less activated and/ or more tolerogenic. Alternatively, it is possible that CTLA-4-expressing Tregs may suppress T effectors by ligating CD80 on the effector T cell, resulting in the inhibition of T-cell proliferation and cytokine production (118).
Treg-mediated cytolysis
In addition, Tregs may also exert their functions by Treg-mediated cytolysis of effector T cells, APCs, or NK cells (119–122). Grossman et al. (119) showed granzyme A expression in human Tregs and demonstrated that human Tregs can utilize granzyme A and perforin to kill target cells. Consistent with these data, Noelle and colleagues (120) first demonstrated that granzyme B-deficient Tregs induced less effector cell apoptosis than wildytpe Tregs. Further work has shown that Treg-mediated killing of APCs occurs in a granzyme B and perforin-dependent fashion (121). The cytolytic function of Tregs may be regulated by the inflammatory microenvironment. Granzyme B was not detected in naive Tregs, but was expressed in CD4+Foxp3+ Tregs from tumor-challenged mice (122). Furthermore, tumor-exposed Tregs induced cytolysis of NK cells and CD8+ T cells in a granzyme B and perforin-dependent fashion.
Regulatory T-cell plasticity
With an arsenal of suppressive strategies, Tregs are a prime candidate for the immunotherapy of autoimmune diseases. However, growing evidence suggests that Tregs are not stable and can be reprogrammed into competent effector cells, revealing plasticity of the regulatory T-cell lineage (123, 124). With many Tregs bearing autoreactive TCRs, stabilization of the Treg phenotype is essential to avoid potential complications of Tregs serving as a reservoir of autoreactive T-effector cells (125). Williams et al. (126) demonstrate that sustained expression of Foxp3 maintains the Foxp3-dependent developmental program of Tregs. Interestingly, when Komatsu et al. (127) transferred Foxp3+ Tregs into lymphopenic hosts, a fraction of the cells became Foxp3− and no longer suppressed T-effector cell proliferation. Moreover, the Foxp3− (former Tregs) began to produce IL-2 and IFN-γ. The instability of Tregs may be determined, in part, by the inflammatory milieu. Pasare et al.(128) convincingly demonstrated that TLR stimulation of dendritic cells could result in the production of proinflammatory factors (such as IL-6) that could block the suppressive activity of Tregs. Furthermore, studies in mouse models of multiple sclerosis (MS) and diabetes report decreased Foxp3 expression and Treg dysfunction within the inflamed target organ (129–131). Wan et al. (130) demonstrated that Tregs from mice genetically engineered to express low levels of Foxp3 lose suppressive activity and simultaneously gain effector cell functions (IL-2, IL-4, IFN-γ production). Decreased Foxp3 expression may be directly linked to decreased Treg function, as Egawa et al. (132) reported that Foxp3 can exert a positive feedback loop on its own expression, mediated by the transcription factor Runx1. Ablation of Runx1 leads to decreased Foxp3 expression by Tregs. Interestingly, a mutation in the Runx1 binding site within the enhancer element of the PD-1 gene resulted in a defect of PD-1-mediated inhibition of IFN-γ production in patients with MS (133). More recently, Haxhinasto et al. (134) and Sauer et al. (135) demonstrated that blockade of the PI3K/mTOR pathway and truncation of TCR signaling increased Foxp3 expression. As we describe below, these data are consistent with our findings that the PD-L1: PD-1 pathway limits T-cell stimulation and promotes the differentiation and maintenance of Foxp3+ Tregs by blocking the Akt/mTOR pathway and augmenting PTEN expression (136).
PD-L1-mediated regulation of Tregs
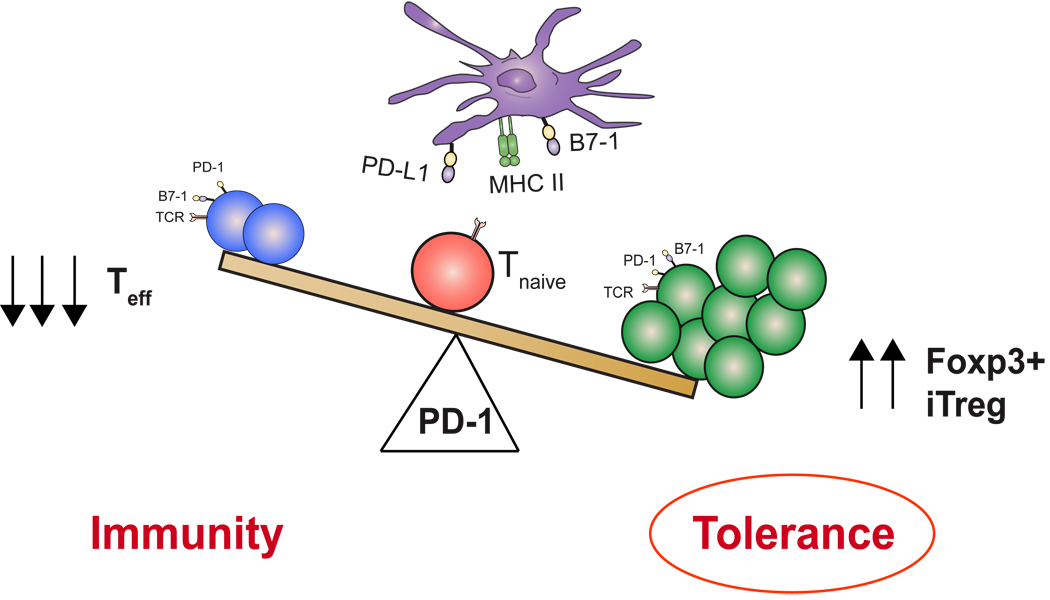
Tregs and the PD-1: PD-L pathway are both critical to terminating immune responses. Elimination of either one leads to uncontrolled T-cell responses, resulting in autoimmunity. Previous studies by our group and others have shown that ligation of PD-1 by either PD-L1 or PD-L2 attenuates effector T-cell proliferation, cytokine secretion, and survival. We and others have questioned whether the PD-1: PD-L pathway has broader control of T-cell responses, specifically interrogating whether Treg frequency or function can be augmented by the ligation of PD-1. We found that in the presence of anti-CD3 and TGF-β, PD-L1-Ig can induce a profound increase in the de novo generation of CD4+Foxp3+ Tregs from naive CD4+ T cells (136) (Fig. 3). Moreover, experiments with PD-L1-expressing APCs resulted in minimal conversion of naive CD4+ T cells to iTreg cells, showing the essential role for PD-L1 during iTreg induction. PD-L1-Ig also can enhance Foxp3 expression and suppressive function of established iTregs. Further engagement of Foxp3+ iTregs by PD-L1-Ig results in better maintenance of Foxp3 expression and enhanced suppressive activity at low Treg to T-effector cell ratios. In mechanistic studies, we demonstrated that PD-L1-Ig induces iTregs from naive T cells by attenuation of Akt-mTOR signaling and concomitant upregulation of PTEN. Although the effects of rapamycin and mTOR inhibition on the development of Treg cells have been well reported (134, 135, 137–139), we first demonstrated how a naturally occurring inhibitory molecule blocks the Akt/mTOR cascade to preferentially push naive T cells toward an induced-Treg fate.
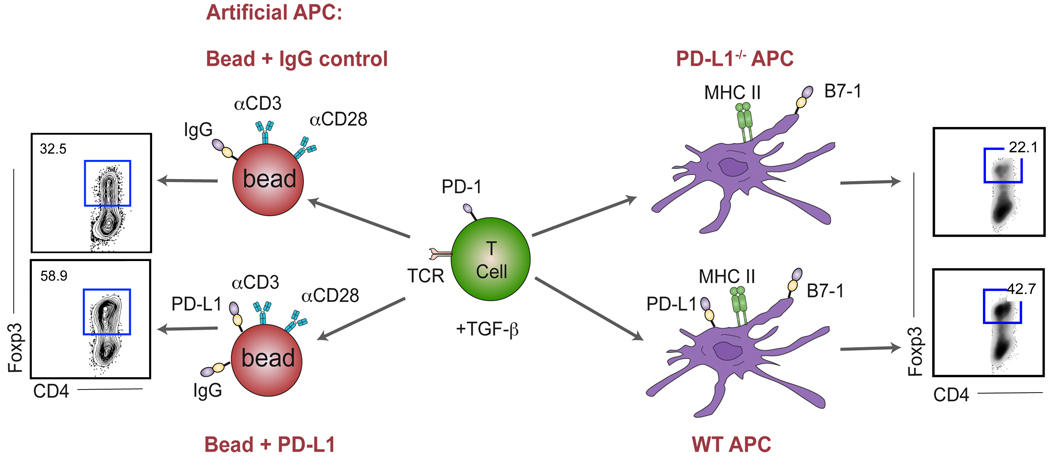
Fig. 3. PD-L1-mediated conversion of naive T cells to regulatory T cells.
PD-1 upregulation upon TCR stimulation mediates PD-1: PD-L1 interaction between the T cell and the APC (or artificial APC consisting of epoxy bead coated with anti-CD3, anti-CD28, PD-L1-Ig and control-Ig) in the presence of TGF-β. When PD-L1 is present on the bead, iTreg conversion is enhanced approximately twofold (left). Similarly, a twofold enhancement of conversion is observed when using WT APCs as compared to PD-L1−/− APCS (right). Adapted from Francisco et al., J Exp Med 2009.
As both nTregs and iTregs express PD-1 and PD-L1, the expression of ligand and receptor on the same cell has some interesting implications. APC encounter of a naive T cell induces expression of PD-1 upon TCR stimulation (Fig. 4). PD-L1 expression on the APC or a newly encountered Treg has the potential to drive the differentiation of a naive T cell toward an iTreg. Therefore, Tregs may further assist Treg-mediated infectious tolerance. However, not all naive cells become Tregs. To permit latitude for T-cell differentiation into multiple T-cell subtypes, cell-intrinsic counter-regulation must exist in naive T cells. Whether Tregs can restrain the magnitude of effector responses by inducing the contra-conversion of T-effectors toward Tregs upon PD-L1: PD-1 interactions remains to be determined.
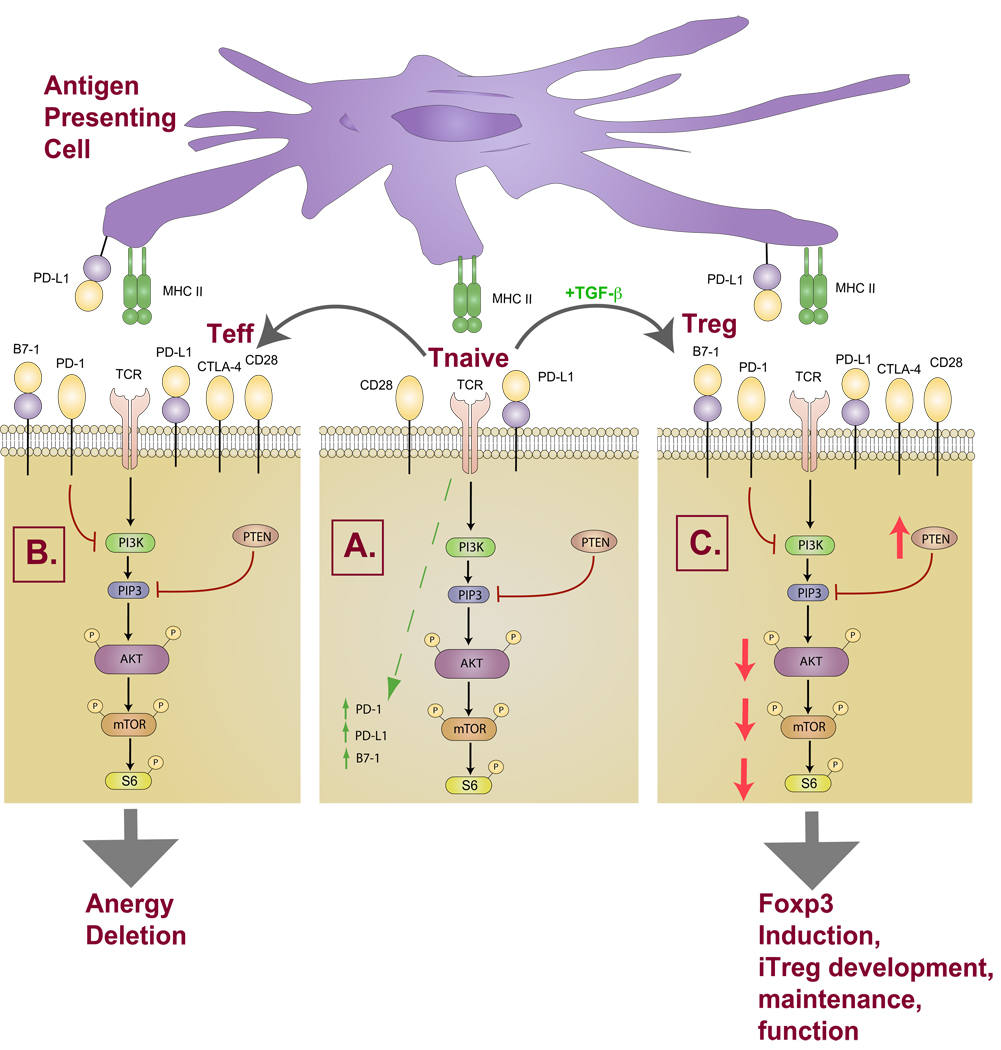
Fig. 4. Differential fates of naive T cells following PD-1 ligation.
