Abstract
Our recent work in endothelial cells and human atherosclerotic plaque showed that overexpression of glutathione-S-tranferases (GSTs) in endothelium protects against oxidative damage from aldehydes such as 4-HNE. Nuclear factor (NF)-κB plays a crucial role during inflammation and immune responses by regulating the expression of inducible genes such as inducible nitric oxide synthase (iNOS). 4-HNE induces apoptosis and affects NF-κB mediated gene expression, but conflicting results on 4-HNE’s effect on NF-κB have been reported. We compared the effect of 4-HNE on iNOS and the NF-κB pathway in control mouse pancreatic islet endothelial (MS1) cells and those transfected with mGSTA4, a α-class GST with highest activity toward 4-HNE. When treated with 4-HNE, mGSTA4-transfected cells showed significant upregulation of iNOS and nitric oxide (NO) through (NF)-κB (p65) translocation in comparison with wild-type or vector-transfected cells. Immunohistochemical studies of early human plaques showed lower 4-HNE content and upregulation of iNOS, which we take to indicate that GSTA4-4 induction acts as an enzymatic defense against high levels of 4-HNE, since 4-HNE accumulated in more advanced plaques, when detoxification and exocytotic mechanisms are likely to be overwhelmed. These studies suggest that GSTA4-4 may play an important defensive role against atherogenesis through detoxification of 4-HNE and upregulation of iNOS.
Keywords: Atherosclerosis, Nitric oxide, Inducible nitric oxide synthase, Glutathione-S-transferase, NF-κB
Introduction
Atherosclerosis, the commonest cause of death in industrialized countries, has for many years been linked to cigarette smoking, and more recently to air pollution. Atherosclerosis is a complex process mediated in large part by inflammatory and oxidative mechanisms, including lipid peroxidation. One of the most plentiful products of lipid peroxidation is the α, β-unsaturated carbonyl, 4-hydroxynonenal (4-HNE), which is believed to be responsible for many of the cytopathological effects observed during inflammatory and oxidative stress (Minekura et al., 2001; Uchida, 2003; Awasthi et al., 2003). Lysine residues modified by 4-HNE have been identified in human and experimental atherosclerotic lesions (Palinski et al., 1989; Napoli et al., 1997). We recently found that the GST isozyme hGSTA4-4, which detoxifies 4-HNE and exhibits high activity in vascular tissue, acts as a major defense against oxidative stress in an endothelial cell line, and is upregulated in endothelial cells overlaying the earliest stages of the human atherosclerotic plaque (Yang et al., 2004). This, and our earlier work in isolated vascular smooth muscle cells (He et al., 1999, 1998), suggests that oxidative damage induced by 4-HNE is an important step in the process of atherogenesis, and induction of 4-HNE-metabolizing GSTs in the vascular wall is protective of endothelial and vascular smooth muscle cells undergoing oxidative stress.
Atherosclerosis may involve many stress-mediated cell signaling processes. Previous studies have suggested the physiological importance of 4-HNE homeostasis to cell cycle signaling (Awasthi et al., 2003; Yang et al., 2004). NF-κB, a pleiotropic transcription factor, has been suggested to play an important role in gene regulation during the inflammatory and immune reactions that promote atherosclerosis (Hayden and Ghosh, 2004). NF-κB regulates the inducible expression of a variety of genes involved in inflammatory and immune responses, including iNOS. It is well known that NO plays a key role in mediating blood pressure and vascular tone. Reduced bioavailability of NO is thought to be one of the central factors common to cardiovascular disease, although it is unclear whether this is a cause or result of endothelial dysfunction (Naseem, 2005). 4-HNE may modulate arterial wall NO production in atherosclerotic lesions, and has been shown to suppress NO production and iNOS expression through inhibition of the NF-κB activation pathway in vascular smooth muscle cells, macrophages and monocytes (Hattori et al., 2001; Liu et al., 2001; Page et al.,1999). The effect of 4-HNE on this pathway in endothelial cells, and the potential for endothelial protection by GSTs, are still unexplored, however.
It is our underlying hypothesis that GSTs, and especially GSTA4-4, functions as a protective mechanism against oxidative stress such as occurs in the early atherosclerotic process. In this study, we sought to define the effects of 4-HNE on iNOS expression and the related NF-κB activation pathway in the endothelial cell. We also investigated whether overexpression of mGSTA4-4, a mouse homologue of GSTs in endothelial cells, can prevent the effects of 4-HNE on iNOS. To further explore the role of GSTA4-4, iNOS and 4-HNE during atherogenesis, human aortic atherosclerotic plaques in early stages, and adjacent normal vascular wall, were analyzed for iNOS and 4-HNE expression.
Materials and methods
Materials
Dulbecco’s modified eagle’s medium (DMEM), penicillin–streptomycin solution (p/s), HEPES, trypsin, hank’s balanced salt solution (HBSS) and fetal calf serum (FCS) were purchased from Gibco (Grand Island, NY). Antibodies to p65, IκB-α, phosphor-IκB-α, eNOS and iNOS; secondary polyclonal antibodies goat anti-rabbit, goad anti-mouse; NF-κB inhibitor and NF-κB control were purchased from Santa Cruz Biotechnology (Santa Cruz Biotechnology, Santa Cruz, CA). Phosphor-p65 (p-p65) antibody was purchased from Cell Signaling Technology, Inc (Danvers, MA). Anti-actin antibody was purchased from Sigma (Saint Louis, MO).
Cell culture
The MS1 cell line (ATCC, Manassas, VA) is an immortalized endothelial cell line originally isolated from pancreatic islet; we have previously characterized the cells in studies of oxidative stress in vitro (Yang et al., 2004). Cells were grown as an adhesive culture as previously described in DMEM containing 4.5 g/l glucose, with 4 mM L-glutamine adjusted to contain 3.7 g/l sodium bicarbonate, supplemented with 5% FCS and 1% (v/v) p/s at 37 °C in a humidified atmosphere of 10% CO2.
Transfection of mGSTA4
A plasmid pRC/CMV (a gift of Dr. P. Zimniak, University of Arkansas for Medical Sciences, Little Rock, AK) constructed with mGSTA4 cDNA (Zimniak et al., 1997). pRC/CMV-mGSTA4 or the vector alone was transfected into MS1 cells using liposome-based Lipofectin® reagent (GIBCO BRL Life Technologies, Grand Island, NY). Stable transfectants were isolated by selection in DMEM containing 3500 μg/ml G418 for approximately 2 weeks and maintained in DMEM containing 1750 μg/ml G418.
Western blot analysis
Cellular protein estimated by the method of Bradford (Bradford, 1976) was resolved by SDS-PAGE and was electrophoretically transferred onto PVDF membranes. 4–20% Tris–Glycine precast ready gels or 4–12% NuPage Bis–Tris gel (Invitrogen Life Tech, Carlsbad, CA) were used for SDS-PAGE. The blots were developed by SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL).
Purification of mGSTA4-4 and enzyme assay
Purification of total GSTs from MS1 cultured cells was performed by glutathione (GSH) affinity chromatography as described by Yang et al. (Yang et al., 2001) and GST activity toward 4-HNE was determined as described previously (Singhal et al., 1992). One unit of GST activity was defined as the amount of the enzyme catalyzing the conjugation of 1 μmol of 4-HNE with GSH per minute at 30 °C.
Detection of iNOS
Wild type (WT), vector-transfected (VT) or mGSTA4-transfected (mGSTA4) MS1 cells (106) were cultured on 100 mm dishes in 10 ml DMEM containing 5% FCS for 24 h. Cells were treated with 20 μM 4-HNE for 1 h or 2 h in serum free DMEM. Control cells were treated for 2 h with 1) medium containing serum or 2) serum-free medium containing same amount of solvent (containing 0.1% ethanol in original diluents) as used in 4-HNE exposures. In same experiment, cells were also treated with a range of concentrations (10–30 μM) of 4-HNE for 2 h; in this range and time period, 4-HNE caused no appreciable cytotoxicity or cell death in MS1 cells (Grune et al., 1994; Fazio et al., 1993; Yang et al., 2004). Control cells were treated as above but with serum-free medium only. Cells were harvested by scraping into cell lysis buffer containing 50 mM Tris–HCl (pH 7.5), 4 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, 50 mM Sodium Fluoride, 1:100 protease inhibitor cocktail (Sigma), PMSF 20 μg/ml, 10 μg/ml trypsin inhibitor, 1% NP-40, 1:100 phosphatase inhibitor cocktail (Sigma), and 150 mM NaCl. iNOS was detected by Western blot analysis.
