Abstract
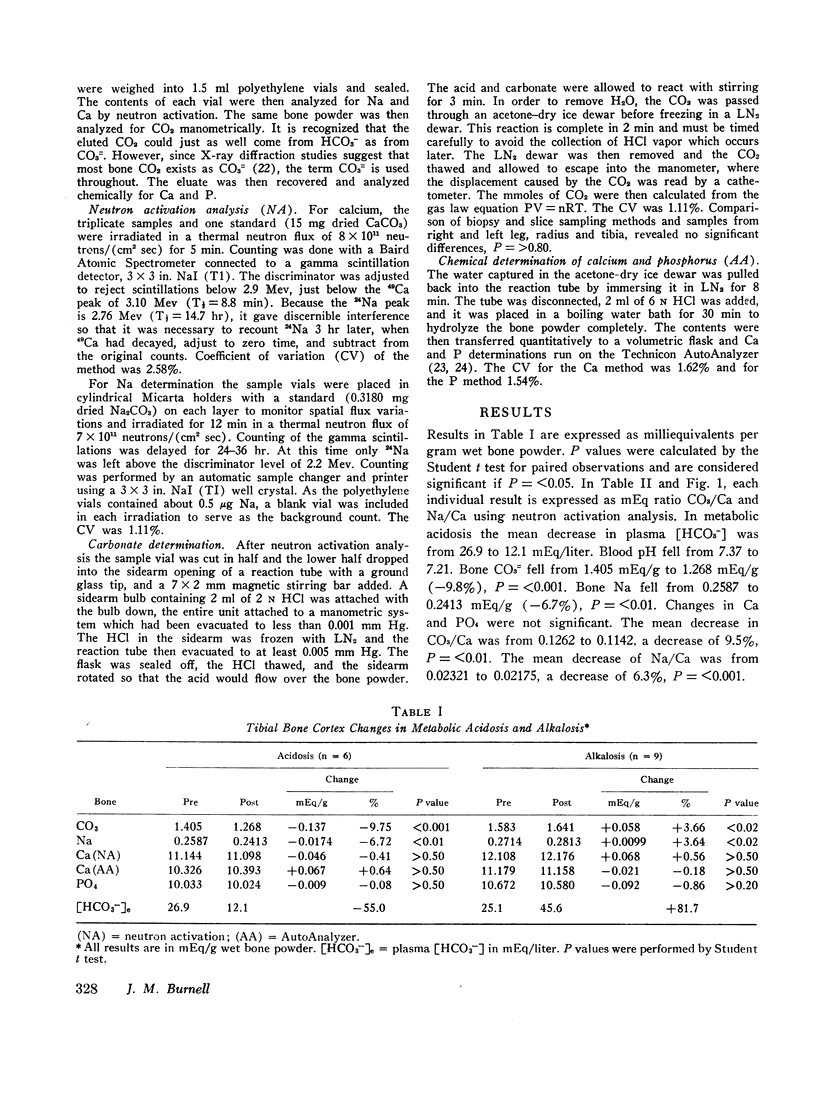
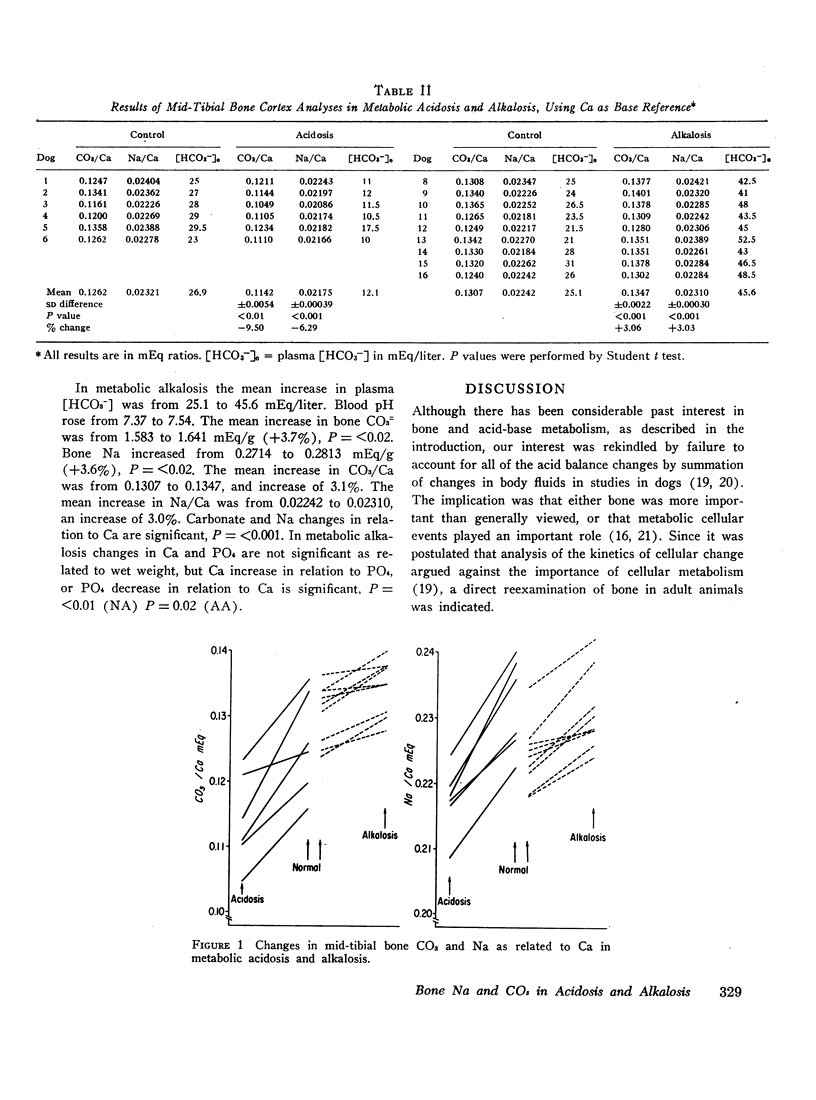
Metabolic acidosis and alkalosis were produced in adult dogs over 5- to 10-day periods. Midtibial cortical bone was analyzed for calcium, sodium, phosphorus, and carbonate. In acidosis bone CO3/Ca decreased 9.5% and bone Na/Ca decreased 6.3%. In alkalosis bone CO3/Ca increased 3.1% and bone Na/Ca increased 3.0%.
