Abstract
Multiple developmental phenotypes have been associated with duplication in the 15q11-13 region. Recently, the 15q11-13 duplication has been associated with a distinct pattern of mitochondrial abnormality that includes a deficiency in complex III. This report describes the third case with this duplication and a similar pattern of mitochondrial dysfunction. Genetic studies performed on this case rules out the previously suggested role of the UBE3A gene. It is proposed that interactions of the duplicated SNRPN gene with nuclear respiratory factor-1 (NRF-1) could result in destabilization of mitochondrial complex formation and activation of apoptosis under metabolic stress, resulting in the pattern of abnormalities found in the current and previously reported cases. In light of the frequency of this duplication in children with developmental dishabilles the wider implication of the association between this duplication and mitochondrial dysfunction needs to be considered.
Keywords: 15q11-13 duplication, mitochondrial disorders, autism
Introduction
Many genetic and metabolic etiologies of autism have been identified but few account for a majority of cases. Over the past decade a number of clinical reports have linked mitochondrial dysfunction with autism. Mitochondrial dysfunction was first confirmed to be associated with autism by muscle biopsy in the HEADD syndrome (hypotonia, epilepsy, autism, and developmental delay)1. Recent studies have suggested that mitochondrial dysfunction may be present in a substantial proportion of children with autism. For example, 65% of children with autism who were referred for neurometabolic work-up demonstrated an oxidative phosphorylation defect2 and a population-based study in older autism patients from Portugal found that 7.2% of patients with autism could be classified as having a ‘definite’ mitochondrial respiratory chain disorder and 20% had elevated serum lactic acid3.
Investigations into the genetic linkage between mitochondrial disorders and autism have been limited. For example, given that autism is particularly prevalent in siblings Kent et al.4 investigated the potential contribution of mitochondrial deoxyribonucleic acid (DNA) inheritance through maternal lineage. Unfortunately no simple association between mitochondrial haplogroups and autism was found.4 However, recently an interesting association between a common genetic duplication and a distinct mitochondrial abnormality has been reported. Filipek et al.5 reported two cases of a duplication in the 15q11-13 region associated with autism, a relative complex III deficiency, enzymatic indicators of mitochondrial proliferation and atypical metabolic markers. The importance of this finding is potentially wide-ranging as multiple developmental phenotypes are associated with duplication in the 15q11-13 region, most notably Angelman and Prader-Willi syndromes.6 Importantly, the association between these phenotypes and mitochondrial disease has not been well studied. Children with this duplication demonstrate a high rate of the autism phenotype and 1–2% of children diagnosed with autism demonstrate this duplication, further suggesting that this duplication and its association with mitochondrial dysfunction may be significant.7
In this communication, we report the third case documenting the association between duplication of the 15q11.2-13 region and mitochondrial dysfunction. The subtle biomarkers associated with this unique pattern of mitochondrial abnormalities are highlighted in the case and the previously reported two cases. In addition, the potential specific molecular mechanism responsible for the mitochondrial abnormalities is discussed.
Case Description
The patient presented at six months of age with developmental delay and clusters of paroxysmal movements. Antiepileptic drug therapy with topiramate (3.4 mg/kg/day) and zonisamide (8.4 mg/kg/day) did not improve the frequency or severity of events. Increased seizure activity resulted in a hospital admission. An electroencephalogram demonstrated an intermittent 4–5Hz occipital dominant rhythm with diffuse intermixed delta and theta activity, multifocal spike and wave discharges throughout the left hemisphere and independent spike and wave discharges in the right parietal and occipital areas. A cluster of infantile spasms was associated with an electrodecremental event followed by fast beta activity. Levetiracetam (30mg/kg/day) was added and topiramate was discontinued with good response.