Assay of NF-κB activation pathway
2×106 WT, VT/mGSTA4-transfected cells were cultured on 150 mm dishes in 25 ml DMEM containing 5% FCS for 24 h. Cells were treated with normal DMEM, serum free DMEM or 4-HNE 20 μM in serum free DMEM for 1 h or 2 h treatment. The protein of nuclear and cytosolic fractions were extracted by using hypotonic lysis buffer and nuclear extraction buffer included in a NF-κB p65 Active ELISA kit (Imgenex, San Diego, CA) (Kim and Stadtman, 1997). Western blot analysis for p65, IκB-α, and phosphor-IκB-α were performed.
Confocal laser microscopy of NF-κB (p65) translocation
WT and VT/mGSTA4-transfected cells (1×105) were cultured on Lab-Tek chambered coverglasses (Nalge Nunc International, NY, USA) in 1 ml DMEM containing 5% FCS for 24 h (approx. 70–80% confluence). Cells were treated with 20 μM 4-HNE for 2 h; control cells were treated with equivalent amounts of solvent in serum free DMEM. After thoroughly washing with PBS, the cells were fixed in 4% paraformaldehyde and 0.2% picric acid at 4 °C for 1 h. Nonspecific antibody binding sites were blocked with 10% normal goat serum (in PBS) with 1% BSA for 1 h at 37 °C. The cells were then incubated with rabbit polyclonal anti-p65 antibodies (Santa Cruz, CA, USA) at a final dilution of 1:50 at 4 °C overnight. After washing with PBS, the cells were incubated with 5 μg/ml goat anti-rabbit IgG FITC-conjugated antibodies at room temperature for 1 h, washed with PBS (5 min ×5), and incubated in DAPI (2 μg/ml; Molecular Probes, Eugene, OR) at 37 °C for 10 min. After a final wash with PBS (5 min×5), microscopic slides were evaluated by confocal microscopy using LSM 510 UV META inverted microscope (Carl Zeiss, Jena, Germany) with a 63× Plan Apo chromatic objective.
Nitrite assay
Nitrite accumulation, as an indicator of NO synthesis, was measured in the cell culture medium of confluent WT and VT/mGSTA4-transfected MS1 cells with a nitric oxide ( ) detection kit (Stressgen, British Columbia, Canada). Cells were grown on 24-well plates and treated with 20 μM 4-HNE for 0, 1 h or 2 h in serum free medium. Control cells (0) were treated with serum free medium containing equivalent amounts of solvent. Nitrite was quantified colorimetrically after adding Griess reagent to samples. Absorbance at 540 nm was determined by using Model 680 microplate reader (BIO-RAD, C A, USA). Nitrite concentrations were calculated by comparison with the absorbance of standard solutions of sodium nitrite.
Analysis of reactive oxygen species (ROS) by immunofluorescent microscopy
WT and VT/mGSTA4-transfected cells (1 ×105) were cultured on Lab-Tek chambered coverglasses (Nalge Nunc International, NY) in 1 ml DMEM containing 5% FCS for 24 h (70–80% confluence) and treated with 20 μM 4-HNE for 1 h or 2 h in serum free medium; controls were treated with equivalent amount of solvent. After washing with PBS three times at 5 min interval, 0.2 mM 2′,7′-Dichlorodihydrofluorescein diacetate (DCDHF) (ALEXIS Biochemicals, CA) in ethanol was added in the wells, and incubated at room temperature for 30 min, then washed in PBS for three times at 10 min interval. Fluorescence images were taken by LSM 510 UV META inverted microscope (Carl Zeiss, Jena, Germany) with excitation at 488 nm and long-pass detection at 530 nm.
Effect of NF-κB inhibitor on iNOS expression
A synthetic inhibitor of NF-κB which contains NLS residues 360–369 of p50 (sequence: AAVALLPAVLLALLAPVQRKRQKLMP) was used to block NF-κB active complex into the nucleus. An inactive NF-κB inhibitor (sequence: AAVALLPAVLLALLAPVQRDGQKLMP) was used as a negative control. VT/mGSTA4-transfected cells (2 ×106) were grown on 150 mm dishes in DMEM containing 5% FCS and incubated for 24 h in 37 °C or until 90% confluent. After changing to serum free medium, cells were treated with either NF-κB inhibitor or inactive control at 50 μg/ml in serum free DMEM and incubated for 3 h (37 °C). 20 μM 4-HNE was then added and cells were incubated for one h (37 °C). Control cells without 4-HNE treatment were left in serum free medium containing inhibitor or inactive control. Nuclear and cytosolic protein was extracted by using nuclear extraction buffer included in NF-κB p65 Active ELISA kit (Imgenex, San Diego, CA). Western blot analysis was performed to examine iNOS and p65 expression.
Immunohistochemical localization of 4-HNE and iNOS in human atherosclerotic plaques
Full-thickness segments of ascending aorta were procured fresh from 7 human donor hearts at the time of cardiac transplantation under an approved human subject protocol (IRB#99-395, University of Texas Medical Branch). The donors included 5 males and 2 females ranging in age from 17–54 years (mean: 33). Aortic segments were rapidly fixed in 10% neutral-buffered formalin solution and atherosclerotic plaques from 1–4 mm in greatest dimension identified grossly and processed with rare lipid streaks and randomly-sampled adjacent areas of normal-appearing aorta for routine light microscopy. Sections (4 μm) were stained with hematoxylin and eosin (H+E) and atherosclerotic plaques graded according to established criteria. (Stary et al., 1995) From 2 to 6 plaques were identified in each patient’s aortic sample, giving an approximate total of 32 plaques of varying ages examined. Lesions and adjacent sections of selected lesions were immunostained for 4-HNE and iNOS with goat anti-HNE antiserum (Alpha Diagnostic, San Antonio, TX), and rabbit anti-iNOS polyclonal antibodies (Santa Cruz Biotechnology., Santa Cruz, CA). ABC kit (Vectors, Burlingame, CA) and diaminobenzidine immunohistochemical methods were used (Yang et al., 2004); negative controls consisted of omission of primary antibody and use of pre-immune serum as primary antibody.
Statistical analysis
Values are reported as either mean±S.D. or mean±S.E. Means were compared using one way ANOVA for three groups or Student’s paired t-test for two groups (Sigma Stat, Jandel Scientific Software, San Rafaelm CA). Statistical significance was assumed at p<0.05.
Results
Overexpression of mGSTA4-4 in MS1 endothelial cells prevents effect of 4-HNE on iNOS and NO production stimulated by serum withdrawal
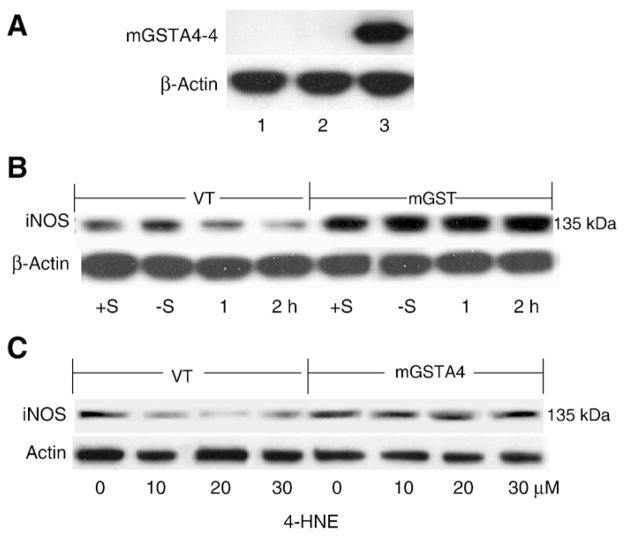
The overexpression of mGSTA4-4 activity in mGSTA4-transfected cells was confirmed by Western blot analysis and enzyme assay (represented as one of three separate experiments). As shown in Fig. 1A, mGSTA4-4 expression was clearly detectable in mGSTA4-transfected cells, but undetectable in WT or VT-transfected MS1 endothelial cells, consistent with GST activity assay, in which the total GST activity of mGSTA4-transfected cells (16.2±3.5 U/min mg of cell protein) was approximately four fold higher toward 4-HNE compared to WT (4.1±0.65 U/min mg of cell protein) or VT (4.3±0.95 U/min mg of cell protein), p<0.05.
