Abstract
In response to a peripheral infection, innate immune cells produce pro-inflammatory cytokines that act on the brain to cause sickness behaviour. When activation of the peripheral immune system continues unabated, such as during systemic infections, cancer or autoimmune diseases, the ensuing immune signalling to the brain can lead to an exacerbation of sickness and the development of symptoms of depression in vulnerable individuals. These phenomena might account for the increased prevalence of clinical depression in physically ill people. Inflammation is therefore an important biological event that might increase the risk of major depressive episodes, much like the more traditional psychosocial factors.
Anyone who has experienced a viral or bacterial infection knows what it means to feel sick. The behaviour of sick people changes dramatically; they often feel feverish and nauseated, ignore food and beverages, and lose interest in their physical and social environments. They tire easily and their sleep is often fragmented. In addition, they feel depressed and irritable, and can experience mild cognitive disorders ranging from impaired attention to difficulties in remembering recent events. Despite their negative impact on well-being, these symptoms of sickness are usually ignored. They are viewed as uncomfortable but banal components of infections1.
Sickness is a normal response to infection, just as fear is normal in the face of a predator. It is characterized by endocrine, autonomic and behavioural changes and is triggered by soluble mediators that are produced at the site of infection by activated accessory immune cells. These mediators are known as pro-inflammatory cytokines, and include interleukin-1α and β (IL-1α and IL-1β), tumour necrosis factor-α (TNF-α) and interleukin-6 (IL-6). They coordinate the local and systemic inflammatory response to microbial pathogens. However, these peripherally produced cytokines also act on the brain to cause the aforementioned behavioural symptoms of sickness. Recently, it has been suggested that ‘sickness behaviour’2,3, a term used to describe the drastic changes in subjective experience and behaviour that occur in physically ill patients and animals, is an expression of a previously unrecognized motivational state. It is responsible for re-organizing perceptions and actions to enable ill individuals to cope better with an infection4.
During the last five years, it has been established that pro-inflammatory cytokines induce not only symptoms of sickness, but also true major depressive disorders in physically ill patients with no previous history of mental disorders. Some of the mechanisms that might be responsible for inflammation-mediated sickness and depression have now been elucidated. These findings suggest that the brain–cytokine system, which is in essence a diffuse system, is the unsuspected conductor of the ensemble of neuronal circuits and neurotransmitters that organize physiological and pathological behaviour. In this Review we discuss how the brain engenders sickness behaviour in response to peripheral infections. We then review the evidence that pro-inflammatory cytokines can also trigger the development of depression in vulnerable individuals, and the possible underlying mechanisms. Finally, we discuss how these actions of cytokines in the brain might have a role in at least part of the increased prevalence of depression in people with physical illness5.
Immune signals from periphery to brain
The brain has long been considered an ‘immune-privileged’ organ but this immune status is far from absolute and varies with age and brain region6. Moreover, the brain contains immune cells, such as macrophages and dendritic cells, which are present in the choroid plexus and meninges. Brain parenchymal macrophages, known as microglial cells, are more quiescent in comparison with other tissue macrophages but can respond to inflammatory stimuli by producing pro-inflammatory cytokines and prostaglandins. In addition, both neuronal and non-neuronal brain cells express receptors for these mediators7.
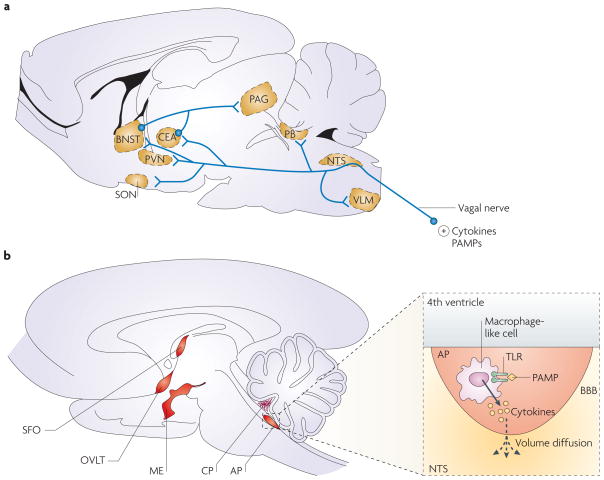
The brain monitors peripheral innate immune responses by several means that act in parallel (FIG. 1). One pathway involves afferent nerves: locally produced cytokines activate primary afferent nerves, such as the vagal nerves during abdominal and visceral infections8,9 and the trigeminal nerves during oro-lingual infections10. In a second, humoral pathway, Toll-like receptors (TLRs) on macrophage-like cells residing in the circumventricular organs and the choroid plexus respond to circulating pathogen-associated molecular patterns by producing pro-inflammatory cytokines11. As the circumventricular organs lie outside the blood–brain barrier, these cytokines can enter the brain by volume diffusion12. A third pathway comprises cytokine transporters at the blood–brain barrier: pro-inflammatory cytokines overflowing in the systemic circulation can gain access to the brain through these saturable transport systems13. Finally, a fourth pathway involves IL-1 receptors that are located on perivascular macrophages and endothelial cells of brain venules14,15. Activation of these IL-1 receptors by circulating cytokines results in the local production of prostaglandin E2.
Figure 1. Pathways that transduce immune signals from the periphery to the brain.
The brain and the immune system communicate through different pathways. a | In the neural pathway, peripherally produced pathogen-associated molecular patterns (PAMPs) and cytokines activate primary afferent nerves, such as the vagal nerves during abdominal and visceral infections8,9 and the trigeminal nerves during oro-lingual infections10. Vagal afferents project to the nucleus tractus solitarius (NTS), and from there to the parabrachial nucleus (PB), the ventrolateral medulla (VLM), the hypothalamic paraventricular and supraoptic nuclei (PVN, SON), the central amygdala (CEA) and the bed nucleus of the stria terminalis (BNST). These last two structures form part of the extended amygdala, which projects to the periaqueductal grey (PAG). b | The humoral pathway involves circulating PAMPs that reach the brain at the level of the choroid plexus (CP) and the circumventricular organs11, including the median eminence (ME), organum vasculosum of the laminae terminalis (OVLT), area postrema (AP) and suprafornical organ (SFO). In the circumventricular organs, PAMPs induce the production and release of pro-inflammatory cytokines by macrophage-like cells expressing Toll-like receptors (TLRs). As the circumventricular organs lie outside the blood–brain barrier (BBB), these cytokines still need to reach the brain. They do so by mechanisms that are still unknown, but involve volume diffusion12.
Engagement of these immune-to-brain communication pathways ultimately leads to the production of pro-inflammatory cytokines by microglial cells. This process requires the convergent action of two events with different time courses: the activation of the rapid afferent neural pathway, and a slower propagation of the cytokine message within the brain. Activation of the neural pathway (FIG. 1) probably sensitizes target brain structures for the production and action of cytokines that propagate from the circumventricular organs and the choroid plexus into the brain16. This way the brain forms an ‘image’ of the peripheral innate immune response that is similar in its elementary molecular components to the response in the periphery. The main difference is that this brain image does not involve an invasion of immune cells into the parenchyma and is not distorted by tissue damage that occurs at the site of infection.
The brain circuitry that mediates the various behavioural actions of cytokines remains elusive (FIG. 1). The social withdrawal that characterizes cytokine-induced sickness behaviour is unlikely to be mediated by the same brain areas as those underlying other responses to infection such as reduced food consumption17 or activation of the hypothalamus–pituitary–adrenal axis18. Ultimately, the site of action of the cytokine message depends on the localization of cytokine receptors or receptors for intermediates such as prostaglandins E2. These cytokine receptors are difficult to visualize on membranes because the number of receptor sites per cell is very low19 and they are easily internalized. Nevertheless, IL-1 receptors were first localized in the granule cell layer of the dentate gyrus, the pyramidal cell layer of the hippocampus and the anterior pituitary gland20. More recently, they were identified in endothelial cells of brain venules throughout the brain, at a high density in the preoptic and supraoptic areas of the hypothalamus and the sub-fornical organ, and a lower density in the paraventricular hypothalamus, cortex, nucleus of the solitary tract and ventrolateral medulla14.
Although the search for neuronal receptors currently dominates research in this field, the possibility of non-neuronal actions should not be neglected as cytokines potently modulate the functioning of endothelial and glial cells.
Cytokines and sickness behaviour
The main pro-inflammatory cytokines involved in sickness behaviour are IL-1β and TNF-α. Systemic administration of lipopolysaccharide (LPS) induces the expression of IL-1β and other pro-inflammatory cytokine mRNAs and proteins in the brain21–25. This expression occurs at doses of LPS that do not cause sepsis.
