Abstract
CB1 and CB2 receptors are activated by a plethora of cannabinoid compounds, be they endogenously-produced, plant-derived or synthetic. These receptors are expressed by microglia, astrocytes and astrocytomas, and their activation regulates these cells’ differentiation, functions and viability. Recent studies show that glial cells also express cannabinoid-like receptors, and that their activation regulates different cell functions, but also control cell viability. This review summarizes this evidence, and discusses how selective compounds targeting cannabinoid-like receptors constitute promising therapeutics to manage neuroinflammation and eradicate malignant astrocytomas. Importantly, the selective targeting of cannabinoid-like receptors should provide therapeutic relieve without inducing the typical psychotropic effects and possible addictive properties associated with the use of Δ9-tetrahydrocannabinol, the main psychotropic ingredient produced by the plant Cannabis sativa.
Cannabis sativa contains over 60 phytocannabinoids, at least three of which are bioactive: Δ9-tetrahydrocannabinol (THC), cannabinol (CBN) and cannabidiol (CBD) (Turner et al. 1980). THC induces psychotropic effects by activating CB1 receptors expressed by neurons (Huestis et al. 2001); but this compound also modifies essential central and peripheral physiological processes by activating CB1 and CB2 receptors expressed by glial cells and peripheral cells (Howlett et al. 2002). Few reports exist on the effects of CBN and CBD on neurons and glial cells, but these compounds are known to reduce peripheral inflammatory responses and blood pressure by interacting with CB2 and cannabinoid-like (CB-like) receptors expressed by immune and vascular endothelial cells (Costa et al. 2004; Herring and Kaminski 1999; Járai et al. 1999; Malfait et al. 2000; Perez-Reyes et al. 1973). Thus, distinct cannabinoid compounds will regulate different physiological processes, and this segregation in bioactivity is mediated by specific receptor types. In fact, during the last twenty years, the field of cannabinoid research has focused on understanding the molecular mechanisms mediating the actions of various cannabinoids in many cell types, with the aim of generating pharmacological and genetic tools that will selectively target CB1 and CB2 receptors to better understand their involvement in various pathophysiological functions.
This review is divided in three sections. In the first section, I will review our current understanding of the pharmacology of cannabinoids at CB1, CB2 and CB-like receptors, as well as mention the signal transduction pathways that these receptors couple to. In the second section, I will review what is known about CB1 and CB2 receptor expression in microglia, astrocytes and astrocytomas, and how activation of these receptors affects these cells’ function and viability. In the final section, I will focus on several landmark studies that have provided evidence for the expression of CB-like receptors in microglia, astrocytes and astrocytomas, and how their activation regulates their function and viability. Because of the clear therapeutic value of targeting CB-like receptors, as well as the academic challenge raised by the elucidation of their molecular identity and mode of action, the studies reviewed herein provide the foundation for a very exciting chapter of research in glial cell biology.
Cannabinoid pharmacology at molecularly-identified receptors
Although the pharmacology and bioactivity of cannabinoids has been extensively studied, many basic questions remain unanswered. For instance, while most of the bioactivity associated with cannabinoids is thought to be mediated through CB1 and CB2, thorough pharmacological studies indicate that cannabinoids regulate cell functions independently of these two molecularly-identified receptors (Begg et al. 2005; Kreitzer and Stella 2009). There are three possible mode of action for cannabinoids to regulate cell function independently of CB1 and CB2: 1) cannabinoids may regulate cell function independently of a protein target (e.g. by changing cell membrane fluidity) (Howlett and Mukhopadhyay 2000; Maingret et al. 2001; Oz 2006) 2) cannabinoids may interact with proteins that do not directly transduce signals (e.g. by inhibiting dopamine and adenosine transport) (Carrier et al. 2006; Price et al. 2007), and/or 3) cannabinoids may produce their effects through other receptors, some of which have already been molecularly identified, other that remain orphan. While the first and second possibilities constitute interesting venues to investigate, I have chosen to focus this review on the third possibility: the evidence for novel, non-CB1/2, receptors engaged by cannabinoids; but before reviewing this evidence, I thought that it would be important to provide a brief overview of cannabinoid chemistry and pharmacology.
The five classes of cannabinoid compounds
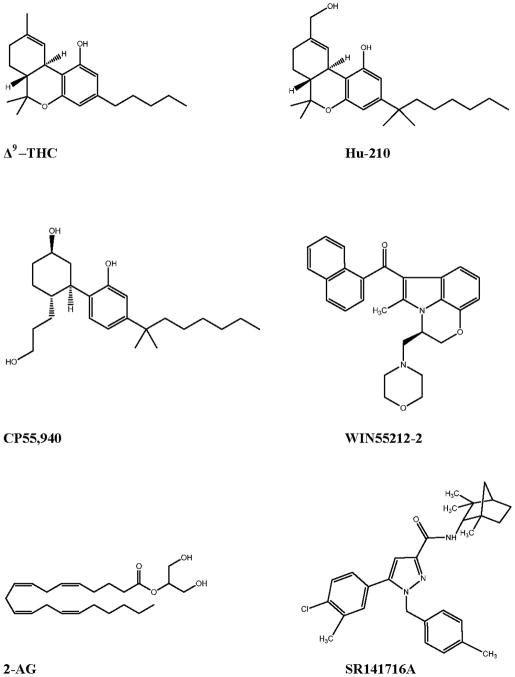
The chemistry and pharmacology of cannabinoid compounds is rich, consisting of a vast array of CB1 and CB2 selective and non-selective agonists and antagonists. Currently, there are five classes of cannabinoid compounds. The first class encompasses the classical cannabinoids, which are tricyclic-dibenzopyran derivatives isolated from the plant Cannabis sativa (including THC) or close synthetic analogues, such as HU-210 (Figure 1a and b). These compounds bind non-selectively to CB1 and CB2. The second class of compounds consists of non-classical cannabinoids, which are structurally similar to the classical cannabinoids, but are AC-bicyclic and ACD-tricyclic analogues lacking the dihydropyran ring. The prototype for this class is CP-55940 (CP), a full agonist at both CB1 and CB2 (Figure 1c). Aminoalkylindoles make up the third class of compounds, the prototypical compound being WIN55,212-2 (WIN), a full agonist at both CB receptors that exhibits ≈ two fold higher affinity toward CB2 over CB1 (Felder et al. 1995a). It is important to note that aminoalkylindoles are structurally quite distinct from the classical and non-classical cannabinoid compounds, as this will become relevant when referring to CB-like receptors activated by these compounds (Figure 1d). The fourth class of cannabinoid ligands encompasses derivatives of arachidonic acid, which are the endogenous ligands for cannabinoid receptors and some CB-like receptors. These endogenous cannabinoids (eCBs) include anandamide (arachidonoylethanolamide, AEA) and 2-arachidonoylglycerol (2-AG), which behave as partial and full agonists at CB1 and CB2 receptors, respectively (Figure 1e). The fifth class consists of the diarylpyrazole compounds, including SR141716, which is referred to as rimonabant (Figure 1f) (Rinaldi-Carmona et al. 1994). These compounds are inverse agonists at CB receptors, and have been extremely useful when testing the involvement of CB1 and CB2 in various pathophysiological processes.
Figure 1.
Chemical structure of prototypical cannabinoids
A “rule of thumb” to consider when assessing the biological effects produced by cannabinoid compounds is that they typically exhibit nanomolar affinities at CB1 and CB2 receptors, and thus testing them at concentrations higher than 100 nM (i.e. concentrations that are two orders of magnitude above their affinity) will likely produce off-target effects, including activating (or antagonizing) the CB-like receptors outlined in this review.
CB1 receptors
CB1 receptors are expressed at high level throughout the brain by many different classes of neurons, and are expressed at lower levels by glial cells and many peripheral cell types (Howlett et al. 2002; Matsuda et al. 1990; Tsou et al. 1998) (Figure 2). They are more abundant in GABAergic interneurons than in glutamatergic principal neurons (Marsicano et al. 2003; Uchigashima et al. 2007). Yet, the classical cannabinoid effects produced in mice, which is often referred to the “tetrad” (Howlett et al. 2002), are mediated by the less abundant CB1 receptors expressed by glutamatergic principal neurons, as demonstrated by an elegant study of mice lacking CB1 in either GABAergic interneurons or glutamatergic principal neurons (Monory et al. 2007). A recent study showed that the THC-mediated deficits of long-term memory are mediated through the more abundant CB1 receptors on GABAergic interneurons (Puighermanal et al. 2009).
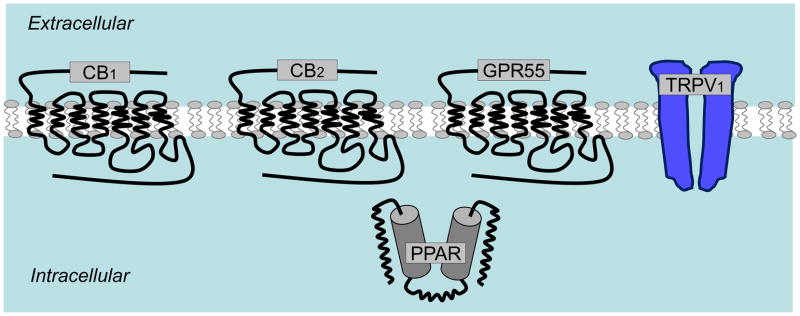
Figure 2.
Molecularly identified receptors activated by cannabinoids
CB1 receptors couple to Gi/o proteins, and modulate the activity of numerous ion channels and second messengers (Straiker and Mackie 2006). It is likely that their acute versus sustained stimulation will differentially modulate cell functions. For example, the acute activation of neuronal CB1 receptors for milliseconds to seconds inhibits presynaptic N-type calcium channels and activates inwardly rectifying potassium channels, thereby reducing neurotransmisson and controlling intrinsic excitability (Henry and Chavkin 1995; Mackie and Hille 1992; Mackie et al. 1995; Marinelli et al. 2009). On the other hand, their sustained activation for minutes to hours stimulates intracellular signals, such as ERK, that in turn modify the activity of enzymes and the expression of specific genes (e.g. brain derived neurotrophic factor (BDNF) (Marsicano et al. 2003)). Importantly, several studies have shown that THC may also function as an antagonist at CB1 receptors (if these are endogenously active), implying that THC might produce some of its biological effect by acting as an antagonist (Kelley and Thayer 2004).
CB2 receptors
CB2 receptors also couple to Gi proteins, but most likely not to Go proteins (Glass and Northup 1999; Munro et al. 1993). They share 44% protein identity with CB1 but display a distinct pharmacological profile and expression pattern (Felder et al. 1995b; Galiègue et al. 1995). Many laboratories have reported that healthy brain tissue does not express CB2, with the exception of a small population of neurons located in the brain stem and possibly the cerebellum (Carlisle et al. 2002; Derocq et al. 1995; Galiègue et al. 1995; Munro et al. 1993; Schatz et al. 1997; Skaper et al. 1996; Sugiura et al. 2000; Van Sickle et al. 2005). A couple of reports claimed that almost all neurons in healthy mouse brain express CB2 receptors, but these reports did not include the required negative controls for assessing immunostaining specificity, in particular the parallel immunostaining of the same brain areas using tissue from CB2 knockout mice (Gong et al. 2006; Onaivi et al. 2008; Onaivi et al. 2006).
While Julian Romero’s group has shown that perivascular microglia express the CB2 receptor in non-inflamed human brain (Nunez et al. 2004), it is important to emphasize that CB2 receptor expression can be induced in many immune cells, including in microglia (see below). Thus, under neuroinflammatory conditions, CB2 may be up-regulated by select cell populations within the brain. Another situation where CB2 levels might increase in inflamed brain parenchyma is as a result of the invasion of peripheral immune cells expressing CB2 receptors, for example peripheral T cells (Maresz et al. 2007).