(A) Upon TCR engagement of a naive T cell, PD-1 and PD-L1 are upregulated. (B) The PD-L1 pathway inhibits the downstream PI3K/AKT pathway in naive T cells resulting in functional inactivation of naive T cells and inhibition of effector T-cell differentiation and function. (C) In the presence of TGF-β, the PD-1 pathway attenuates the PI3K/AKT pathway, preferentially biasing naive T-cell programming towards iTreg development and survival.
As loss of PTEN augments PD-L1 expression, the PD-1 pathway may negatively regulate its own functions (40). Hence, increasing PTEN expression via PD-1 signaling may result in reduced PD-L1 expression and serve as a negative feedback loop to downregulate Treg development and/ or function. This putative mechanism allows broader T-cell diversity, ensuring that the appropriate T-cell subsets are poised for rapid response to immune challenge.
Since Tregs may mediate suppression by altering stable interactions with effector T cells, the PD-1: PD-L pathway also may control the complicated dynamic interactions among Tregs, T-effector cells, and APCs (Fig. 5). Recently, Bluestone and colleagues (140) reported that PD-1 ligation affects the stability of DC-T-cell contacts by inhibiting TCR-induced stop signals. Two-photon laser-scanning microscopy was used to demonstrate that T cells tolerized for islet antigen did not stably interact with antigen-specific DCs but moved freely in the pancreatic lymph nodes. Using blocking antibodies for PD-L1 or PD-1, Fife et al. (140) elegantly showed that continued interactions between PD-L1 and PD-1 inhibited TCR-mediated stop signals. In our studies using bead-bound anti-CD3 plus PD-L1-Ig to induce Treg differentiation, we similarly observed that increasing concentrations of PD-L1-Ig reduced T-cell clustering. In contrast, bead-bound anti-CD3 plus control-Ig induced stable T-cell clusters during Treg differentiation (L. Francisco, V. Salinas, and A.H. Sharpe, unpublished observations). Hence, the constitutive expression of PD-L1 and PD-1 on Tregs may regulate formation of stable and productive immunological contacts, providing a novel mechanism of suppression utilized by Tregs.
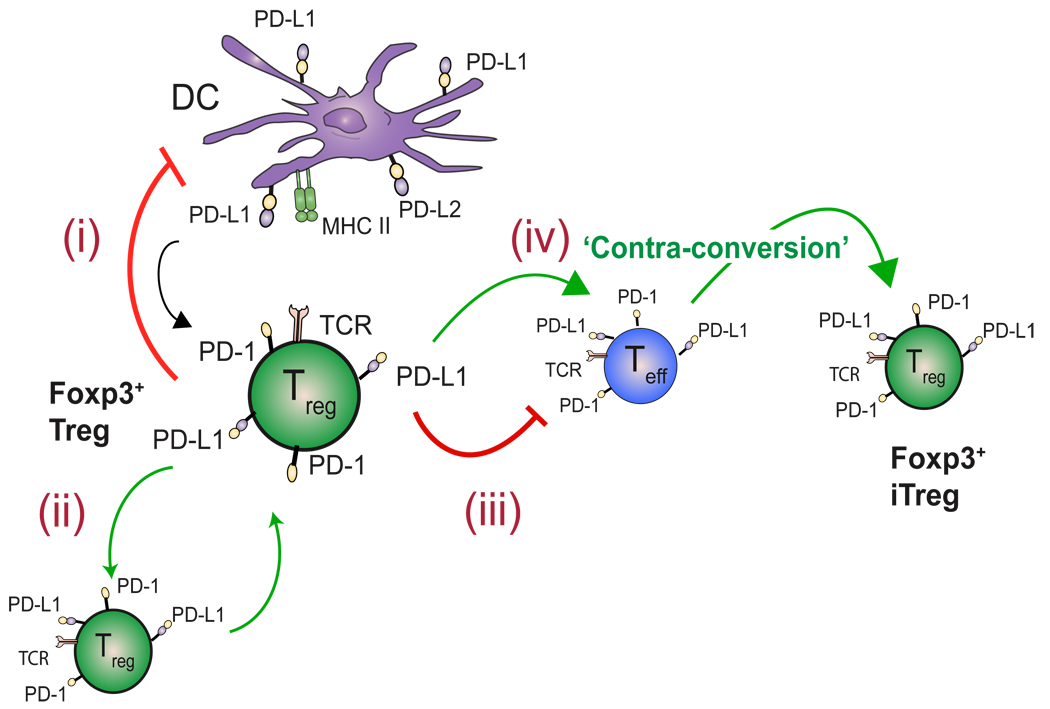
Fig. 5. Functions of the PD-1 pathway on Tregs.
(i) PD-L1-expressing DCs may induce the conversion of naive T cells to Foxp3+ iTregs. Upon interaction with a PD-L-bearing DC, Tregs may condition the DC towards tolerogenicity. (ii) PD-L1-expressing Tregs may sustain Foxp3 Treg expression and effector function in other Tregs. (iii) Tregs can also directly suppress autoreactive T effectors (limit IL-2 production, prevent cell cycle progression, negatively influence stable DC: Teff interactions, etc.) or (iv) promote the contra-conversion of T effectors to iTregs.
PD-L1 expression on Tregs may directly influence the tolerogenicity of DCs, as illustrated by recent adoptive transfer experiments. Antigen-specific CD4+Foxp3− effector T-cell expansion was limited by co-transfer of iTregs and protected mice from autoimmune gastritis (141). Transfer of iTregs reduced T-effector cell priming by DCs originally exposed to iTregs in vivo as compared to unconditioned DCs. iTreg-conditioned DCs also downregulated expression of costimulatory molecules CD80 and CD86, suggesting that Treg suppressive capacity may be due, in part, to decreasing the stimulatory capacity of DCs. While DiPaolo et al. did not find any differences in PD-L1 or PD-L2 expression on DCs influenced by iTregs in vivo, expression of PD-1 was not examined. Chen and colleagues (29) recently have shown that DC expression of PD-1 may negatively regulate DC function and thereby impede immune responses. Thus, PD-L1-expressing iTregs have the potential to indirectly suppress T-effector responses by directly engaging PD-1 on dendritic cells and modulating DC function.
When considering the consequences of uncontrolled immunity, it would be therapeutically advantageous to simultaneously harness two inhibitory modalities (Treg induction and negative costimulation). Not only would ligation of the PD-1 pathway result in diminished effector T cells but also would lead to generation of Tregs that would be restrain pathogenic T cells that have escaped into the periphery (Fig. 6). Tregs induced by the PD-1 pathway may also assist in maintaining immune homeostasis, keeping the threshold for T-cell activation high enough to safeguard against autoimmunity, while simultaneously permitting protective immunity.
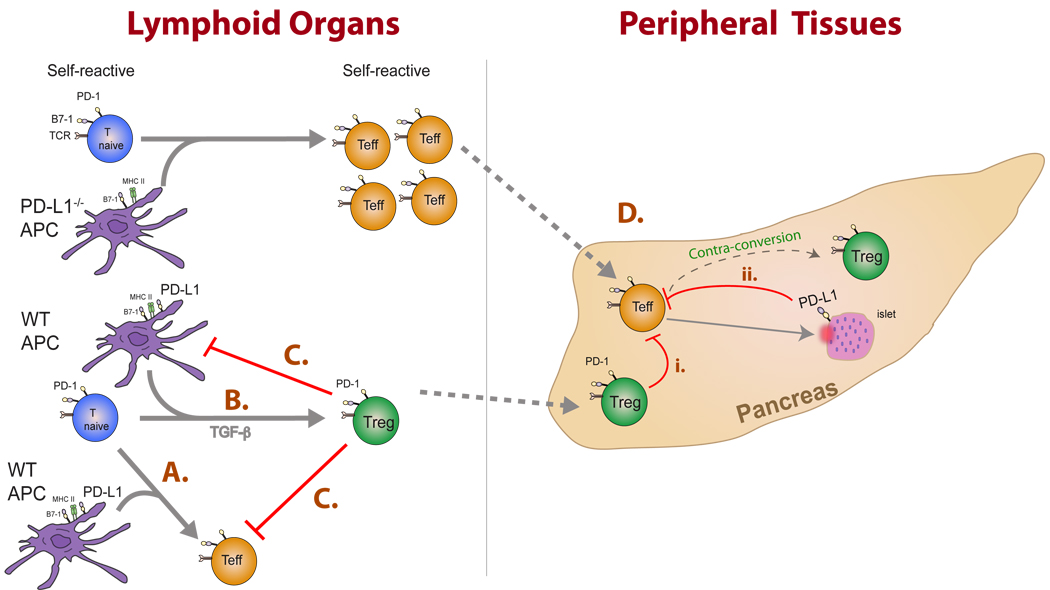
Fig. 6. The PD-1:PD-L pathway can regulate the dynamics between effector and regulatory T cells during multiple stages of autoimmune disease progression.
This figure illustrates consequences of these interactions in lymphoid organs and peripheral tissues. (A) PD-L1-expressing DCs stimulate PD-1 expression on naive T cells. PD-L1: PD-1 interactions limit T-effector cell expansion and survival. (B) In the presence of TGF-β, PD-L1-expressing DCs can promote the development of iTregs. (C) Tregs may restrain the magnitude of the immune response by inhibiting both DC and effector T-cell functions. (D) Activated T effector cells migrate to sites of inflammation where interaction with (i) PD-1-expressing Tregs or (ii) PD-L1 expressing target cells can (a) directly inhibit T-effector responses or may( b) facilitate the ‘contra-conversion’ of T effectors toward iTregs within the target organ. Additional cell types expressing PD-L1 such as the vascular endothelium and stromal cells may also influence T-effector cells but are not depicted in this figure for simplicity.
The PD-1 pathway, Tregs, and autoimmune diseases
Type 1 diabetes
Type 1 diabetes (T1D) is an autoimmune disease, resulting from severe β-cell destruction within the pancreatic islets. The naturally occurring non-obese diabetic (NOD) mouse strain, which spontaneously develops diabetes, has facilitated the understanding of the cellular and molecular mechanisms underlying the pathogenesis of T1D. The disease susceptibility and phenotype of the NOD mouse closely resembles human T1D and therefore is considered one of the most physiological murine models for peripheral tolerance and autoimmunity.