Pharmacological experiments have amply demonstrated that systemic or central administration of IL-1β or TNF-α to rats and mice induces the full spectrum of behavioural signs of sickness in a dose- and time-dependent manner4. In general, animals injected with IL-1β or TNF-α stay in a corner of their home cage in a hunched posture and show little or no interest in their physical and social environment unless they are stimulated. Specifically, they show decreased motor activity, social withdrawal, reduced food and water intake, increased slow-wave sleep and altered cognition (FIG. 2). In addition, they often have increased pain sensitivity, although this can be followed by hypoalgesia at later stages of sickness. IL-1 in the brain also has a pivotal role in the occurrence of fatigue as assessed by decreased resistance to forced exercise on a treadmill26. Finally, IL-1β and TNF-α flatten the diurnal rhythm of activity by decreasing the expression of steady-state mRNAs for clock genes that control the amplitude but not the period of activity rhythms27,28.
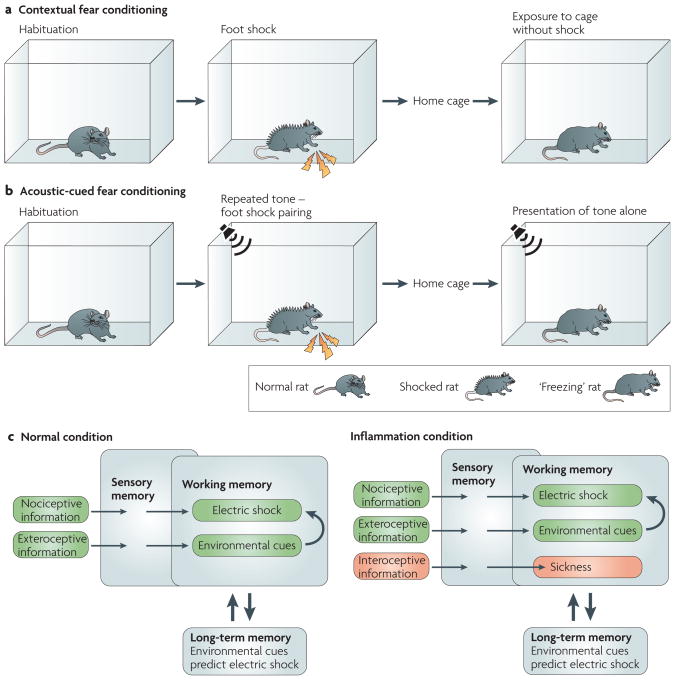
Figure 2. Increased brain cytokine signalling impairs learning and memory.
Studies in animals have demonstrated that acute activation of pro-inflammatory cytokine signalling in the brain in response to peripheral immune activation is associated with deficits in hippocampal-dependent memory such as contextual fear conditioning (a). Rats treated with lipopolysaccharide (LPS) shortly after exposure to an inescapable electric shock show less ‘freezing’ when re-exposed to the cage in which they were previously shocked. However, they still freeze in response to a tone that was previously paired with electric shocks, a phenomenon that is known as auditory-cued fear conditioning and is dependent on the amygdala103 (b). These behavioural data are consistent with the impairing effect of enhanced cytokine signalling on hippocampal long-term potentiation104. In terms of information processing, information about stimulus contingencies and behavioural response outcomes is briefly processed by sensory memory. When attended to, this information is given cognitive meaning in the working memory register before being stored in the long-term memory register (c). The cognitive load theory105 capitalizes on the limited capacity of working memory to handle several pieces of information at the same time. This is not a problem in normal conditions as only a small amount of information needs to be processed by the working memory register. However, the intruding interoceptive sensations of sickness, which are mediated by pro-inflammatory cytokines during an episode of inflammation, are likely to increase the load on working memory and limit the ability to extract information about the temporal contingencies between nociceptive stimuli and exteroceptive environmental stimuli, especially when the exteroceptive stimuli lack salience (that is, diffuse contextual cues versus distinct auditory cues).
In contrast to IL-1β and TNF-α, IL-6 administered systemically or centrally has no behavioural effect despite its ability to induce a fever response4. However, LPS-induced sickness behaviour and hippocampus-mediated cognitive impairment are less noticeable in IL-6-deficient mice than in wild-type controls29. This deficit is caused by a less marked expression of TNF-α and IL-1β in the brain in response to LPS29, indicating that brain IL-6 contributes to the expression of brain cytokines in response to immune stimuli.
Anti-inflammatory cytokines regulate the intensity and duration of sickness behaviour, probably by inhibiting pro-inflammatory cytokine production and attenuating pro-inflammatory cytokine signalling30,31. In particular, central administration of IL-10 or insulin-like growth factor I (IGF-I), a growth factor that behaves like an anti-inflammatory cytokine in the brain, attenuates behavioural signs of sickness induced by centrally injected LPS32,33. This protective effect of IGF-I is more noticeable in TNF-α- than IL-1β-induced sickness behaviour34. These data are consistent with the idea that in the brain, as in systemic organs, the natural balance between pro- and anti-inflammatory cytokines regulates the intensity and duration of the response to immune stimuli.
Corroborating the need for balance between pro- and anti-inflammatory cytokines in the brain are studies in IL-10-deficient mice and in aged or obese mice. Compared to wild-type mice, IL-10-deficient mice respond to intraperitoneal administration of LPS with an exaggerated sickness behaviour that is associated with an increased expression of pro-inflammatory cytokine genes in the brain. These very recent findings have not yet been reported but are supported by an earlier study which showed that LPS-induced fever was exaggerated and prolonged in IL-10-deficient mice35.
Ageing is associated with increased activity of the innate immune system, which at the brain level translates into an enhanced production of pro-inflammatory cytokines, such as IL-6, and a decreased production of anti-inflammatory cytokines, including IL-10 (REFS 36,37). Simultaneously, aged mice show more severe sickness behaviour after administration of LPS38. Macrophages from obese db/db mice, which are a model for type 2 diabetes, respond to LPS with an increased production of IL-1β and a lowered production of IL-1 receptor antagonist and IL-1 receptor II compared with non-diabetic mice. Their more pronounced sickness behaviour in response to LPS and IL-1β injected peripherally or centrally is commensurate with this abnormality at the macrophage level39.
A role for cytokines in depression?
The similarity between the symptoms of cytokine-induced sickness behaviour and depression is striking: in both cases there is a withdrawal from the physical and social environment that is accompanied by pain, malaise and decreased reactivity to reward (anhedonia). Moreover, some components of sickness behaviour, such as a decreased preference for sweet solutions and reduced social exploration, are improved by anti-depressant treatment40. In humans, major depressive disorders develop in roughly a third of patients who are treated with the recombinant human cytokines IL-2 and interferon-α (IFN-α)41. In agreement with these findings, major depressive disorders are more prevalent in patients afflicted with conditions that lead to chronic inflammation (such as cardiovascular diseases, type 2 diabetes and rheumatoid arthritis) than in the general population5. However, the similarity between sickness and depression is only partial; whereas sickness is an adaptive response to infection by pathogens and fully reversible once the pathogen has been cleared, this is not the case for depression. It is possible that depression represents a maladaptive version of cytokine-induced sickness, which could occur when activation of the innate immune response is exacerbated in intensity and/or duration, or that takes place in the context of an increased vulnerability to depression, for example, in individuals with hyperactive corticotrophin-releasing factor (CRH) neuronal circuits42.
A role for cytokines in depression was first proposed by Smith43 in the form of the ‘macrophage theory of depression’ and further studied by Maes in the early 1990s. Building on the observation that patients with severe clinical depression have increased blood concentrations of inflammatory biomarkers, they proposed that depression is associated with an acute-phase response. According to his theory, the pro-inflammatory cytokines that are responsible for this acute-phase reaction also cause various clinical aspects of depression, including hyperactivity of the hypothalamus–pituitary–adrenal axis, disturbed serotonin metabolism and neurovegetative symptoms44. Despite its originality, especially at a time when depression was thought to be associated with decreased rather than increased immunity45, this hypothesis failed to attract the interest of the psychiatry community. Because biomarkers of inflammation in clinically depressed patients are not always elevated, the postulate that common pathophysiological mechanisms link depression to inflammation was limited. Other key components that would support this postulate were also missing, such as a demonstration that stimulation of the immune system induces depression-like disorders; identification of a possible common pathophysiological mechanism between the effects of cytokines in the brain and the neurobiological basis of depression; and proof that decreasing the inflammatory response attenuates symptoms of depression. As discussed below, research in this field has now supplied these key components.
Vegetative, somatic and psychological symptoms of depression
Nearly twenty years ago, when the recombinant human cytokines IL-2 and IFN-α were first used chronically to boost the immune system to eliminate tumours that resisted chemotherapy and radiotherapy, or to clear hepatitis C virus, clinicians noted the occurrence of severe neuropsychiatric changes, including major depressive disorders, after treatment onset in a significant percentage of patients46,47. However, these neuropsychiatric complications were seen as side effects of immunotherapy. It was only eight years ago that the major depressive disorders caused by immunotherapy became a quasi-experimental model to investigate the pathophysiology of cytokine-induced depression. Systematic investigation of the symptoms that developed in cancer and hepatitis C patients receiving immunotherapy confirmed that they were caused by the treatment, and revealed that they fell into two distinct categories: early-onset neurovegetative and somatic symptoms of depression, which all patients display and which include flu-like symptoms, fatigue, anorexia, pain and sleep disorders, and late-onset psychological symptoms of depression that are experienced by up to half of patients and include mild cognitive alterations and symptoms of depressed mood, sometimes accompanied by anxiety and irritability48–50. Pre-treatment with paroxetine, a serotonin-reuptake inhibitor, reduces the psychological symptoms but has little or no effect on the concomitant neurovegetative symptomatology48. The patients who developed psychological symptoms of depression scored higher on a depression scale before immunotherapy was started51 and had an enhanced pituitary–adrenal response following the first injection of IFN-α52, indicating that vulnerability to immunotherapy-induced depression involves both psychological and physiological risk factors.