GPR55
The possibility that GPR55 constitutes the target responsible for some of the effects mediated by cannabinoids has captured an increasing amount of attention. GPR55 was first identified in 1998 by researchers performing homology searches of the amino acid sequences of known GPCRs using BLAST (Basic Local Alignment Search Tool) on publicly available databases (GenBank High Throughput Genome and expressed sequence tag) (Sawzdargo et al. 1999). GPR55 mRNA is expressed in healthy brain (caudate, putamen, hippocampus, thalamic nuclei and midbrain), spleen, intestine and fetal tissue (Sawzdargo et al. 1999). Two patents issued by pharmaceutical companies that followed these initial studies claimed that GPR55 represents a novel CB-like receptor (Drmota et al. 2003; Johns et al. 2007), and yet GPR55 exhibits only 13.5% and 14.4% sequence homology to CB1 and CB2, respectively (Baker et al. 2006). Interestingly, the two patents used different expression systems, and they obtained markedly different pharmacological profiles when applying cannabinoids. For example, one patent claimed that palmitoylethanolamide (PEA), a lipid closely related to AEA, is a high affinity agonist that increases [35S]-GTPγS binding in GPR55-expressing HEK293 cells (Ryberg et al. 2007), whereas the other patent found no activity of PEA at GPR55.
Several laboratories have confirmed that some cannabinoids applied at micromolar concentrations do activate GPR55, as shown by monitoring [Ca2+]i (Henstridge et al. 2009; Lauckner et al. 2008; Oka et al. 2007; Waldeck-Weiermair et al. 2008); but here too the pharmacological profiles reported by these studies were often different. For example, the laboratory of Ken Mackie showed that low micromolar concentrations of THC and CP reliably activates GPR55 (Lauckner et al. 2008), whereas the laboratory of Takayuki Sugiura reported that these compounds do not activate GPR55, but that instead lysophosphatidylinositol (LPI) does (Oka et al. 2007). A recent study performed on GPR55 knockout mice showed that CBD acts as an antagonist at GPR55, in particular by modulating the function of osteoblasts and osteoclasts (Whyte et al. 2009). One point that remains consistent between several laboratories is that AEA and 2-AG (3–30 μM) have no effect on GPR55-induced modulation of [Ca2+]i (Henstridge et al. 2009; Lauckner et al. 2008; Oka et al. 2007). Thus, despite this intriguing series of studies and patents, there is still no reliable understanding of the cannabinoid’s action atGPR55, leaving its overall pharmacological profile ambiguous.
Receptors activated by atypical cannabinoids
Using mice that are genetically deficient for both CB1 and CB2, the laboratory of George Kunos identified a receptor engaged by atypical cannabinoids: activated by both abnormal-cannabidiol (abn-CBD) and O-1602, and antagonized by O-1918 (Járai et al. 1999; Offertáler et al. 2003). These Gi/o protein-coupled receptors are expressed by the endothelial cells on blood vessels, where they increase cGMP production and regulate blood pressure (Begg et al. 2003a; Offertáler et al. 2003). The pharmacological profile of this receptor is distinct from that of CB1 and CB2, since THC, HU-210 and WIN lack efficacy at this receptor, and some commonly used antagonists at CB1 and CB2 (e.g. AM251, SR144528, AM281 and AM630) do not block it (Begg et al. 2003b; Herradon et al. 2007; Ho and Hiley 2003; McCollum et al. 2007; Wagner et al. 1999). The molecular mechanisms and signal transduction pathways involved in the abn-CBD-induced response include Gi/o proteins, PI3 Kinase/Akt, calcium-sensitive potassium channels and NO synthase (Begg et al. 2003b; Ho and Hiley 2003; McCollum et al. 2007). Note that while one report suggested that abn-CBD and O-1602 increases [35S]-GTPγS binding in GPR55-expressing HEK293T cells (Drmota et al. 2003), the biological effects produced by these compounds are unchanged in GPR55 KO mice compared to wild-type control mice, suggesting that GPR55 is not the endothelial receptor mediating the vasodilatory effects of abn-CBD (Johns et al. 2007).
N-arachidonoyl-L-serine (ARA-S) is an interesting – yet understudied – lipid that might constitute an endogenous ligand for the abn-CBD-sensitive receptor. Indeed, while ARA-S binds with minimal affinity to CB1, CB2, and TRPV1, it produces endothelium-dependent vasodilation in rat isolated mesenteric arteries with an EC50 of 550 nM, and this response is blocked by O-1918 (Milman et al. 2006). Remarkably, abn-CBD inhibits neutrophil migration induced by fMLP, and ARA-S antagonizes this response (McHugh et al. 2008).
Receptors activated by palmitoylethanolamide (PEA)
While it is clear that activation of CB1 and CB2 receptors induces analgesia, several lines of evidence show that some of the cannabinoid-induced analgesic responses are not mediated through these two receptor types. A remarkable example involves PEA, which does not activate either CB1 or CB2 receptor types (Showalter et al. 1996), and yet this lipid is a potent analgesic and its effect is blocked bySR144528, the CB2 antagonist (LoVerme et al. 2005). This result may be interpreted in two different ways: either 1) both PEA and SR144528 bind to a novel CB-like receptor type (suggesting that SR144528 is a non-selective antagonist), or 2) PEA binds to a site distinct from the one bound bySR144528 and their subsequent signaling interact.
Peroxisome proliferator-activated receptors (PPARs)
There are three PPAR isotypes: α, δ and γ (with γ being further subdivided as 1, 2 and 3). These isotypes are differentially expressed by distinct cells types, yet they often regulate similar biological functions (e.g. lipid metabolism and inflammation). Generally, PPARs heterodimerize with retinoid X receptors and increase the transcription rate of specific genes upon ligand binding and cofactor recruitment (Burstein 2005; Michalik et al. 2004). Because of the clear therapeutic value of these targets, an area of intense research is to increase our understanding of how endogenous ligands activate these isotypes. Along these lines, PEA has been shown to bind to PPAR-α with relatively high affinity and regulate gene expression, suggesting this receptor might be involved in this lipid’s biological actions (Lo Verme et al. 2005; Sun et al. 2007). Furthermore, genetic deletion of PPAR-α abolishes PEA’s ability to regulate gene expression, as well as its ability to modulate CB2-independent inflammatory responses (LoVerme et al. 2005). These results should be considered in light of the following fact: the ligand-binding domains of PPARs are quite large, and thus these domains may exhibit promiscuous binding to an array of structurally related chemicals (Kliewer et al. 1997). Accordingly, 2-AG and AEA (and other lipid closely related to them) exhibit comparable activities at various PPAR isotypes (Lenman and Fowler 2007; Rockwell et al. 2006). Even THC, CBD, WIN and CP activate PPAR-γ and increase PPAR-γ-dependent transcription (O’Sullivan et al. 2006a; O’Sullivan et al. 2006b; O’Sullivan et al. 2005). Thus, while PEA might induce part of its biological effect through PPAR-α, some phytocannabinoids, synthetic cannabinoids and endogenous lipids related to endocannabinoids might also mediate part of their biological response through PPARs.
Receptors activated by WIN55212-2(WIN)
A CB-like receptor activated by WIN was first identified in brain homogenates. Specifically, WIN (EC50 = 1.8 μM) and AEA (EC50 = 3.6 μM) increase [35S]-GTPγS binding in brain samples prepared from CB1−/− mice, and this response is insensitive to rimonabant (Breivogel et al. 2001; Monory et al. 2002). This binding site is expressed in brain stem, cortex, hippocampus, midbrain, and spinal cord, while being absent in the cerebellum and basal ganglia (Breivogel et al. 2001). Whether this binding site was on neurons and/or glial cells was uncertain. A complication for studying this CB-like receptor is that its expression varies with age and genetic background (Hoffman et al. 2005; Monory et al. 2002). Electrophysiological evidence suggested that this CB-like receptor regulates neurotransmission in adult hippocampus, as first described by Hajos et al. using CB1−/− mice (Hajos et al. 2001). Specifically, WIN inhibited EPSCs (but not IPSCs) at the schaffer collateral synapse in the mouse hippocampal CA1 region (Hajos et al. 2001). This response was also present in rat hippocampal slices, where administration of the CB1 antagonist AM251 and of the vanilloid receptor antagonist capsezapine abolishes the WIN-mediated inhibition of IPSCs, but not EPSCs, indicating that EPSCs might be regulated by a CB-like receptor sensitive to AM251 and capsezapine (Hajos and Freund 2002). Thus, while this receptor represents a very interesting new target regulating neurotransmission, a clear understanding of its pharmacology and cellular expression pattern is still lacking.
Transient receptor potential cation channel, subfamily V, member 1 (TRPV1)
TRPV1 is a ligand-gated nonselective cation channel activated by a wide array of stimuli, including heat greater than 43°C, low pH and the active ingredient of chili peppers, capsaicin (Zygmunt et al. 1999). Recent studies have shown that this ion channel is activated by a growing number of cannabinoids, including THC, CBN, WIN, AEA and rimonabant, although these compounds are only active when applied at micromolar concentrations (De Petrocellis et al. 2008; Jeske et al. 2006; Patwardhan et al. 2006; Zygmunt et al. 2002). Thus, it is possible that a cross-interaction between CB-like receptor activated by WIN and TRV1 exists. An important control that is still lacking in this specific area of research is to show that cannabinoids do indeed activate TRPV1 in vivo, and how this translates in pain sensation.
Glial cells express CB1 and CB2 receptors
CB1 and CB2 receptors in microglia
CB1 and CB2 expression in microglia changes depending on their phenotype and activation profile. In resting microglia, which can only be found in intact healthy brain tissue, CB1 and CB2 expression has not been addressed directly, but one can presume that these cells do not express much – if any – CB1 or CB2. Specifically, when staining healthy brain slices with a CB1 antibody, one does not find labeled cells that exhibit a morphology reminiscent of resting microglia. With regard to CB2, only trace amounts of CB2 mRNA is detectable in healthy brain tissue, suggesting that resting microglia do not express CB2 either (Carlisle et al. 2002; Derocq et al. 1995; Galiègue et al. 1995; Griffin et al. 1999; McCoy et al. 1999; Munro et al. 1993; Schatz et al. 1997; Sugiura et al. 2000).
Microglia in primary cultures are intrinsically activated – or “primed” – because of the procedure involved in transferring these cells into culture (Becher and Antel 1996). Several laboratories have shown that such primed microglia (prepared from human, rat or mouse tissue) do express significant amount of CB2 receptors (Carlisle et al. 2002; Facchinetti et al. 2003; Klegeris et al. 2003; Walter et al. 2003), and that certain pathogens and cytokines further up- or down-regulate this expression (Carayon et al. 1998; Derocq et al. 2000; Gardner et al. 2002; Lee et al. 2001; Rodríguez et al. 2001; Waksman et al. 1999). Rodent microglial cell lines, including BV-2 cells, which inherently exhibit high rates of cell proliferation, also express CB2 receptors (Carrier et al. 2004; Walter et al. 2003).
CB2 receptors are expressed by activated microglia in brain tissue, but depending on the type of neuropathology activating them, their phenotype will vary, as will the presence and amount of CB2 expression. One remarkable example is found in rat spinal cord, where neuropathic pain up-regulates CB2 in microglia, but chronic inflammatory pain does not (Zhang et al. 2003). Another study also demonstrated microglia CB2 receptor up-regulation in vivo in response to an inflammatory challenge (Maresz et al. 2005). Activated microglia in brain tissue from Alzheimer’s and multiple sclerosis (MS) patients express CB2, especially when these cells are associated with the plaques that accumulate in these diseases (Benito et al. 2003; Yiangou et al. 2006), as do the activated microglia found in a simian model of AIDS dementia (Benito et al. 2005). Thus, CB2 receptor up-regulation in activated microglia may occur as a result of specific neuropathologies and neuroinflammatory responses, and this up-regulation most likely depends on the combination of toxins, pathogens, cytokines and molecules encountered by these immune cells and regulating gene expression.