Diabetes develops in NOD mice at 14 to 30 weeks of age and is diagnosed by increased blood glucose levels and insulitis, indicative of pancreatic inflammation and β-cell destruction. Transfer studies of diabetogenic and non-diabetogenic T cells into irradiated mice have shown that T cells from non-diabetic mice can control diabetes (142, 143). These non-pathogenic T cells were CD25 positive and regulated T-effector cell responses (116). As discussed earlier, Tregs utilize a number of different mechanisms to inhibit immune responses, including secretion of anti-inflammatory cytokines (IL-35, IL-10, and TGF-β), killing of effector T cells directly, metabolic inhibition of effector T cells, or modulation of APCs (144). Of these possible modes of suppression, TGF-β has been shown to be important in regulating T1D in the NOD mouse. Tregs cannot control disease progression in TGFβRII knockout NOD mice (145), possibly due to the lack of Treg presentation of surface-bound TGF-β to effector T cells (103), thereby likely impairing immune responses through conversion of effector T cells to Tregs. Additional studies are needed to further elucidate mechanisms of Treg suppression in T1D progression.
The role for Tregs in human T1D is less clear than in mouse models. Several studies have shown either reduced Treg numbers or altered Treg function in human T1D (87, 146, 147). In one recent study the defect in Treg suppression was assessed using in vitro studies of Treg function. Increased IFN-γ production (87, 146) and decreased IL-10 production (87) were seen in cocultures of Tregs and T effectors. However, both populations were obtained from the same individual, making the intrinsic defect of either the Treg population or the T-effector cell population impossible to discern. Other studies have shown that Tregs from diabetic patients have normal suppressive function in similar autologous cocultures of T-effectors and Tregs (148). Moreover, iTregs from T1D patients can regulate T-effector cells from healthy individuals (149). The discrepancy among these studies also may be complicated by different techniques in Treg isolation. Lacking a precise surface-marker for Tregs, isolation of CD4+CD25+ T cells results in a heterogeneous population consisting of both Tregs and T-effector cells. Furthermore, effector T cells from T1D individuals may be intrinsically defective. Schneider et al. (149) found that T-effector cells isolated from T1D patients are not suppressed by iTregs from healthy individuals. The T-effector cells and Tregs used in many of these studies have come from blood. T-effector cells and Tregs in the tissues are likely to be different due to increased T-cell activation thresholds resulting from endothelial and/or parenchymal interactions with inhibitory molecules. Although still unclear, Tregs may factor into disease progression. It is unclear whether a general defect in Treg function exists. Possibly, the pro-inflammatory milieu may ‘tip the balance’ and overwhelm the Tregs. More in depth studies are needed to investigate these issues.
Both PD-1 and PD-L1 have been implicated in regulating the pathogenesis of T1D from studies in mouse models of the disease. PD-L1 expression is seen on β cells of the islets and may function to limit self-reactive T cells at the target tissue. Ansari et al. (55) demonstrated the protective role of PD-L1 interactions in T1D. In this study, administration of blocking antibodies to PD-1 (J43) or PD-L1 (MIH6) drastically accelerated diabetes in the NOD mouse when given at any time point (55). When Martin-Orozco et al. (150) performed parallel studies in the RIP-OVA transfer model of peripheral tolerance (which uses diabetes as a readout for a break in tolerance), they noted a similar acceleration of diabetes after administration of blocking antibodies to PD-1 (J43) or PD-L1 (MIH5). Blocking PD-L2 (clone TY25) does not alter diabetes progression in either NOD mice or in the RIP-OVA transfer model of peripheral tolerance (55, 150). A PD-1: PD-L1 interaction exclusively accelerated diabetes because blockade of either PD-1 or PD-L1 gave virtually identical results. Wang et al. (49) demonstrate a similar acceleration of diabetes in the NOD PD-1−/− mouse. Consistent with these findings, we observed accelerated diabetes in both the NOD and RIP-OVA adoptive transfer models using PD-1-deficient donors and PD-L1-deficient recipients (56, 59). Additionally, we demonstrated that PD-1-deficient T cells are unable to be cross-tolerized in the RIP-OVA adoptive transfer model (59).
Although it is clear that PD-1 plays a critical role in the pathogenesis of T1D, the precise location(s) of PD-1:PD-L interaction remains to be determined. Bone marrow chimera studies have revealed that PD-L1-expressing parenchymal cells control peripheral tolerance in NOD and RIP-OVA models (56, 59). Since PD-L1-expressing radio-resistant cells were critical for peripheral tolerance, it is unlikely that a hematopoietic lineage cell was conferring this tolerance. Both islet cells and vascular endothelial cells within the pancreas express PD-L1 and therefore may be the cells inducing tolerance.
PD-L1 expression on islet cells may protect them from diabetogenic T-cell cytolysis during islet destruction. During the setting of islet transplantation in syngeneic or allogenic settings, PD-L1 has been shown to protect from either autoimmune progression or graft rejection, respectively (56, 151). Studies addressing the function of islet PD-L1 expression using transgenic approaches have yielded conflicting results, perhaps related to differences in genetic background or promoters used. Studies by Subudhi et al. (152) show that in C57BL/6 mice, transgenic overexpression of PD-L1 under the rat insulin promoter causes spontaneous diabetes. When OTI T cells were transferred to RIP-OVA C57BL/6 mice constitutively expressing PD-L1 under the RIP promoter, the recipients develop more severe diabetes than control mice. These findings contrast with recent work in which PD-L1 was overexpressed in diabetic prone NOD mice using a human insulin promoter (153). Overexpression of PD-L1 in NOD mice almost completely protected them from diabetes (154). These results remain to be reconciled with other studies that show the dampening of inflammation by PD-L1. PD-L1 may be simultaneously preventing cytolysis as well as participating in contra-conversion of T-effector cells to Tregs. Additionally, PD-L1 could also have a cell-intrinsic role in the islet cell itself.
PD-L1 not only is found on the islets but is also expressed on the vascular endothelium in the pancreas (154). Endothelial expressed PD-L1 protects from cytolysis but may also act as a final checkpoint before diabetogenic T cells enter the tissue (58). Although controversial, if endothelial cells are capable of endocytosing and presenting antigen, PD-L1 expression by endothelial cells may also aid in downregulating the immune response and participate in contra-conversion of T-effector cells toward a Treg phenotype. In support of this concept, work by Krupnick et al. (155) suggests that endothelial expression of PD-L1 may drive iTreg development using in vitro allograft models. Other stromal cells may also be important in influencing the PD-1:PD-L pathway. Thus, PD-L1 expression within inflamed sites may be essential for the maintenance of peripheral tolerance.
PD-1 signals may be important at multiple checkpoints during the initiation and progression of diabetes. Recently, intravital microscopy studies have revealed that PD-1 ligation truncates the TCR signal in lymph nodes (140). When naive BDC2.5 transgenic T cells were transferred into prediabetic NOD mice that were previously ‘tolerized’ by injection of fixed antigen-pulsed splenocytes and injected with anti-PD-L1 blocking antibodies (MIH5 and MIH6), the duration of TCR signal was much longer under PD-L1 blocking conditions, manifested as reduced movement of the BDC2.5 T cells within the lymph node. The prolonged cell-cell contact during PD-L1 blockade increased IFN-γ production, broke immune tolerance, and resulted in diabetes progression. It is not clear if blockade of the PD-1 signal with PD-L1 blocking antibodies modulated the function or differentiation of Tregs. Since we have shown that PD-1 ligation both diminishes T-cell activation and actively converts T cells towards Tregs, dysregulation of PD-1 signaling can simultaneously enhance Tregs and limit T-effector cells during diabetes progression.
Although the exact function of PD-1 signaling on Treg function in T1D is still unclear, it is possible that PD-1 modulation may change contact time as well as the local cytokine milieu, both of which may alter the differentiation of naive T cells away from the T-effector phenotype and toward Treg cells. It has been shown that modulating dendritic cells to a ‘less responsive’ phenotype can increase the polarization to the Treg subset (156, 157). In one of these studies, treatment of DCs with vitamin D3 increased the PD-L1/CD86 ratio and also led to an increase in Treg function (157). Consistent with the results reported by Fife et al.(140), blocking PD-L1 led to an increase in IFN-γ and less IL-10, which inhibited the tolerogenic ability of Tregs.
It is quite clear that PD-1 engagement by PD-L1 leads to a truncation of the TCR signal and affects cytokine production. The net result of blockade in T1D is a breach of tolerance, by inhibiting both Treg numbers and function. In the case of T1D, further work is needed to elucidate which stages of the autoimmune response are influenced by PD-1. PD-L1 expression in the lymph node, on vascular endothelium, and on the islets all may contribute to tolerance. PD-L1 on other cell types such as stromal cells may also function in similar roles.
Multiple sclerosis
MS is an autoimmune disease in which immune-mediated attack of the brain and spinal cord results in inflammation and demyelination of the central nervous system (CNS). Studies of experimental autoimmune encephalomyelitis (EAE), a murine model of MS that recapitulates a number of immunopathological processes found in human MS, reveal an important role for CD4+ T cells in disease pathogenesis. While a number of studies have shown decreased Treg function in relapsing-remitting MS, Viglietta et al. (91) first reported a functional defect in CD4+CD25high T cells isolated from peripheral blood of MS patients. When tested with in vitro suppression assays, CD4+CD25high Tregs were less effective at suppressing the proliferation and IFN-γ production by effector T cells from either autologous or healthy individuals in the presence of lower concentrations of anti-CD3. The interpretation of many human Treg studies is technically limited by the lack of Treg-specific surface markers. Isolation of CD4+CD25high cells not only results in an enriched population of Tregs but also may include effector T cells as well. To avoid this complication, Michel et al. (158) isolated CD4+CD25high T cells that expressed only low levels of the IL-7Rα chain (CD127) from MS patients and healthy controls. Foxp3 interacts with the CD127 promoter and likely represses its protein expression, resulting in an inverse correlation between Foxp3 and CD127 expression (158, 159). These CD4+CD25highCD127low Tregs represented a more pure population of regulatory cells that were more suppressive than their CD4+CD25highCD127high counterparts (158, 160). Hence, unlike previous reports, Tregs from MS patients, selected on the basis of CD127low expression, exhibit suppressive functions that are comparable to healthy individuals. These data suggest that previous findings of reduced Treg function in MS patients may be due, in part, to CD4+ effector cells within the unfractionated CD4+CD25+ pool. However, it remains unclear whether peripheral blood CD4+CD25highCD127low Tregs are functionally similar to CD4+CD25highCD127low Tregs present in the CNS during inflammation.
Studies of Treg cell function in EAE, reveal critical roles for Tregs in the prevention and amelioration of CNS inflammation by limiting encephalitogenic T-cell expansion and function. Adoptive transfer of CD4+CD25+ Tregs decreased inflammation of the CNS and conferred substantial protection from clinical EAE (161). Depletion of Tregs with an anti-CD25 monoclonal antibody (PC61) rendered naturally resistant B10.S mice susceptible to proteolipid protein (PLP139–151)-induced EAE (162). Furthermore, Treg depletion prior to disease induction facilitated the expansion of PLP139–151-reactive cells, enhanced proinflammatory cytokine production (IL-6, IL-17 and IFN-γ), and increased disease severity in the otherwise resistant mice (163–165). These studies convincingly demonstrated that Tregs are instrumental in providing a protective barrier from immune-mediated tissue damage, thus setting an activation-threshold for autoimmune disease onset.