Cytokine-induced depression in animal models
In order to delineate the mechanisms of cytokine-induced depression using animal models, one must demonstrate that pro-inflammatory cytokines can induce depression-like behaviour in animals. However, the occurrence of cytokine-induced sickness behaviour represents an important bias in these experiments, as some of these behaviours overlap with depression-like behaviour. For example, the reduction in motor activity that develops in sick individuals mimics the enhanced immobility that is supposed to reflect helplessness in inescapable situations, such as in the forced-swim and tail-suspension tests. In the same manner, the considerably reduced appetite of sick animals translates into a decreased intake of rewarding aliments, which mimics depression-associated anhedonia. To prove a role for cytokines in the aetiology of depression it is therefore necessary to show that specific depression-like behaviour in immune-stimulated animals develops independently of obvious performance impairments (behavioural validation) and that it is relieved by antidepressant treatment (pharmacological validation).
Some indication that depression-like behaviour remained after sickness behaviour had resolved came from experiments in which LPS-treated mice displayed increased immobility in the tail-suspension test and in the forced-swim test 24 hours after treatment, a time point when motor activity had returned to normal53 (FIG. 3). In the same experiment, a decrease in preference for a sweetened drinking solution was still apparent when food intake and drinking had normalized.
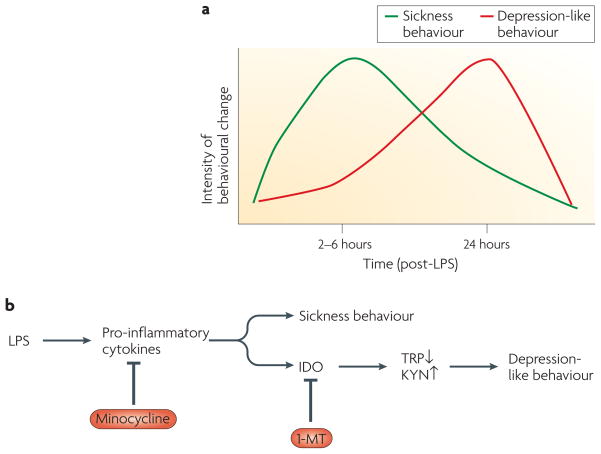
Figure 3. LPS-increased depression-like behaviour in mice.
Peripheral administration of lipopolysaccharide (LPS) induces sickness behaviour that peaks 2 to 6 hours later and gradually wanes (a). Depression-like behaviour, as measured by increased immobility in the forced-swim test or the tail-suspension test and decreased preference for a sweet solution, emerges on this background. The development of sickness behaviour requires activation of pro-inflammatory cytokine signalling in the brain in response to peripheral LPS (b). Some of the pro-inflammatory cytokines that induce sickness behaviour also enhance activity of the ubiquitous indoleamine 2,3 dioxygenase (IDO) that peaks at 24 hours post-LPS. Activation of IDO results in decreased tryptophan (TRP) levels and increased production of kynurenine (KYN) and other tryptophan-derived metabolites. Pre-treatment with the second-generation tetracycline minocycline, which has potent anti-inflammatory effects both at the periphery and in the brain106, blocks both LPS-induced sickness behaviour and depression-like behaviour. By contrast, administration of 1-methyl tryptophan (1-MT), a competitive inhibitor of IDO, blocks LPS-induced depression-like behaviour without altering LPS-induced sickness behaviour.
Another approach is the use of genetic animal models of depression. For instance, fawn-hooded rats display many of the proposed animal equivalents of depression. These rats were more sensitive than normal Sprague-Dawley rats to IL-1β-induced immobility in the forced-swim test54. In terms of pharmacological validation of cytokine-induced depression-like behaviour, pre-treatment with antidepressant drugs abrogated the reduced intake of a sweetened solution in LPS-treated rats40 and the decreased performance of IL-1β-treated rats in a task in which they had to progressively increase their rate of response in order to obtain a sucrose solution reward55. These findings in animal models confirm the observation in humans that activation of the immune system can cause depression.
A role for tryptophan?
Immunotherapy alters the clinical biochemistry of patients; the most revealing sign is a pronounced reduction in plasma levels of tryptophan56, which correlates with the patients’ depression scores 3 weeks into the treatment. Tryptophan is an essential amino acid that is actively transported into the brain for the synthesis of serotonin. The bioavailability of this serotonin precursor determines the rate of serotonin synthesis in the brain; this explains why there has been so much speculation on the relationship between circulating tryptophan levels and mood. However, the only reliable finding to date that links tryptophan levels with depression is the observation that acute tryptophan depletion decreases mood in vulnerable people who have a familial history of major depressive disorders or are drug-free in remission after an episode of major depression57. Thus, it is yet unclear whether the reduction in plasma tryptophan levels in patients receiving immunotherapy is causally linked to any of the psychiatric symptoms they exhibit.
The fall in plasma levels of tryptophan that occurs in patients receiving immunotherapy could be due to activation of the major enzymes that metabolize tryptophan, namely tryptophan 2,3 dioxygenase (TDO) and indoleamine 2,3 dioxygenase (IDO). Both enzymes degrade tryptophan along the kynurenine pathway (BOX 1). TDO is activated by cortisol, but plasma cortisol levels are not elevated in these patients. In contrast, IDO can be directly activated by a number of cytokines, including IFN-γ and TNF-α. IDO is present in accessory immune cells, including macrophages and dendritic cells, and it is expressed in all organs including the brain58.
Box 1. IDO degrades tryptophan through the kynurenine pathway.
Tryptophan is an essential amino acid that is required for protein synthesis and serves as a precursor for serotonin. Normally, the majority of dietary tryptophan (>95%) is oxydatively degraded in the liver through the kynurenine pathway and only a small portion of it is used for the synthesis of serotonin. Tryptophan oxidation is catalyzed by tryptophan dioxygenase (TDO), which generates nicotinamide adenine dinucleotide (NAD) (not shown). Tryptophan oxidation can also occur extrahepatically by the enzyme indoleamine 2,3 dioxygenase (IDO) (see figure). Although tryptophan degradation by IDO is normally negligible, IDO is highly inducible by pro-inflammatory cytokines, including interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α).
Degradation of tryptophan through the kynurenine pathway has important neuropsychiatric implications. Decreased tryptophan concentrations have the potential to influence serotonergic neurotransmission in the brain as tryptophan is the precursor of serotonin and its bioavailability regulates the synthesis of serotonin. In addition, IDO is expressed in the brain so that fluctuations in its enzymatic activity can affect serotonin biosynthesis.
The major metabolite of tryptophan, kynurenine, is readily transported across the blood–brain-barrier into the brain where it can be further metabolized in perivascular macrophages, microglia and astrocytes to generate neuroactive compounds. Kynurenine is degraded along one of two catabolic branches, leading to the formation of either 3-hydroxykynurenine (3-HK) and quinolinic acid (QA) or kynurenic acid (KA). 3-HK generates free-radical species that can cause oxidative stress and lipid peroxidation, whereas QA is an N-methyl-D-aspartate (NMDA) receptor agonist. By contrast, KA is an NMDA receptor antagonist that has been speculated to be neuroprotective. These apparently antagonistic pathways are compartmentalized within the brain: microglia preferentially produce QA, whereas astrocytes produce KA95. In light of recent evidence suggesting a role of heightened glutamate receptor activity in major depression, an imbalance of kynurenine pathway metabolites might underlie inflammation-associated depressive disorders61.
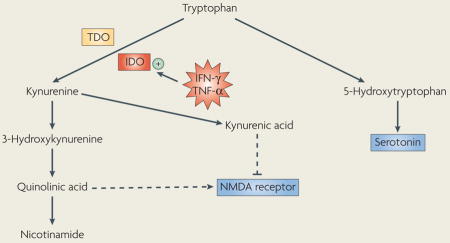
The enzymatic activity of IDO is enhanced in conditions of acute or chronic activation of the immune system, including immunotherapy, acquired immunodeficiency syndrome (AIDS), atherosclerosis and coronary heart disease, rheumatoid arthritis and obesity58. Acute activation of TLR-4 by LPS or of TLR-2 by peptidoglycan increases circulating levels of IFN-γ in mice and potently activates IDO in both the periphery and the brain59. Also in mice, chronic stimulation of the immune system by inoculation with an attenuated form of Mycobacterium bovis induces a sustained elevation in circulating levels of IFN-γ and a chronic activation of IDO. Both of these events are associated with depression-like behaviour60. Blocking IDO activation by abrogating the expression of the cytokines that induce this enzyme, or by direct inhibition of the enzyme, reduced LPS-induced depression-like behaviour (FIG. 3) (J. C. O’Connor, M. A. Lawson, C. Andre, J. Lestage, N. Castanon, K. W. Kelley & R. Dantzer, unpublished data), but the respective roles of peripheral and brain IDO in these effects remains to be investigated.