Fig. 1.
mGSTA4-4 Expression and iNOS upregulation in mGSTA4-transfected MS1 cells. Panel A: mGSTA4-4 expression by Western blot analysis. 50 μg cell lysates were loaded on 4–12% SDS-PAGE. The primary polyclonal rabbit-anti-mGSTA4-4 antibodies were applied at 1:4000 dilutions, the secondary goat-anti-rabbit antibodies were diluted 1:20,000. The blots were developed by SuperSignal West Pico Chemiluminescent Substrate. Lanes 1, 2, 3 represent the WT, VT-transfected and mGSTA4-transfected cells, respectively. Panel B: iNOS expression at 1 h or 2 h after 4-HNE (20 μM). Two controls, +S and −S, indicate plus or minus serum in DMEM without 4-HNE treatment. Panel C: iNOS expression in cells treated with different concentrations of 4-HNE in serum free DMEM for 2 h. “0” indicates controls in serum free DMEM containing equivalent solvent (2 h). For Western blot analysis, primary antibody of rabbit anti-iNOS was diluted at 1:100, and secondary goat-anti-rabbit was used at 1:10,000 dilutions in both panels. Protein was loaded at 25–50 μg. All figures are representative of three reproducible results.
Previous studies have suggested that serum withdrawal activates iNOS and NO formation in macrophages and that this effect is inhibited by 4-HNE. (Liu et al., 2001) To investigate the role of 4-HNE in this process, and the potential protective effect of mGSTA4-4, we stimulated cultured WT and VT/mGSTA4-transfected MS1 cells in serum free medium and treated with 20 μM 4-HNE for different times, or with different concentrations of 4-HNE for a fixed period of time (2 h). Consistent with previous studies iNOS was inhibited by 4-HNE in WT and VT cells, but the inhibitory effect of 4-HNE was attenuated in mGSTA4-transfected cells (Figs. 1B and C) (represented as one of four reproducible data) However this effect of 4-HNE on iNOS expression in control MS1 cells lasted only 2 h and decreased at 4 h after treatment (data not shown).
Because eNOS-derived NO plays a major role in maintaining normal vascular tone in endothelial cells, we wanted to address whether its level was affected by 4-HNE treatment and serum starvation and contributed to NO production under this condition. To this end, Western blot of eNOS expression was carried out. No difference was observed in its expression between cell lines at any time or following the same exposures and manipulations performed for iNOS in this study (eNOS data not shown).
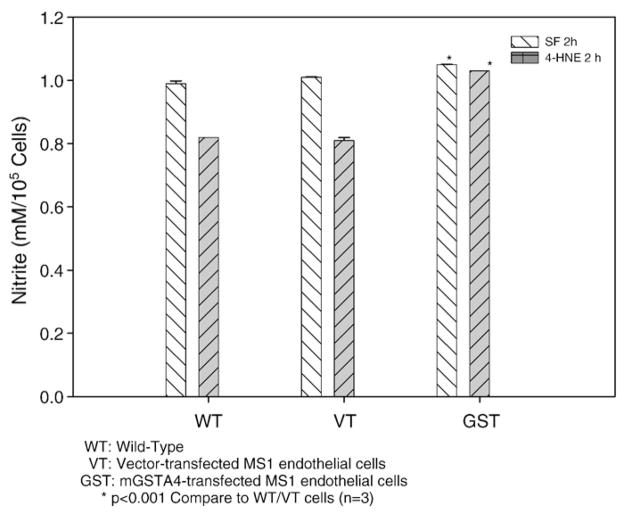
To determine the effect of 4-HNE on serum withdrawal induced nitrite production as an indicator of NO level and the role of GST, we measured nitrite accumulation in the culture medium under various conditions (represented as three reproducible data). As shown in Fig. 2, mGSTA4-transfected cell showed higher nitrite levels as an indicator of NO production when compared to WT or VT-transfected cells (p<0.001, n=3) in serum free medium with or without 4-HNE treatment. No significant difference was observed between WT and VT cells. Nitrite production was obviously inhibited by 4-HNE treatment at 2 h (versus no treatment) in WT or VT cells (p<0.001), but this pattern was not shown in mGSTA4-transfected cells. Taken together these results indicate that 4-HNE inhibits stimulation of NO in serum starved cells and that overexpression of mGSTA4-4 alleviates this effect, most likely by metabolizing 4-HNE, as shown in previous studies (Yang et al., 2004, 2003). These results correlate with the cellular expression of iNOS (Figs. 1B, C). Other genes related to the NF-κB pathway might be involved, and cannot be excluded by this study; furthermore, it has been suggested that 4-HNE serves as a crucial signaling molecule by modulating gene expression {Patrick et al., 2005 96/id}.
Fig. 2.
Effect of mGSTA4 transfection on nitrite production triggered by serum depletion and 4-HNE treatment. Aliquots of cells from WT, VT/mGSTA4-transfected MS1 cells were incubated at a density of 1×105 cells/ml DMEM in 24-well plate until near confluent. Nitrite production was measured in these cells in serum free medium without 4-HNE treatment and after 2 h treatment with 20 μM 4-HNE in serum free medium. The values are the mean±S.E. of three determinations and are representative of three separate experiments. *p<0.001 compared to the value as shown in WT or VT-transfected cells.
mGSTA4 transfection activates NF-κB p65 translocation which is inhibited by 4-HNE
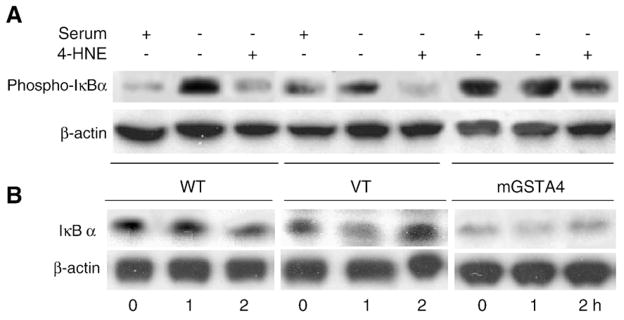
Ample evidence exists that iNOS is modulated by NF-κB and its related pathways (Agusti et al., 2004; Hattori et al., 2001; Liu et al., 2001; Morris et al., 2003;Yan et al., 1999; Connelly et al., 2001). To determine whether NF-κB is involved in the signaling of 4-HNE and mGSTA4-4 regulated iNOS expression, we examined the effect of serum withdrawal, 4-HNE treatment, and mGSTA4 transfection on NF-κB (p65) and its nuclear translocation assessed by Western blot analysis and confocal laser microscopy. Data shown (Fig. 3A) represent one of three reproducible experiments. Overall, mGSTA4-transfected cells showed much higher amounts of p65 translocation into nucleus upon addition of serum, serum withdrawal, or 2 h after treatment with 4-HNE. Markedly greater induction of nuclear phosphor-p65 was observed in mGSTA4-transfected cells, including after 4-HNE treatment, when compared to VT-transfected cells. This consistently greater cytoplasmic-to-nuclear translocation and nuclear phosphor-p65, even following exposure to 4-HNE, suggests that the protective effect of GSTA4-4 transfection (and changes shown in iNOS and NO) are at least partially mediated through the NF-κB signaling pathway, and perhaps through cellular 4-HNE levels. In addition, confocal immunofluorescent assay confirmed increased translocation of p65 into nucleus in mGSTA4-transfected cells in serum free medium without 4-HNE or 2 h after 4-HNE treatment when compared to WT or VT-transfected cells (Fig. 3B).
Fig. 3.