During EAE, an accumulation of Tregs in the CNS has consistently been seen during disease resolution (129, 161, 165, 166). McGeachy et al. (165) demonstrated central roles for Tregs during the induction and effector phases of EAE. Low numbers of adoptively transferred Tregs protect recipient mice from disease induction, whereas depletion of Tregs prevents recovery from EAE and further exacerbates disease progression (165). Moreover, CNS-derived Tregs were capable of suppressing both naive CD4+CD25− cells and CNS-derived CD4+CD25− cells. However, the interpretation of the functional capacity of CNS-derived Tregs is limited by their lack of antigen specificity and also by the phenotype of CNS-derived CD4+CD25− T cells. As T cells generally upregulate CD25 upon activation, the isolation of CD4+CD25− cells from the CNS as putative effector cells may not accurately represent an encephalitogenic effector population from an inflamed CNS. In contrast, using Foxp3-GFP reporter mice and MOG-tetramers to further define antigen-specific Tregs and T effector cells, Korn et al. (129) demonstrated that although Tregs are critical for regulating naïve MOG-specific T cells, they may not be as effective at regulating MOG-reactive T effectors within the target site. Tregs are often tested ex vivo against non-encephalitogenic effector cells. Korn et al. (129) suggest that antigen-specific encephalitogenic effector cells derived from sites of inflammation are distinct and respond differently to Tregs than non-encephalitogenic effector T cells. When CNS-infiltrating tetramer-positive Tregs were combined with MOG tetramer-reactive T effector cells, Tregs could no longer maintain suppressive function, at least in part owing to the production of pro-inflammatory cytokines (IL-6 and TNF-α) that may overwhelm the suppressive capacity of Tregs (128, 129, 167). Hence, the total milieu in which ‘Treg meets T-effector meets cytokines’, affects disease resolution. How inhibitory cytokines and molecules alter this balance remains to be investigated.
PD-1 and its ligands are expressed on a variety of cell types within the CNS. PD-1 is constitutively expressed in retinal neurons of naive mice, while PD-L1 and PD-L2 are found on retinal neurons under inflammation conditions (168). Similarly, during active EAE, meningial infiltrates express PD-1, PD-L1, and PD-L2 (38), suggesting a role for the PD-1 pathway in regulating inflammatory processes within the CNS. PD-L1 is also expressed on astrocytes and vascular endothelial cells and is induced by IL-12 on CD11b+ APCs in mice with EAE (169). This PD-L1 expression is due to IFN-γ, since it is abrogated in IFN-γdeficient mice. Studies with IL-12p35 deficient mice, demonstrated that IL-12 suppresses EAE in C57BL/6 mice (170). Furthermore, microglial cell expression of PD-L1 is regulated by IFN-γ (171). Together, these data implicate the PD-L1:PD-1 pathway as central to disease progression. In addition, studies of German MS patients revealed a mutation found in the enhancer element of the PD-1 gene that affects Runx1 binding and results in a decreased ability of PD-1 to inhibit IFN-γ production. As Runx1 augments Foxp3 expression, this mutation may also result in decreased Foxp3 expression by Tregs (133).
Initial studies describing PD-1 and PD-L2 as critical to EAE pathogenesis used neutralizing antibodies specific for PD-1 (J43) or PD-L2 (TY25) monoclonal antibodies during disease induction (172). Salama et al. (172) showed that PD-L1 or PD-L2 blockade led to the expansion of MOG-reactive T cells, increased lymphocytic infiltration of the CNS, and ultimately, accelerated disease onset and severity. Studies with PD-1 and PD-L-deficient mice indicate that PD-1 and PD-L1, but not PD-L2, are predominantly responsible for regulating disease severity (173, 174). PD-1 and PD-L1-deficient mice may have intrinsic deficits in regulation. Restimulation of T cells from PD1−− and PDL1−/− with active disease resulted in copius production of IL-17 and IFN-γ as compared to WT mice, suggesting that T cells with deficient PD-1 signaling may be preferentially polarized toward effector T-cell differentiation (174).
A number of reports have indicated a role for regulatory T cells in EAE. Adoptive transfer studies have identified critical functions for PD-L1 on both the transferred T cells and in the recipient animal (173). As these studies were done with CD4+ enriched cells, it is likely that regulatory populations expressing PD-L1 may be directly converting or suppressing effector CD4+ T cells via PD-1 ligation, since PD-1 and PD-L1 are highly expressed on Tregs (60). Moreover, host expression of PD-L1 may contribute to the peripheral conversion of transferred T cells toward an iTreg phenotype, a notion supported by the observation that vascular endothelial expression of PD-L1 may induce Treg cell populations (155). More direct support for a role of the PD-1:PD-L1 pathway in iTreg induction came from studies in EAE identifying that PD-1 is directly modulated by pertussis toxin (PT) administration (175). Pertussis toxin was originally thought to increase the permeability of the blood brain barrier prior to disease onset in EAE (176, 177). PT was also shown to induce P-selectin on pial vessels and enhance adhesion of activated T cells (178, 179). Importantly, PT administration decreased the frequency and function of Tregs and increased Th1 and Th2 responses (180–182). Wang et al. (175) showed PD-1-deficient mice given MOG/CFA without pertussis toxin develop fulminant disease, owing to a reduced iTreg frequency. This is in marked contrast to WT mice which do not develop EAE in the absence of PT. Splenocytes from WT mice immunized with MOG/CFA in the absence of PT had a two- to three-fold increase in Foxp3+Treg frequency compared with PD-1-deficient mice. In vitro conversion of CD4+ T cells into iTregs and their subsequent suppressive activity was PD-1 dependent. Although pertussis treatment does not alter the expression of the Treg phenotypic markers CD45RB, CD103, GITR, and CTLA-4, PT directly downregulates PD-1 on Tregs, underscoring a central role for PD-1 in iTreg development and supplying a mechanism for the immunological adjuvancy of PT in multiple strains of mice (175, 181).
These numerous studies clearly demonstrate that the interplay between the PD-1 pathway and Tregs is instrumental in regulating inflammation within the CNS. That neurons express PD-L1 (and PD-L2) upon inflammation and produce TGF-β is intriguing in light of evidence describing that encephalitogenic T cells are converted to iTregs upon neuronal encounter (166, 168). When neurons isolated from 7-day-old mice were co-cultured with encephalitogenic T cells, this resulted in an outgrowth of a CD4+ T-cell population that expressed Foxp3, CD25, TGF-β1, and CTLA-4 and could suppress encephalitogenic T cells and inhibit EAE. It is thus plausible that encephalitogenic effectors T cells encounter neurons and stimulate their PD-L1 expression. Neuronal PD-L1 then drives iTreg conversion from effector T cells, effectively restraining CNS inflammation and spreading infectious tolerance.
Inflammatory bowel disease
Inflammatory bowel disease (IBD) is a group of inflammatory conditions of the small intestine and colon. The major types of IBD are Crohn’s disease and ulcerative colitis. Chronic inflammation of the intestinal mucosa is thought to result from an aberrant immune response toward commensal bacteria. Normally, the intestinal micro-environment exists in a delicate immunological equilibrium, providing tolerance to resident intestinal flora and protection from potential harmful pathogens. To limit inflammation, the gut has evolved multiple safeguards for maintaining immune homeostasis, one of which is provided by Tregs. As a site of daily entry for exogenous oral antigen, gut-resident Tregs are well poised to reinforce intestinal tolerance. That Tregs are central to the prevention of autoimmunity and control of colitis and gastritis in vivo is evident in both humans and mice, as illustrated by the IPEX syndrome, which is accompanied by severe enteritis and the wasting disease that develops in Scurfy mice, unless treated with wildtype Tregs. In a murine T-cell transfer model of colitis, Uhrig et al. (183) showed that CD4+CD25+Foxp3+ Tregus can prevent and ameliorate intestinal inflammation. Foxp3+CD4+CD25+ cells were found to accumulate in the colon and secondary lymphoid organs of mice in experimentally induced colitis when T-effector cells were transferred in the presence or absence of Tregs. Similarly, colon samples from patients with ulcerative colitis or Crohn’s disease revealed an increase in Foxp3+ cells, suggesting that Tregs may traffic to and/ or develop within inflammatory sites as a compensatory mechanism to resolve inflammation.
As a site where pathogens are continuously encountered, the GI tract must regularly maintain and replenish Treg populations specific for enteric flora to preserve tolerance. In fact, antigens found in the intestinal microenvironment distinctly shape the repertoire of Tregs found in the gut. Orally fed antigens induce Foxp3+ iTregs predominantly within the gut-associated lymphoid tissue (GALT) due to specialized mucosal CD103+ DCs (184, 185). Moreover, Lantrop et al. (186) find that Tregs within the mesenteric lymph nodes express unique TCR sequences compared with Tregs isolated from non-gut associated peripheral lymph nodes.
While induction of Tregs and anergy or deletion of autoreactive T cells occurs in studies of oral tolerance in mice, results of humans studies are not as clear (187). In patients with ulcerative colitis and Crohn’s disease, a breakdown in orally induced mucosal suppression is observed (188). When IBD patients and healthy individuals were fed keyhole limpet hemocyanin (KLH) prior to subcutaneous immunization and boosting, KLH feeding led to the suppression of T-cell proliferation in healthy control individuals. In contrast, IBD patients fed KLH showed significantly augmented T-cell proliferation, indicating defective gastrointestinal immunosuppression.
Another proposed mechanism for the breakdown of mucosal tolerance is that individuals with IBD may not only have reduced numbers of iTregs but that these Tregs are simply inundated by effector T cells and the cytokines they produce. Increased expression of the proinflammatory cytokines IL-12, IL-17, IL-21, IL-23, and IFN-γ is seen in inflamed IBD mucosa. Eastaff-Leung et al. (189) associate IBD with a reduced ratio of Treg to Th17 cells in peripheral blood. This reduction in the Tregs to Th17 cell ratio in the peripheral blood may reflect Treg trafficking and/or sequestration within the inflamed gut as elevated expression of both Foxp3 and IL-17 is seen in the mucosa of IBD patients. Intestinal biopsies revealed elevated levels of IL-6 and IL-1β transcripts, both of which have been implicated in preventing effective Treg suppression of Th17 cells during active autoimmune disease (IBD and EAE) (129, 189). It is possible that other subsets of Treg cells that do not express Foxp3 [such as IL-10-producing Type 1 regulatory T cells (Tr1) and orally induced TGF-β-producing Tregs (Th3)] may regulate tolerance at mucosal sites and provide another barrier of protection from autoimmune attack. However, a discussion of these Foxp3− cells is beyond the scope of this review.
That PD-L1 is highly expressed on intestinal epithelial cells of IBD patients compared to healthy individuals suggests an important role for the PD-L1: PD-1 pathway in regulating mucosal tolerance in situ (190). In a mixed leukocyte reaction (MLR) of IECs from IBD patients and control donor T cells, addition of an anti-PD-L1 blocking antibody (MIH-2) resulted in increased IFN-γ production. Because the MLR was performed with both CD4+ and CD8+ peripheral blood T cells, PD-L1 blockade may have blocked the IEC-induced generation of iTregs and thereby resulted in the increased IFN-γ production by effector T cells. PD-L1 blockade could simultaneously directly abrogate effector cell inhibition.