Despite its dramatic effects on circulating levels of tryptophan, activation of IDO by cytokines does not necessarily induce depression-like behaviour through alterations in the metabolism of serotonin. Indeed, blockade of IDO activation attenuated LPS-induced depression-like behaviour in mice, as mentioned above, but this effect was independent of any consistent action on LPS-induced increases in serotonin turnover. An alternative explanation for the involvement of IDO in the pathophysiology of depression-like behaviour is that degradation of tryptophan along the kynurenine pathway generates compounds that act as either agonists (for example, quinolinic acid and 3 hydroxy-kynurenine) or antagonists (for example, kynurenic acid) of the NMDA (N-methyl-D-aspartate) receptor (BOX 1). The net result is probably an alteration in glutamatergic neurotransmission that could trigger the necessary conditions for the development of depression61.
Alternative mechanisms for cytokine-induced depression
Although current clinical and experimental data strongly point toward the involvement of IDO in the development of inflammation-associated major depressive disorders, it is probably not the sole mechanism. For instance, there is evidence that LPS and pro-inflammatory cytokines increase tryptophan uptake in the brain and enhance serotonin turnover62. Furthermore, IL-1β and TNF-α stimulate serotonin uptake in mouse midbrain and striatal synaptosomes, and these effects are mediated by activation of the p38 mitogen-activated protein (MAP) kinase63. IFN-α decreases the expression of serotonin receptor 1A in various non-neuronal cell lines and this effect is antagonized by co-incubation with two classical antidepressant drugs, desipramine and fluoxetine64. These findings indicate that cytokines might modulate serotonergic neurotransmission by mechanisms other than the IDO-mediated decrease in tryptophan levels and the generation of neuroactive tryptophan metabolites.
Non-serotonergic mechanisms might also be involved in immunotherapy-induced clinical depression. A hyperactive hypothalamus–pituitary–adrenal axis is often associated with clinical depression65. Pro-inflammatory cytokines acutely and potently activate the hypothalamus–pituitary–adrenal axis. This effect is usually attributed to increased production of CRH66, although vasopressin takes precedence over CRH67 when inflammation is chronic. In biological psychiatry, CRH and vasopressin have a long history as candidate neuropeptides for accounting for symptoms of depression68,69.
The molecular mechanisms responsible for the increased production and release of CRH in clinically depressed patients are still being explored. In conditions of chronic inflammation, pro-inflammatory cytokines can cause glucocorticoid receptor resistance in immunocytes and their cellular targets through the induction of the MAP kinases c-jun N-terminal kinase (JNK) and p38 (REF. 45). In addition, pro-inflammatory cytokines seem to promote expression of the β isoform of the glucocorticoid receptor that is inactive but still able to bind its ligand70. At the hypothalamic level, this cytokine-dependent glucocorticoid receptor resistance can explain the reduced ability of glucocorticoids to down-regulate the production of CRH. At the level of peripheral and central innate immune cells, the normally inhibitory effect of glucocorticoids on cytokine production and action would no longer be operative, setting the condition for a feed-forward cascade that would result in an ever-increasing production of pro-inflammatory cytokines. This increased inflammatory response in the brain results in a decreased inhibitory feedback on CRH by glucocorticoids, thereby intensifying the stress-response system71.
Taken together, these findings indicate that pro-inflammatory cytokines can cause depression by several mechanisms, including activation of IDO. This leads to the generation of neuroactive tryptophan metabolites and glucocorticoid receptor resistance, which amplifies the inflammatory response and leads to excessive production of CRH.
Neuroanatomy of cytokine-induced depression
The search for a possible neuroanatomical basis of cytokine-induced depression has focused on the brain circuits that are involved in emotion processing and psychomotor retardation, both of which are altered in patients with clinical depression. Neuroimaging data of clinically depressed patients show decreased baseline activity in the frontal and temporal cortex and the insula, and increased activity in the cerebellum, subcortical and limbic regions72. In agreement with this, cancer patients treated with IFN-α display decreased glucose metabolism in the dorsal prefrontal cortex but increased glucose metabolism in the cerebellum and basal ganglia73. None of these changes correlated with scores of depressed mood, probably because these studies were carried out at an early stage of IFN-α therapy when neurovegetative symptoms predominate over depressed mood. However, hypermetabolism in the left putamen and nucleus accumbens significantly correlated with fatigue and lack of energy. In patients receiving IFN-α for the treatment of hepatitis C, the fatigue and impaired concentration that are induced by the treatment were not associated with changes in activity of parietal and occipital brain regions, as assessed by functional magnetic resonance imaging73. However, IFN-α-treated patients showed a greater activation of the anterior cingulate cortex during a high-demand visuo-spatial attention task74. This hyperactivity was highly correlated with the number of task-related errors in IFN-α-treated patients despite the lack of any effect of IFN-α on the number of errors. These data are consistent with the greater difficulty of IFN-α-treated patients to regulate their emotions50. However, the possible relationship between hyperactivity of the anterior cingulate cortex and depressed mood was not investigated in this study.
In animal studies, variation in the expression of the immediate early gene c-fos has been used to map brain areas that are involved in cytokine-induced depression-like behaviour. LPS increased c-fos expression in the paraventricular hypothalamus and bed nucleus of the stria terminalis, but abolished the increased expression of c-fos that is normally observed in the motor, cingular and piriform cortex, locus coeruleus and nucleus accumbens of mice when they are exposed to new cages with fresh bedding75. The same differential effects were observed on MAP kinase activation in the cingular cortex and paraventricular hypothalamus75. The changes in c-fos expression were observed at the peak of LPS-induced sickness behaviour (3.5 hours post-LPS) and could therefore be explained by the interference of sickness with the ability of mice to move around and explore their surroundings. Nevertheless, the affected brain structures might be involved in the reduction of positively-motivated behaviour that is characteristic of cytokine-induced depression. In order to avoid this ambiguity, a neuroanatomical characterization of the brain areas that mediate cytokine-induced depression-like behaviour was carried out later, when sickness had dissipated but depression-like behaviour was still apparent53. For this purpose, the expression of FosB and its truncated splice variant ΔFosB were measured, both of which have a longer half-life than c-fos and accumulate during repeated or long-lasting stimulation53. As expected, c-fos expression was transiently expressed in the brain areas that coordinate the behavioural, endocrine and autonomic components of LPS-induced sickness. However, c-fos expression remained elevated 24 hours post-LPS in most of the structures of the extended amygdala and several hypothalamic areas in which a delayed increase in FosB/ΔFosB immunoreactivity was observed. FosB/ΔFos B was also elevated in the hippocampus.
Although these observations are indirect and qualitative as far as the relationship with LPS-induced depression-like behaviour is concerned, they point to a possible role of the hippocampus, extended amygdala and hypothalamus in the pathophysiology of cytokine-induced depression. This would be consistent with the proposed implication of these brain areas in affective disorders76, but it does not necessarily mean that there is a full overlap between the brain areas that are involved in major depressive disorders and those that are activated in cytokine-induced depression. Indeed, in a model of localized bronchopulmonary immune activation, only a specific subset of serotonergic neurons in the interfascicular region of the dorsal raphe nucleus were activated, as assessed by increased c-fos expression77. This subpopulation of serotonergic neurons is not the same as that which responds to anxiety-inducing stimuli. The origin of this neuroanatomical specificity is unknown but could be related to a more general difference in the way the brain processes interoceptive versus exteroceptive information78.
Implications for depression in medically ill people
A growing amount of clinical data point to the importance of the relationship between inflammation and depression in physically ill patients and in conditions that are associated with increased activity of the innate immune system, including ageing and obesity. For instance, the prevalence of co-morbid depression in patients with coronary heart disease, a disease in which inflammation is now recognized as a major contributing factor, is three times higher than in the general population79. Depression has long been known to be a risk factor for subsequent cardiac events and mortality, which is usually explained by the detrimental effects of depression on illness behaviour including adherence to treatment. However, this traditional view of the relationship between inflammation and morbidity/mortality in physically ill patients is challenged by the new hypothesis, set out in this Review, that depression can actually be caused by inflammation in vulnerable patients41 (FIG. 4).
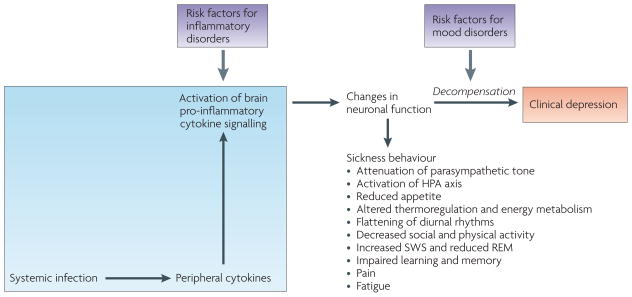
Figure 4. Depression as a consequence of decompensation of the mechanisms that regulate sickness.