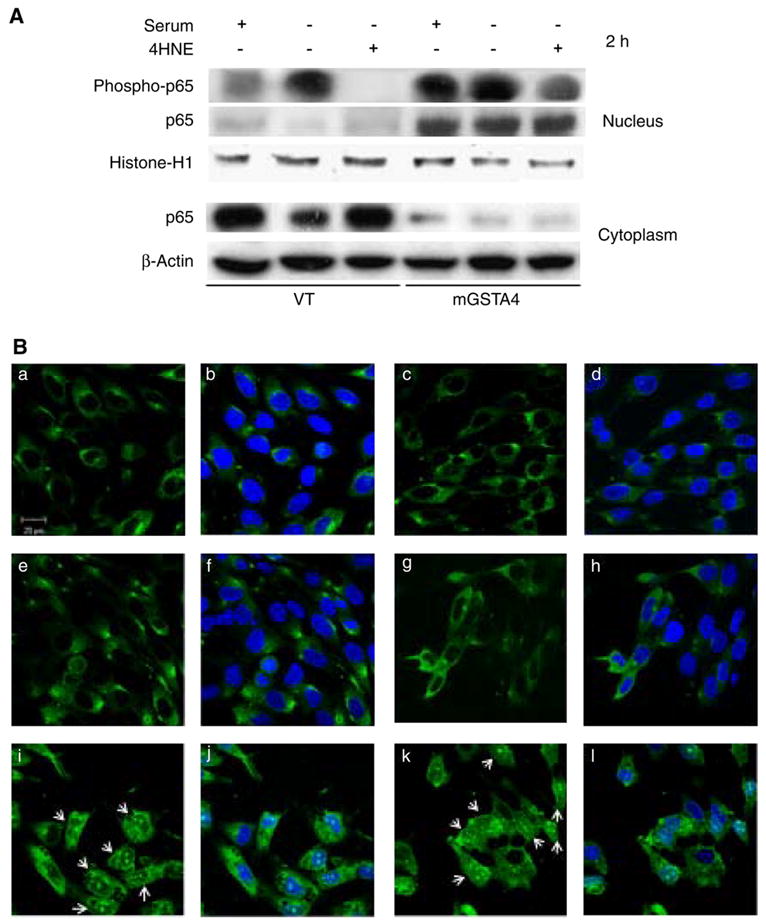
Effect of mGSTA4 Transfection on activation and translocation of NF-κB. Panel A: Western blot analysis of NF-κB (p65) and phosphorylated p65 (p-p65) in nuclear and cytoplasmic proteins. Cells were treated with 20 μM 4-HNE for 1 h or 2 h, control cells were treated with serum free medium without 4-HNE, nuclear and cytoplasmic proteins were extracted from total cell pellets as described in Materials and methods section. 50 μg proteins was loaded in each lane and after electrophoresis probed with mouse monoclonal anti-p65 antibody at 1:200 and rabbit polyclonal anti-p-p65 at 1:100. Polyclonal mouse anti-histone antibody (1:1000) was used to reprobe the membrane as loading control of nuclear protein, polyclonal rabbit anti-actin antibody (1:5000) was used as loading control of cytoplasmic protein. Panel B: Confocal laser microscopic analysis of p65 translocation. The upper (a, b, c, d), middle (e, f, g, h) and lower (i, j, k, l) rows are WT, VT and mGSTA4-transfected cells, respectively. The left two columns (a, b, e, f, i, j) are cells treated with serum free DMEM for 2 h, the right two columns (c, d, g, h, k, l) are cells treated with 20 μM 4-HNE in serum free DMEM for 2 h. The green fluorescent images are confocal immunolocalization of p65 stained with rabbit anti-p65 polyclonal antibodies at 1:50 and FITC-conjugated goat-anti-rabbit IgG at 5 μg/ml. The rest of blue staining images made with DAPI and overlapped with fluorescent images. The arrows indicate NF-κB (p65) translocated into nucleus in mGSTA4-transfected cells both in 4-HNE treated and serum free conditions. Confocal images were taken with a LSM 510 UV META inverted microscope (Carl Zeiss, Jena, Germany) with 63× Plan Apo chromatic objective; excitation was at 488 nm to view green fluorescence, and 460 nm to view blue DAPI image. All figures are representative of three separate experiments with similar results.
mGSTA4 transfection activates p-IκB-α which are modulated by serum withdrawal and 4-HNE
Inactive NF-κB is located in the cytosol, bound to its inhibitory protein, IκB. Dissociation of NF-κB from IκB is a critical step in NF-κB activation that leads to translocation of NF-κB to the nucleus, enabling DNA binding and transactivation (Gilmore, 1999; Hayden and Ghosh, 2004). The key event for NF-κB activation is phosphorylation of two serine residues at the N terminus of IκB (Ser 32 and Ser 36 for IkB-a) by IκB kinase (IKK) (Larson et al., 1999). To determine whether the effect of mGSTA4-4 modulated activation of NF-κB was through phosphorylation of IκB-α, we examined the expression of IκB-α and phosphor-IκB-α (p-IκB-α) in cytoplasmic fraction by Western blot analysis in the WT and VT/mGSTA4-transfected MS1 cells (represented as one of three separate experiments). As shown in Fig. 4A, mGSTA4 transfection activated IκB-α phosphorylation in cytoplasm, whereas this induction was inhibited by the addition of 4-HNE (20 μM) in WT and VT cells, 4-HNE had little apparent effect in mGSTA4-transfected cells. Results presented in Fig. 4B showed that degradation of IκB-α in mGSTA4-transfected cells is enhanced compared with that in WT or VT cell.
Fig. 4.
Effect of mGSTA4-transfection on IκB-α degradation and phosphorylation. Panel A: Phosphorylation of IκB-α in cytoplasm regulated by serum free and 4-HNE. Cells were treated with normal or minus serum DMEM (+/−S) and 20 μM 4-HNE for 2 h. 50 μg protein from cytoplasm were loaded into 4–12% SDS-PAGE. Monoclonal mouse-anti-phospho-IκB-α was used as primary antibodies at 1:100 dilutions. Panel B: IκB-α degradation by mGSTA4-4. Cells were treated with 20 μM 4-HNE in serum free medium for 1 h or 2 h, control cells were treated with serum free medium containing equivalent amount of solvent for 2 h. 50 μg total cell lysates were resolved into 4–12% NuPage SDS-PAGE. Monoclonal mouse-anti-IκB-α as primary antibodies were used at 1:100 dilutions. Secondary goat-anti-mouse polyclonal antibodies were used for both at 1:10,000 dilutions. WT: wild-type; VT: vector-transfected cells; mGSTA4: mGSTA4-transfected cells. These data are representative of three reproducible experiments.
Upregulation of iNOS by mGSTA4-4 is inhibited by NF-κB inhibitor
To further confirm that upregulation of iNOS by mGSTA transfection occurs through the NF-κB activation pathway, we used an NF-κB inhibitor (a synthetic inhibitor for NF-κB which contains NLS residues 360–369 of p50) to block the induction of NF-κB in VT and mGSTA4-transfected cells, and examined the expression of iNOS in the cytoplasm and p65 in nucleus by Western blot analysis. Data shown represent three reproducible experiments. The results of these experiments showed that in VTcells, in the absence of 4-HNE, NF-κB inhibitor did not significantly downregulate the expression of iNOS (Figs. 5A and B) although it clearly downregulated p65 (Fig. 5A, p<0.05). In mGSTA4-transfected cells not treated with 4-HNE, however, there was a dramatic inhibition of iNOS (p<0.05) when compared to the appropriate control. In mGSTA4-transfected cells treated with 4-HNE, a non-significant, but slight apparent reduction was seen in iNOS (Figs. 5A and B).
Fig. 5.
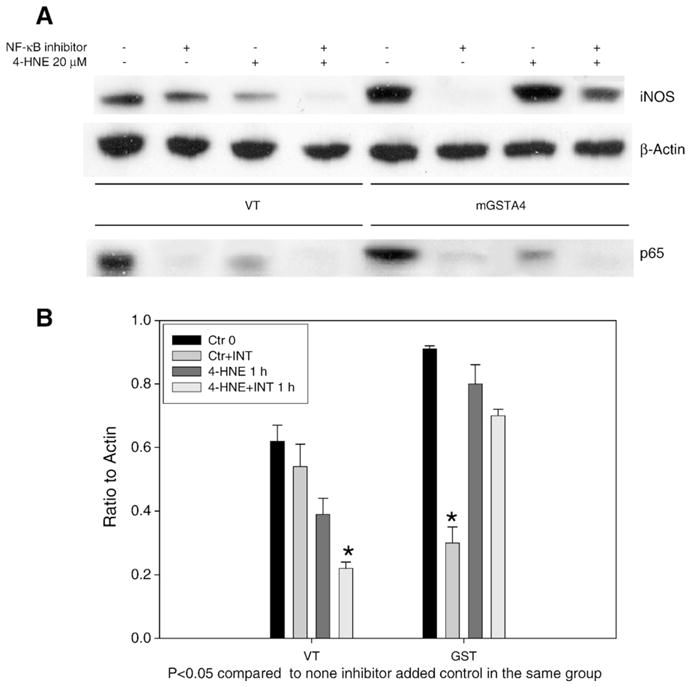
Effects of NF-κB inhibitor on iNOS and p65 expression. Panel A: Western blot of iNOS and p65 expression in presence of NF-κB inhibitor. VT/mGSTA4-transfected MS1 cells were grown in 100 mm dishes. Cells were treated with NF-κB inhibitor for 3 h, and then 20 μM 4-HNE for 1 h. Symbols (−/+) indicate minus or plus 4-HNE or NF-κB inhibitor (50 μg/ml). Cytoplasmic and nuclear protein were extracted as described in section 2 and loaded into SDS-PAGE for iNOS and p65 expression, respectively. Primary antibodies of rabbit anti-iNOS were diluted at 1:100, primary mouse monoclonal anti-p65 antibodies were used at 1:200. Panel B: The quantification of the Western blots of iNOS. Ratios of iNOS band density to β-actin as control are shown. Experiments were repeated three times, data shown in panel B represents the mean±SE of triplicate samples. Asterisk indicates p <0.05 when compared to matched cells without inhibitor treatment.