Experiments in murine models of colitis support a role for the PD-1: PD-L1 pathway in controlling regulatory cells and have identified a subpopulation of regulatory CD4+CD25-PD-1+T cells that can inhibit the development of colitis. PD-1 expression on non-colitogenic T cells isolated from spleens of naive mice provides the potential for recognition of PD-L1 by PD-1-expressing T cells, resulting in the induction of a subpopulation of Tregs (191). These CD4+CD25-PD-1+ T cells suppressed colitis in severe combined immunodeficient (SCID) mice given CD4+CD45RBhigh effector T cells. These PD-1+ T cells were hypoproliferative in response to anti-CD3 stimulation and expressed Foxp3 transcripts. Curiously, addition of an anti-CTLA-4 monoclonal antibody but not anti-PD-1 monoclonal antibody modestly restored the proliferation of effector T cells. This may be due to the inability of the anti-PD-1 blocking monoclonal antibody (RMP 1–14) to recognize its PD-1-binding epitope, as these CD4+CD25−PD-1+ T cells were previously sorted with another anti-PD-1 monoclonal antibody (RMP 1–30). While not proven in these experiments, the constitutive expression of PD-1 on these T cells may have directly facilitated their conversion to iTregs following engagement by PD-L1. More recently, studies from the same group have found that colitogenic CD4+ T cells become dysfunctional upon successive adoptive transfers and resemble exhausted T cells seen during chronic viral infections (192). In sequential transfers of lamina propria T cells from colitic mice, CD4+CD45RBhigh cells transferred disease to new SCID mice. However, with each successive transfer, the duration before disease onset gradually increased and by the seventh transfer, the incidence of colitis decreased. LP CD4+ T cells from non-colitic mice transferred over seven times with colitic lamina propria T cells became ‘non-colitogenic’. These non-colitogenic T CD4+ T cells expressed high levels of PD-1, modest levels of Foxp3, and suppressed colitis in SCID mice co-transferred with CD4+CD45RBhigh effector cells. Together with reports correlating PD-1 as a marker for T-cell exhaustion during viral infection (10, 193), these data suggest that some exhausted CD4+PD-1+ T cells may become iTregs upon stimulation through the PD-L1: PD-1 pathway, thus representing another means of de novo iTreg conversion.
Rheumatoid arthritis
Rheumatoid arthritis (RA) is characterized by immune-mediated inflammation of the synovium, a thin tissue that lines the capsule of diarthrodial joints. Both T cells and B cells are required for the production of pathogenic autoantibodies (e.g. rheumatoid factor and anti-citrulline antibodies) and the resulting immune complexes that initiate joint-restricted inflammation. The synovial lining is transformed into a destructive pannus that degrades cartilage and bone, resulting in joint deformities. The importance of CD4+ Tregs in the pathogenesis of arthritis has been illustrated in mouse models where Treg depletion led to exacerbated disease and adoptive transfer experiments where Tregs prevented and treated established disease (194–196). Recently, Wright et al. (197) developed a system where antigen-specific Tregs accumulate within the inflamed joint and can inhibit T-effector cells and joint destruction in the absence of systemic immune suppression. Using an ovalbumin-induced arthritis model, mice were immunized with methylated BSA (mBSA) and intra-articularly rechallenged with mBSA or mBSA plus ovalbumin (OVA) in one knee and with mBSA alone in the contralateral knee. Tregs retrovirally engineered towards OVA specificity were transferred just prior to rechallenge and led to an increase in Tregs and decrease in Th17 cells in the OVA-challenged knee compared to the control knee. Moreover, treatment with antigen-specific Tregs resulted in reduced knee swelling and ameliorated joint damage in the immunized knee.
Despite convincing evidence in mouse models of arthritis, the role of Tregs in human rheumatic diseases is not yet clear. While some groups describe compromised function of Tregs in RA, Tregs actively suppress responder T cells and regulate inflammation in juvenile idiopathic arthritis (93, 198). The difficulty in assessing human Treg numbers and function in human rheumatologic diseases is underscored by the lack of a Treg-specific biomarker found on the cell surface enabling their uniform isolation. Thus, differences in human studies may be related to diverse Treg populations as well as diverse patient populations. Despite differences in Treg function reported in various forms of arthritis, there is a consensus that CD4+CD25+Foxp3+ Tregs are enriched in the synovial fluid of individuals with RA, almost all of whom were treated with various disease modifying anti-rheumatic drugs prior to analyses (92, 199–201). One of the most effective treatments for RA today is TNF blockade. Interestingly, Ehrenstein et al. (198) reported that anti-TNF treatment of RA increases peripheral blood Tregs and restores their suppressive function. Further work by Valencia et al. (167) elegantly demonstrated that TNF-α inhibits natural and induced Tregs due to TNFRII signaling. CD4+CD25high Tregs isolated from patients with active RA had decreased Foxp3 expression and poor suppressive capacity. Moreover, evaluation of Tregs from RA patients pre- and post-infliximab (anti-TNF-α) treatment, revealed increased Foxp3 levels and increased Treg suppressive function following TNF-α blockade. Thus, while it remains to be determined whether chronic inflammation results in defective Tregs or if defective Treg function contributes to the development of RA, accumulating evidence suggests that TNF-α blockade enhances Treg cell numbers and function, underscoring a central role for Tregs in the pathogenesis of RA.
Studies by Nadkarni and colleagues (202) demonstrated that patients treated with anti-TNF-α therapy have an increase in a distinct CD4+CD25highFoxp3+CD62L− subpopulation of Tregs. In vitro, infliximab plus anti-CD3/anti-CD28 stimulation of CD4+CD25− T cells (isolated from peripheral blood of patients with active RA) induced the development of CD4+CD25highFoxp3+CD62L− Tregs. Interestingly, infliximab induction of Tregs was abrogated by anti-TGF-β neutralizing monoclonal antibodies, raising the question of whether infliximab potentiates the induction of iTregs by influencing TGF-β. In fact, phenotypically anergic CD4+PD-1+ T cells are enriched in the joints of RA patients (203). These cells express CTLA-4, are CD45Rblow, and have reduced IL-2 production. While these cells share characteristics with Tregs, their suppressive capacity remains untested.
High expression of both PD-L1 and PD-1 is found in synovial T cells and macrophages of individuals with RA currently not under immunosuppressive treatment (204). Yet despite high levels of inhibitory molecules, persistent activation of T cells remained. Wan et al. (204) identified an alternative splice variant of PD-1 (PD-1Δex3) present within the synovial fluid of these RA patients. PD-1Δex3 results in a soluble form of PD-1 (sPD-1) that could antagonize the inhibitory effects of membrane-bound PD-1 on T cells. In vitro studies using recombinant PD-1-Ig showed enhanced proliferation of T cells from RA patients compared with control Ig, suggesting that the endogenously found sPD-1 competitively binds PD-L1 and negates immunosuppression mediated by membrane-bound PD-1 during RA. It is thus possible that this soluble PD-1 isoform may block PD-L1: PD-1 interactions and have deleterious consequences towards the maintenance of induced-Tregs and iTreg function within the joints of RA patients.
Collagen II (CII)-induced arthritis (CIA) is a murine experimental model of human RA, where intradermal administration of type II collagen results in joint swelling, often progressing to articular cartilage destruction and ankylosis of the joints. PD-L1 can regulate CII-reactive T cells and joint destruction (205). Pretreatment of mice with intraperitoneal treatment of PD-L1-Ig inhibited the development of CIA assessed clinically and histologically. While Wang et al. (205) observed reduced inflammation, the efficacy of PD-L1-Ig treatment may be influenced by route of administration. Rather than intraperitoneal injection of PD-L1-Ig, direct injection into the joint may increase the local concentration of PD-L1-Ig and provide a critical anti-inflammatory barrier at the site of destruction. Mechanistically, PD-L1-Ig treatment reduced serum levels of IL-17 and IL-23 and inhibited CII-specific splenocyte proliferation. As bulk splenocytes may contain multiple T-cell subsets, it is conceivable that PD-L1 could be acting (i) on naive T cells, regulating their conversion toward iTregs, which in turn, could further condition DCs towards tolerogenicity, (ii) on existing Tregs, by both promoting their maintenance and suppressor function, and directly limiting antigen-specific T effector cells, or (iii) by inducing the contra-conversion of T-effector cells to Tregs (Fig. 5). These findings not only point to a pivotal role for PD-L1 in RA but also suggest that PD-L1 may be a rational target for RA therapy.
Concluding remarks
PD-1 and its ligands have critical, multifaceted roles in regulating the balance among T-cell activation, tolerance, and immune-mediated tissue damage. One of the most remarkable aspects of the PD-1:PD-L pathway is the many ways by which it can thwart self-reactive T cells and protect against autoimmunity. As highlighted in this review, PD-1 and its ligands defend against potentially pathogenic self-reactive effector T cells not only by inhibiting the activation and function of autoreactive T cells, but also by promoting the development and function of Tregs. Thus, this pathway regulates the dynamic balance between potentially pathogenic self-reactive T cells and Tregs, tipping the balance toward restraint of effector cell responses (Fig. 7). The expression of PD-L1 on non-hematopoietic cells as well as hematopoietic cells endows PD-L1 with the capacity to promote Treg development and enhance Treg function in lymphoid organs and tissues that are targets of autoimmune attack. Thus, this pathway can participate in immune homeostasis and protect against immune-mediated tissue damage in variety of microenvironments. At sites where TGF-β is present, such as the placenta and the eye, PD-L1 may play a role in mediating immune privilege, by promoting de novo generation of Tregs, since there are synergistic effects between PD-L1 and TGF-β̣ in promoting Treg development (136). The function of this pathway in promoting Treg development and sustaining and enhancing Treg function has therapeutic as well as fundamental implications. There is great interest in producing regulatory T cells ex vivo as a therapy for autoimmune diseases and transplant rejection (206). However, because Tregs can exhibit functional plasticity and make proinflammatory cytokines at the site of inflammation with detrimental consequences (123, 124), it is crucial to develop approaches to sustain and promote Treg suppressive function. The development of PD-L1 agonists may provide a therapeutic opportunity to sustain and enhance Treg function, while concurrently inhibiting the expansion and functions of activated T-effector cells.
Fig. 7. Mechanisms of PD-1-mediated T-cell tolerance.
PD-L1 ligation of PD-1 following TCR stimulation results in two possible T-cell fates: diminished T-effector responses and augmented iTreg development, thus tipping the balance towards immunologic tolerance.
We have focused this review on the roles of PD-1 and its ligands in controlling T-effector and Treg cell dynamics in autoimmune diseases, but this pathway also can exert important inhibitory signals that result in ineffective anti-tumor and anti-microbial immunity. These effects may be mediated at least in part by inducing Treg development and sustaining Treg function. PD-L1 is expressed on a wide variety of tumors, and high levels of PD-L1 expression strongly correlate with unfavorable prognosis in a number of cancers (14). Increased numbers of Foxp3+ T cells within tumors also correlates with a poor prognosis. PD-L1 expression by tumor cells may induce and maintain iTregs in the tumor microenvironment, thereby augmenting the suppression of anti-tumor T-cell responses and allowing tumor progression. Increased Tregs also are present in chronic infections (207) and correlate with a lack of sterilizing immunity. There are studies that indicate PD-L1 may exert inhibitory effects in persistent infections (e.g. Helicobacter pylori) (208, 209) by promoting iTreg development and maintaining iTreg function, as well as by inhibiting anti-microbial effector T-cell function. Further studies are needed to better understand how the effects of PD-1 and its ligands on Treg cell development and function contribute to the inhibition of effective anti-tumor and anti-microbial immunity.
While we have focused mainly on how PD-1 and its ligands can regulate T-cell responses and the impact of this pathway on T-cell tolerance and autoimmunity, there is clearly a role for the PD-1 pathway on other cell types. Since both PD-1 and PD-L1 are expressed on T cells (including follicular T-helper cells), B cells, macrophages, and some types of DCs, PD-L1 and PD-1 have the potential to regulate tolerance in a number of ways. There are many potential interactions between PD-1 and its ligands, since PD-L1 and PD-L2 may signal bidirectionally by engaging PD-1 on T cells and by delivering signals into PD-L-expressing cells. Recent work indicates that PD-1:PD-L interactions can regulate B-cell responses (210), but whether PD-1 or PD-L1 on the T or B cell or PD-L2 on the B cells is involved is not yet clear. Further work is needed to determine whether this pathway can control pathogenic B-cell responses in a cell intrinsic or cell extrinsic fashion. Recent studies also indicate potential direct regulation of DC and macrophages by the PD-1 pathway (29, 42). In particular, PD-1 on macrophages may contribute to macrophage dysfunction and anergy. These findings suggest that the potential therapeutic benefit of modulating this pathway might be much broader than previously appreciated.