Sickness behaviour in response to an infectious episode is normally reversible owing to the ability of the immune system to clear infectious pathogens and to the recovery mechanisms that oppose the production and action of pro-inflammatory cytokines, both in the periphery and the brain. Clinical evidence shows that depression can develop on a background of sickness with which it shares many of the neurovegetative and psychological components. Studies in animals show the same phenomenon. Decompensation of the mechanisms that regulate sickness behaviour can occur in vulnerable patients whose inflammatory response is more intense because the balance between pro- and anti-inflammatory mediators is shifted towards inflammation (for example, hyperproduction of tumour necrosis factor-α (TNF-α), insufficient production of interleukin (IL)-10 and glucocorticoid resistance). It can also occur in patients whose brain sensitivity to immune-mediated events is higher because of disturbed neurotransmitter metabolism, for example, less efficient serotonergic functioning owing to homozygosity for the short allele of the serotonin transporter gene. HPA, hypothalamus–pituitary–adrenal; SWS, slow-wave sleep; REM, rapid-eye movement sleep.
Testing this hypothesis in the clinic has been difficult owing to the lack of unanimously accepted biomarkers of inflammation, excessive reliance on psychiatric diagnosis of major depression, inadequacy of psychological scales of depression (especially for measurement of neurovegetative symptoms), lack of widely recognized vulnerability factors and the predominance of cross-sectional over longitudinal investigations. Despite these difficulties, a few attempts are under way to treat symptoms of depression with anti-inflammatory drugs. Encouraging results have been obtained by blocking TNF-α in patients with psoriasis80 and from the administration of COX2 inhibitors to patients with major depression81. However, such studies are certainly premature in the absence of sufficient knowledge on the pathophysiological mechanisms linking inflammation to sickness and depression.
In general, it is easier to link inflammation to depression when depression is considered as a continuous dimensional variable rather than a categorical entity, as this allows one to take into consideration moderate and subclinical levels of depressed mood5. For instance, a prospective study of 267 85-year-old subjects with no psychiatric history revealed that elevated biomarkers of inflammation preceded the onset of depressed mood in an aged population with no psychiatric history82.
To explain how inflammation can modulate depressed mood, it is useful to come back to animal data. Aged mice are more sensitive than young adults to not only LPS-induced sickness behaviour but also to LPS-induced depression-like behaviour, and this is associated with an exaggerated inflammatory response in the brain83. However, in the absence of an acute immune stimulation, aged mice do not differ from young adults in their behaviour in animal models of depression. In other words, their increased inflammatory status remains behaviourally silent until it is challenged. This condition is very similar to the one observed in mouse models of chronic neurodegenerative disease, in which a peripheral immune activation is necessary to reveal the primed state of the microglial compartment (BOX 2). Translated back to the clinical world, the implication is that fluctuations in the neurovegetative, somatic and psychological dimensions of depression are more likely to parallel fluctuations in inflammatory status under conditions of activation of the innate immune system (for example, when the microbial burden increases) than at baseline. The probability of detecting an association between inflammation and depression should therefore be much higher in a longitudinal than in a cross-sectional study, which is consistent with the available literature.
Box 2. Mechanisms of enhanced response to systemic inflammation.
Conditions of chronic inflammation exacerbate the sickness and depression-like behaviours that develop in response to acute peripheral inflammation. This phenomenon can be seen in murine models of prion disease96,97, type 2 diabetes39,98 and normal ageing98, and might be due to an effect called ‘priming’. For example, in macrophages that have previously been exposed to interferon-γ (IFN-γ) (the priming stimulus), exposure to a triggering stimulus, such as lipopolysaccharide (LPS), leads to an exaggerated production of pro-inflammatory cytokines. Priming of macrophages involves several molecular mechanisms99, including upregulation of Toll-like receptors (TLRs) and accessory molecules (for example, CD14 for TLR-4); increased expression of intracellular signalling components that are essential for most TLRs, such as the adaptor protein MyD88 and downstream activation of nuclear factor-kappa B (NFκB); and synergy of transcription factors on TLR promoters.
Although microglia can be primed in conditions of chronic inflammation, there is more to this concept than what was originally defined as priming of macrophages. For example, increased production of interleukin (IL)-1β in aged mice is associated with decreased production of the anti-inflammatory cytokine IL-10 (REF. 37). In obese mice, LPS induces more IL-1β but less IL-1 receptor antagonist and IL-1 receptor II (REF. 39) than in non-obese mice. Both of these phenomena can be explained by the concept of phenotypic heterogeneity of microglial cell populations100. Another complicating factor is that exaggerated sickness responses can occur because of an increase in the number or type of leukocytes in the brain. For example, chronic neurodegenerative diseases are associated with increasing numbers of microglia100. Furthermore, dendritic cells and T-lymphocytes gradually invade the healthy aging brain101. Mast cells can also release pre-formed pro-inflammatory cytokines in response to TLR ligands following their rapid migration from blood to the perivascular space of the brain parenchyma, especially in the non-specific sensory thalamus102.
Future directions
The findings described here indicate that we are starting to understand why we feel sick and behave accordingly when we are ill. We now also recognize that inflammation is an important biological event that increases the risk of occurrence of major depressive episodes, much like the more traditional psychosocial factors such as the death of a loved one. Importantly, the rapid increase in knowledge about immune-to-brain communication must be translated into clinical practice.
In the clinic, symptoms of sickness (for example, fatigue, reduced appetite, sleep disorders, altered mood and cognition) are well known to have a negative impact on the quality of life of patients with chronic inflammatory disorders, but not much can be done to alleviate these symptoms. Controlled studies are necessary to validate the putative beneficial value of various nutriments and intervention (for example, physical exercise) on the symptoms of sickness. Such studies can now be carried out at the preclinical and clinical levels by not only evaluating clinically relevant end-points (for example, alleviation of fatigue or depressed mood) but also by taking into account intermediate mechanisms using biomarkers of inflammation. If confirmed in the clinic, the efficacy of compounds targeting IDO and inflammatory mediators for the alleviation of symptoms of depression will open new opportunities for drug development. However, as such compounds have the potential to compromise resistance to infection, targets in the brain should be preferred over peripheral ones.
At the basic science level, it must be recognized that research into cytokine-induced sickness behaviour is still in its infancy. For example, the neurobiological mechanisms underlying the behavioural effects of pro-inflammatory cytokines have rarely been investigated in enough detail to be able to relate a given behavioural effect of a cytokine to a specific action in a well-defined area(s) in the brain29. Micropharmacology experiments that use both in vivo dialysis and approaches that target inflammatory mediators in specific brain areas need to be implemented in order to define the cause–effect relationships more clearly. The field will also benefit from newly-developed technologies that use approaches based on genomic biology. Furthermore, as behaviour has a temporal component, techniques that enable continuous monitoring of biologic phenomena have considerable advantages over techniques that require euthanasia of animals at specific time points. In particular, optical recording of either neuronal activity in vivo using voltage-sensitive dye imaging84 or activation patterns of molecules labelled with genetically encoded, green-fluorescent-protein-based indicators (for example, COX2 (REF. 85) and IDO86) could enlighten our understanding of the neural basis of cytokine-induced sickness and depression-like behaviour.
Very little is known about the contribution of immune-cell trafficking in the brain to the activation of pro-inflammatory cytokine signalling and its behavioural consequences, even though this is likely to be important in CNS diseases. For example, depression is a highly prevalent co-morbid condition in multiple sclerosis87. This autoimmune neuroinflammatory disease can be modelled in rodents by a condition known as experimental allergic encephalomyelitis (EAE) that is associated with clear signs of sickness and depression-like behaviour88,89. Elucidation of the temporal dynamics of immune cell recruitment in the brains of mice with EAE90,91 provides a valuable tool for studying the contribution of leukocyte trafficking to the activation of cytokine signalling in the brain and to the development of sickness and depression-like behaviour in this model.
A consideration of the possible recruitment of pro-inflammatory cytokines by non-infectious stimuli, such as hypoxia92, through the release of catecholamines is also quite likely to provide new insights into the patho-physiology of the increased prevalence of depression in individuals with obstructive sleep apnea93 or chronic obstructive pulmonary disease94.
Finally, the demonstration of a possible contribution of the immune system to the development of depression is likely to open new avenues in psychopathology. The cytokine theory of depression is certainly attractive for a field that is short of real innovations. However, the identification of the intracellular molecular mechanisms that are at the origin of the association between inflammation and depression will provide valuable targets for the development of new antidepressant drugs only if the activation of brain pro-inflammatory cytokine signalling is proven to represent the final common pathway for the various conditions that lead to depression. This task is still in its infancy.