Previous attempts to account for changes in net acid balance by summation of extra- and intracellular acid-base changes have uniformly resulted in about 40-60% of acid gained or lost being “unaccounted for.” If it is assumed that changes in tibial cortex reflect changes in the entire skeletal system, changes in bone CO3= are sufficiently large to account for the “unaccounted for” acid change without postulating changes in cellular metabolic acid production.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER S., ROY A., RELMAN A. S. INTRACELLULAR ACID-BASE REGULATION. I. THE RESPONSE OF MUSCLE CELLS TO CHANGES IN CO2 TENSION OR EXTRACELLULAR BICARBONATE CONCENTRATION. J Clin Invest. 1965 Jan;44:8–20. doi: 10.1172/JCI105129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler S. The role of pH, PCO2, and bicarbonate in regulating rat diaphragm citrate content. J Clin Invest. 1970 Sep;49(9):1647–1655. doi: 10.1172/JCI106382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGSTROM W. H. The relationship of sodium and potassium to carbonate in bone. J Biol Chem. 1954 Feb;206(2):711–715. [PubMed] [Google Scholar]
- Burnell J. M., Dawborn J. K. Acid-base parameters in potassium depletion in the dog. Am J Physiol. 1970 Jun;218(6):1583–1589. doi: 10.1152/ajplegacy.1970.218.6.1583. [DOI] [PubMed] [Google Scholar]
- Burnell J. M. In vivo response of muscle to changes in CO2 tension or extracellular bicarbonate. Am J Physiol. 1968 Dec;215(6):1376–1383. doi: 10.1152/ajplegacy.1968.215.6.1376. [DOI] [PubMed] [Google Scholar]
- Clancy R. L., Brown E. B., Jr In vivo CO-2 buffer curves of skeletal and cardiac muscle. Am J Physiol. 1966 Dec;211(6):1309–1312. doi: 10.1152/ajplegacy.1966.211.6.1309. [DOI] [PubMed] [Google Scholar]
- GOODMAN A. D., LEMANN J., Jr, LENNON E. J., RELMAN A. S. PRODUCTION, EXCRETION, AND NET BALANCE OF FIXED ACID IN PATIENTS WITH RENAL ACIDOSIS. J Clin Invest. 1965 Apr;44:495–506. doi: 10.1172/JCI105163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye M., Frueh A. J., Silverman M., Henderson J., Thibault T. A study of vertebral bone powder from patients with chronic renal failure. J Clin Invest. 1970 Mar;49(3):442–453. doi: 10.1172/JCI106253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraml M. A semi-automated determination of phospholipids. Clin Chim Acta. 1966 Apr;13(4):442–448. doi: 10.1016/0009-8981(66)90235-x. [DOI] [PubMed] [Google Scholar]
- LEMANN J., Jr, LENNON E. J., GOODMAN A. D., LITZOW J. R., RELMAN A. S. THE NET BALANCE OF ACID IN SUBJECTS GIVEN LARGE LOADS OF ACID OR ALKALI. J Clin Invest. 1965 Apr;44:507–517. doi: 10.1172/JCI105164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemann J., Jr, Litzow J. R., Lennon E. J. The effects of chronic acid loads in normal man: further evidence for the participation of bone mineral in the defense against chronic metabolic acidosis. J Clin Invest. 1966 Oct;45(10):1608–1614. doi: 10.1172/JCI105467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORMAN N. The participation of bone in the sodium and potassium metabolism of the rat. II. The effect of variation of electrolyte intake, acidosis and alkalosis. Acta Physiol Scand. 1963 Apr;57:373–383. doi: 10.1111/j.1748-1716.1963.tb02600.x. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ W. B., JENSON R. L., RELMAN A. S. The disposition of acid administered to sodium-depleted subjects: the renal response and the role of the whole body buffers. J Clin Invest. 1954 Apr;33(4):587–597. doi: 10.1172/JCI102930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWAN R. C., PITTS R. F. Neutralization of infused acid by nephrectomized dogs. J Clin Invest. 1955 Feb;34(2):205–212. doi: 10.1172/JCI103073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloerb P. R., Blackburn G. L., Grantham J. J. Carbon dioxide dissociation curve in potassium depletion. Am J Physiol. 1967 Apr;212(4):953–956. doi: 10.1152/ajplegacy.1967.212.4.953. [DOI] [PubMed] [Google Scholar]
- WADDELL W. J., BUTLER T. C. Calculation of intracellular pH from the distribution of 5,5-dimethyl-2,4-oxazolidinedione (DMO); application to skeletal muscle of the dog. J Clin Invest. 1959 May;38(5):720–729. doi: 10.1172/JCI103852. [DOI] [PMC free article] [PubMed] [Google Scholar]