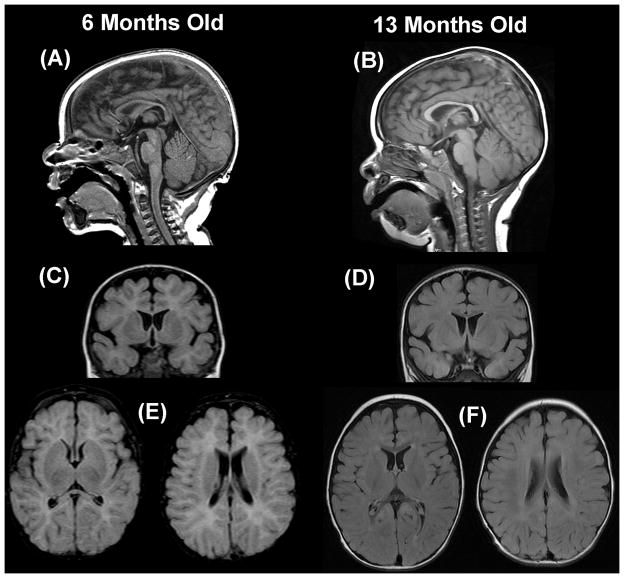
Magnetic resonance imaging of the brain demonstrated delayed myelination, thin corpus callosum, increased extra-axial space and underdevelopment of the operculum (Figure 1). A chromosomal micro-array revealed a gain in the subcentromeric and proximal regions of the long arm of chromosome 15q11.2-13 encompassing a 4.395 megabase segment. Florescent in-situ hybridization confirmed this duplication in the patient but found no evidence of chromosome 15q duplication in the parents. Metabolic abnormalities over a five day hospitalization are presented in Table 1. A lumbar puncture demonstrated normal 5-methyltetrahydrofolate, lactic acid, 5-hydroxyindoleacetic acid, homovanillic acid, 3-O-methydopa, neopterin, tetrahydrobiopterin.
Figure 1.
T2 fluid attenuated inverse recovery magnetic resonance imaging of the patient’s brain at 6 and 15 months. Mid-sagittal images demonstrate that the corpus callosum is thin at 6 months (A) but a normal at 13 months (B). Excessive extra-axial space and underdevelopment of the operculum is seen on coronal and axial images at 6 months (C, E) but not 13 months (D, F).
Table 1.
Metabolic Abnormalities During Hospitalization.
| Hospital Day | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Carbon Dioxide (mEq/L) | 14, 19 | 18 | ||
| AST (U/L) | 38 | |||
| Lactate (mMol/L) | 2.4 | 1.9 | ||
| Pyruvate (mMol/L) | 0.152 | |||
| Ammonia (mcg/dL) | 258 | 441 | 138 | |
| Alanine (uMol) | 304 | |||
| Lysine (uMol) | 84 | |||
| Alanine/Lysine Ratio (<2.5) | 3.6 | |||
| Urine Organic Acids (Present) | succinic, 4-OH-PHE-Acetic, aconitic, hippuric, citric, 2-oxoglutaric | |||
At 5 months, a muscle biopsy was consistent with mitochondrial dysfunction (Table 2). The electron microscopy demonstrated abnormal mitochondrial morphology and degeneration. The increase in citrate synthase from electron transport chain enzyme analysis suggested mitochondrial proliferation. The decreased corrected Rotenone sensitive complex I+III (23%) and complex II+III (33%) combined with normal corrected complex I (50%) and complex II (83%) suggested a specific defect in complex III. With the muscle biopsy result, the patient was started on a mitochondrial cocktail consisting of carnitine (17mg/kg/day), coenzyme Q10 (4mg/kg/day), niacin (9mg/kg/day) and thiamine (9mg/kg/day) and further genetic testing was performed. No common mitochondrial DNA point mutations or deletions were detected, including those responsible for mitochondrial encephalopathy, lactic acidosis and stroke (MELAS), myoclonic epilepsy and ragged red fibers (MERRF), neuropathy, ataxia, retinitis pigmentosa (NARP), Leigh syndrome, Leber’s optic neuropathy, Kearns-Sayre syndrome, chronic progressive external ophthalmoplegia (CPEO), and mitochondrial DNA deletion syndrome (Mitochondrial DNA screening panel, Baylor Medical Genetic Laboratory, Houston, TX). Additionally, an oligonucleotide array comparative genomic hybridization analysis demonstrated no deletions or duplications in approximately 180 nuclear genes, including genes involved in mitochondrial DNA biogenesis, maintenance, transcription and translation, or respiratory chain complex assembly (MitoMetSM, Baylor Medical Genetics Laboratory, Houston, TX).
Table 2.