Activation of microglial CB2 receptors by cannabinoids regulates their immune-related functions. For example, activation of CB2 increases microglial cell proliferation and migration, while reducing the release of detrimental factors, including TNFα and free radicals (Carrier et al. 2004; Dirikoc et al. 2007; Eljaschewitsch et al. 2006; Ramirez et al. 2005; Walter et al. 2003). Thus, the overall effect of stimulating CB2 receptors in microglia would be that higher numbers of “less harmful” microglia should accumulate at lesion sites.
CB1 receptors are expressed by microglial cells in culture, at least when prepared from mollusk, mouse and rat, but not when prepared from human (Carlisle et al. 2002; Facchinetti et al. 2003; Klegeris et al. 2003; Molina-Holgado et al. 2002a; Sinha et al. 1998; Stefano et al. 1996; Waksman et al. 1999; Walter et al. 2003). How these receptors regulate microglial cell function is controversial. For example, CP acting at CB1 increases NO production from mollusk microglia (Stefano et al. 1996), but inhibits the LPS-induced release of NO from rat microglia (Waksman et al. 1999). The latter effect of CP on NO production is only partially antagonized by rimonabant applied at micromolar concentrations, which calls into question the true involvement of CB1 receptors in this response (Stefano et al. 1996; Waksman et al. 1999).
In summary, several laboratories have shown that microglial cells express CB1 and CB2 receptors, and that activation of these receptors under cell culture conditions regulates specific immune-related functions carried by these cells. The question that remains to be addressed here is: Does that activation of these receptors expressed by microglia in situ affect neuroinflammation in vivo, and if so how?
CB1 receptors in astrocytes
Few studies have addressed the expression profile and functional significance of CB receptors in astrocytes. Astrocytes in culture prepared from different species may vary in their phenotype, and accordingly rat astrocytes in culture express CB1, whereas mouse astrocytes in culture do not (Antel and Becker 1997; Bouaboula et al. 1995; Sánchez et al. 1998a). Astrocytic CB1 activation controls their metabolic functions: for example activation of CB1 receptors on rat astrocytes increase the rate of glucose oxidation and ketogenesis, two mechanisms involved in the energy supply of the brain (Blázquez et al. 1999; Sánchez et al. 1998b). Activation of astrocytic CB1 receptors tempers these cells ability to produce inflammatory mediators, including NO production induced by lipopolysaccharide and IL-1β (Blázquez et al. 1999; Molina-Holgado et al. 2002b; Sánchez et al. 1998b; Sheng et al. 2005). Astrocytes in situ express CB1 (Moldrich and Wenger 2000; Rodríguez et al. 2001; Salio et al. 2002), and accordingly astrocytic CB1 receptors and endocannabinoids mediate some of the neuron/astrocyte interactions measured in adult mice brain slices (Navarrete and Araque 2008).
Considering the ability of astrocytic CB1 receptors to regulate energy metabolism and mediate neuronglia interactions, one can speculate about their possible pathophysiolgical role. The majority of the perivascular astrocytes in adult rat brain express CB1 (70% of which are localized on the plasmalemma of both their cell body and the filamentous glial processes) (Rodríguez et al. 2001). Because perivascular astrocytes are pivotally located and involved in supplying energy from blood to neurons in an activity-dependent manner (Magistretti 2009), one hypothesis is that astrocytic CB1 receptors regulate energy supply to neurons. Accordingly, rat brain energy metabolism is increase after exposure to AEA and THC (Costa and Colleoni 2000). Thus, while understudied, CB1 receptors expressed by astrocytes might be involved in a rich array of fundamental regulatory functions.
CB1 and CB2 receptors in astrocytomas
In the mid-1990s, the French pharmaceutical company Sanofi-Aventis Research reported that CB1 receptors is present in various human glioma cell lines, as well as explants of human tumors with various degrees of malignancy (Bouaboula et al. 1995; Bouaboula et al. 1996). Accordingly, cannabinoid agonists active ERK and krox-24in human glioma cell lines in culture, both of which are antagonized by rimonabant applied a nanomolar concentrations (Bouaboula et al. 1995). Shortly after these publications, the group of Manuel Guzman undertook a series of studies testing the hypothesis that cannabinoids may serve as powerful anti-tumoral agents in the treatment of astrocytomas. A sub-clone of the rat glioma cell line C6, namely C6.9, was sensitive to THC administration, but this toxic effect was not antagonized by either rimonabant orSR144528 alone, but only by their combination, suggesting that activation of either receptor type is sufficient to induce apoptosis in astrocytomas (Galve-Roperh et al. 2000). Autophagy and p8, a stress-regulated mediator of cell fate, constitute key players in THC-induced apoptosis of these C6 cells (Carracedo et al. 2006; Salazar et al. 2009). Building upon these results, the Guzman laboratory tested the anti-tumoral properties of cannabinoids on C6 cells stereotactically injected into rat brain. Highly invasive tumors formed within 2 weeks of the injection of C6 cells, and untreated tumor-bearing animals did not survive beyond 3 weeks (Galve-Roperh et al. 2000). Intratumoral administration of either THC or WIN slowed the progression of these malignant tumors, increasing mean survival times post-inoculation (Galve-Roperh et al. 2000). These results were quite spectacular: in 25% of the cannabinoid-treated cases, gliomas were completely eradicated, and these rats survived beyond study observations (Galve-Roperh et al. 2000). Furthermore, cannabinoid treatment did not damage neighboring healthy tissue. To date, whether these in vivo therapeutic effects of THC and WIN are truly mediated by CB1 and/or CB2 has not been addressed directly. This is an important point, since the stereotaxic injection of cannabinoids is likely to result in quite high local concentrations. Thus, extending these experiments by testing selective CB1 and CB2 agonists (or co-administering selective antagonists) would help determine the relative involvement of these receptor types in this very promising therapeutic effect of cannabinoids.
A controversial question that is central to this line of research is whether or not CB2 is expressed in brain tumor tissue (Bouaboula et al. 1995; Bouaboula et al. 1996; Sánchez et al. 2001). Although pharmacological data suggest that brain tumor tissue does expresses CB2 receptors, the equivalent corresponding expression data obtained using commercially available antibodies have long been more difficult to interpret. To corroborate their findings, Guzman and colleagues reported that both C6 cells and human glioma tissues express CB1 and CB2 receptor mRNA and protein (Sánchez et al. 2001), contradicting an earlier study that found no CB2 mRNA in a panel of human astrocytoma cell lines and tissues (as assessed by RT-PCR) (Bouaboula et al. 1995). A more recent study reported the qPCR and immunohistochemical analyses of biopsies from 37 human astrocytomas of varying malignancy (Held-Feindt et al. 2006). In summary, a large body of evidence supports the chemotherapeutic value of cannabinoids acting on CB1 and CB2 on astrocytomas in culture, and these results still need to be further investigated in vivo.
CB-like receptors in glial cells
Microglia express CB-like receptors
Many of the early studies assessing the role of cannabinoid receptors in microglial cells in culture indicate that high concentrations of cannabinoid agonists are required to affect their immune-related functions, suggesting that these compounds are not acting through CB1 and CB2, but rather through CB-like receptors. For example, only micromolar concentrations of the potent synthetic cannabinoid agonists CP, WIN and HU210 inhibit cytokine release from rat microglia in primary culture, and this response is not stereo selective, nor is it fully blocked by micromolar concentrations of rimonabant (Facchinetti et al. 2003; Puffenbarger et al. 2000). Because such high concentrations of cannabinoids are required to modulate microglial cell function, it is still difficult to fully interpret these early results, as well as clearly identify the CB-like receptor type involved in the cannabinoid-induced regulation of cytokine release from microglia. Thus, these early studies should be revisited, for example by using microglia lacking CB1 and CB2, and/or treating these cells with compounds now known to selectively activate and antagonize specific types of CB-like receptors. Obvious candidate receptors that should be tested are GPR55, PPARγ and TRPV1 (Bernardo et al. 2005; Kim et al. 2006; Petrova et al. 1999; Pietr et al. 2009). With regard to GPR55, a recent study showed that both mouse microglia in primary culture and BV-2 cells express GPR55 mRNA, and that its endogenous agonist, LPI, activates ERK in these cells, demonstrating that this receptor is indeed functional in these cells (Pietr et al. 2009). Furthermore, GPR55 mRNA and LPI-induced ERK activation are differentially regulated by LPS and IFNγ, and this change in expression parallels that of CB2 mRNA (Pietr et al. 2009), suggesting that GPR55 and CB2 expression might be concomitantly regulated by particular pathogens and cytokines.
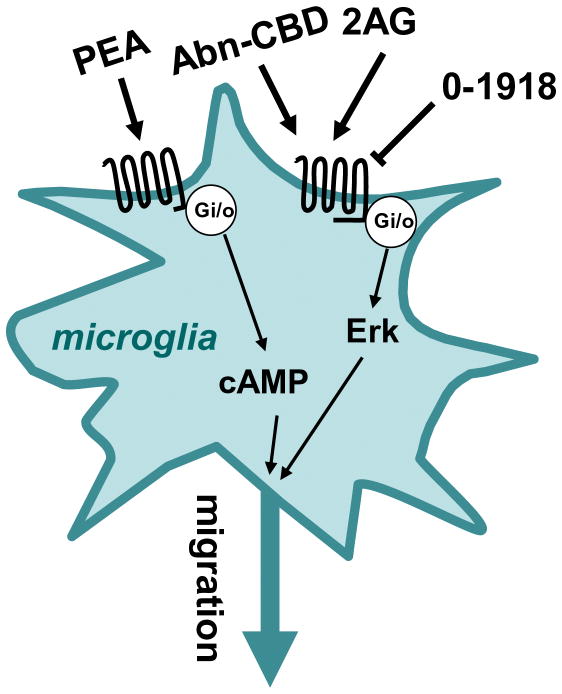
Several laboratories found pharmacological evidence for the presence of abn-CBD receptors in microglia and BV-2 cells (Figure 3). Activation of this receptor by micromolar concentrations of abn-CBD, 2-AG or arachidonylcyclopropylamide stimulates BV-2 cell migration by activating chemotaxis (a response that depends on Gi/o proteins and ERK, and is antagonized by O-1918 and CBD) (Franklin and Stella 2003; Walter et al. 2003). Microglial cell activation in organotypic hippocampal slice cultures is also regulated by abn-CBD receptors. Organotypic slices constitute an attractive model to study microglial cell activation and migration in the presence of neurons and astrocytes, as well as in the absence of blood-borne monocytes and T lymphocytes. Thus, using this model, one can ask if a given compound can directly modulate microglial cell function, and if this modulation affects excitotoxicity independently of peripheral immune cells? In an initial study, Kreutz et al. showed that 3 μM THC and 10 nM 2-AG reduce NMDA-associated activation of microglia in the dentate gyrus (as assessed by ILB4 staining and counting cells that exhibit an amoeboid shape) (Kreutz et al. 2007). The CB2 antagonist AM630 applied at 10 μM partially blocked the THC response and yet enhanced the 2-AG response, raising questions about the involvement of CB2 in this response produced by 2-AG. When testing how these compounds affect excitotoxicity (as assessed by propidium iodide staining), THC slightly increased NMDA-induced toxicity, while 2-AG had a protective effect. Here too, the THC response was only partially blocked by AM630, whereas the 2-AG protective response was insensitive. One interesting side conclusion from this first study is that there is no correlation between microglial cell activation and excitotoxicity in this model, since THC decreases microglial cell activation and yet increases toxicity. Could the presence of invading monocytes and T cells influence how THC modulates microglia and excitotoxicity? This question remains open, but there is reason to expect the answer is “yes”, since THC can reduce cell damage by directly tempering invading T cells (Maresz et al. 2007). In a follow-up study from the same laboratory, Kreutz et al. focused on the effect of 2-AG in organotypic slices and found that this eCB acts through abn-CBD receptors (since 2-AG reduces both microglial cell activation and excitotoxicity, and this response is blocked by O-1918 and CBD, and mimicked by abn-CBD) (Kreutz et al. 2009). Thus, abn-CBD receptors expressed by microglia represent a valid target to temper the detrimental properties of these brain macrophages.