Acknowledgments
The authors’ work described here is supported by grants from the National Institute of Mental Health (NIMH), the National Institute on Aging (NIA) and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). R.D. (R01 MH 079829 and R01 MH 71349), K.W.K. (R01 MH 51569 and R01 AG 029573) R.W.J. (R01 AG 023580, AG 0616710, MH 069148 and R21 DA 024443) and G.G.F (R01 DK 064862). The authors thank R. -M. Bluthe, N. Castanon, S. Laye, P. Parnet, J. P. Konsman, J. Lestage, L. Capuron, C. Dantzer and their Ph.D. students for their valuable contribution to many of the results and concepts presented in this Review.
- Accessory immune cells
Cells such as macrophages and dendritic cells that are required for, but do not actually mediate, adaptive immune responses of T and B lymphocytes
- Motivational state
A central state that re-organizes perception and action
- Inflammation
A response of tissues to injury or irritation that is characterized by pain, swelling, redness and heat
- Physical illness
An infectious, autoimmune or oncogenic disease in which physical rather than psychological symptoms of the diseased tissue or organ predominate
- Choroid plexus
A capillary bed that is covered by transporting ependymal cells and that protrudes into the cerebral ventricles. The ependymal cells are responsible for producing cerebral spinal fluid
- Meninges
The three protective layers of tissue that surround the brain and spinal cord
- Prostaglandin
Cellular communication molecule synthesized from arachidonic acid. Specific compounds are designated by adding a letter to indicate the type of substituents found on the hydrocarbon skeleton and a subscript to indicate the number of double bonds in the hydrocarbon skeleton
- Vagal nerve
The 10th pair of cranial nerves that innervates the pharynx, larynx and visceral organs. It contains more afferent than efferent nerve fibres and projects from the medulla oblongata in the brain stem to the colon
- Toll-like receptor
Highly conserved membrane spanning receptor that recognizes pathogenic molecules that are distinct from host antigens (collectively referred to as pathogen-associated molecular patterns)
- Circumventricular organs
Structures that surround the brain ventricles and are devoid of a functional blood–brain barrier because of fenestrated capillaries
- Blood–brain barrier
A series of structures that limit the penetration and diffusion of circulating water-soluble substances into the brain and include tight junctions between endothelial cells of brain capillaries, a dense network of astrocytes, a reduced volume of extracellular milieu and efflux pumps
- Volume diffusion
A form of neurotransmission that involves the diffusion in the extracellular space of neurotransmitters that are normally released from neurons. Volume diffusion permits neurotransmitters and cytokines to reach extrasynaptic receptors
- Parenchyma
The tissue of an organ, in this case the brain, that supports its functions and is distinct from supporting and connective tissue
- Anti-inflammatory cytokines
Together with specific cytokine inhibitors and soluble cytokine receptors, these are immunoregulatory molecules that down-regulate the pro-inflammatory cytokine response
- Innate immune system
Part of the immune system that is responsible for natural immunity and is geared toward efficiently recognizing pathogenic molecules independently of any prior exposure
- Acute-phase response
The reaction that develops in response to an injury. It is mediated by pro-inflammatory cytokines and is characterized by a local response (inflammation) and a systemic component, which includes production of acute phase proteins by hepatocytes, fever and profound changes in lipid, protein and carbohydrate metabolism
- Neurovegetative
This term refers to phenomena that are visceral and controlled by the autonomic nervous system. In the case of depression, neurovegetative symptoms include sleep disturbances, change in appetite and decreased energy
- Depression-like behaviour
Behaviour displayed by laboratory animals that mimics some features of clinical depression. These include, among others, helplessness and anhedonia. Depression-like behaviour is normally alleviated by antidepressant drugs
- Glucocorticoid receptor resistance
This occurs despite normal or excessive concentrations of glucocorticoids. It is sometimes caused by loss-of-function mutations in the glucocorticoid receptor and, more commonly, by events that occur during chronic inflammation, ultimately leading to a reduction in the ability of glucocorticoids to translocate into the nucleus
- Psychomotor retardation
A generalized slowing of physical and mental activity, frequently occurring as a symptom of severe depression
- Co-morbidity
The presence of one or more diseases in addition to a primary disease
- Illness behaviour
In health psychology, illness behaviour refers to any behaviour undertaken by an individual who feels ill in order to relieve that experience and to better understand the meaning of disease symptoms. It is profoundly influenced by the social context and psychological factors, and manifests itself by denial or amplification of symptoms, attributional processes, a search for medical information, decisions for entering or leaving the health care system, and adherence to treatment
Footnotes
DATABASES
Entrez Gene: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene
c-fos | FosB
UniProtKB: http://ca.expasy.org/sprot
COX2 | CRH | IDO | IFN-α | IFN-γ | IL-1α | IL-2 | IL-6 | IL-1β | serotonin receptor 1A | TDO | TNF-α
FURTHER INFORMATION
Robert Dantzer’s homepage:
References
- 1.Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. The original description of sickness behaviour, its relationship with fever and adaptive value. [DOI] [PubMed] [Google Scholar]
- 3.Dantzer R, Kelley KW. Stress and immunity: an integrated view of relationships between the brain and the immune system. Life Sci. 1989;44:1995–2008. doi: 10.1016/0024-3205(89)90345-7. [DOI] [PubMed] [Google Scholar]
- 4.Dantzer R. Cytokine-induced sickness behavior: where do we stand? Brain Behav Immun. 2001;15:7–24. doi: 10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- 5.Steptoe A. Depression and Physical Illness. Cambridge University Press; Cambridge: 2007. [Google Scholar]
- 6.Galea I, Bechmann I, Perry VH. What is immune privilege (not)? Trends Immunol. 2007;28:12–18. doi: 10.1016/j.it.2006.11.004. A review of how the brain immune response differs from that in other organs. [DOI] [PubMed] [Google Scholar]
- 7.Dantzer R. In: Psychoneuroimmunology. Ader R, editor. Elsevier; Amsterdam: 2007. pp. 271–280. [Google Scholar]
- 8.Bluthe RM, et al. Lipopolysaccharide induces sickness behaviour in rats by a vagal mediated mechanism. C R Acad Sci III. 1994;317:499–503. The first demonstration that section of the vagus nerves blocks immune-to-brain communication and abrogates lipopolysacccharide-induced sickness behaviour without compromising the peripheral immune response. [PubMed] [Google Scholar]
- 9.Watkins LR, et al. Neurocircuitry of illness-induced hyperalgesia. Brain Res. 1994;639:283–299. doi: 10.1016/0006-8993(94)91742-6. [DOI] [PubMed] [Google Scholar]
- 10.Romeo HE, Tio DL, Rahman SU, Chiappelli F, Taylor AN. The glossopharyngeal nerve as a novel pathway in immune-to-brain communication: relevance to neuroimmune surveillance of the oral cavity. J Neuroimmunol. 2001;115:91–100. doi: 10.1016/s0165-5728(01)00270-3. [DOI] [PubMed] [Google Scholar]
- 11.Quan N, Whiteside M, Herkenham M. Time course and localization patterns of interleukin-1beta messenger RNA expression in brain and pituitary after peripheral administration of lipopolysaccharide. Neuroscience. 1998;83:281–293. doi: 10.1016/s0306-4522(97)00350-3. [DOI] [PubMed] [Google Scholar]
- 12.Vitkovic L, et al. Cytokine signals propagate through the brain. Mol Psychiatry. 2000;5:604–615. doi: 10.1038/sj.mp.4000813. [DOI] [PubMed] [Google Scholar]
- 13.Banks WA. The blood-brain barrier in psychoneuroimmunology. Neurol Clin. 2006;24:413–419. doi: 10.1016/j.ncl.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Konsman JP, Vigues S, Mackerlova L, Bristow A, Blomqvist A. Rat brain vascular distribution of interleukin-1 type-1 receptor immunoreactivity: relationship to patterns of inducible cyclooxygenase expression by peripheral inflammatory stimuli. J Comp Neurol. 2004;472:113–129. doi: 10.1002/cne.20052. [DOI] [PubMed] [Google Scholar]
- 15.Schiltz JC, Sawchenko PE. Distinct brain vascular cell types manifest inducible cyclooxygenase expression as a function of the strength and nature of immune insults. J Neurosci. 2002;22:5606–5618. doi: 10.1523/JNEUROSCI.22-13-05606.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dantzer R, Konsman JP, Bluthe RM, Kelley KW. Neural and humoral pathways of communication from the immune system to the brain: parallel or convergent? Auton Neurosci. 2000;85:60–65. doi: 10.1016/S1566-0702(00)00220-4. [DOI] [PubMed] [Google Scholar]