In these experiments, p65 was markedly inhibited by NF-κB inhibitor in both 4-HNE-treated and non-treated cells (Figs. 5A and B). These results imply that other pathways may also be involved in the mechanism of iNOS upregulation.
Inhibition of ROS formation by mGSTA4-transfection
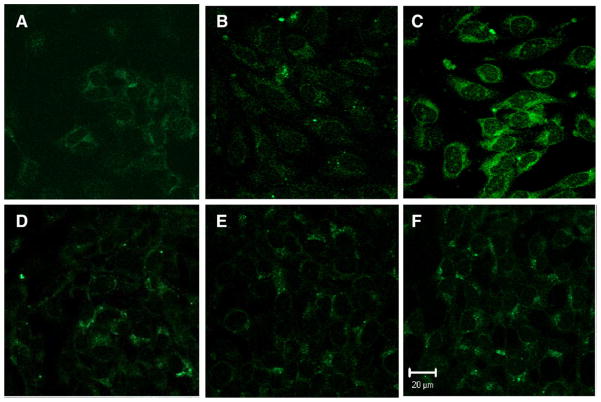
Because NF-κB regulates iNOS and the derived NO has pro- or anti-inflammatory action on vascular wall (Connelly et al., 2001; Naseem, 2005; Yogo et al., 2000), to determine if the upregulation of iNOS and NO in endothelial cells by overexpression of mGSTA4-4 has a vascular protective effect, we measured ROS formation including peroxynitrite (ONOO−), a main oxidant produced by NO reaction with superoxide (O−) and hydrogen peroxide (H2O2). For this purpose, we used DCDHF, a cell permeable, sensitive indicator of ROS formation. DCDHF can be directly oxidized by peroxynitrite, or by H2O2 in the present of peroxidase, to a highly fluorescent product dichlorofluorescein (DCF) (Ischiropoulos et al., 1999). Because this method is not specific for substrate, however, and because of the rapid interconversion of ROS, data are best described as an indication of ROS production (Anrather et al., 2006). Our results (Fig. 6) show that mGSTA4-transfected cells produce much less ROS when compared to the vector-transfected cells following serum withdrawal alone, or after 4-HNE treatment in serum-free medium (1 h or 2 h). These results indicate that upregulation of iNOS and NO by overexpression of mGSTA4-4 in endothelial cells play a protective role against oxidative stress. In addition, even in the face of increased nitrite generation as a indicator of NO, mGSTA4-4 is capable of reducing ROS formation, probably by reducing overall lipid peroxidation, intracellular oxidants, and resulting peroxidative damage through metabolizing, i.e., detoxifying, 4-HNE.
Fig. 6.
ROS formation was attenuated by overexpression of mGSTA4-4 in endothelial cells. VT/mGSTA4-transfected MS1 cells (1×105) were cultured into Lab-Tek chambered coverglass (Nalge Nunc International, NY, USA) until near confluent. Confocal images were taken with a LSM 510 UV META inverted microscope (Carl Zeiss, Jena, Germany) with 63× Plan Apo chromatic objectives. The upper panel and lower panel are VT-transfected and mGSTA4-transfected endothelial cells, respectively. A and D are control cells without 4-HNE treatment; B and E show 4-HNE (20 μM) treatment for 2 h; C and F show 4-HNE treatment for 4 h. DCDHF-DA (ALEXIS Biochemicals, CA) was used to detect the ROS, including H2O2 and peroxynitrite, formation. The results are representative of two reproducible experiments.
4-HNE and iNOS immunolocalization in early and late human atherosclerotic plaques
In aortic sections from the two youngest individuals (age 17 and 19 years) multiple lipid streaks (stage 2 plaque; foam cells only (Stary et al., 1995)) but few plaques consistent with stage 3 were observed; no stage 4 or greater plaques were seen. In aortic sections of the five other patients (ages 23–54) lipid streaks as well as atherosclerotic “fibrous” plaques (stage 3; two to three mm in greatest dimension; plentiful foams cells; proliferating vascular smooth muscle cells) and stage 4 (rare cholesterol clefts, small necrotic core) were observed; a total of approx. 30 plaques of grades 3–4 were studied. No overtly advanced (grades 5–6) or complicated (exhibiting intra-plaque hemorrhage, plaque fissuring, mural thrombosis or calcification) plaques were found.
In early fibrous and fibro-fatty stage 3 atherosclerotic plaques (Figs. 7A and B) iNOS immunochemical staining was increased in plaque cells including foam cells and proliferating vascular smooth muscle cells, and in endothelial cells overlying the plaque. Deep to the internal elastic lamina iNOS staining was not evident and fatty streaks showed no or minimal iNOS staining (Fig. 7C). Early plaques showed no staining for 4-HNE (Fig. 7D). In areas adjacent to plaque, even with a few millimeters distance, essentially no staining for either iNOS or 4-HNE were evident (Figs. 7E and F).
Fig. 7.
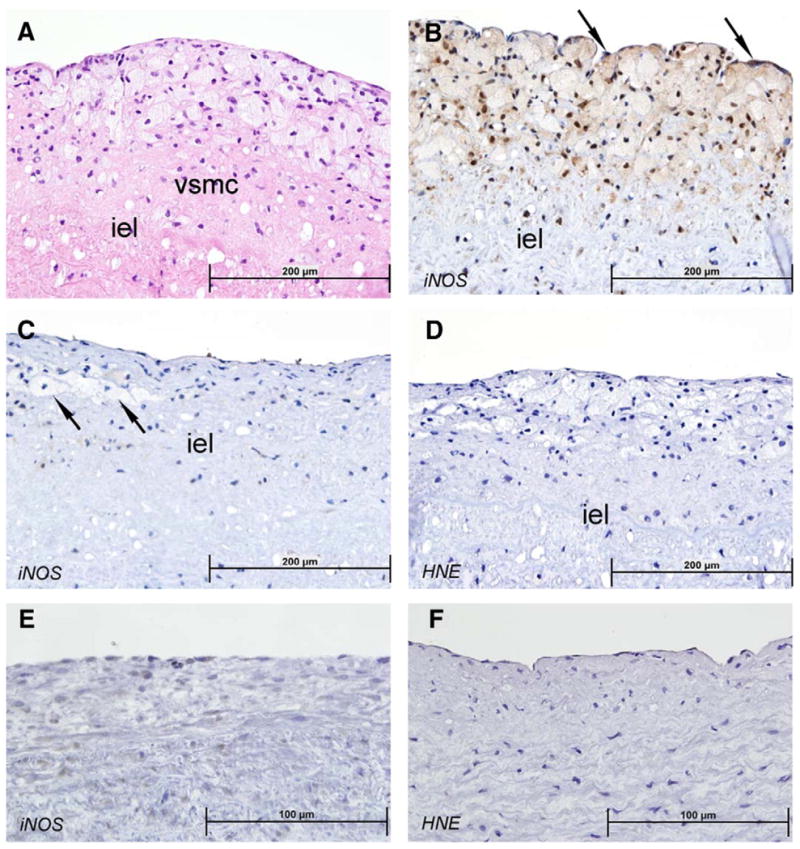
Earliest human atherosclerotic fibro-fatty plaques and lipid streaks; iNOS and 4-HNE staining. Hematoxylin and eosin stain of aortic arch from 23 year old male (A) shows stage 3 fibro-fatty plaque above internal elastin lamina (iel); proliferating vascular smooth muscle cells (VSMC) and infiltrating inflammatory macrophages (foam cells) are evident. Immunostain for iNOS of similar stage 3 plaque (B) shows marked positivity in inflammatory cells, proliferating vascular smooth muscle cells and endothelial cells overlying the plaque (arrow). Stage 2 atherosclerotic plaque, or lipid streak (C) from 19 year old male shows no staining for iNOS in foam cells (arrows). Immunostain for 4-HNE (D) shows minimal staining in stage 3 fibro-fatty plaque similar to those shown in A and B. Normal aortic wall at slightly higher power (E+F) in area approx 1–2 mm from a stage 3 plaque shows essentially negative staining for iNOS (E) and 4-HNE (F) in endothelial cells, subendothelial area, or VSMC. Sections (4 μm) were immunostained for 4-HNE and iNOS with goat anti-HNE antiserum (Alpha Diagnostic, San Antonio, TX), and rabbit anti-iNOS polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). See Methods for details.