Figure 3.
Cannabinoid-like receptors expressed by microglia. PEA activates a Gi/o-protein coupled receptor that inhibits adenylyl cyclase activity, Abn-CBD and 2-AG activate, while O-1918 antagonizes, a Gi/o-protein coupled receptor that activates Erk. Both reduction in cAMP and activation of Erk stimulate microglial cell migration.
My laboratory showed that PEA activates a receptor expressed by BV-2 cells with an EC50 of 6 nM (Franklin et al. 2003) (Figure 3). This receptor couples to Gi/o, and its ability to reduce intracellular concentrations of cAMP results in an increase in AEA-stimulated cell migration, without affecting 2-AG-stimulated migration, cell proliferation, release of NO and phagocytosis of particles (Franklin et al. 2003). Increasing our understanding of how PEA regulates microglial cell function might turn out to be therapeutically important, because many neuroinflammatory responses, including those induced by cerebral ischemia, are associated with increased PEA accumulation (Berger et al. 2004; Degn et al. 2007; Franklin et al. 2003; Schäbitz et al. 2002). Thus, PEA and its action on molecularly-identified and/or orphan receptors expressed by microglia might be involved in fine-tuning the recruitment of these macrophages toward the brain areas that have been injured.
Together, these studies suggest that microglial cell function, namely their migration and ability to release cytokines and control excitotoxic insults, is regulated by compounds that target CB-like receptors. While much work is still required to understand the details of these processes, the series of studies reviewed here suggest a new therapeutic paradigm: one that uses non-psychotropic cannabinoid compounds as chemical platforms, and targets CB-like receptors to temper the neuronal damage induced by excitotoxicity.
Astrocytes express CB-like receptors
The first evidence for the presence of CB-like receptors in astrocytes came from a landmark study carried-out by Venance et al (Venance et al. 1995). Specifically, these authors showed that AEA(5 μM), but not CP (1 μM) or WIN (5 μM), inhibits gap junctions in striatal astrocytes in culture, a response not antagonized by rimonabant (0.5 μM) (Figure 4). Interestingly, this AEA response is found in astrocytes prepared from embryonic mouse striatum, but not in astrocytes prepared from embryonic mouse cortex, hippocampus and brain stem, suggesting differential expression of this particular CB-like receptor in these glial cells (Venance et al. 1995). In a follow-up study, the same laboratory measured cAMP levels in striatal astrocytes in culture and showed that AEA inhibits the cAMP accumulation induced by isoproterenol (IC50s = 0.6 μM), a response also insensitive to rimonabant (Sagan et al. 1999). Interestingly, the authors showed that striatal astrocytes do not bind [3H]-rimonabant, nor are these cells immunostained by an antibody recognizing CB1 receptors, indicating that these particular cells in culture do no express significant amount of CB1 receptors (Sagan et al. 1999). The absence of functional CB1 receptors in astrocytes in culture had previously been suggested (Jung et al. 1997). Here two points are worth noting. First, as we saw above, some laboratories did detect CB1 receptors in astrocytes in culture, but this expression could depend on the brain area that these cells were prepared from. Since nowadays CB1−/− mice are readily available, the presence or absence of functional CB1 receptors in cultured astrocytes could be settled by using this unbiased genetic tool. Second, whether the inhibition of cAMP production by AEA mediates its ability to block gap junctions remains unknown, but could also be easily tested.
Figure 4.
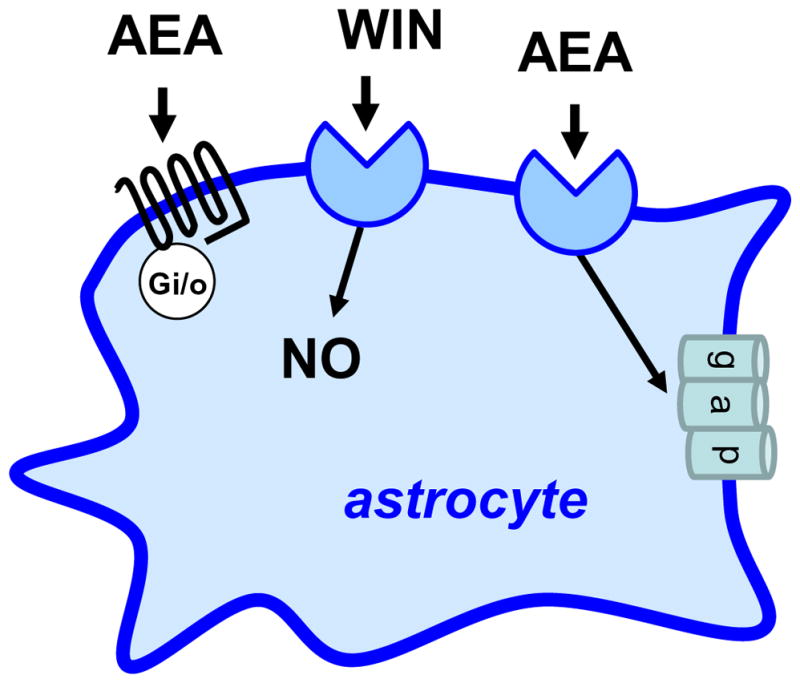
Cannabinoid-like receptors expressed by astrocytes. Anandamide (AEA) activates a Gi/o-protein coupled receptor that inhibits adenylyl cyclase activity, WIN regulates nitric oxide (NO) production through an unknown receptor, and anandamide closes gap junction through an unknown receptor.
Additional evidence for the presence of CB-like receptors in astrocytes come from studies showing that WIN (10 μM) inhibits NO production stimulated by IL-1β, a response partially blocked by rimonabant (10 μM) and insensitive to SR144528 (10 μM) (Sheng et al. 2005) (Figure 4). AEA also stimulates glycine transport into astrocytes (EC50 = 14 μM), a response not mimicked by WIN (50 μM) (Pearlman et al. 2003). Thus, several independent laboratories reported the presence of CB-like receptors in astrocytes in culture; but their presence and functional significance in vivo still need to be directly tested, an endeavor that is eased by the genetic and pharmacological tools that are now available.
Astrocytomas express CB-like receptors
In 1998, the laboratory of Manuel Guzman showed that 1 μM THC reduces the proliferation of C6 rat glioma cells in culture, while having no effect on neurons or astrocytes in primary culture (Sánchez et al. 1998a). Importantly, co-administration of rimonabant failed to block this cannabinoid-induced cytotoxicity (Sánchez et al. 1998a). This result may be interpreted as follow: either 1) CB1 activation is not involved or 2) activation of either CB1 or CB2 is sufficient. This toxic effect of THC on C6 cells involves apoptosis, as inferred by the DNA fragmentation and changes in membrane morphology (Sánchez et al. 1998a). The laboratory of Christopher Fowler showed that CP, AEA, 2-AG and JWH-015 (a non-selective CB2 agonist) also inhibit C6 cell proliferation (Fowler et al. 2003; Jacobsson et al. 2001). In this case, while the anti-proliferative effects of AEA and 2-AG are blocked by cannabinoid receptor antagonists, the anti-proliferative effects of CP and JWH-015 are not (Jacobsson et al. 2001). Interestingly, AEA induces apoptosis in a variety of human glioma cell lines through yet another mechanism, one involving TRPV1 and lipid rafts (Bari et al. 2005; Contassot et al. 2004; Jacobsson et al. 2001; Jonsson et al. 2003; Maccarrone et al. 2000). Several other CB-like compounds have been shown to induce apoptosis in C6 cells, including the eCB analogue stearoylethanolamide, but the molecular target of this particular lipid remains unknown (Ellert-Miklaszewska et al. 2005; Maccarrone et al. 2002).
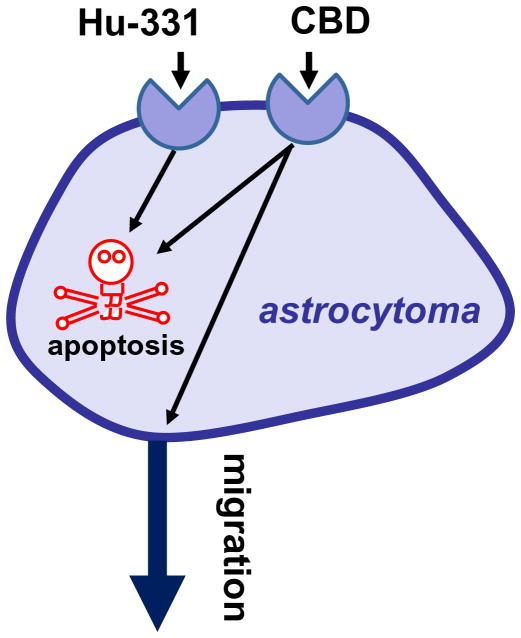
The laboratory of Daniela Parolaro reported that CB-like receptors engaged by CBD regulate the migration and viability of glioma cells. CBD applied at 3 μM inhibits the migration of U87 glioma cells toward their conditioned media (Vaccani et al. 2005) (Figure 5). This response is not antagonized by either SR141617, SR144528 or capsazepine, and is not blocked by PTX pretreatment, clearly ruling-out the involvement of CB1, CB2 and TRPV1 in this response (Vaccani et al. 2005). Whether CBD acts as either an agonist inhibiting migration or an antagonist blocking a chemoattractant present in the conditioned media is unknown. CBD applied at much higher concentrations, here 25 μM, kills U87 and U373 glioma cells in culture, and this response is not antagonized by SR141617, SR144528 or capsazepine, nor is it blocked by PTX pretreatment (Massi et al. 2004). The molecular mechanism mediating this effect involves caspase-3 activation, cytochrome c release, caspase-9 and caspase-8 activation, and the production of reactive oxygen species (Massi et al. 2006). Remarkably, CBD delivered i.p. reduces by 50% the growth of U87 implanted subcutaneously in athymic nude mice (Massi et al. 2004).
Figure 5.
Cannabinoid-like receptors expressed by astrocytomas. Hu-311 induces apoptosis through an unknown receptor, and CBD both induces apoptosis and inhibits cell migration.
The laboratory of Raphael Mechoulam reported the anti-proliferative properties of the oxidized products of some cannabis constituents (e.g. Hu-331, an oxidized product of CBD) (Figure 5) (Kogan et al. 2004). Treating the glioblastoma cell line SNB-19 in culture with Hu-331 at 25 μg/ml for 3 days killed more than 90% of the cells (Kogan et al. 2004). This effect is not mediated by CB receptors because these compounds do not bind these receptors, but do involve redox cycling, DNA damage, inhibition of topoisomerase, protein damage, and/or lipid peroxidation. Indeed, these effects are typically induced by compounds that contain quinine groups, such as adriamycin and daunorubicin, both of which have been in clinical use for treatment of solid tumors for over 30 years (Gewirtz 1999). Mechoulam’s group has cogently shown that HU-331 most likely acts by inhibiting topoisomerase II (Kogan et al. 2007).
In summary for this subsection, compounds that activate or antagonize CB-like receptors block some of the most fundamental processes involved in the progression of brain tumors, namely their migration and proliferation. While the exact molecular targets and signal transduction mechanisms mediating these therapeutic effects still need to be determined, these initial studies provide the required stepping stone for developing novel cannabinoid-based chemotherapeutic agents that lack the controversial effects associated with the use and abuse of THC.