- 17.Reyes TM, Sawchenko PE. Involvement of the arcuate nucleus of the hypothalamus in interleukin-1-induced anorexia. J Neurosci. 2002;22:5091–5099. doi: 10.1523/JNEUROSCI.22-12-05091.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ericsson A, Kovacs KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci. 1994;14:897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dinarello CA. Interleukin-1. Cytokine Growth Factor Rev. 1997;8:253–265. doi: 10.1016/s1359-6101(97)00023-3. [DOI] [PubMed] [Google Scholar]
- 20.Parnet P, Kelley KW, Bluthe RM, Dantzer R. Expression and regulation of interleukin-1 receptors in the brain. Role in cytokines-induced sickness behavior. J Neuroimmunol. 2002;125:5–14. doi: 10.1016/s0165-5728(02)00022-x. [DOI] [PubMed] [Google Scholar]
- 21.van Dam AM, Brouns M, Louisse S, Berkenbosch F. Appearance of interleukin-1 in macrophages and in ramified microglia in the brain of endotoxin-treated rats: a pathway for the induction of non-specific symptoms of sickness? Brain Res. 1992;588:291–296. doi: 10.1016/0006-8993(92)91588-6. The first demonstration that peripherally administered lipopolysaccharide induces the expression of IL-1β in the brain. [DOI] [PubMed] [Google Scholar]
- 22.Laye S, Parnet P, Goujon E, Dantzer R. Peripheral administration of lipopolysaccharide induces the expression of cytokine transcripts in the brain and pituitary of mice. Brain Res Mol Brain Res. 1994;27:157–162. doi: 10.1016/0169-328x(94)90197-x. [DOI] [PubMed] [Google Scholar]
- 23.Quan N, Stern EL, Whiteside MB, Herkenham M. Induction of pro-inflammatory cytokine mRNAs in the brain after peripheral injection of subseptic doses of lipopolysaccharide in the rat. J Neuroimmunol. 1999;93:72–80. doi: 10.1016/s0165-5728(98)00193-3. [DOI] [PubMed] [Google Scholar]
- 24.Gatti S, Bartfai T. Induction of tumor necrosis factor-alpha mRNA in the brain after peripheral endotoxin treatment: comparison with interleukin-1 family and interleukin-6. Brain Res. 1993;624:291–294. doi: 10.1016/0006-8993(93)90090-a. [DOI] [PubMed] [Google Scholar]
- 25.Breder CD, et al. Regional induction of tumor necrosis factor alpha expression in the mouse brain after systemic lipopolysaccharide administration. Proc Natl Acad Sci USA. 1994;91:11393–11397. doi: 10.1073/pnas.91.24.11393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carmichael MD, et al. Role of brain IL-1beta on fatigue after exercise-induced muscle damage. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1344–1348. doi: 10.1152/ajpregu.00141.2006. [DOI] [PubMed] [Google Scholar]
- 27.Ohdo S, Koyanagi S, Suyama H, Higuchi S, Aramaki H. Changing the dosing schedule minimizes the disruptive effects of interferon on clock function. Nature Med. 2001;7:356–360. doi: 10.1038/85507. [DOI] [PubMed] [Google Scholar]
- 28.Cavadini G, et al. TNF-{alpha} suppresses the expression of clock genes by interfering with E-box-mediated transcription. Proc Natl Acad Sci USA. 2007 doi: 10.1073/pnas.0701466104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sparkman NL, et al. Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. J Neurosci. 2006;26:10709–10716. doi: 10.1523/JNEUROSCI.3376-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heyen JR, Ye S, Finck BN, Johnson RW. Interleukin (IL)-10 inhibits IL-6 production in microglia by preventing activation of NF-kappaB. Brain Res Mol Brain Res. 2000;77:138–147. doi: 10.1016/s0169-328x(00)00042-5. [DOI] [PubMed] [Google Scholar]
- 31.Strle K, et al. Novel activity of an anti-inflammatory cytokine: IL-10 prevents TNFalpha-induced resistance to IGFI in myoblasts. J Neuroimmunol. doi: 10.1016/j.jneuroim.2007.05.003. in the press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bluthe RM, et al. Central injection of IL-10 antagonizes the behavioural effects of lipopolysaccharide in rats. Psychoneuroendocrinology. 1999;24:301–311. doi: 10.1016/s0306-4530(98)00077-8. [DOI] [PubMed] [Google Scholar]
- 33.Dantzer R, Gheusi G, Johnson RW, Kelley KW. Central administration of insulin-like growth factor-1 inhibits lipopolysaccharide-induced sickness behavior in mice. Neuroreport. 1999;10:289–292. doi: 10.1097/00001756-199902050-00015. [DOI] [PubMed] [Google Scholar]
- 34.Bluthe RM, Kelley KW, Dantzer R. Effects of insulin-like growth factor-I on cytokine-induced sickness behavior in mice. Brain Behav Immun. 2006;20:57–63. doi: 10.1016/j.bbi.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leon LR, Kozak W, Rudolph K, Kluger MJ. An antipyretic role for interleukin-10 in LPS fever in mice. Am J Physiol. 1999;276:R81–89. doi: 10.1152/ajpregu.1999.276.1.R81. [DOI] [PubMed] [Google Scholar]
- 36.Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. J Neuroimmunol. 1999;93:139–148. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]
- 37.Ye SM, Johnson RW. An age-related decline in interleukin-10 may contribute to the increased expression of interleukin-6 in brain of aged mice. Neuroimmunomodulation. 2001;9:183–192. doi: 10.1159/000049025. [DOI] [PubMed] [Google Scholar]
- 38.Huang Y, Henry CJ, Dantzer R, Johnson RW, Godbout JP. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Connor JC, et al. IL-1beta-mediated innate immunity is amplified in the db/db mouse model of type 2 diabetes. J Immunol. 2005;174:4991–4997. doi: 10.4049/jimmunol.174.8.4991. [DOI] [PubMed] [Google Scholar]
- 40.Yirmiya R, et al. Cytokines, “depression due to a general medical condition” and antidepressant drugs. Adv Exp Med Biol. 1999;461:283–316. doi: 10.1007/978-0-585-37970-8_16. [DOI] [PubMed] [Google Scholar]
- 41.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. An excellent review of the clinical features of cytokine-induced depression and its possible mechanisms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nemeroff CB, Vale WW. The neurobiology of depression: inroads to treatment and new drug discovery. J Clin Psychiatry. 2005;66(Suppl 7):5–13. [PubMed] [Google Scholar]
- 43.Smith RS. The macrophage theory of depression. Med Hypotheses. 1991;35:298–306. doi: 10.1016/0306-9877(91)90272-z. [DOI] [PubMed] [Google Scholar]
- 44.Maes M, Smith R, Scharpe S. The monocyte-T-lymphocyte hypothesis of major depression. Psychoneuroendocrinology. 1995;20:111–116. doi: 10.1016/0306-4530(94)00066-j. [DOI] [PubMed] [Google Scholar]
- 45.Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007;21:374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 46.Denicoff KD, et al. The neuropsychiatric effects of treatment with interleukin-2 and lymphokine-activated killer cells. Ann Intern Med. 1987;107:293–300. doi: 10.7326/0003-4819-107-2-293. [DOI] [PubMed] [Google Scholar]
- 47.Renault PF, et al. Psychiatric complications of long-term interferon alfa therapy. Arch Intern Med. 1987;147:1577–1580. [PubMed] [Google Scholar]
- 48.Capuron L, et al. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 49.Capuron L, Ravaud A, Dantzer R. Early depressive symptoms in cancer patients receiving interleukin 2 and/or interferon alfa-2b therapy. J Clin Oncol. 2000;18:2143–2151. doi: 10.1200/JCO.2000.18.10.2143. [DOI] [PubMed] [Google Scholar]
- 50.Constant A, et al. Mood alterations during interferon-alfa therapy in patients with chronic hepatitis C: evidence for an overlap between manic/hypomanic and depressive symptoms. J Clin Psychiatry. 2005;66:1050–1057. doi: 10.4088/jcp.v66n0814. [DOI] [PubMed] [Google Scholar]
- 51.Capuron L, Ravaud A. Prediction of the depressive effects of interferon alfa therapy by the patient’s initial affective state. N Engl J Med. 1999;340:1370. doi: 10.1056/NEJM199904293401716. [DOI] [PubMed] [Google Scholar]
- 52.Capuron L, et al. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am J Psychiatry. 2003;160:1342–1345. doi: 10.1176/appi.ajp.160.7.1342. [DOI] [PubMed] [Google Scholar]
- 53.Frenois F, et al. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology. 2007;32:516–531. doi: 10.1016/j.psyneuen.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simmons DA, Broderick PA. Cytokines, stressors, and clinical depression: augmented adaptation responses underlie depression pathogenesis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:793–807. doi: 10.1016/j.pnpbp.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 55.Merali Z, Brennan K, Brau P, Anisman H. Dissociating anorexia and anhedonia elicited by interleukin-1beta: antidepressant and gender effects on responding for “free chow” and “earned” sucrose intake. Psychopharmacology (Berl) 2003;165:413–418. doi: 10.1007/s00213-002-1273-1. [DOI] [PubMed] [Google Scholar]
- 56.Capuron L, et al. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry. 2002;7:468–473. doi: 10.1038/sj.mp.4000995. A landmark paper demonstrating that repeated activation of the immune system by systemic administration of IL-2 and IFN-α to cancer patients induces a drastic fall in plasma tryptophan levels that is positively correlated to the depression scores. [DOI] [PubMed] [Google Scholar]
- 57.Ruhe HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry. 2007;12:331–359. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- 58.Wirleitner B, Neurauter G, Schrocksnadel K, Frick B, Fuchs D. Interferon-gamma-induced conversion of tryptophan: immunologic and neuropsychiatric aspects. Curr Med Chem. 2003;10:1581–1591. doi: 10.2174/0929867033457179. [DOI] [PubMed] [Google Scholar]