Muscle Biopsy Histology and Enzymatic Complex Activities.
| Activities | Complexes | Patient (% Normal; Corrected) | Normal (SD) |
|---|---|---|---|
| NADH Fericyanide dehydrogenase | I | 328 (77%; 50%) | 426 (254) |
| NADH cytochrome c reductase | I + III | ||
| Total | 53.7 (56%; 37%) | 95.2 (36.7) | |
| Rotenone sensitive | 15.3 (35%; 23%) | 43.3 (21.0) | |
| Succinate dehydrogenase | II | 18.4 (127%; 83%) | 14.5 (7.37) |
| Succinate cytochrome c reductase | II+III | 12.4 (52%; 33%) | 24.6 (11.9) |
| Citrate synthase | 421 (152%; 100%) | 227 (135) | |
| Muscle Histology | Slightly irregular muscle fiber size with some hypertrophic fibers and some fibers contained slightly increased sarcoplasmic lipid. | ||
| Muscle Electron Microscopy | Some elongated mitochondria with mild cristal irregularities. Few mitochondria undergoing degeneration. Some lipid-like deposits in the subsarcolemmal regions. | ||
The patient was the only product of a non-consanguinity Hispanic union (30-year-old G4 P3-0-1-3 mother and 34-year-old father). Gestation was complicated by diabetes requiring insulin control. Four ultrasounds during the pregnancy were unremarkable. The child was born at term via C-section with a birth weight was 10 pounds 6 ounces (>90%) and length of 21.75 inches (>90%). The child was observed for ten days due to transient respiratory distress and suspected sepsis. Two newborn screens were normal.
Family history was significant. A paternal aunt and female paternal second cousin (paternal grandmother’s brother’s daughter) died in the neonatal period from unexplained causes. A female paternal second cousin had a diagnosis of autism (paternal grandmother’s brother’s daughter, different brother than above). A maternal female first cousin (maternal uncle’s daughter) carried a diagnosis of cerebral palsy. A half-sister was diagnosed with intellectual impairment and speech delay.
Neurological follow-up was conducted at 7 and 13 months of age. Dysmorphology included left preauricular pit and almond-shaped eyes with slight epicanthal folds. Skin examination demonstrated four small (1–3mm) hyperpigmented macules on the legs and buttocks and one 1 cm diameter hypopigmented spot on his right upper shoulder. Occipital-frontal circumference, weight and height followed the standardized growth curve at approximately the 50th, 75th and 75th percentiles, respectively across the initial hospitalization and follow-up examinations.
At 7 months of age, developmental assessment was 4 month equivalent for language, motor and social skills as accessed by the Denver Developmental Screening Test II (Denver Developmental Materials, Inc., Denver, CO). Neurological examination was significant for moderate axial and appendicular hypotonia. Levetiracetam was increased to 45mg/kg/day.
At 13-month-old the patient continued to have frequent seizures characterized by eyes rolling up into his head along with bilateral arm and right leg flexion. Developmentally he remained substantially delayed. His social, fine motor and language development was estimated to be at a 5 month level while his gross motor developmental was estimated to be at an 8 month level. Neurological examination demonstrated intermittent right esotropia, intermittent myoclonus, moderate axial and appendicular hypotonia and mild left hemiplegia. Despite appearing alert and awake, he did not demonstrate any spontaneous interaction or movement and did not orient to novel stimuli. A routine electroencephalogram demonstrated intermittent bitemporal sharp transients, left hemisphere low amplitude spike and wave discharges maximal over the left temporal region and left temporal slowing. Oxcarbazepine was started for further seizure control.
Brain stem auditory evoked potentials demonstrated minimal bilateral delayed of the V wave suggesting conduction delay between the rostral brainstem (lateral lemniscus) and caudal midbrain (inferior colliculi). Repeat magnetic resonance imaging demonstrated improvement in brain growth and resolution of myelination delay (Figure 1).
Discussion
This is the third case to demonstrate the association between 15q11.2-13 duplication and mitochondrial dysfunction. The pattern of mitochondrial dysfunction, relatively decreased complex III function and enzymatic evidence of mitochondrial proliferation, in this case matches the pattern of mitochondrial abnormalities of the previous reported cases. Atypical laboratory abnormalities in this case, including increase in aspartate aminotransferase, alanine-to-lysine ratio and ammonia, match other cases reported with complex deficiency and autism.5,8,9 At his point in development, it is too early to diagnose this child with autism, but this child has a high probability to be diagnosed with autism in the not too distant future.