Conclusions and possible future directions
The studies outlined in this review were carried-out over the recent decades and suggest the following testable hypothesis: the pharmacological targeting of CB-like receptors in vivo should reduce the production of pro-inflammatory cytokines by microglia, reduce the recruitment of these macrophages towards brain lesions, as well as block the propagation and overall viability of malignant astrocytomas. While very exciting, these original results now need to be extended to more elaborate animal models of neuroinflammation and brain tumors. Ideally, follow-up studies would test if the compounds targeting CB-like receptors are easy to deliver, do not produce overt side-effects, eradicate malignant cells while sparing healthy cells, and prevent the accumulation of harmful pro-inflammatory cytokines and immune cells while promoting the recruitment of repair immune cells. While these expectations might seem overly ambitious, the data already available for some of the compounds targeting CB-like receptors are quite promising. Taking CBD as an example: this plant-derived compound does not induce the typical psychotropic effects induced by THC in humans (Hollister 1973; Perez-Reyes et al. 1973). CBD inhibits the migration of both astrocytomas and microglia, so treating individuals with this compound could reduce the infiltration of astrocytomas into healthy brain parenchyma, and also reduce the recruitment of microglia toward the tumor mass. The latter effect is important to achieve since microglia release pro-angiogenic factors that favor tumor growth (Condeelis and Pollard 2006). An additional advantage of CBD is that it directly kills astrocytomas when applied at high concentrations. When considering the use of cannabinoids to treat malignant tumors, some laboratories have attempted to isolate the non-psychotropic therapeutic effects ascribed to CB2 receptor activation, while eliminating the psychotropic and addictive properties ascribed to CB1 receptor activation (Sánchez et al. 2001). However, since only extremely high amounts of cannabinoids are required to become toxic to healthy cells (Iversen 2000), their delivery at relatively high concentrations, either orally, or even directly into tumors, should not be overlooked. Indeed, stereotactic injection of chemotherapeutic compounds directly into human brain tumor masses constitutes a routine procedure for neurosurgeons, and thus cannabinoids can easily be delivered by this technique (Guzman et al. 2006). Taken together, the studies outlined in this review suggest that stereotactic injection of high concentrations of CBD could constitute a useful regimen for neurosurgeons to use in the treatment of malignant astrocytomas and of excessive/chronic neuroinflammation. Such a treatment could provide therapeutic effects both directly, by killing the astrocytoma and limiting its propagation, and indirectly, by reducing the accumulation of activated microglia or invading peripheral immune cells.
The fact that non-psychotropic cannabinoids acting through CB-like receptors affect such fundamental processes involved in microglial cell activation and astrocytoma propagation constitutes, in my opinion, one of the most exciting areas of research in our search for new chemotherapeutic agents to treat malignant brain tumors and new anti-inflammatory agents to temper the damage linked to chronic neuroinflammation. Furthermore, the curative properties of cannabinoids do not overlap with currently available medicines, and therefore cannabinoid-based treatments constitute a new therapeutic platform. Yet, in order to gain broad public support, cannabinoid-based therapies will need to transcend the drug of abuse stigma by remaining devoid of THC-related adverse effects, which include dizziness, disorientation, alterations in temporal perception, memory impairment, anxiety and possible addiction. Therefore, it is important to rapidly increase our pharmacological and molecular understanding of CB-like receptors in general, and in glial cells in particular, so that we can exploit their desirable properties for therapeutic gain.
Acknowledgments
To Susan Fung and William Marrs from my laboratory for critical reading of this manuscript, and to NIH (DA 14486 and DA 21285) for grant support.
References
- Antel J, Becker B. Clinical Neuroimmunology. Montreal: Blackwell Sciences; 1997. Central Nervous System-Immune Interactions: Contribution to neurologic disease and recovery; pp. 26–39. [Google Scholar]
- Baker D, Pryce G, Davies WL, Hiley CR. In silico patent searching reveals a new cannabinoid receptor. Trends Pharmacol Sci. 2006;27(1):1–4. doi: 10.1016/j.tips.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Bari M, Battista N, Fezza F, Finazzi-Agro A, Maccarrone M. Lipid rafts control signaling of type-1 cannabinoid receptors in neuronal cells. Implications for anandamide-induced apoptosis. J Biol Chem. 2005;280(13):12212–20. doi: 10.1074/jbc.M411642200. [DOI] [PubMed] [Google Scholar]
- Becher B, Antel JP. Comparison of phenotypic and functional properties of immediately ex vivo and cultured human adult microglia. Glia. 1996;18:1–10. doi: 10.1002/(SICI)1098-1136(199609)18:1<1::AID-GLIA1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Begg M, Mo F-M, Offertáler L, Bátkai S, Pacher P, Razdan RK, Lovinger DM, Kunos G. G protein-coupled endothelial receptor for atypical cannabinoid ligands modulates a Ca2+-dependent K+ current. The Journal of Biological Chemistry. 2003a;278(46):46188–46194. doi: 10.1074/jbc.M307258200. [DOI] [PubMed] [Google Scholar]
- Begg M, Mo FM, Offertaler L, Batkai S, Pacher P, Razdan RK, Lovinger DM, Kunos G. G protein-coupled endothelial receptor for atypical cannabinoid ligands modulates a Ca2+-dependent K+ current. J Biol Chem. 2003b;278(46):46188–94. doi: 10.1074/jbc.M307258200. [DOI] [PubMed] [Google Scholar]
- Begg M, Pacher P, Batkai S, Osei-Hyiaman D, Offertaler L, Mo FM, Liu J, Kunos G. Evidence for novel cannabinoid receptors. Pharmacol Ther. 2005;106(2):133–45. doi: 10.1016/j.pharmthera.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Benito C, Kim WK, Chavarria I, Hillard CJ, Mackie K, Tolon RM, Williams K, Romero J. A glial endogenous cannabinoid system is upregulated in the brains of macaques with simian immunodeficiency virus-induced encephalitis. J Neurosci. 2005;25(10):2530–6. doi: 10.1523/JNEUROSCI.3923-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito C, Núnez E, Tolón RM, Carrier EJ, Rábano A, Hillard CJ, Romero J. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaques-associated glia in Alzheimer’s disease brains. The Journal of Neuroscience. 2003;23(35):11136–11141. doi: 10.1523/JNEUROSCI.23-35-11136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger C, Schmid PC, Schabitz WR, Wolf M, Schwab S, Schmid HH. Massive accumulation of N-acylethanolamines after stroke. Cell signalling in acute cerebral ischemia? J Neurochem. 2004;88(5):1159–67. doi: 10.1046/j.1471-4159.2003.02244.x. [DOI] [PubMed] [Google Scholar]
- Bernardo A, Ajmone-Cat MA, Gasparini L, Ongini E, Minghetti L. Nuclear receptor peroxisome proliferator-activated receptor-gamma is activated in rat microglial cells by the anti-inflammatory drug HCT1026, a derivative of flurbiprofen. J Neurochem. 2005;92(4):895–903. doi: 10.1111/j.1471-4159.2004.02932.x. [DOI] [PubMed] [Google Scholar]
- Blázquez C, Sánchez C, Daza A, Galve-Roperh I, Guzmán M. The stimulation of ketogenesis by cannabinoids in cultured astrocytes defines carnitine palmitoyltransferase I as a new ceramide-activated enzyme. The Journal of Neurochemistry. 1999;72:1759–1768. doi: 10.1046/j.1471-4159.1999.721759.x. [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Bourrié B, Rinaldi-Carmona M, Shire D, Le Fur G, Casellas P. Stimulation of cannabinoid receptor CB1 induces krox-24 expression in human astrocytoma cells. The Journal of Biological Chemistry. 1995;270:13973–13980. doi: 10.1074/jbc.270.23.13973. [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Poinot-Chazel C, Marchand J, Canat X, Bourrié B, Rinaldi-Carmona M, Calandra B, Le Fur G, Casellas P. Signaling pathway associated with stimulation of CB2 peripheral cannabinoid receptor. European Journal of Biochemistry. 1996;237:704–11. doi: 10.1111/j.1432-1033.1996.0704p.x. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Griffin G, Di Marzo V, Martin BR. Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol Pharmacol. 2001;60(1):155–63. [PubMed] [Google Scholar]
- Burstein S. PPAR-gamma: a nuclear receptor with affinity for cannabinoids. Life Sci. 2005;77(14):1674–84. doi: 10.1016/j.lfs.2005.05.039. [DOI] [PubMed] [Google Scholar]
- Carayon P, Marchand J, Dussossoy D, Derocq J-M, Jbilo O, Bord A, Bouaboula M, Galiègue S, Mondière P, Pénarier G, et al. Modulation and functional involvement of CB2 peripheral cannabinoid receptors during B-cell differentiation. Blood. 1998;92:3605–3615. [PubMed] [Google Scholar]
- Carlisle SJ, Marciano-Cabral F, Staab A, Ludwick C, Cabral GA. Differential expression of the CB2 cannabinoid receptor by rodent macrophages and macrophage-like cells in relation to cell activation. Int Immunopharm. 2002;2:69–82. doi: 10.1016/s1567-5769(01)00147-3. [DOI] [PubMed] [Google Scholar]
- Carracedo A, Lorente M, Egia A, Blazquez C, Garcia S, Giroux V, Malicet C, Villuendas R, Gironella M, Gonzalez-Feria L, et al. The stress-regulated protein p8 mediates cannabinoid-induced apoptosis of tumor cells. Cancer Cell. 2006;9(4):301–12. doi: 10.1016/j.ccr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Carrier EJ, Auchampach JA, Hillard CJ. Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression. Proc Natl Acad Sci U S A. 2006;103(20):7895–900. doi: 10.1073/pnas.0511232103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier EJ, Kearn CS, Barkmeier AJ, Breese NM, Yang W, Nithipatikom K, Pfister SL, Campbell WB, Hillard CJ. Cultured rat microglial cells synthesize the endocannabinoid 2-arachidonylglycerol, which increases proliferation via a CB2 receptor-dependent mechanism. Mol Pharmacol. 2004;65(4):999–1007. doi: 10.1124/mol.65.4.999. [DOI] [PubMed] [Google Scholar]
- Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–6. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Contassot E, Wilmotte R, Tenan M, Belkouch MC, Schnuriger V, de Tribolet N, Burkhardt K, Dietrich PY. Arachidonylethanolamide induces apoptosis of human glioma cells through vanilloid receptor-1. J Neuropathol Exp Neurol. 2004;63(9):956–63. doi: 10.1093/jnen/63.9.956. [DOI] [PubMed] [Google Scholar]
- Costa B, Colleoni M. Changes in rat brain energetic metabolism after exposure to anandamide or Đ9-tetrahydrocannabinol. European Journal of Pharmacology. 2000;395:1–7. doi: 10.1016/s0014-2999(00)00170-9. [DOI] [PubMed] [Google Scholar]
- Costa B, Colleoni M, Conti S, Parolaro D, Franke C, Trovato AE, Giagnoni G. Oral anti-inflammatory activity of cannabidiol, a non-psychoactive constituent of cannabis, in acute carrageenan-induced inflammation in the rat paw. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:74–9. doi: 10.1007/s00210-004-0871-3. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Vellani V, Schiano-Moriello A, Marini P, Magherini PC, Orlando P, Di Marzo V. Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J Pharmacol Exp Ther. 2008;325(3):1007–15. doi: 10.1124/jpet.107.134809. [DOI] [PubMed] [Google Scholar]