Recent work has advanced our understanding of the dynamics between the PD-1 pathway and Tregs, adding to the mechanisms by which this pathway exerts its inhibitory functions. With Treg plasticity being a major concern for the therapeutic use of Tregs in vivo, manipulating PD-L1:PD-1 interactions may provide a novel approach for maintaining and enhancing Treg function. This pathway may be a particularly attractive therapeutic target in autoimmune diseases, because the development of PD-1 agonists could deliver the necessary ‘one-two punch’ to protect against self-reactivity: (i) augmenting iTreg function and concomitantly (ii) suppressing the expansion and functions of activated effector T cells.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (to A.H.S.), from the National Multiple Sclerosis Society (to L.M.F.) and National Institutes of Health training grant (to A.H.S., P.T.S.).
References
- 1.van Noort JM, van Sechel A, Boon J, Boersma WJ, Polman CH, Lucas CJ. Minor myelin proteins can be major targets for peripheral blood T cells from both multiple sclerosis patients and healthy subjects. J Neuroimmunol. 1993;46:67–72. doi: 10.1016/0165-5728(93)90234-p. [DOI] [PubMed] [Google Scholar]
- 2.Lohmann T, Leslie RD, Londei M. T cell clones to epitopes of glutamic acid decarboxylase 65 raised from normal subjects and patients with insulin-dependent diabetes. J Autoimmun. 1996;9:385–389. doi: 10.1006/jaut.1996.0052. [DOI] [PubMed] [Google Scholar]
- 3.Lafferty KJ, Andrus L, Prowse SJ. Role of lymphokine and antigen in the control of specific T cell responses. Immunol Rev. 1980;51:279–314. doi: 10.1111/j.1600-065x.1980.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz RH, Mueller DL, Jenkins MK, Quill H. T-cell clonal anergy. Cold Spring Harb Symp Quant Biol. 1989;54:605–610. doi: 10.1101/sqb.1989.054.01.072. [DOI] [PubMed] [Google Scholar]
- 5.Sharpe AH. Mechanisms of costimulation. Immunol Rev. 2009;229:5–11. doi: 10.1111/j.1600-065X.2009.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 7.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 8.Nurieva RI, Liu X, Dong C. Yin-Yang of costimulation: crucial controls of immune tolerance and function. Immunol Rev. 2009;229:88–100. doi: 10.1111/j.1600-065X.2009.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 11.Crawford A, Wherry EJ. The diversity of costimulatory and inhibitory receptor pathways and the regulation of antiviral T cell responses. Curr Opin Immunol. 2009;21:179–186. doi: 10.1016/j.coi.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaufmann DE, Walker BD. PD-1 and CTLA-4 inhibitory cosignaling pathways in HIV infection and the potential for therapeutic intervention. J Immunol. 2009;182:5891–5897. doi: 10.4049/jimmunol.0803771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 14.Driessens G, Kline J, Gajewski TF. Costimulatory and coinhibitory receptors in anti-tumor immunity. Immunol Rev. 2009;229:126–144. doi: 10.1111/j.1600-065X.2009.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson RH, et al. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1757–1761. doi: 10.1158/1078-0432.CCR-06-2599. [DOI] [PubMed] [Google Scholar]
- 16.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 18.Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci USA. 2001;98:13866–13871. doi: 10.1073/pnas.231486598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riley JL. PD-1 signaling in primary T cells. Immunol Rev. 2009;229:114–125. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheppard KA, et al. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004;574:37–41. doi: 10.1016/j.febslet.2004.07.083. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, et al. Structural and functional analysis of the costimulatory receptor programmed death-1. Immunity. 2004;20:337–347. doi: 10.1016/s1074-7613(04)00051-2. [DOI] [PubMed] [Google Scholar]
- 22.Nishimura H, et al. Developmentally regulated expression of the PD-1 protein on the surface of double-negative (CD4-CD8-) thymocytes. Int Immunol. 1996;8:773–780. doi: 10.1093/intimm/8.5.773. [DOI] [PubMed] [Google Scholar]
- 23.Polanczyk MJ, Hopke C, Vandenbark AA, Offner H. Estrogen-mediated immunomodulation involves reduced activation of effector T cells, potentiation of Treg cells, and enhanced expression of the PD-1 costimulatory pathway. J Neurosci Res. 2006;84:370–378. doi: 10.1002/jnr.20881. [DOI] [PubMed] [Google Scholar]
- 24.Agata Y, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 25.Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J Exp Med. 2006;203:2223–2227. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinter AL, et al. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol. 2008;181:6738–6746. doi: 10.4049/jimmunol.181.10.6738. [DOI] [PubMed] [Google Scholar]
- 27.Oestreich KJ, Yoon H, Ahmed R, Boss JM. NFATc1 regulates PD-1 expression upon T cell activation. J Immunol. 2008;181:4832–4839. doi: 10.4049/jimmunol.181.7.4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho HY, Lee SW, Seo SK, Choi IW, Choi I. Interferon-sensitive response element (ISRE) is mainly responsible for IFN-alpha-induced upregulation of programmed death-1 (PD-1) in macrophages. Biochim Biophys Acta. 2008;1779:811–819. doi: 10.1016/j.bbagrm.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Yao S, et al. PD-1 on dendritic cells impedes innate immunity against bacterial infection. Blood. 2009;113:5811–5818. doi: 10.1182/blood-2009-02-203141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 31.Freeman GJ, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Latchman Y, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 33.Tseng SY, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee SJ, et al. Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-gamma-induced upregulation of B7-H1 (CD274) FEBS Lett. 2006;580:755–762. doi: 10.1016/j.febslet.2005.12.093. [DOI] [PubMed] [Google Scholar]
- 36.Marzec M, et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1) Proc Natl Acad Sci USA. 2008;105:20852–20857. doi: 10.1073/pnas.0810958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gong AY, et al. MicroRNA-513 regulates B7-H1 translation and is involved in IFN-gamma-induced B7-H1 expression in cholangiocytes. J Immunol. 2009;182:1325–1333. doi: 10.4049/jimmunol.182.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang SC, et al. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol. 2003;33:2706–2716. doi: 10.1002/eji.200324228. [DOI] [PubMed] [Google Scholar]
- 39.Liu J, et al. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-γ and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 2007;110:296–304. doi: 10.1182/blood-2006-10-051482. [DOI] [PubMed] [Google Scholar]
- 40.Parsa AT, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X, Lewkowich IP, Kohl G, Clark JR, Wills-Karp M, Kohl J. A protective role for C5a in the development of allergic asthma associated with altered levels of B7-H1 and B7-DC on plasmacytoid dendritic cells. J Immunol. 2009;182:5123–5130. doi: 10.4049/jimmunol.0804276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang X, et al. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci USA. 2009;106:6303–6308. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carter L, et al. PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur J Immunol. 2002;32:634–643. doi: 10.1002/1521-4141(200203)32:3<634::AID-IMMU634>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 44.Nurieva R, et al. T-cell tolerance or function is determined by combinatorial costimulatory signals. EMBO J. 2006;25:2623–2633. doi: 10.1038/sj.emboj.7601146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parry RV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bennett F, et al. Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation: attenuation of ICOS, IL-4, and IL-21, but not CD28, IL-7, and IL-15 responses. J Immunol. 2003;170:711–718. doi: 10.4049/jimmunol.170.2.711. [DOI] [PubMed] [Google Scholar]
- 47.Yamazaki T, Akiba H, Koyanagi A, Azuma M, Yagita H, Okumura K. Blockade of B7-H1 on macrophages suppresses CD4+ T cell proliferation by augmenting IFN-gamma-induced nitric oxide production. J Immunol. 2005;175:1586–1592. doi: 10.4049/jimmunol.175.3.1586. [DOI] [PubMed] [Google Scholar]
- 48.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 49.Wang J, Yoshida T, Nakaki F, Hiai H, Okazaki T, Honjo T. Establishment of NOD-Pdcd1−/− mice as an efficient animal model of type I diabetes. Proc Natl Acad Sci USA. 2005;102:11823–11828. doi: 10.1073/pnas.0505497102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishimura H, Honjo T, Minato N. Facilitation of beta selection and modification of positive selection in the thymus of PD-1-deficient mice. J Exp Med. 2000;191:891–898. doi: 10.1084/jem.191.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keir ME, Freeman GJ, Sharpe AH. PD-1 regulates self-reactive CD8+ T cell responses to antigen in lymph nodes and tissues. J Immunol. 2007;179:5064–5070. doi: 10.4049/jimmunol.179.8.5064. [DOI] [PubMed] [Google Scholar]
- 52.Blank C, Brown I, Marks R, Nishimura H, Honjo T, Gajewski TF. Absence of programmed death receptor 1 alters thymic development and enhances generation of CD4/CD8 double-negative TCR-transgenic T cells. J Immunol. 2003;171:4574–4581. doi: 10.4049/jimmunol.171.9.4574. [DOI] [PubMed] [Google Scholar]
- 53.Zucchelli S, Holler P, Yamagata T, Roy M, Benoist C, Mathis D. Defective central tolerance induction in NOD mice: genomics and genetics. Immunity. 2005;22:385–396. doi: 10.1016/j.immuni.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 54.Probst HC, McCoy K, Okazaki T, Honjo T, van den Broek M. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat Immunol. 2005;6:280–286. doi: 10.1038/ni1165. [DOI] [PubMed] [Google Scholar]
- 55.Ansari MJ, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 2003;198:63–69. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keir ME, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodig N, et al. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol. 2003;33:3117–3126. doi: 10.1002/eji.200324270. [DOI] [PubMed] [Google Scholar]
- 58.Grabie N, et al. Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8+ T-cell mediated injury in the heart. Circulation. 2007;116:2062–2071. doi: 10.1161/CIRCULATIONAHA.107.709360. [DOI] [PubMed] [Google Scholar]
- 59.Keir ME, Freeman GJ, Sharpe A. PD-1 regulates self-reactive CD8+ T cell responses to antigen in lymph nodes and tissues. J Immunol. 2007;179:5064–5070. doi: 10.4049/jimmunol.179.8.5064. [DOI] [PubMed] [Google Scholar]
- 60.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25+ regulatory cells from human peripheral blood express very high levels of CD25 ex vivo. Novartis Found Symp. 2003;252:67–88. doi: 10.1002/0470871628.ch6. discussion -91, 106–114. [DOI] [PubMed] [Google Scholar]
- 61.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 62.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sakaguchi S, Toda M, Asano M, Itoh M, Morse SS, Sakaguchi N. T cell-mediated maintenance of natural self-tolerance: its breakdown as a possible cause of various autoimmune diseases. J Autoimmun. 1996;9:211–220. doi: 10.1006/jaut.1996.0026. [DOI] [PubMed] [Google Scholar]
- 64.Mason D, Powrie F. Control of immune pathology by regulatory T cells. Curr Opin Immunol. 1998;10:649–655. doi: 10.1016/s0952-7915(98)80084-8. [DOI] [PubMed] [Google Scholar]
- 65.Bennett CL, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 66.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 67.Brunkow ME, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 68.Wildin RS, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 69.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 70.Chen W, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rubtsov YP, Rudensky AY. TGFbeta signalling in control of T-cell-mediated self-reactivity. Nat Rev Immunol. 2007;7:443–453. doi: 10.1038/nri2095. [DOI] [PubMed] [Google Scholar]