In their cases, Filipek et al.5 proposed that the increase mitochondrial proliferation, as indexes by increased citrate synthase, was an adaptive response to a complex III deficiency. They also suggested that UBE3A and ATP10C genes could be involved in this disorder. However, the oligonucleotide array comparative genomic hybridization analysis ruled out duplication of the UBE3A gene and the ATP10C gene has not been shown to be involved in mitochondrial function. Indeed, there may be a more direct reason for mitochondrial proliferation as well as the pattern of changes in complex activity described in these cases. The DNase I hypersensitive site within the SNRPN gene, a gene within the 15q11.2-13 region, interacts with several regulatory proteins, including nuclear respiratory factor-1 (NRF-1)10. NRF-1 is a transcription factor that acts on nuclear genes encoding respiratory complexes, and components of the mitochondrial transcription and replication machinery11. A disruption in expression of these genes results in both an increase and decrease in gene expression and disrupts the critical balance between the maintenance of respiratory complex subunits and a means for their import and correct assemble12,13.
NRF-1 has been found to regulate SDH2, one of the four nuclear-encoded genes for complex II. Thus, overexpression of NRF-1 could very well lead to over expression of Complex II as appears apparent in the case presented as well as in case #2 presented by Filipek et al5. Overexpression of one or more complexes could upregulate other Kreb cycle enzymes, such as citrate synthase, without necessarily resulting in mitochondrial proliferation. Indeed, the electron microscopy did not show mitochondrial proliferation.
In cell culture, under serum-deprived conditions, overexpression of NRF-1 leads to enlarged electron-dense mitochondria with disruption of the mitochondrial membrane and apoptotic cell death13. Elongated mitochondria with mild cristal irregularities and mitochondria degeneration was reported on the electron microscopy in the current case. The electron microscopy may be a milder representation of what is seen with overexpression of NRF-113. Milder findings would be expected since the electron microscopy of this case does not represent deprived conditions. The sensitivity of NRF-1 overexpressed cells to apoptosis under deprived conditions could be translated clinically to cell dysfunction and death under metabolic stress. In this manner, metabolic stress could result in a mild myopathy causing an increase in creatine kinase. This could explain the increased creatine kinase in Filipek et al.5 case #1. Since children with autism have an increased incidence of mitochondrial disorders3,14, particularly complex disorders2,9, this could explain the increase in creatine kinase reported in children with autism as a group8,9.
The case presented, the cases of Filiipek et al.5 and the case of Poling et al.8 demonstrate the role of subtle biomarkers in the diagnosis of mitochondrial disorders, specifically electron transport chain complex abnormalities. Filipek et al.5 case #2 and the current case demonstrate moderate hyperammonemia with a non-diagnostic pattern of amino acids abnormalities. Filipek et al.5 case #1 like Poling et al.8 reported an abnormally increased creatine kinase. The current case as well as Poling et al.8 report inconsistent lactic acidosis, and increased aspartate aminotransferase and alanine-to-lysine ratio. Poling et al.8 demonstrated that these non-specific subtle biomarkers (creatine kinase and aspartate aminotransferase) were, on average, abnormally elevated in a group of autism children. Elevation in these subtle biomarkers in autism is consistent with the fact that children with autism have an increased incidence of mitochondrial disorders3,14, particularly complex disorders2. The true rate of these subtle metabolic abnormalities in mitochondrial disorders is not known since, in general, they are probably underreported or not examined in most cases of mitochondrial disorders.
The autism phenotype demonstrates a much greater prevalence in Prader-Willi syndrome associated with maternal uniparental disomy as compared to other causes of Prader-Willi syndrome, including paternally inherited deletions or translocations or imprinting mutations or deletions of the Prader–Willi syndrome Imprinting Center6. The fact that 15q11.2-13 duplications for both patients with autism presented by Fillipek et al.5 were maternally inherited suggests that overlap may exist with the genes associated with the autism phenotype in Prader-Willi syndrome. If these are also the genes resulting in mitochondrial dysfunction, it is possible that some cases of Prader-Willi may also be manifest abnormalities in genes associated with mitochondrial dysfunction. Under normal conditions, the SNRPN gene is exclusively transcribed from the paternal chromosomal. Thus, if this was the gene involved in mitochondrial dysfunction, the duplicated maternally inherited SNRPN gene would need to be abnormally transcribed. Clearly the role of mitochondrial genes in autism and other developmental disorders with abnormalities of the 15q11-13 region needs to be studied in more detail. Unfortunately, the risks associated with muscle biopsy limits our ability diagnoses a definite mitochondria disorder in many patients. Future clinical research will hopefully provide more accurate biomarkers for mitochondrial disorders.