- Degn M, Lambertsen KL, Petersen G, Meldgaard M, Artmann A, Clausen BH, Hansen SH, Finsen B, Hansen HS, Lund TM. Changes in brain levels of N-acylethanolamines and 2-arachidonoylglycerol in focal cerebral ischemia in mice. J Neurochem. 2007;103(5):1907–16. doi: 10.1111/j.1471-4159.2007.04892.x. [DOI] [PubMed] [Google Scholar]
- Derocq J-M, Jbilo O, Bouaboula M, Ségui M, Clère C, Casellas P. Genomic and functional changes induced by the activation of the peripheral cannabinoid receptor CB2 in the promyelocytic cells HL-60. The Journal of Biological Chemistry. 2000;275:15621–15628. doi: 10.1074/jbc.275.21.15621. [DOI] [PubMed] [Google Scholar]
- Derocq J-M, Ségui M, Marchand J, Le Fur G, Casellas P. Cannabinoids enhance human B-cell growth at low nanomolar concentrations. FEBS letters. 1995;369:177–182. doi: 10.1016/0014-5793(95)00746-v. [DOI] [PubMed] [Google Scholar]
- Dirikoc S, Priola SA, Marella M, Zsurger N, Chabry J. Nonpsychoactive cannabidiol prevents prion accumulation and protects neurons against prion toxicity. J Neurosci. 2007;27(36):9537–44. doi: 10.1523/JNEUROSCI.1942-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drmota TP, Greasley, Groblewski T. Screening assays for cannabinoid-ligand-type modulators of GPR55. GSK; US: 2003. [Google Scholar]
- Eljaschewitsch E, Witting A, Mawrin C, Lee T, Schmidt PM, Wolf S, Hoertnagl H, Raine CS, Schneider-Stock R, Nitsch R, et al. The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron. 2006;49(1):67–79. doi: 10.1016/j.neuron.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Ellert-Miklaszewska A, Kaminska B, Konarska L. Cannabinoids down-regulate PI3K/Akt and Erk signalling pathways and activate proapoptotic function of Bad protein. Cell Signal. 2005;17(1):25–37. doi: 10.1016/j.cellsig.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Facchinetti F, Del Giudice E, Furegato S, Passarotto M, Leon A. Cannabinoids ablate release of TNFα in rat microglial cells stimulated with lypopolysaccharide. Glia. 2003;41:161–168. doi: 10.1002/glia.10177. [DOI] [PubMed] [Google Scholar]
- Felder CC, Joyce KE, Briley EM, Mansouri J, Mackie K, Blond O, Lai Y, Ma AL, Mitchell RL. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol Pharmacol. 1995a;48(3):443–50. [PubMed] [Google Scholar]
- Felder CC, Joyce KE, Briley EM, Mansouri J, Mackie K, Blond O, Lai Y, Ma AL, Mitchell RL. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Molecular Pharmacology. 1995b;48:443–450. [PubMed] [Google Scholar]
- Fowler CJ, Jonsson KO, Andersson A, Juntunen J, Jarvinen T, Vandevoorde S, Lambert DM, Jerman JC, Smart D. Inhibition of C6 glioma cell proliferation by anandamide, 1-arachidonoylglycerol, and by a water soluble phosphate ester of anandamide: variability in response and involvement of arachidonic acid. Biochem Pharmacol. 2003;66(5):757–67. doi: 10.1016/s0006-2952(03)00392-7. [DOI] [PubMed] [Google Scholar]
- Franklin A, Parmentier-Batteur S, Walter L, Greenberg DA, Stella N. Palmitoylethanolamide increases after focal cerebral ischemia and potentiates microglial cells motility. The Journal of Neuroscience. 2003 August 29;23(21):7767–7775. doi: 10.1523/JNEUROSCI.23-21-07767.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin A, Stella N. Arachidonylcyclopropylamide increases microglial cell migration through cannabinoid CB2 and abnormal-cannabidiol-sensitive receptors. European Journal of Pharmacology. 2003;474:195–198. doi: 10.1016/s0014-2999(03)02074-0. [DOI] [PubMed] [Google Scholar]
- Galiègue S, Mary S, Marchand J, Dussossoy D, Carrière D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. European Journal of Biochemistry. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Galve-Roperh I, Sánchez C, Cortés ML, Gómez del Pulgar T, Izquierdo M, Guzmán M. Anti-tumoral action of cannabinoids: involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nature Medicine. 2000;6:313–319. doi: 10.1038/73171. [DOI] [PubMed] [Google Scholar]
- Gardner B, Zu LX, Sharma S, Liu Q, Makriyannis A, Tashkin D, Dubinett SM. Autocrine and paracrine regulation of lymphocyte CB2 receptor expression by TGF-β. Biochemical and biophysical research communication. 2002;290:91–96. doi: 10.1006/bbrc.2001.6179. [DOI] [PubMed] [Google Scholar]
- Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57(7):727–41. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- Glass M, Northup JK. Agonist selective regulation of G proteins by cannabinoid CB(1) and CB(2) receptors. Mol Pharmacol. 1999;56(6):1362–9. doi: 10.1124/mol.56.6.1362. [DOI] [PubMed] [Google Scholar]
- Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, Uhl GR. Cannabinoid CB2 receptors: Immunohistochemical localization in rat brain. Brain Res. 2006;1071(1):10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Griffin G, Wray EJ, Tao Q, McAllister SD, Rorrer WK, Aung MM, Martin BR, Abood ME. Evaluation of the cannabinoid CB2 receptor-selective antagonist, SR144528: further evidence for cannabinoid CB2 receptor absence in the rat central nervous system. Eur J Pharmacol. 1999;377(1):117–25. doi: 10.1016/s0014-2999(99)00402-1. [DOI] [PubMed] [Google Scholar]
- Guzman M, Duarte MJ, Blazquez C, Ravina J, Rosa MC, Galve-Roperh I, Sanchez C, Velasco G, Gonzalez-Feria L. A pilot clinical study of Delta9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme. Br J Cancer. 2006;95(2):197–203. doi: 10.1038/sj.bjc.6603236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos N, Freund TF. Pharmacological separation of cannabinoid sensitive receptors on hippocampal excitatory and inhibitory fibers. Neuropharmacology. 2002;43(4):503–10. doi: 10.1016/s0028-3908(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Hajos N, Ledent C, Freund TF. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience. 2001;106(1):1–4. doi: 10.1016/s0306-4522(01)00287-1. [DOI] [PubMed] [Google Scholar]
- Held-Feindt J, Dorner L, Sahan G, Mehdorn HM, Mentlein R. Cannabinoid receptors in human astroglial tumors. J Neurochem. 2006;98(3):886–93. doi: 10.1111/j.1471-4159.2006.03911.x. [DOI] [PubMed] [Google Scholar]
- Henry DJ, Chavkin C. Activation of inwardly rectifying potassium channels (GIRK1) by co-expressed rat brain cannabinoid receptors in Xenopus oocytes. Neuroscience Lettres. 1995;186:91–94. doi: 10.1016/0304-3940(95)11289-9. [DOI] [PubMed] [Google Scholar]
- Henstridge CM, Balenga NA, Ford LA, Ross RA, Waldhoer M, Irving AJ. The GPR55 ligand L-alpha-lysophosphatidylinositol promotes RhoA-dependent Ca2+ signaling and NFAT activation. FASEB J. 2009;23(1):183–93. doi: 10.1096/fj.08-108670. [DOI] [PubMed] [Google Scholar]
- Herradon E, Martin MI, Lopez-Miranda V. Characterization of the vasorelaxant mechanisms of the endocannabinoid anandamide in rat aorta. Br J Pharmacol. 2007;152(5):699–708. doi: 10.1038/sj.bjp.0707404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring AC, Kaminski NE. Cannabinol-mediated inhibition of nuclear factor-κB, cAMP response element-binding protein, and interleukin-2 secretion by activated thymocytes. The Journal of Pharmacology and Experimental Therapeutics. 1999;291:1156–1163. [PubMed] [Google Scholar]
- Ho WS, Hiley CR. Vasodilator actions of abnormal-cannabidiol in rat isolated small mesenteric artery. Br J Pharmacol. 2003;138(7):1320–32. doi: 10.1038/sj.bjp.0705160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AF, Macgill AM, Smith D, Oz M, Lupica CR. Species and strain differences in the expression of a novel glutamate-modulating cannabinoid receptor in the rodent hippocampus. Eur J Neurosci. 2005;22(9):2387–91. doi: 10.1111/j.1460-9568.2005.04401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollister LE. Cannabidiol and cannabinol in man. Experientia. 1973;29(7):825–6. doi: 10.1007/BF01946311. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Mukhopadhyay S. Cellular signal transduction by anandamide and 2-arachidonoylglycerol. Chem Phys Lipids. 2000;108(1–2):53–70. doi: 10.1016/s0009-3084(00)00187-0. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, Frank RA. Blockade of effects of smoked marijuana by the CBl-selective cannabinoid receptor antagonist SR141716. Arc Gen Psychiatry. 2001;58:322–328. doi: 10.1001/archpsyc.58.4.322. [DOI] [PubMed] [Google Scholar]
- Iversen LL. The Science of Marijuana. New York: Oxford University Press; 2000. [Google Scholar]
- Jacobsson SO, Wallin T, Fowler CJ. Inhibition of rat C6 glioma cell proliferation by endogenous and synthetic cannabinoids. Relative involvement of cannabinoid and vanilloid receptors. J Pharmacol Exp Ther. 2001;299(3):951–9. [PubMed] [Google Scholar]
- Járai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, Zimmer AM, Bonner TI, Buckley NE, Mezey E, et al. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proceedings of the National Academy of Sciences. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeske NA, Patwardhan AM, Gamper N, Price TJ, Akopian AN, Hargreaves KM. Cannabinoid WIN 55,212–2 regulates TRPV1 phosphorylation in sensory neurons. J Biol Chem. 2006;281(43):32879–90. doi: 10.1074/jbc.M603220200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns DG, Behm DJ, Walker DJ, Ao Z, Shapland EM, Daniels DA, Riddick M, Dowell S, Staton PC, Green P, et al. The novel endocannabinoid receptor GPR55 is activated by atypical cannabinoids but does not mediate their vasodilator effects. Br J Pharmacol. 2007;152(5):825–31. doi: 10.1038/sj.bjp.0707419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson KO, Andersson A, Jacobsson SO, Vandevoorde S, Lambert DM, Fowler CJ. AM404 and VDM 11 non-specifically inhibit C6 glioma cell proliferation at concentrations used to block the cellular accumulation of the endocannabinoid anandamide. Arch Toxicol. 2003;77(4):201–7. doi: 10.1007/s00204-002-0435-6. [DOI] [PubMed] [Google Scholar]
- Jung M, Calassi R, Rinaldi-Carmona M, Chardenot P, Le Fur G, Soubrié P, Oury-Donat F. Characterization of CB1 receptors on rat neuronal cell cultures: binding and functional studies using the selective receptor antagonist SR 141716A. The Journal of Neurochemistry. 1997;68:402–409. doi: 10.1046/j.1471-4159.1997.68010402.x. [DOI] [PubMed] [Google Scholar]
- Kelley BG, Thayer SA. Delta 9-tetrahydrocannabinol antagonizes endocannabinoid modulation of synaptic transmission between hippocampal neurons in culture. Neuropharmacology. 2004;46(5):709–15. doi: 10.1016/j.neuropharm.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Kim SR, Kim SU, Oh U, Jin BK. Transient receptor potential vanilloid subtype 1 mediates microglial cell death in vivo and in vitro via Ca2+-mediated mitochondrial damage and cytochrome c release. J Immunol. 2006;177(7):4322–9. doi: 10.4049/jimmunol.177.7.4322. [DOI] [PubMed] [Google Scholar]
- Klegeris A, Bissonnette CJ, McGeer PL. Reduction of human monocytic cell neurotoxicity and cytokine secretion by ligands of the cannabinoid-type CB2 receptor. British Journal of Pharmacology. 2003;139:775–786. doi: 10.1038/sj.bjp.0705304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lenhard JM, et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci U S A. 1997;94(9):4318–23. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan NM, Rabinowitz R, Levi P, Gibson D, Sandor P, Schlesinger M, Mechoulam R. Synthesis and antitumor activity of quinonoid derivatives of cannabinoids. J Med Chem. 2004;47(15):3800–6. doi: 10.1021/jm040042o. [DOI] [PubMed] [Google Scholar]