- 72.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 73.Davidson TS, DiPaolo RJ, Andersson J, Shevach EM. Cutting edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J Immunol. 2007;178:4022–4026. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- 74.Lohr J, Knoechel B, Abbas AK. Regulatory T cells in the periphery. Immunol Rev. 2006;212:149–162. doi: 10.1111/j.0105-2896.2006.00414.x. [DOI] [PubMed] [Google Scholar]
- 75.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 76.Takahashi T, et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 77.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lyon MF, Peters J, Glenister PH, Ball S, Wright E. The scurfy mouse mutant has previously unrecognized hematological abnormalities and resembles Wiskott-Aldrich syndrome. Proc Natl Acad Sci USA. 1990;87:2433–2437. doi: 10.1073/pnas.87.7.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kanangat S, et al. Disease in the scurfy (sf) mouse is associated with overexpression of cytokine genes. Eur J Immunol. 1996;26:161–165. doi: 10.1002/eji.1830260125. [DOI] [PubMed] [Google Scholar]
- 80.Blair PJ, Bultman SJ, Haas JC, Rouse BT, Wilkinson JE, Godfrey VL. CD4+CD8− T cells are the effector cells in disease pathogenesis in the scurfy (sf) mouse. J Immunol. 1994;153:3764–3774. [PubMed] [Google Scholar]
- 81.Clark LB, Appleby MW, Brunkow ME, Wilkinson JE, Ziegler SF, Ramsdell F. Cellular and molecular characterization of the scurfy mouse mutant. J Immunol. 1999;162:2546–2554. [PubMed] [Google Scholar]
- 82.Schubert LA, Jeffery E, Zhang Y, Ramsdell F, Ziegler SF. Scurfin (FOXP3) acts as a repressor of transcription and regulates T cell activation. J Biol Chem. 2001;276:37672–37679. doi: 10.1074/jbc.M104521200. [DOI] [PubMed] [Google Scholar]
- 83.Huter EN, Punkosdy GA, Glass DD, Cheng LI, Ward JM, Shevach EM. TGF-beta-induced Foxp3(+) regulatory T cells rescue scurfy mice. Eur J Immunol. 2008;38:1814–1821. doi: 10.1002/eji.200838346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thornton AM, Donovan EE, Piccirillo CA, Shevach EM. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol. 2004;172:6519–6523. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- 85.Thornton AM, Piccirillo CA, Shevach EM. Activation requirements for the induction of CD4+CD25+ T cell suppressor function. Eur J Immunol. 2004;34:366–376. doi: 10.1002/eji.200324455. [DOI] [PubMed] [Google Scholar]
- 86.Tang Q, et al. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 87.Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI. Defective suppressor function in CD4(+)CD25(+) T-cells from patients with type 1 diabetes. Diabetes. 2005;54:92–99. doi: 10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]
- 88.Asseman C, Fowler S, Powrie F. Control of experimental inflammatory bowel disease by regulatory T cells. Am J Respir Crit Care Med. 2000;162:S185–S189. doi: 10.1164/ajrccm.162.supplement_3.15tac9. [DOI] [PubMed] [Google Scholar]
- 89.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bour-Jordan H, Salomon BL, Thompson HL, Szot GL, Bernhard MR, Bluestone JA. Costimulation controls diabetes by altering the balance of pathogenic and regulatory T cells. J Clin Invest. 2004;114:979–987. doi: 10.1172/JCI20483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cao D, van Vollenhoven R, Klareskog L, Trollmo C, Malmstrom V. CD25brightCD4+ regulatory T cells are enriched in inflamed joints of patients with chronic rheumatic disease. Arthritis Res Ther. 2004;6:R335–R346. doi: 10.1186/ar1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.de Kleer IM, et al. CD4+CD25bright regulatory T cells actively regulate inflammation in the joints of patients with the remitting form of juvenile idiopathic arthritis. J Immunol. 2004;172:6435–6443. doi: 10.4049/jimmunol.172.10.6435. [DOI] [PubMed] [Google Scholar]
- 94.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 95.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3− expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 96.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 97.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 98.Suri-Payer E, Cantor H. Differential cytokine requirements for regulation of autoimmune gastritis and colitis by CD4(+)CD25(+) T cells. J Autoimmun. 2001;16:115–123. doi: 10.1006/jaut.2000.0473. [DOI] [PubMed] [Google Scholar]
- 99.Maynard CL, et al. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3− precursor cells in the absence of interleukin 10. Nat Immunol. 2007;8:931–941. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- 100.Rubtsov YP, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 101.Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol. 2008;9:632–640. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 102.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Piccirillo CA, et al. CD4(+)CD25(+) regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta1 production and responsiveness. J Exp Med. 2002;196:237–246. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 106.Andersson J, et al. CD4+ FoxP3+ regulatory T cells confer infectious tolerance in a TGF-beta-dependent manner. J Exp Med. 2008;205:1975–1981. doi: 10.1084/jem.20080308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tran DQ, et al. Analysis of adhesion molecules, target cells, and role of IL-2 in human FOXP3+ regulatory T cell suppressor function. J Immunol. 2009;182:2929–2938. doi: 10.4049/jimmunol.0803827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Collison LW, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 109.Bardel E, Larousserie F, Charlot-Rabiega P, Coulomb-L'Hermine A, Devergne O. Human CD4+ CD25+ Foxp3+ regulatory T cells do not constitutively express IL-35. J Immunol. 2008;181:6898–6905. doi: 10.4049/jimmunol.181.10.6898. [DOI] [PubMed] [Google Scholar]
- 110.Tang Q, et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tadokoro CE, et al. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J Exp Med. 2006;203:505–511. doi: 10.1084/jem.20050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Cutting edge: human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172:4676–4680. doi: 10.4049/jimmunol.172.8.4676. [DOI] [PubMed] [Google Scholar]
- 113.Serra P, et al. CD40 ligation releases immature dendritic cells from the control of regulatory CD4+CD25+ T cells. Immunity. 2003;19:877–889. doi: 10.1016/s1074-7613(03)00327-3. [DOI] [PubMed] [Google Scholar]
- 114.Takahashi T, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Salomon B, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 117.Wing K, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 118.Paust S, Cantor H. Regulatory T cells and autoimmune disease. Immunol Rev. 2005;204:195–207. doi: 10.1111/j.0105-2896.2005.00247.x. [DOI] [PubMed] [Google Scholar]
- 119.Grossman WJ, Verbsky JW, Tollefsen BL, Kemper C, Atkinson JP, Ley TJ. Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood. 2004;104:2840–2848. doi: 10.1182/blood-2004-03-0859. [DOI] [PubMed] [Google Scholar]
- 120.Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174:1783–1786. doi: 10.4049/jimmunol.174.4.1783. [DOI] [PubMed] [Google Scholar]
- 121.Zhao DM, Thornton AM, DiPaolo RJ, Shevach EM. Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood. 2006;107:3925–3932. doi: 10.1182/blood-2005-11-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cao X, et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27:635–646. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 123.Yang XO, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Joetham A, et al. Plasticity of regulatory T cells: subversion of suppressive function and conversion to enhancement of lung allergic responses. J Immunol. 2008;180:7117–7124. doi: 10.4049/jimmunol.180.11.7117. [DOI] [PubMed] [Google Scholar]
- 125.Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 126.Williams LM, Rudensky AY. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat Immunol. 2007;8:277–284. doi: 10.1038/ni1437. [DOI] [PubMed] [Google Scholar]
- 127.Komatsu N, Mariotti-Ferrandiz ME, Wang Y, Malissen B, Waldmann H, Hori S. Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc Natl Acad Sci USA. 2009;106:1903–1908. doi: 10.1073/pnas.0811556106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 129.Korn T, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 131.Tang Q, et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Egawa T, Tillman RE, Naoe Y, Taniuchi I, Littman DR. The role of the Runx transcription factors in thymocyte differentiation and in homeostasis of naive T cells. J Exp Med. 2007;204:1945–1957. doi: 10.1084/jem.20070133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kroner A, et al. A PD-1 polymorphism is associated with disease progression in multiple sclerosis. Ann Neurol. 2005;58:50–57. doi: 10.1002/ana.20514. [DOI] [PubMed] [Google Scholar]
- 134.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sauer S, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci USA. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Francisco LM, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177:8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 138.Strauss L, Whiteside TL, Knights A, Bergmann C, Knuth A, Zippelius A. Selective survival of naturally occurring human CD4+CD25+Foxp3+ regulatory T cells cultured with rapamycin. J Immunol. 2007;178:320–329. doi: 10.4049/jimmunol.178.1.320. [DOI] [PubMed] [Google Scholar]
- 139.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fife BT, et al. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009;10:1185–1192. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.DiPaolo RJ, Brinster C, Davidson TS, Andersson J, Glass D, Shevach EM. Autoantigen-specific TGFbeta-induced Foxp3+ regulatory T cells prevent autoimmunity by inhibiting dendritic cells from activating autoreactive T cells. J Immunol. 2007;179:4685–4693. doi: 10.4049/jimmunol.179.7.4685. [DOI] [PubMed] [Google Scholar]
- 142.Boitard C, Yasunami R, Dardenne M, Bach JF. T cell-mediated inhibition of the transfer of autoimmune diabetes in NOD mice. J Exp Med. 1989;169:1669–1680. doi: 10.1084/jem.169.5.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Herbelin A, Gombert JM, Lepault F, Bach JF, Chatenoud L. Mature mainstream TCR alpha beta+CD4+ thymocytes expressing L-selectin mediate "active tolerance" in the nonobese diabetic mouse. J Immunol. 1998;161:2620–2628. [PubMed] [Google Scholar]
- 144.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Green EA, Gorelik L, McGregor CM, Tran EH, Flavell RA. CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-beta-TGF-beta receptor interactions in type 1 diabetes. Proc Natl Acad Sci USA. 2003;100:10878–10883. doi: 10.1073/pnas.1834400100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Brusko TM, Wasserfall CH, Clare-Salzler MJ, Schatz DA, Atkinson MA. Functional defects and the influence of age on the frequency of CD4+ CD25+ T-cells in type 1 diabetes. Diabetes. 2005;54:1407–1414. doi: 10.2337/diabetes.54.5.1407. [DOI] [PubMed] [Google Scholar]
- 147.Kukreja A, et al. Multiple immuno-regulatory defects in type-1 diabetes. J Clin Invest. 2002;109:131–140. doi: 10.1172/JCI13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Putnam AL, Vendrame F, Dotta F, Gottlieb PA. CD4+CD25high regulatory T cells in human autoimmune diabetes. J Autoimmun. 2005;24:55–62. doi: 10.1016/j.jaut.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 149.Schneider A, Rieck M, Sanda S, Pihoker C, Greenbaum C, Buckner JH. The effector T cells of diabetic subjects are resistant to regulation via CD4+ FOXP3+ regulatory T cells. J Immunol. 2008;181:7350–7355. doi: 10.4049/jimmunol.181.10.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Martin-Orozco N, Wang YH, Yagita H, Dong C. Cutting Edge: Programmed death (PD) ligand-1/PD-1 interaction is required for CD8+ T cell tolerance to tissue antigens. J Immunol. 2006;177:8291–8295. doi: 10.4049/jimmunol.177.12.8291. [DOI] [PubMed] [Google Scholar]
- 151.Lee I, et al. Blocking the monocyte chemoattractant protein-1/CCR2 chemokine pathway induces permanent survival of islet allografts through a programmed death-1 ligand-1-dependent mechanism. J Immunol. 2003;171:6929–6935. doi: 10.4049/jimmunol.171.12.6929. [DOI] [PubMed] [Google Scholar]