Acknowledgments
This study was supported by NS046565 to Dr. Richard E. Frye.
Footnotes
There are no conflicts of interest to declare. This study in not under review elsewhere.
Statement of conflict of interest: Dr. Frye is the director of the medically-based Autism clinic at University of Texas. Dr. Frye provides expert opinion for child neurology cases for both the plaintiff and defendant, including those in which autism is suspected and vaccine injury cases. All proceeds from such work are provided to the Department of Pediatrics at University of Texas with some of these funds supporting Dr. Frye’s research.
References
- 1.Fillano JJ, Goldenthal MJ, Rhodes CH, Marin-Garcia J. Mitochondrial dysfunction in patients with hypotonia, epilepsy, autism, and developmental delay: HEADD syndrome. J Child Neurol. 2002;17:435–439. doi: 10.1177/088307380201700607. [DOI] [PubMed] [Google Scholar]
- 2.Shoffner J, Hyams L, Langley G. 60th Annual Meeting of the American Academy of Neurology. Chicago: 2008. Oxidative Phosphorylation (OXPHOS) Defects in Children with Autistic Spectrum Disorders. [Google Scholar]
- 3.Oliveira G, Diogo L, Grazina M, et al. Mitochondrial dysfunction in autism spectrum disorders: a population-based study. Developmental medicine and child neurology. 2005;47:185–189. doi: 10.1017/s0012162205000332. [DOI] [PubMed] [Google Scholar]
- 4.Kent L, Gallagher L, Elliott HR, Mowbray C, Chinnery PF. An investigation of mitochondrial haplogroups in autism. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:987–989. doi: 10.1002/ajmg.b.30687. [DOI] [PubMed] [Google Scholar]
- 5.Filipek PA, Juranek J, Smith M, et al. Mitochondrial dysfunction in autistic patients with 15q inverted duplication. Annals of neurology. 2003;53:801–804. doi: 10.1002/ana.10596. [DOI] [PubMed] [Google Scholar]
- 6.Veltman MW, Craig EE, Bolton PF. Autism spectrum disorders in Prader-Willi and Angelman syndromes: a systematic review. Psychiatric genetics. 2005;15:243–254. doi: 10.1097/00041444-200512000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nature reviews. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poling JS, Frye RE, Shoffner J, Zimmerman AW. Developmental regression and mitochondrial dysfunction in a child with autism. Journal of child neurology. 2006;21:170–172. doi: 10.2310/7010.2006.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weissman JR, Kelley RI, Bauman ML, Cohen BH, Murray KF, Mitchell RL, Kern RL, Natowicz MR. Mitochondrial disease in autism spectrum disorder patients: a cohort analysis. PLoS ONE. 2008;3:e3815. doi: 10.1371/journal.pone.0003815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez-Jato S, Nicholls RD, Driscoll DJ, Yang TP. Characterization of cis- and transacting elements in the imprinted human SNURF-SNRPN locus. Nucleic acids research. 2005;33:4740–4753. doi: 10.1093/nar/gki786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gleyzer N, Vercauteren K, Scarpulla RC. Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Molecular and cellular biology. 2005;25:1354–1366. doi: 10.1128/MCB.25.4.1354-1366.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schapira AH, Cock HR. Mitochondrial myopathies and encephalomyopathies. European journal of clinical investigation. 1999;29:886–898. doi: 10.1046/j.1365-2362.1999.00540.x. [DOI] [PubMed] [Google Scholar]
- 13.Morrish F, Giedt C, Hockenbery D. c-MYC apoptotic function is mediated by NRF-1 target genes. Genes & development. 2003;17:240–255. doi: 10.1101/gad.1032503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oliveira G, Ataide A, Marques C, et al. Epidemiology of autism spectrum disorder in Portugal: prevalence, clinical characterization, and medical conditions. Developmental medicine and child neurology. 2007;49:726–733. doi: 10.1111/j.1469-8749.2007.00726.x. [DOI] [PubMed] [Google Scholar]