In late fibrofatty plaques and plaques with necrotic cores (Fig. 8) iNOS staining was again noted in foam cells and proliferating vascular smooth muscle cells but not in extracellular matrix or necrotic core. iNOS staining was also found in adjacent vascular smooth muscle cells underlying plaque. Immunochemical staining for 4-HNE was heavy in cells comprising late plaques and was evident in endothelial cells overlying plaques but not in endothelial cells in areas adjacent to plaque. Patchy, weaker staining for 4-HNE was also noted in necrotic core (Fig. 8D).
Fig. 8.
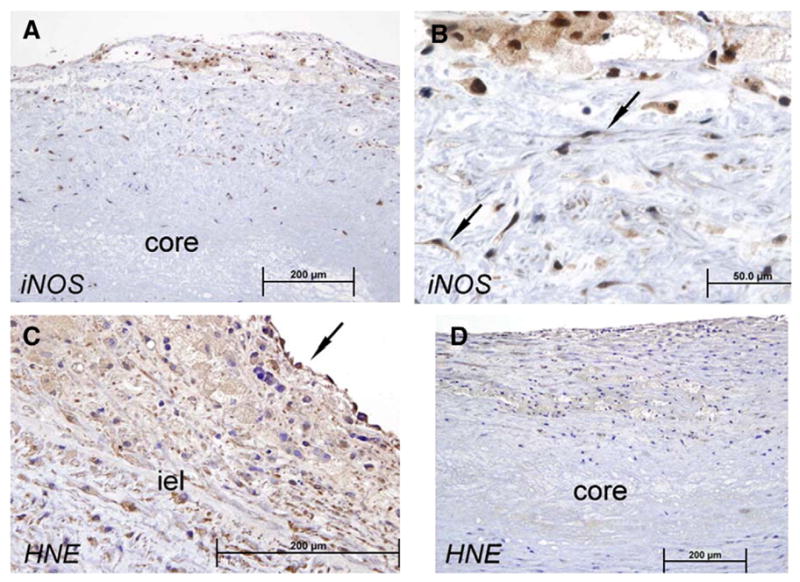
Later stage human atherosclerotic fibro-fatty plaques; iNOS and 4-HNE staining. More advanced stage 3 and 4 plaques from 47 year old woman and 54 year old man show larger intense staining for iNOS (A, B) evident in cells of plaque including macrophages (foam cells) and proliferating vascular smooth muscle cells (B, arrow). 4-HNE also stains intensely in plaque cells and in vascular smooth muscle cells underlying plaque (C); note faint staining in acellular necrotic core of more advanced stage 4 plaque (D). Sections (4 μm) were immunostained for 4-HNE and iNOS with goat anti-HNE antiserum (Alpha Diagnostic, San Antonio, TX), and rabbit anti-iNOS polyclonal antibodies (Santa Cruz, CA). See Methods for details.
Discussion
The modern theory of atherogenesis, or the “response to injury hypothesis” (Ross, 1999), states that atherosclerosis is a response to an insult upon the arterial wall, especially the endothelium. Recently, atherosclerosis has come to be recognized as active and inflammatory, rather than simply a passive process of lipid infiltration or a reactive process following injury (Langheinrich and Bohle, 2005). Endothelial cell damage and dysfunction induced by ROS are thought to represent an important part of the process of atherosclerotic lesion formation. Based on these assumptions, a great deal of both basic and clinical study has focused on the therapeutic possibility of altering ROS or other forms of oxidative stress in the vascular wall. Unfortunately, the general consensus to date is that no anti-oxidant therapy exists which has been shown to have a beneficial effect on the progression of atherosclerotic plaque, or the visceral sequelae of atherosclerotic occlusive vascular disease.
A group of immunologically related α-class mammalian GSTs which utilize 4-HNE as their preferred substrate has been proposed to be a major cellular defense system against oxidative injury by products of lipid peroxidation. This group of GSTs has been of special interest to our laboratory because of their possible role in protection against oxidative stress induced injury of cardiovascular tissues. Recent studies from this and other laboratories have shown that the GST isozyme known as GSTA4-4 plays an important role in protection of endothelial cells (Yang et al., 2004) and vascular smooth muscle cells (He et al., 1999) against 4-HNE induced oxidative stress and apoptosis; hence, in the present study we elucidated the effect of GSTA4-4 on NO and iNOS and showed that their upregulation is signaled through NF-κB (p65) nuclear translocation.
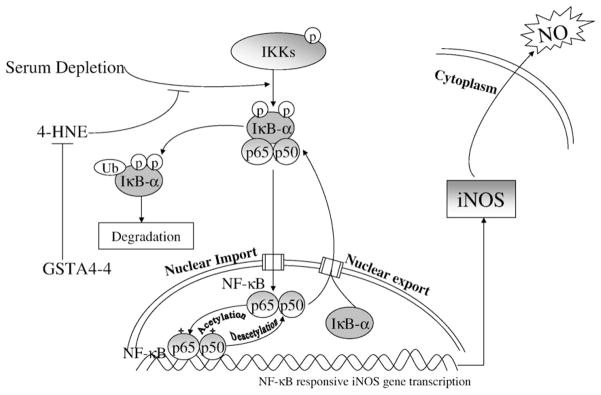
Our data also showed that serum withdrawal alone induces iNOS. This effect of serum withdrawal may reflect the response of endothelial cells to a mild degree of extracellular stress, thus maintaining cell homeostasis, as shown previously by Liu et al. in macrophages (Liu et al., 2001). Furthermore, we found that NO production in serum-stressed cells is attenuated by 4-HNE in WT and VT-transfected cells, but in cells overexpressing GSTA4-4 the effect of 4-HNE on iNOS and NO production was prevented, indicating a role for GSTA4-4 in maintaining homeostasis of NO in the endothelial cell. The proposed mechanism of GSTs and 4-HNE on iNOS, NO through NF-κB transduction pathway is shown diagrammatically in Fig. 9.
Fig. 9.
Proposed effect of GSTA4-4 on 4-HNE and serum depletion in NF-κB signal transduction pathway. The solid arrow → indicate a positive effect or induction on the downstream pathway, the symbol ⊣ indicates suppressive effect. The central role of GSTA4-4 mediated through NF-κB pathway is by conjugation of 4-HNE, adapted from EMD Chemicals, Inc. website.
It is generally assumed that the major NOS isotype responsible for the improvement of endothelial function during atherogenesis is the constitutively expressed eNOS. However, under chronic proinflammatory conditions, iNOS isotype is also expressed in endothelial and other cell types (Wilcox et al., 1997). Expression of iNOS has often been assumed to play a toxic role associated with local tissue destruction, for example, during endothelial dysfunction in chronic inflammatory conditions (Steiner et al., 1997; Buttery et al., 1996). Other data suggest a protective role of iNOS derived NO against oxidative stress in endothelium (Hemmrich et al., 2003). In vivo studies indicate that eNOS and iNOS have different vasculoprotective actions against vascular lesion formation: NO derived from eNOS inhibits neointimal formation, whereas NO derived from iNOS suppresses the development of constrictive remodeling (Yogo et al., 2000). Antioxidants, such as SOD, catalase and glutathione can affect the bioavailability of NO (Naseem, 2005; Patel et al., 2000), but the influence of antioxidants on NO-related cell signaling is unclear.
Our studies suggest that GSTs participate in iNOS and NO regulation through the well described NF-κB signaling pathway. Inducible transcription factors regulate immediate and long-lived cellular responses necessary for an organism’s adaptation to the environment. Such responses are mediated to a large degree through changes in gene expression. One transcription factor that serves as a key responder to changes in the environment is NF-κB, an evolutionarily conserved signaling module that plays a critical role in many biological processes (Hayden and Ghosh, 2004), and which has both pro-apoptotic and anti-apoptotic effects(Gilmore, 1999). 4-HNE has been shown to modulate the NF-κB-signaling pathway, which plays an important role in gene regulation during inflammatory and immune responses. Page, et al (Page et al., 1999) have shown that 4-HNE prevents NF-κB activation and tumor necrosis factor expression by inhibiting IκB phosphorylation. In later studies, Ji, et al. (Ji et al., 2001) and Donath, et al. (Donath et al., 2002) demonstrated that HNE inhibits IκB kinase and subsequent IκB degradation, which prevents NF-κB activation and nuclear localization. Hattori, et al has shown that 4-HNE prevents NO production in vascular smooth muscle cells by inhibiting NF-κB-dependent transcriptional activation of iNOS induced by LPS and IFN-γ (Hattori et al., 2001). Liu et al. (Liu et al., 2001) showed that 4-HNE inhibits iNOS and NO synthesis through selective suppression of IκB phosphorylation, independent of p38 kinase. On the other hand, results from other investigators (Ruef et al., 2001; Herbst et al., 1999) have shown conflicting effects of 4-HNE on NF-κB. We presume that the concentration or the time course of 4-HNE treatment, and serum conditions in the medium might be factors that result in variability in such observations.