- 152.Subudhi SK, et al. Local expression of B7-H1 promotes organ-specific autoimmunity and transplant rejection. J Clin Invest. 2004;113:694–700. doi: 10.1172/JCI19210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang CJ, et al. Protective role of programmed death 1 ligand 1 (PD-L1)in nonobese diabetic mice: the paradox in transgenic models. Diabetes. 2008;57:1861–1869. doi: 10.2337/db07-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Eppihimer MJ, et al. Expression and regulation of the PD-L1 immunoinhibitory molecule on microvascular endothelial cells. Microcirculation. 2002;9:133–145. doi: 10.1038/sj/mn/7800123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Krupnick AS, et al. Murine vascular endothelium activates and induces the generation of allogeneic CD4+25+Foxp3+ regulatory T cells. J Immunol. 2005;175:6265–6270. doi: 10.4049/jimmunol.175.10.6265. [DOI] [PubMed] [Google Scholar]
- 156.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Unger WW, Laban S, Kleijwegt FS, van der Slik AR, Roep BO. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: differential role for PD-L1. Eur J Immunol. 2009;39:3147–3159. doi: 10.1002/eji.200839103. [DOI] [PubMed] [Google Scholar]
- 158.Michel L, et al. Patients with relapsing-remitting multiple sclerosis have normal Treg function when cells expressing IL-7 receptor alpha-chain are excluded from the analysis. J Clin Invest. 2008;118:3411–3419. doi: 10.1172/JCI35365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liu W, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Seddiki N, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- 162.Reddy J, et al. Myelin proteolipid protein-specific CD4+CD25+ regulatory cells mediate genetic resistance to experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2004;101:15434–15439. doi: 10.1073/pnas.0404444101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Reddy J, et al. Cutting edge: CD4+CD25+ regulatory T cells contribute to gender differences in susceptibility to experimental autoimmune encephalomyelitis. J Immunol. 2005;175:5591–5595. doi: 10.4049/jimmunol.175.9.5591. [DOI] [PubMed] [Google Scholar]
- 164.Stephens LA, Gray D, Anderton SM. CD4+CD25+ regulatory T cells limit the risk of autoimmune disease arising from T cell receptor crossreactivity. Proc Natl Acad Sci USA. 2005;102:17418–17423. doi: 10.1073/pnas.0507454102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J Immunol. 2005;175:3025–3032. doi: 10.4049/jimmunol.175.5.3025. [DOI] [PubMed] [Google Scholar]
- 166.Liu Y, Teige I, Birnir B, Issazadeh-Navikas S. Neuron-mediated generation of regulatory T cells from encephalitogenic T cells suppresses EAE. Nat Med. 2006;12:518–525. doi: 10.1038/nm1402. [DOI] [PubMed] [Google Scholar]
- 167.Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108:253–261. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chen L, et al. Constitutive neuronal expression of the immune regulator, programmed death 1 (PD-1), identified during experimental autoimmune uveitis. Ocul Immunol Inflamm. 2009;17:47–55. doi: 10.1080/09273940802491884. [DOI] [PubMed] [Google Scholar]
- 169.Cheng X, Zhao Z, Ventura E, Gran B, Shindler KS, Rostami A. The PD-1/PD-L pathway is up-regulated during IL-12-induced suppression of EAE mediated by IFN-gamma. J Neuroimmunol. 2007;185:75–86. doi: 10.1016/j.jneuroim.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gran B, et al. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J Immunol. 2002;169:7104–7110. doi: 10.4049/jimmunol.169.12.7104. [DOI] [PubMed] [Google Scholar]
- 171.Magnus T, et al. Microglial expression of the B7 family member B7 homolog 1 confers strong immune inhibition: implications for immune responses and autoimmunity in the CNS. J Neurosci. 2005;25:2537–2546. doi: 10.1523/JNEUROSCI.4794-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Salama AD, et al. Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J Exp Med. 2003;198:71–78. doi: 10.1084/jem.20022119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Latchman YE, et al. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci USA. 2004;101:10691–10696. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Carter LL, et al. PD-1/PD-L1, but not PD-1/PD-L2, interactions regulate the severity of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2007;182:124–134. doi: 10.1016/j.jneuroim.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 175.Wang C, Li Y, Proctor TM, Vandenbark AA, Offner H. Down-modulation of programmed death 1 alters regulatory T cells and promotes experimental autoimmune encephalomyelitis. J Neurosci Res. 2010;88:7–15. doi: 10.1002/jnr.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Linthicum DS, Munoz JJ, Blaskett A. Acute experimental autoimmune encephalomyelitis in mice. I. Adjuvant action of Bordetella pertussis is due to vasoactive amine sensitization and increased vascular permeability of the central nervous system. Cell Immunol. 1982;73:299–310. doi: 10.1016/0008-8749(82)90457-9. [DOI] [PubMed] [Google Scholar]
- 177.Bruckener KE, el Baya A, Galla HJ, Schmidt MA. Permeabilization in a cerebral endothelial barrier model by pertussis toxin involves the PKC effector pathway and is abolished by elevated levels of cAMP. J Cell Sci. 2003;116:1837–1846. doi: 10.1242/jcs.00378. [DOI] [PubMed] [Google Scholar]
- 178.Racke MK, Hu W, Lovett-Racke AE. PTX cruiser: driving autoimmunity via TLR4. Trends Immunol. 2005;26:289–291. doi: 10.1016/j.it.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 179.Kerfoot SM, Norman MU, Lapointe BM, Bonder CS, Zbytnuik L, Kubes P. Reevaluation of P-selectin and alpha 4 integrin as targets for the treatment of experimental autoimmune encephalomyelitis. J Immunol. 2006;176:6225–6234. doi: 10.4049/jimmunol.176.10.6225. [DOI] [PubMed] [Google Scholar]
- 180.Cassan C, et al. Pertussis toxin reduces the number of splenic Foxp3+ regulatory T cells. J Immunol. 2006;177:1552–1560. doi: 10.4049/jimmunol.177.3.1552. [DOI] [PubMed] [Google Scholar]
- 181.Chen X, Winkler-Pickett RT, Carbonetti NH, Ortaldo JR, Oppenheim JJ, Howard OM. Pertussis toxin as an adjuvant suppresses the number and function of CD4+CD25+ T regulatory cells. Eur J Immunol. 2006;36:671–680. doi: 10.1002/eji.200535353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mielcarek N, Hornquist EH, Johansson BR, Locht C, Abraham SN, Holmgren J. Interaction of Bordetella pertussis with mast cells, modulation of cytokine secretion by pertussis toxin. Cell Microbiol. 2001;3:181–188. doi: 10.1046/j.1462-5822.2001.00106.x. [DOI] [PubMed] [Google Scholar]
- 183.Uhlig HH, et al. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006;177:5852–5860. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lathrop SK, Santacruz NA, Pham D, Luo J, Hsieh CS. Antigen-specific peripheral shaping of the natural regulatory T cell population. J Exp Med. 2008;205:3105–3117. doi: 10.1084/jem.20081359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232–259. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kraus TA, Toy L, Chan L, Childs J, Mayer L. Failure to induce oral tolerance to a soluble protein in patients with inflammatory bowel disease. Gastroenterology. 2004;126:1771–1778. doi: 10.1053/j.gastro.2004.03.076. [DOI] [PubMed] [Google Scholar]
- 189.Eastaff-Leung N, Mabarrack N, Barbour A, Cummins A, Barry S. Foxp3(+) Regulatory T Cells, Th17 Effector Cells, and Cytokine Environment in Inflammatory Bowel Disease. J Clin Immunol. 2010;30:80–89. doi: 10.1007/s10875-009-9345-1. [DOI] [PubMed] [Google Scholar]
- 190.Nakazawa A, et al. The expression and function of costimulatory molecules B7H and B7-H1 on colonic epithelial cells. Gastroenterology. 2004;126:1347–1357. doi: 10.1053/j.gastro.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 191.Totsuka T, et al. Regulation of murine chronic colitis by CD4+CD25− programmed death-1+ T cells. Eur J Immunol. 2005;35:1773–1785. doi: 10.1002/eji.200425109. [DOI] [PubMed] [Google Scholar]
- 192.Totsuka T, et al. Immunosenescent colitogenic CD4(+) T cells convert to regulatory cells and suppress colitis. Eur J Immunol. 2008;38:1275–1286. doi: 10.1002/eji.200737914. [DOI] [PubMed] [Google Scholar]
- 193.Day CL, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 194.Morgan ME, et al. CD25+ cell depletion hastens the onset of severe disease in collagen-induced arthritis. Arthritis Rheum. 2003;48:1452–1460. doi: 10.1002/art.11063. [DOI] [PubMed] [Google Scholar]
- 195.Frey O, et al. The role of regulatory T cells in antigen-induced arthritis: aggravation of arthritis after depletion and amelioration after transfer of CD4+CD25+ T cells. Arthritis Res Ther. 2005;7:R291–R301. doi: 10.1186/ar1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Morgan ME, et al. Effective treatment of collagen-induced arthritis by adoptive transfer of CD25+ regulatory T cells. Arthritis Rheum. 2005;52:2212–2221. doi: 10.1002/art.21195. [DOI] [PubMed] [Google Scholar]
- 197.Wright GP, et al. Adoptive therapy with redirected primary regulatory T cells results in antigen-specific suppression of arthritis. Proc Natl Acad Sci USA. 2009;106:19078–19083. doi: 10.1073/pnas.0907396106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ehrenstein MR, et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200:277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Raghavan S, et al. FOXP3 expression in blood, synovial fluid and synovial tissue during inflammatory arthritis and intra-articular corticosteroid treatment. Ann Rheum Dis. 2009;68:1908–1915. doi: 10.1136/ard.2008.100768. [DOI] [PubMed] [Google Scholar]
- 200.Cao D, et al. FOXP3 identifies regulatory CD25bright CD4+ T cells in rheumatic joints. Scand J Immunol. 2006;63:444–452. doi: 10.1111/j.1365-3083.2006.001755.x. [DOI] [PubMed] [Google Scholar]
- 201.Mottonen M, Heikkinen J, Mustonen L, Isomaki P, Luukkainen R, Lassila O. CD4+ CD25+ T cells with the phenotypic and functional characteristics of regulatory T cells are enriched in the synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol. 2005;140:360–367. doi: 10.1111/j.1365-2249.2005.02754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nadkarni S, Mauri C, Ehrenstein MR. Anti-TNF-alpha therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-beta. J Exp Med. 2007;204:33–39. doi: 10.1084/jem.20061531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hatachi S, et al. CD4+ PD-1+ T cells accumulate as unique anergic cells in rheumatoid arthritis synovial fluid. J Rheumatol. 2003;30:1410–1419. [PubMed] [Google Scholar]
- 204.Wan B, et al. Aberrant regulation of synovial T cell activation by soluble costimulatory molecules in rheumatoid arthritis. J Immunol. 2006;177:8844–8850. doi: 10.4049/jimmunol.177.12.8844. [DOI] [PubMed] [Google Scholar]
- 205.Wang G, Hu P, Yang J, Shen G, Wu X. The effects of PDL-Ig on collagen-induced arthritis. Rheumatol Int. 2009 doi: 10.1007/s00296-009-1249-0. 10.1007/s00296-009-1249-0. [DOI] [PubMed] [Google Scholar]
- 206.Roncarolo MG, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7:585–598. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 207.Belkaid Y. Role of Foxp3−positive regulatory T cells during infection. Eur J Immunol. 2008;38:918–921. doi: 10.1002/eji.200738120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Beswick EJ, Pinchuk IV, Das S, Powell DW, Reyes VE. Expression of the programmed death ligand 1, B7-H1, on gastric epithelial cells after Helicobacter pylori exposure promotes development of CD4+ CD25+ FoxP3+ regulatory T cells. Infect Immun. 2007;75:4334–4341. doi: 10.1128/IAI.00553-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Das S, Suarez G, Beswick EJ, Sierra JC, Graham DY, Reyes VE. Expression of B7-H1 on gastric epithelial cells: its potential role in regulating T cells during Helicobacter pylori infection. J Immunol. 2006;176:3000–3009. doi: 10.4049/jimmunol.176.5.3000. [DOI] [PubMed] [Google Scholar]
- 210.Haas KM, Poe JC, Tedder TF. CD21/35 promotes protective immunity to Streptococcus pneumoniae through a complement-independent but CD19-dependent pathway that regulates PD-1 expression. J Immunol. 2009;183:3661–3671. doi: 10.4049/jimmunol.0901218. [DOI] [PMC free article] [PubMed] [Google Scholar]