- Kogan NM, Schlesinger M, Priel E, Rabinowitz R, Berenshtein E, Chevion M, Mechoulam R. HU-331, a novel cannabinoid-based anticancer topoisomerase II inhibitor. Mol Cancer Ther. 2007;6(1):173–83. doi: 10.1158/1535-7163.MCT-06-0039. [DOI] [PubMed] [Google Scholar]
- Kreitzer FR, Stella N. The therapeutic potential of novel cannabinoid receptors. Pharmacol Ther. 2009;122(2):83–96. doi: 10.1016/j.pharmthera.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutz S, Koch M, Bottger C, Ghadban C, Korf HW, Dehghani F. 2-Arachidonoylglycerol elicits neuroprotective effects on excitotoxically lesioned dentate gyrus granule cells via abnormal-cannabidiol-sensitive receptors on microglial cells. Glia. 2009;57(3):286–94. doi: 10.1002/glia.20756. [DOI] [PubMed] [Google Scholar]
- Kreutz S, Koch M, Ghadban C, Korf HW, Dehghani F. Cannabinoids and neuronal damage: differential effects of THC, AEA and 2-AG on activated microglial cells and degenerating neurons in excitotoxically lesioned rat organotypic hippocampal slice cultures. Exp Neurol. 2007;203(1):246–57. doi: 10.1016/j.expneurol.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Lauckner JE, Jensen JB, Chen HY, Lu HC, Hille B, Mackie K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc Natl Acad Sci U S A. 2008;105(7):2699–704. doi: 10.1073/pnas.0711278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SF, Newton C, Widen R, Friedman H, Klein TW. Differential expression of cannabinoid CB2 receptor mRNA in mouse immune cell subpopulation and following B cell stimulation. European Journal of Pharmacology. 2001;423:235–241. doi: 10.1016/s0014-2999(01)01122-0. [DOI] [PubMed] [Google Scholar]
- Lenman A, Fowler CJ. Interaction of ligands for the peroxisome proliferator-activated receptor gamma with the endocannabinoid system. Br J Pharmacol. 2007;151(8):1343–51. doi: 10.1038/sj.bjp.0707352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Verme JL, Fu J, Astarita G, La Rana G, Russo R, Calignano A, Piomelli D. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Molecular Pharmacology. 2005;67(1):15–19. doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]
- LoVerme J, La Rana G, Russo R, Calignano A, Piomelli D. The search for the palmitoylethanolamide receptor. Life Sci. 2005;77(14):1685–98. doi: 10.1016/j.lfs.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Lorenzon T, Bari M, Melino G, Finazzi-Agrò A. Anandamide induces apoptosis in human cells via vanilloid receptors. The Journal of Biological Chemistry. 2000;275(41):31938–31945. doi: 10.1074/jbc.M005722200. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Pauselli R, Di Rienzo M, Finazzi-Agrò A. Binding, degradation and apoptotic activity of stearoylethanolamide in rat C6 glioma cells. Biochemical Journal. 2002;366:137–144. doi: 10.1042/BJ20020438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K, Hille B. Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proceedings of the National Academy of Sciences. 1992;89:3825–3829. doi: 10.1073/pnas.89.9.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K, Lai Y, Westenbroek R, Mitchell R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptors. The Journal of Neuroscience. 1995;15:6552–6561. doi: 10.1523/JNEUROSCI.15-10-06552.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ. Neuroscience. Low-cost travel in neurons. Science. 2009;325(5946):1349–51. doi: 10.1126/science.1180102. [DOI] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lazdunski M, Honore E. The endocannabinoid anandamide is a direct and selective blocker of the background K(+) channel TASK-1. EMBO J. 2001;20(1–2):47–54. doi: 10.1093/emboj/20.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfait AM, Gallily R, Sumariwalla PF, Malik AS, Andreakos E, Mechoulam R, Feldmann M. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proceedings of the National Academy of Sciences. 2000;97:9561–9566. doi: 10.1073/pnas.160105897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresz K, Carrier EJ, Ponomarev ED, Hillard CJ, Dittel BN. Modulation of the cannabinoid CB receptor in microglial cells in response to inflammatory stimuli. J Neurochem. 2005 doi: 10.1111/j.1471-4159.2005.03380.x. [DOI] [PubMed] [Google Scholar]
- Maresz K, Pryce G, Ponomarev ED, Marsicano G, Croxford JL, Shriver LP, Ledent C, Cheng X, Carrier EJ, Mann MK, et al. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nat Med. 2007;13(4):492–7. doi: 10.1038/nm1561. [DOI] [PubMed] [Google Scholar]
- Marinelli S, Pacioni S, Cannich A, Marsicano G, Bacci A. Self-modulation of neocortical pyramidal neurons by endocannabinoids. Nat Neurosci. 2009;12(12):1488–90. doi: 10.1038/nn.2430. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Grazia Cascio M, Ortega Gutiérrez S, Van der Stelt M, et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- Massi P, Vaccani A, Bianchessi S, Costa B, Macchi P, Parolaro D. The non-psychoactive cannabidiol triggers caspase activation and oxidative stress in human glioma cells. Cell Mol Life Sci. 2006;63(17):2057–66. doi: 10.1007/s00018-006-6156-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massi P, Vaccani A, Ceruti S, Colombo A, Abbracchio MP, Parolaro D. Antitumor effects of cannabidiol, a nonpsychoactive cannabinoid, on human glioma cell lines. J Pharmacol Exp Ther. 2004;308(3):838–45. doi: 10.1124/jpet.103.061002. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- McCollum L, Howlett AC, Mukhopadhyay S. Anandamide-mediated CB1/CB2 cannabinoid receptor--independent nitric oxide production in rabbit aortic endothelial cells. J Pharmacol Exp Ther. 2007;321(3):930–7. doi: 10.1124/jpet.106.117549. [DOI] [PubMed] [Google Scholar]
- McCoy KL, Matveyeva M, Carlisle SJ, Cabral GA. Cannabinoid inhibition of the processing of intact lysozyme by macrophages: evidence for CB2 receptor participation. The Journal of Pharmacology and Experimental Therapeutics. 1999;289:1620–1625. [PubMed] [Google Scholar]
- McHugh D, Tanner C, Mechoulam R, Pertwee RG, Ross RA. Inhibition of human neutrophil chemotaxis by endogenous cannabinoids and phytocannabinoids: evidence for a site distinct from CB1 and CB2. Mol Pharmacol. 2008;73(2):441–50. doi: 10.1124/mol.107.041863. [DOI] [PubMed] [Google Scholar]
- Michalik L, Desvergne B, Wahli W. Peroxisome-proliferator-activated receptors and cancers: complex stories. Nat Rev Cancer. 2004;4(1):61–70. doi: 10.1038/nrc1254. [DOI] [PubMed] [Google Scholar]
- Milman G, Maor Y, Abu-Lafi S, Horowitz M, Gallily R, Batkai S, Mo FM, Offertaler L, Pacher P, Kunos G, et al. N-arachidonoyl L-serine, an endocannabinoid-like brain constituent with vasodilatory properties. Proc Natl Acad Sci U S A. 2006;103(7):2428–33. doi: 10.1073/pnas.0510676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldrich G, Wenger T. Localization of the CB1 cannabinoid receptor in the rat brain. An immunohistochemical study. Peptides. 2000;21:1735–1742. doi: 10.1016/s0196-9781(00)00324-7. [DOI] [PubMed] [Google Scholar]
- Molina-Holgado E, Vela JM, Arévalo-Martin A, Almazán G, Molina-Holgado F, Borrell J, Guaza C. Cannabinoids promote oligodendrocyte progenitor survival: involvement of cannabinoid receptor and phosphatidyllinositol-3 kinase/Akt signaling. The Journal of Neuroscience. 2002a;22(22):9742–9753. doi: 10.1523/JNEUROSCI.22-22-09742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Holgado F, Molina-Holgado E, Guaza C, Rothwell NJ. Role of CB1 and CB2 receptors in the inhibitory effects of cannabinoids on lipopolysaccharide-induced nitric oxide release in astrocytes cultures. Journal of Neuroscience Research. 2002b;67:829–836. doi: 10.1002/jnr.10165. [DOI] [PubMed] [Google Scholar]
- Monory K, Blaudzun H, Massa F, Kaiser N, Lemberger T, Schutz G, Wotjak CT, Lutz B, Marsicano G. Genetic dissection of behavioural and autonomic effects of Delta(9)-tetrahydrocannabinol in mice. PLoS Biol. 2007;5(10):e269. doi: 10.1371/journal.pbio.0050269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monory K, Tzavara ET, Lexime J, Ledent C, Parmentier M, Borsodi A, Hanoune J. Novel, not adenylyl cyclase-coupled cannabinoid binding site in cerebellum of mice. Biochem Biophys Res Commun. 2002;292(1):231–5. doi: 10.1006/bbrc.2002.6635. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Navarrete M, Araque A. Endocannabinoids mediate neuron-astrocyte communication. Neuron. 2008;57(6):883–93. doi: 10.1016/j.neuron.2008.01.029. [DOI] [PubMed] [Google Scholar]
- Nunez E, Benito C, Pazos MR, Barbachano A, Fajardo O, Gonzalez S, Tolon RM, Romero J. Cannabinoid CB2 receptors are expressed by perivascular microglial cells in the human brain: an immunohistochemical study. Synapse. 2004;53(4):208–13. doi: 10.1002/syn.20050. [DOI] [PubMed] [Google Scholar]
- O’Sullivan SE, Bennet AJ, Kendall DA, Randall MD. Cannabinoids and peroxisome proliferator-activated receptor gamma (PPARgamma) Proc Intl Cannbinoid Res Soc. 2006a (abstract) [Google Scholar]
- O’Sullivan SE, Kendall DA, Randall MD. Further characterization of the time-dependent vascular effects of delta9-tetrahydrocannabinol. J Pharmacol Exp Ther. 2006b;317(1):428–38. doi: 10.1124/jpet.105.095828. [DOI] [PubMed] [Google Scholar]
- O’Sullivan SE, Tarling EJ, Bennett AJ, Kendall DA, Randall MD. Novel time-dependent vascular actions of Delta9-tetrahydrocannabinol mediated by peroxisome proliferator-activated receptor gamma. Biochem Biophys Res Commun. 2005;337(3):824–31. doi: 10.1016/j.bbrc.2005.09.121. [DOI] [PubMed] [Google Scholar]
- Offertáler L, Mo F, Bátkai S, Liu J, Begg M, Razdan RK, Martin BR, Bukoski RD, Kunos G. Selective Ligands And Cellular Effectors Of A G Protein-Coupled Endothelial Cannabinoid Receptor. Molecular Pharmacology. 2003;63:699–705. doi: 10.1124/mol.63.3.699. [DOI] [PubMed] [Google Scholar]
- Oka S, Nakajima K, Yamashita A, Kishimoto S, Sugiura T. Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem Biophys Res Commun. 2007;362(4):928–34. doi: 10.1016/j.bbrc.2007.08.078. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Meozzi PA, Myers L, Perchuk A, Mora Z, Tagliaferro PA, Gardner E, et al. Brain neuronal CB2 cannabinoid receptors in drug abuse and depression: from mice to human subjects. PLoS ONE. 2008;3(2):e1640. doi: 10.1371/journal.pone.0001640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Perchuk A, Meozzi PA, Myers L, Mora Z, Tagliaferro P, Gardner E, et al. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann N Y Acad Sci. 2006;1074:514–36. doi: 10.1196/annals.1369.052. [DOI] [PubMed] [Google Scholar]
- Oz M. Receptor-independent effects of endocannabinoids on ion channels. Curr Pharm Des. 2006;12(2):227–39. doi: 10.2174/138161206775193073. [DOI] [PubMed] [Google Scholar]