Atherosclerosis continues to be the number one cause of death in industrialized nations today. The atherosclerotic process has been linked to smoking, environmental smoke exposure, and exposure to air pollution, especially with regard to particulate matter. In the present study, we examined early human atherosclerotic plaques obtained from the aortas of young heart-transplant donors in an attempt to establish the relevance of our experimental cellular findings to this important human disease. Our previous study of early-stage atherosclerotic plaques (Yang et al., 2004) showed that GST A4-4 was markedly upregulated during early (stages 2–4) plaque formation in endothelial cells overlying plaque, whereas GST A4-4 protein was not demonstrable in normal endothelial cells. Vascular smooth muscle cells proliferating in the early plaque also expressed high GSTA4-4, as did vascular smooth muscle immediately underlying plaque, further supporting the concept that GSTs respond to oxidative stress in the early inflammatory and proliferative stage of atherogenesis. These findings are consistent with our previous studies of experimentally induced atherosclerotic-like lesions (He et al., 1999; Misra et al., 1995).
In the present study, the expression of iNOS in multiple cell types in the very earliest stage of plaque intracellular, the lipid streak (stage 2) of, suggests that GST A4-4 may play a defensive role during oxidative stress by lowering intracellular aldehyde content (specifically, 4-NHE). It is of interest that the cells of the earliest plaque did not express 4-HNE immunohistochemically, suggesting that excessive aldehydes (such as 4-HNE), or aldehyde adducts, do not accumulate until the later, more advanced plaque (Fig. 9.) when detoxification and exocytotic mechanisms are overwhelmed. Furthermore, 4-HNE was found in small pools of necrotic core, where it might be expected to accumulate.
In summary, we demonstrate for the first time an important effect of GSTs on upregulation of iNOS and NO production by activating phosphorylation of IκB-α, and, thus, the translocation of NF-κB into nucleus. We suggest that NO derived from iNOS, which is upregulated through NF-κB activation in endothelial cells, may play a protective role in the earlier stages of the atherosclerotic process. Our present data also indicate that 4-HNE inhibits iNOS and NO production by downregulating the NF-κB translocation pathway, resulting in increased ROS-mediated oxidative damage, perhaps mediated through peroxynitrite, which may contribute to chronic inflammation in the endothelium. In our experiments, mGSTA4-4 abrogates these adverse effects on isolated endothelial cells, presumably by depleting 4-HNE as well as by other, unknown means of reducing oxidative stress. Thus, the GSTs provide protection against oxidative stress in vitro, and are likely to be an important defense mechanism against oxidants that act as atherogens. We suggest that manipulations of these intracellular defenses could be a potential therapeutic target in future clinical attempts to moderate the progression of atherosclerosis. Further studies are needed, however, to completely elucidate the role of iNOS, 4-HNE and GSTs in different stages of atherosclerotic plaque development in both experimental models and human atherosclerosis.
Acknowledgments
This work was supported by NIH grants HL 65416 to PJB, ES-012171 and IES 00676 to YCA, and NIEHS Center Grant ES06676. The authors thank Eugene P Knutson for assistance with confocal immunofluorescent staining and imaging.
References
- Agusti A, Morla M, Sauleda J, Saus C, Busquets X. NF-kappaB activation and iNOS upregulation in skeletal muscle of patients with COPD and low body weight. Thorax. 2004;59:483–487. doi: 10.1136/thx.2003.017640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anrather J, Racchumi G, Iadecola C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J Biol Chem. 2006;281:5657–5667. doi: 10.1074/jbc.M506172200. [DOI] [PubMed] [Google Scholar]
- Awasthi YC, Sharma R, Cheng JZ, Yang Y, Sharma A, Singhal SS, Awasthi S. Role of 4-hydroxynonenal in stress-mediated apoptosis signaling. Mol Aspects Med. 2003;24:219–230. doi: 10.1016/s0098-2997(03)00017-7. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buttery LD, Springall DR, Chester AH, Evans TJ, Standfield EN, Parums DV, Yacoub MH, Polak JM. Inducible nitric oxide synthase is present within human atherosclerotic lesions and promotes the formation and activity of peroxynitrite. Lab Invest. 1996;75:77–85. [PubMed] [Google Scholar]
- Connelly L, Palacios-Callender M, Ameixa C, Moncada S, Hobbs AJ. Biphasic regulation of NF-kappa B activity underlies the pro- and anti-inflammatory actions of nitric oxide. J Immunol. 2001;166:3873–3881. doi: 10.4049/jimmunol.166.6.3873. [DOI] [PubMed] [Google Scholar]
- Donath B, Fischer C, Page S, Prebeck S, Jilg N, Weber M, da CC, Neumeier D, Miethke T, Brand K. Chlamydia pneumoniae activates IKK/I kappa B-mediated signaling, which is inhibited by 4-HNE and following primary exposure. Atherosclerosis. 2002;165:79–88. doi: 10.1016/s0021-9150(02)00198-3. [DOI] [PubMed] [Google Scholar]
- Fazio VM, Rinaldi M, Ciafre S, Barrera G, Farace MG. Control of neoplastic cell proliferation and differentiation by restoration of 4-hydroxynonenal physiological concentrations. Mol Aspects Med. 1993;14:217–228. doi: 10.1016/0098-2997(93)90008-2. [DOI] [PubMed] [Google Scholar]
- Gilmore TD. The Rel/NF-kappaB signal transduction pathway: introduction. Oncogene. 1999;18:6842–6844. doi: 10.1038/sj.onc.1203237. [DOI] [PubMed] [Google Scholar]
- Grune T, Siems WG, Zollner H, Esterbauer H. Metabolism of 4-hydroxynonenal, a cytotoxic lipid peroxidation product, in Ehrlich mouse ascites cells at different proliferation stages. Cancer Res. 1994;54:5231–5235. [PubMed] [Google Scholar]
- Hattori Y, Hattori S, Kasai K. 4-hydroxynonenal prevents NO production in vascular smooth muscle cells by inhibiting nuclear factor-kappaB-dependent transcriptional activation of inducible NO synthase. Arterioscler Thromb Vasc Biol. 2001;21:1179–1183. doi: 10.1161/hq0701.092135. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- He N, Singhal SS, Awasthi S, Zhao T, Boor PJ. Role of glutathione S-transferase 8-8 in allylamine resistance of vascular smooth muscle cells in vitro. Toxicol Appl Pharmacol. 1999;158:177–185. doi: 10.1006/taap.1999.8700. [DOI] [PubMed] [Google Scholar]
- He NG, Awasthi S, Singhal SS, Trent MB, Boor PJ. The role of glutathione S-transferases as a defense against reactive electrophiles in the blood vessel wall. Toxicol Appl Pharmacol. 1998;152:83–89. doi: 10.1006/taap.1998.8511. [DOI] [PubMed] [Google Scholar]
- Hemmrich K, Suschek CV, Lerzynski G, Kolb-Bachofen V. iNOS activity is essential for endothelial stress gene expression protecting against oxidative damage. J Appl Physiol. 2003;95:1937–1946. doi: 10.1152/japplphysiol.00419.2003. [DOI] [PubMed] [Google Scholar]
- Herbst U, Toborek M, Kaiser S, Mattson MP, Hennig B. 4-Hydroxynonenal induces dysfunction and apoptosis of cultured endothelial cells. J Cell Physiol. 1999;181:295–303. doi: 10.1002/(SICI)1097-4652(199911)181:2<295::AID-JCP11>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H, Gow A, Thom SR, Kooy NW, Royall JA, Crow JP. Detection of reactive nitrogen species using 2,7-dichlorodihydrofluorescein and dihydrorhodamine 123. Methods Enzymol. 1999;301:367–373. doi: 10.1016/s0076-6879(99)01100-3. [DOI] [PubMed] [Google Scholar]