- 59.Lestage J, Verrier D, Palin K, Dantzer R. The enzyme indoleamine 2, 3-dioxygenase is induced in the mouse brain in response to peripheral administration of lipopolysaccharide and superantigen. Brain Behav Immun. 2002;16:596–601. doi: 10.1016/s0889-1591(02)00014-4. [DOI] [PubMed] [Google Scholar]
- 60.Moreau M, et al. Bacille Calmette-Guerin inoculation induces chronic activation of peripheral and brain indoleamine 2, 3-dioxygenase in mice. J Infect Dis. 2005;192:537–544. doi: 10.1086/431603. [DOI] [PubMed] [Google Scholar]
- 61.Muller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry. 2007;12:988–1000. doi: 10.1038/sj.mp.4002006. [DOI] [PubMed] [Google Scholar]
- 62.Dunn AJ, Swiergiel AH, de Beaurepaire R. Cytokines as mediators of depression: what can we learn from animal studies? Neurosci Biobehav Rev. 2005;29:891–909. doi: 10.1016/j.neubiorev.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 63.Zhu CB, Blakely RD, Hewlett WA. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology. 2006;31:2121–2131. doi: 10.1038/sj.npp.1301029. [DOI] [PubMed] [Google Scholar]
- 64.Cai W, et al. Interferon-alpha-induced modulation of glucocorticoid and serotonin receptors as a mechanism of depression. J Hepatol. 2005;42:880–887. doi: 10.1016/j.jhep.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 65.Pariante CM. Depression, stress and the adrenal axis. J Neuroendocrinol. 2003;15:811–812. doi: 10.1046/j.1365-2826.2003.01058.x. [DOI] [PubMed] [Google Scholar]
- 66.Berkenbosch F, van Oers J, del Rey A, Tilders F, Besedovsky H. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science. 1987;238:524–526. doi: 10.1126/science.2443979. [DOI] [PubMed] [Google Scholar]
- 67.Grinevich V, et al. Hypothalamic pituitary adrenal axis and immune responses to endotoxin in rats with chronic adjuvant-induced arthritis. Exp Neurol. 2002;178:112–123. doi: 10.1006/exnr.2002.8022. [DOI] [PubMed] [Google Scholar]
- 68.Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev. 2005;4:141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 69.Holsboer F. Corticotropin-releasing hormone modulators and depression. Curr Opin Investig Drugs. 2003;4:46–50. [PubMed] [Google Scholar]
- 70.Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2007;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- 72.Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp. 2007 doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Capuron L, et al. Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301362. [DOI] [PubMed] [Google Scholar]
- 74.Capuron L, et al. Anterior cingulate activation and error processing during interferon-alpha treatment. Biol Psychiatry. 2005;58:190–196. doi: 10.1016/j.biopsych.2005.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stone EA, Lehmann ML, Lin Y, Quartermain D. Depressive behavior in mice due to immune stimulation is accompanied by reduced neural activity in brain regions involved in positively motivated behavior. Biol Psychiatry. 2006;60:803–811. doi: 10.1016/j.biopsych.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 76.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 77.Lowry CA, et al. Identification of an immune-responsive mesolimbocortical serotonergic system: potential role in regulation of emotional behavior. Neuroscience. 2007;146:756–772. doi: 10.1016/j.neuroscience.2007.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 79.Frasure-Smith N, Lesperance F. Depression and coronary artery disease. Herz. 2006;31(Suppl 3):64–68. [PubMed] [Google Scholar]
- 80.Tyring S, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- 81.Muller N, et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry. 2006;11:680–684. doi: 10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]
- 82.van den Biggelaar AH, et al. Inflammation and interleukin-1 signaling network contribute to depressive symptoms but not cognitive decline in old age. Exp Gerontol. 2007;42:693–701. doi: 10.1016/j.exger.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 83.Godbout JP, et al. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology. doi: 10.1038/sj.npp.1301649. in the press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Airan RD, et al. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science. 2007;317:819–823. doi: 10.1126/science.1144400. [DOI] [PubMed] [Google Scholar]
- 85.Sheng H, et al. Transforming growth factor-beta1 enhances Ha-ras-induced expression of cyclooxygenase-2 in intestinal epithelial cells via stabilization of mRNA. J Biol Chem. 2000;275:6628–6635. doi: 10.1074/jbc.275.9.6628. [DOI] [PubMed] [Google Scholar]
- 86.Babcock TA, Carlin JM. Transcriptional activation of indoleamine dioxygenase by interleukin 1 and tumor necrosis factor alpha in interferon-treated epithelial cells. Cytokine. 2000;12:588–594. doi: 10.1006/cyto.1999.0661. [DOI] [PubMed] [Google Scholar]
- 87.Siegert RJ, Abernethy DA. Depression in multiple sclerosis: a review. J Neurol Neurosurg Psychiatry. 2005;76:469–475. doi: 10.1136/jnnp.2004.054635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pollak Y, Ovadia H, Orion E, Weidenfeld J, Yirmiya R. The EAE-associated behavioral syndrome: I. Temporal correlation with inflammatory mediators. J Neuroimmunol. 2003;137:94–99. doi: 10.1016/s0165-5728(03)00075-4. [DOI] [PubMed] [Google Scholar]
- 89.Pollak Y, Ovadia H, Orion E, Yirmiya R. The EAE-associated behavioral syndrome: II. Modulation by anti-inflammatory treatments. J Neuroimmunol. 2003;137:100–108. doi: 10.1016/s0165-5728(03)00073-0. [DOI] [PubMed] [Google Scholar]
- 90.McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nature Med. 2005;11:335–339. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- 91.Miller SD, McMahon EJ, Schreiner B, Bailey SL. Antigen presentation in the CNS by myeloid dendritic cells drives progression of relapsing experimental autoimmune encephalomyelitis. Ann NY Acad Sci. 2007;1103:179–191. doi: 10.1196/annals.1394.023. [DOI] [PubMed] [Google Scholar]
- 92.Johnson DR, O’Connor JC, Hartman ME, Tapping RI, Freund GG. Acute hypoxia activates the neuroimmune system, which diabetes exacerbates. J Neurosci. 2007;27:1161–1166. doi: 10.1523/JNEUROSCI.4560-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ohayon MM. The effects of breathing-related sleep disorders on mood disturbances in the general population. J Clin Psychiatry. 2003;64:1195–1200. doi: 10.4088/jcp.v64n1009. quiz, 1274–1276. [DOI] [PubMed] [Google Scholar]
- 94.Borson S, Claypoole K, McDonald GJ. Depression and chronic obstructive pulmonary disease: treatment trials. Semin Clin Neuropsychiatry. 1998;3:115–130. [PubMed] [Google Scholar]
- 95.Schwarcz R. The kynurenine pathway of tryptophan degradation as a drug target. Curr Opin Pharmacol. 2004;4:12–17. doi: 10.1016/j.coph.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 96.Combrinck MI, Perry VH, Cunningham C. Peripheral infection evokes exaggerated sickness behaviour in pre-clinical murine prion disease. Neuroscience. 2002;112:7–11. doi: 10.1016/s0306-4522(02)00030-1. [DOI] [PubMed] [Google Scholar]
- 97.Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25:9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Godbout JP, et al. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- 99.Schroder K, Sweet MJ, Hume DA. Signal integration between IFNgamma and TLR signalling pathways in macrophages. Immunobiology. 2006;211:511–524. doi: 10.1016/j.imbio.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 100.Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nature Rev Immunol. 2007;7:161–167. doi: 10.1038/nri2015. A presentation of the concept of priming of the microglial compartment during chronic brain inflammation and its role in the exaggerated response to systemic infections. [DOI] [PubMed] [Google Scholar]
- 101.Stichel CC, Luebbert H. Inflammatory processes in the aging mouse brain: participation of dendritic cells and T-cells. Neurobiol Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 102.Silverman AJ, Sutherland AK, Wilhelm M, Silver R. Mast cells migrate from blood to brain. J Neurosci. 2000;20:401–408. doi: 10.1523/JNEUROSCI.20-01-00401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain Behav Immun. 1998;12:212–229. doi: 10.1006/brbi.1998.0524. [DOI] [PubMed] [Google Scholar]
- 104.Vereker E, O’Donnell E, Lynch MA. The inhibitory effect of interleukin-1beta on long-term potentiation is coupled with increased activity of stress-activated protein kinases. J Neurosci. 2000;20:6811–6819. doi: 10.1523/JNEUROSCI.20-18-06811.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sweller J. Cognitive load during problem solving: effects on learning. Cognitive Sci. 1988;12:257–285. [Google Scholar]
- 106.Blum D, Chtarto A, Tenenbaum L, Brotchi J, Levivier M. Clinical potential of minocycline for neurodegenerative disorders. Neurobiol Dis. 2004;17:359–366. doi: 10.1016/j.nbd.2004.07.012. [DOI] [PubMed] [Google Scholar]