- Patwardhan AM, Jeske NA, Price TJ, Gamper N, Akopian AN, Hargreaves KM. The cannabinoid WIN 55,212–2 inhibits transient receptor potential vanilloid 1 (TRPV1) and evokes peripheral antihyperalgesia via calcineurin. Proc Natl Acad Sci U S A. 2006;103(30):11393–8. doi: 10.1073/pnas.0603861103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman RJ, Aubrey KR, Vandenberg RJ. Arachidonic acid and anandamide have opposite modulatory actions at the glycine transporter, GLYT1a. The Journal of Neurochemistry. 2003;84:592–601. doi: 10.1046/j.1471-4159.2003.01549.x. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes M, Timmons MC, Davis KH, Wall ME. A comparison of the pharmacological activity in man of intravenously administrered Đ9-tetrahydrocannabinol, cannabinol, and cannabidiol. Experientia (Basel) 1973;29:1368–1369. doi: 10.1007/BF01922823. [DOI] [PubMed] [Google Scholar]
- Petrova TV, Akama KT, Van Eldik LJ. Cyclopentenone prostaglandins suppress activation of microglia: down regulation of inducible nitric-oxide synthase by 15-deoxy-Đ12,14-prostaglandin J2. Proceedings of the National Academy of Sciences. 1999;96:4668–4673. doi: 10.1073/pnas.96.8.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietr M, Kozela E, Levy R, Rimmerman N, Lin YH, Stella N, Vogel Z, Juknat A. Differential changes in GPR55 during microglial cell activation. FEBS Lett. 2009;583(12):2071–6. doi: 10.1016/j.febslet.2009.05.028. [DOI] [PubMed] [Google Scholar]
- Price DA, Owens WA, Gould GG, Frazer A, Roberts JL, Daws LC, Giuffrida A. CB1-independent inhibition of dopamine transporter activity by cannabinoids in mouse dorsal striatum. J Neurochem. 2007;101(2):389–96. doi: 10.1111/j.1471-4159.2006.04383.x. [DOI] [PubMed] [Google Scholar]
- Puffenbarger RA, Boothe AC, Cabral GA. Cannabinoids inhibit LPS-inducible cytokine mRNA expression in rat microglial cells. Glia. 2000;29:58–69. [PubMed] [Google Scholar]
- Puighermanal E, Marsicano G, Busquets-Garcia A, Lutz B, Maldonado R, Ozaita A. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nat Neurosci. 2009;12(9):1152–8. doi: 10.1038/nn.2369. [DOI] [PubMed] [Google Scholar]
- Ramirez BG, Blazquez C, Gomez del Pulgar T, Guzman M, de Ceballos ML. Prevention of Alzheimer’s disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci. 2005;25(8):1904–13. doi: 10.1523/JNEUROSCI.4540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Neliat G, Caput D, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350(2–3):240–4. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- Rockwell CE, Snider NT, Thompson JT, Vanden Heuvel JP, Kaminski NE. Interleukin-2 suppression by 2-arachidonyl glycerol is mediated through peroxisome proliferator-activated receptor gamma independently of cannabinoid receptors 1 and 2. Mol Pharmacol. 2006;70(1):101–11. doi: 10.1124/mol.105.019117. [DOI] [PubMed] [Google Scholar]
- Rodríguez JJ, Mackie K, Pickel VM. Ultrastructural localization of the CB1 cannabinoid receptor in μ-opioid receptor patches of the rat caudate putamen nucleus. The Journal of Neuroscience. 2001;21:823–833. doi: 10.1523/JNEUROSCI.21-03-00823.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjogren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152(7):1092–101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagan S, Venance L, Torrens Y, Cordier J, Glowinski J, Giaume C. Anandamide and WIN 55212–2 inhibit cyclic AMP formation through G-protein-coupled receptors distinct from CB1 cannabinoid receptors in cultured astrocytes. European Journal of Neuroscience. 1999;11:691–699. doi: 10.1046/j.1460-9568.1999.00480.x. [DOI] [PubMed] [Google Scholar]
- Salazar M, Carracedo A, Salanueva IJ, Hernandez-Tiedra S, Lorente M, Egia A, Vazquez P, Blazquez C, Torres S, Garcia S, et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J Clin Invest. 2009;119(5):1359–72. doi: 10.1172/JCI37948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salio C, Doly S, Fischer J, Franzoni MF, Conrath M. Neuronal and astrocytic localization of the cannabinoid receptor-1 in the dorsal horn of the rat spinal cord. Neuroscience Lettres. 2002;329:13–16. doi: 10.1016/s0304-3940(02)00549-9. [DOI] [PubMed] [Google Scholar]
- Sánchez C, de Ceballos ML, Gómez del Pulgar T, Rueda D, Corbacho C, Velasco G, Galve-Roperh I, Huffman JW, Ramón y Cajal S, Guzman M. Inhibition of glioma growth In Vivo by selective activation of the CB2 cannabinoid receptor. Cancer Research. 2001;61:5784–5789. [PubMed] [Google Scholar]
- Sánchez C, Galve-Roperh I, Canova C, Brachet P, Guzmán M. Đ9-tetrahydrocannabinol induces apoptosis in C6 glioma cells. FEBS letters. 1998a;436:6–10. doi: 10.1016/s0014-5793(98)01085-0. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Galve-Roperh I, Rueda D, Guzman M. Involvement of sphingomyelin hydrolysis and the mitogen-activated protein kinase cascade in the Đ9-tetrahydrocannabinol-induced stimulation of glucose metabolism in primary astrocytes. Molecular Pharmacology. 1998b;54:834–843. doi: 10.1124/mol.54.5.834. [DOI] [PubMed] [Google Scholar]
- Sawzdargo M, Nguyen T, Lee DK, Lynch KR, Cheng R, Heng HH, George SR, O’Dowd BF. Identification and cloning of three novel human G protein-coupled receptor genes GPR52, PsiGPR53 and GPR55: GPR55 is extensively expressed in human brain. Brain Res Mol Brain Res. 1999;64(2):193–8. doi: 10.1016/s0169-328x(98)00277-0. [DOI] [PubMed] [Google Scholar]
- Schäbitz W-R, Giuffrida A, Berger C, Aschoff A, Schwaninger M, Schwab S, Piomelli D. Release of fatty acid amides in a patient with hemispheric stroke. Stroke. 2002;33:2112–2114. doi: 10.1161/01.str.0000023491.63693.18. [DOI] [PubMed] [Google Scholar]
- Schatz AR, Lee M, Condie RB, Pulaski JT, Kaminski NE. Cannabinoid receptors CB1 and CB2: a characterization of expression and adenylate cyclase modulation within the immune system. Toxicol Appl Pharmacol. 1997;142:278–287. doi: 10.1006/taap.1996.8034. [DOI] [PubMed] [Google Scholar]
- Sheng WS, Hu S, Min X, Cabral GA, Lokensgard JR, Peterson PK. Synthetic cannabinoid WIN55,212–2 inhibits generation of inflammatory mediators by IL-1beta-stimulated human astrocytes. Glia. 2005;49(2):211–9. doi: 10.1002/glia.20108. [DOI] [PubMed] [Google Scholar]
- Showalter VM, Compton DR, Martin BR, Abood ME. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J Pharmacol Exp Ther. 1996;278(3):989–99. [PubMed] [Google Scholar]
- Sinha D, Bonner TI, Bhat NR, Matsuda LA. Expression of the CB1 cannabinoid receptor in macrophage-like cells from brain tissue: immunochemical characterization by fusion protein antibodies. Journal of Neuroimmunology. 1998;82:13–21. doi: 10.1016/S0165-5728(97)00181-1. [DOI] [PubMed] [Google Scholar]
- Skaper SD, Buriani A, Dal Toso R, Petrelli L, Romanello S, Facci L, Leon A. The ALIAmide palmitoylethanolamide and cannabinoids, but not anandamide, are protective in a delayed postglutamate paradigm of excitotoxic death in cerebellar granule neurons. Proceedings of the National Academy of Sciences. 1996;93:3984–9. doi: 10.1073/pnas.93.9.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano GB, Liu Y, Goligorsky MS. Cannabinoid receptors are coupled to nitric oxide release in invertebrate immunocytes, microglia, and human monocytes. The Journal of Biological Chemistry. 1996;271:19238–19242. doi: 10.1074/jbc.271.32.19238. [DOI] [PubMed] [Google Scholar]
- Straiker A, Mackie K. Cannabinoids, electrophysiology, and retrograde messengers: challenges for the next 5 years. AAPS J. 2006;8(2):E272–6. doi: 10.1007/BF02854897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Kishimoto S, Miyashita T, Nakane S, Kodaka T, Suhara Y, Takayama H, Waku K. Evidence that 2-arachidonylglycerol but not N-palmitpylethanolamine or anandamide is the physiological ligand for the cannabinoid CB2 receptor. The Journal of Biological Chemistry. 2000;275:605–612. doi: 10.1074/jbc.275.1.605. [DOI] [PubMed] [Google Scholar]
- Sun Y, Alexander SP, Garle MJ, Gibson CL, Hewitt K, Murphy SP, Kendall DA, Bennett AJ. Cannabinoid activation of PPAR alpha; a novel neuroprotective mechanism. Br J Pharmacol. 2007;152(5):734–43. doi: 10.1038/sj.bjp.0707478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Turner CE, Elsohly MA, Boeren EG. Constituents of Cannabis sativa L. XVII. A review of the natural constituents. J Nat Prod. 1980;43(2):169–234. doi: 10.1021/np50008a001. [DOI] [PubMed] [Google Scholar]
- Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci. 2007;27(14):3663–76. doi: 10.1523/JNEUROSCI.0448-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccani A, Massi P, Colombo A, Rubino T, Parolaro D. Cannabidiol inhibits human glioma cell migration through a cannabinoid receptor-independent mechanism. Br J Pharmacol. 2005;144(8):1032–6. doi: 10.1038/sj.bjp.0706134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310(5746):329–32. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Venance L, Piomelli D, Glowinski J, Giaume C. Inhibition by anandamide of gap junctions and intercellular calcium signalling in striatal astrocytes. Nature. 1995;376:590–594. doi: 10.1038/376590a0. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Varga K, Jarai Z, Kunos G. Mesenteric vasodilation mediated by endothelial anandamide receptors. Hypertension. 1999;33(1 Pt 2):429–34. doi: 10.1161/01.hyp.33.1.429. [DOI] [PubMed] [Google Scholar]
- Waksman Y, Olson JM, Carlisle SJ, Cabral GY. The central cannabinoid receptor (CB1) mediates inhibition of nitric oxide production by rat microglial cells. The Journal of Pharmacology and Experimental Therapeutics. 1999;288:1357–1366. [PubMed] [Google Scholar]
- Waldeck-Weiermair M, Zoratti C, Osibow K, Balenga N, Goessnitzer E, Waldhoer M, Malli R, Graier WF. Integrin clustering enables anandamide-induced Ca2+ signaling in endothelial cells via GPR55 by protection against CB1-receptor-triggered repression. J Cell Sci. 2008;121(Pt 10):1704–17. doi: 10.1242/jcs.020958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter L, Franklin A, Witting A, Wade C, Xie Y, Kunos G, Mackie K, Stella N. Non-psychotropic cannabinoid receptors regulate microglial cell migration. The Journal of Neuroscience. 2003;23(4):1398–1405. doi: 10.1523/JNEUROSCI.23-04-01398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte LS, Ryberg E, Sims NA, Ridge SA, Mackie K, Greasley PJ, Ross RA, Rogers MJ. The putative cannabinoid receptor GPR55 affects osteoclast function in vitro and bone mass in vivo. Proc Natl Acad Sci U S A. 2009;106(38):16511–6. doi: 10.1073/pnas.0902743106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiangou Y, Facer P, Durrenberger P, Chessell IP, Naylor A, Bountra C, Banati RR, Anand P. COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol. 2006;6:12. doi: 10.1186/1471-2377-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Hoffert C, Vu K, Groblewski T, Ahmad S, O’Donnell D. Induction of CB2 receptors expression in the rat spinal cord of neuropathic but not inflammatory chonic pain model. European Journal of Neuroscience. 2003;17:2750–2754. doi: 10.1046/j.1460-9568.2003.02704.x. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Andersson DA, Högestätt ED. Đ9-tetrahydrocannabinol and cannabinol activate capsaicin-sensitive sensory nerves via a CB1 and CB2 cannabinoid receptor-independent mechanism. The Journal of Neuroscience. 2002;22:4720–4727. doi: 10.1523/JNEUROSCI.22-11-04720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H-H, Sørgard M, Di Marzo V, Julius D, Högestätt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]