- Ji C, Kozak KR, Marnett LJ. IkappaB kinase, a molecular target for inhibition by 4-hydroxy-2-nonenal. J Biol Chem. 2001;276:18223–18228. doi: 10.1074/jbc.M101266200. [DOI] [PubMed] [Google Scholar]
- Kim IY, Stadtman TC. Inhibition of NF-kappaB DNA binding and nitric oxide induction in human T cells and lung adenocarcinoma cells by selenite treatment. Proc Natl Acad Sci U S A. 1997;94:12904–12907. doi: 10.1073/pnas.94.24.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langheinrich AC, Bohle RM. Atherosclerosis: humoral and cellular factors of inflammation. Virchows Arch. 2005;446:101–111. doi: 10.1007/s00428-004-1180-4. [DOI] [PubMed] [Google Scholar]
- Larson CJ, Li YW, Mercurio F, Muller R. Functional characterization of a purified, recombinant NF-kappaB/IkappaB complex. Biochem Biophys Res Commun. 1999;260:691–698. doi: 10.1006/bbrc.1999.0949. [DOI] [PubMed] [Google Scholar]
- Liu W, Kato M, Itoigawa M, Murakami H, Yajima M, Wu J, Ishikawa N, Nakashima I. Distinct involvement of NF-kappaB and p38 mitogen-activated protein kinase pathways in serum deprivation-mediated stimulation of inducible nitric oxide synthase and its inhibition by 4-hydroxynonenal. J Cell Biochem. 2001;83:271–280. doi: 10.1002/jcb.1234. [DOI] [PubMed] [Google Scholar]
- Minekura H, Kumagai T, Kawamoto Y, Nara F, Uchida K. 4-Hydroxy-2-nonenal is a powerful endogenous inhibitor of endothelial response. Biochem Biophys Res Commun. 2001;282:557–561. doi: 10.1006/bbrc.2001.4586. [DOI] [PubMed] [Google Scholar]
- Misra P, Srivastava SK, Singhal SS, Awasthi S, Awasthi YC, Boor PJ. Glutathione S-transferase 8-8 is localized in smooth muscle cells of rat aorta and is induced in an experimental model of atherosclerosis. Toxicol Appl Pharmacol. 1995;133:27–33. doi: 10.1006/taap.1995.1123. [DOI] [PubMed] [Google Scholar]
- Morris KR, Lutz RD, Choi HS, Kamitani T, Chmura K, Chan ED. Role of the NF-kappaB signaling pathway and kappaB cis-regulatory elements on the IRF-1 and iNOS promoter regions in mycobacterial lipoarabinomannan induction of nitric oxide. Infect Immun. 2003;71:1442–1452. doi: 10.1128/IAI.71.3.1442-1452.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C, D’Armiento FP, Mancini FP, Postiglione A, Witztum JL, Palumbo G, Palinski W. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia Intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J Clin Invest. 1997;100:2680–2690. doi: 10.1172/JCI119813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseem KM. The role of nitric oxide in cardiovascular diseases. Mol Aspects Med. 2005;26:33–65. doi: 10.1016/j.mam.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Page S, Fischer C, Baumgartner B, Haas M, Kreusel U, Loidl G, Hayn M, Ziegler-Heitbrock HW, Neumeier D, Brand K. 4-Hydroxynonenal prevents NF-kappaB activation and tumor necrosis factor expression by inhibiting IkappaB phosphorylation and subsequent proteolysis. J Biol Chem. 1999;274:11611–11618. doi: 10.1074/jbc.274.17.11611. [DOI] [PubMed] [Google Scholar]
- Palinski W, Rosenfeld ME, Yla-Herttuala S, Gurtner GC, Socher SS, Butler SW, Parthasarathy S, Carew TE, Steinberg D, Witztum JL. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci U S A. 1989;86:1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RP, Levonen A, Crawford JH, rley-Usmar VM. Mechanisms of the pro- and anti-oxidant actions of nitric oxide in atherosclerosis. Cardiovasc Res. 2000;47:465–474. doi: 10.1016/s0008-6363(00)00086-9. [DOI] [PubMed] [Google Scholar]
- Patrick B, Li J, Jeyabal PV, Reddy PM, Yang Y, Sharma R, Sinha M, Luxon B, Zimniak P, Awasthi S, Awasthi YC. Depletion of 4-hydroxynonenal in hGSTA4-transfected HLE B-3 cells results in profound changes in gene expression. Biochem Biophys Res Commun. 2005;334:425–432. doi: 10.1016/j.bbrc.2005.06.099. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Ruef J, Moser M, Bode C, Kubler W, Runge MS. 4-hydroxynonenal induces apoptosis, NF-kappaB-activation and formation of 8-isoprostane in vascular smooth muscle cells. Basic Res Cardiol. 2001;96:143–150. doi: 10.1007/s003950170064. [DOI] [PubMed] [Google Scholar]
- Singhal SS, Saxena M, Ahmad H, Awasthi S, Haque AK, Awasthi YC. Glutathione S-transferases of human lung: characterization and evaluation of the protective role of the alpha-class isozymes against lipid peroxidation. Arch Biochem Biophys. 1992;299:232–241. doi: 10.1016/0003-9861(92)90269-3. [DOI] [PubMed] [Google Scholar]
- Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis American Heart Association. Arterioscler Thromb Vasc Biol. 1995;15:1512–1531. doi: 10.1161/01.atv.15.9.1512. [DOI] [PubMed] [Google Scholar]
- Steiner L, Kroncke K, Fehsel K, Kolb-Bachofen V. Endothelial cells as cytotoxic effector cells: cytokine-activated rat islet endothelial cells lyse syngeneic islet cells via nitric oxide. Diabetologia. 1997;40:150–155. doi: 10.1007/s001250050656. [DOI] [PubMed] [Google Scholar]
- Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- Wilcox JN, Subramanian RR, Sundell CL, Tracey WR, Pollock JS, Harrison DG, Marsden PA. Expression of multiple isoforms of nitric oxide synthase in normal and atherosclerotic vessels. Arterioscler Thromb Vasc Biol. 1997;17:2479–2488. doi: 10.1161/01.atv.17.11.2479. [DOI] [PubMed] [Google Scholar]
- Yan ZQ, Sirsjo A, Bochaton-Piallat ML, Gabbiani G, Hansson GK. Augmented expression of inducible NO synthase in vascular smooth muscle cells during aging is associated with enhanced NF-kappaB activation. Arterioscler Thromb Vasc Biol. 1999;19:2854–2862. doi: 10.1161/01.atv.19.12.2854. [DOI] [PubMed] [Google Scholar]
- Yang Y, Cheng JZ, Singhal SS, Saini M, Pandya U, Awasthi S, Awasthi YC. Role of glutathione S-transferases in protection against lipid peroxidation Over-expression of hGSTA2-2 in K562 cells protects against hydrogen peroxide-induced apoptosis and inhibits JNK and caspase 3 activation. J Biol Chem. 2001;276:19220–19230. doi: 10.1074/jbc.M100551200. [DOI] [PubMed] [Google Scholar]
- Yang Y, Sharma R, Sharma A, Awasthi S, Awasthi YC. Lipid peroxidation and cell cycle signaling: 4-hydroxynonenal, a key molecule in stress mediated signaling. Acta Biochim Pol. 2003;50:319–336. [PubMed] [Google Scholar]
- Yang Y, Yang Y, Trent MB, He N, Lick SD, Zimniak P, Awasthi YC, Boor PJ. Glutathione-S-transferase A4-4 modulates oxidative stress in endothelium: possible role in human atherosclerosis. Atherosclerosis. 2004;173:211–221. doi: 10.1016/j.atherosclerosis.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Yogo K, Shimokawa H, Funakoshi H, Kandabashi T, Miyata K, Okamoto S, Egashira K, Huang P, Akaike T, Takeshita A. Different vasculoprotective roles of NO synthase isoforms in vascular lesion formation in mice. Arterioscler Thromb Vasc Biol. 2000;20:E96–E100. doi: 10.1161/01.atv.20.11.e96. [DOI] [PubMed] [Google Scholar]
- Zimniak L, Awasthi S, Srivastava SK, Zimniak P. Increased resistance to oxidative stress in transfected cultured cells overexpressing glutathione S-transferase mGSTA4-4. Toxicol Appl Pharmacol. 1997;143:221–229. doi: 10.1006/taap.1996.8070. [DOI] [PubMed] [Google Scholar]