Abstract
In Drosophila melanogaster, the small interfering RNA (siRNA) pathway is triggered by exogenous double-stranded RNA (dsRNA) or upon viral infection. This pathway requires Dicer-2 (Dcr-2) in association with a dsRNA binding protein (dsRBP) called R2D2. A potentially distinct siRNA pathway, which requires Dcr-2 in association with a different dsRBP, called Loquacious (Loqs), is activated by endogenous dsRNA derived from transposons, structured loci and overlapping transcripts. Here, we show that different sources of dsRNA enter a common siR-NA pathway that requires R2D2 and Loqs. R2D2 and loqs mutants show impaired silencing triggered by injection of exogenous dsRNA or by artificial and natural expression of endogenous dsRNA. In addition, we show that these dsRBPs function sequentially and non-redundantly in collaboration with Dcr-2. Loqs is primarily required for dsRNA processing while R2D2 is essential for the subsequent loading of siRNAs into effector Ago-RISC complexes.
RNA interference (RNAi) uses small non-coding RNAs to regulate post-transcriptional gene expression. Within somatic animal cells, at least two different species of small RNAs are found: siRNAs and microRNAs (miRNAs) 1. In Drosophila, the biogenesis and action of miRNAs and siRNAs require distinct machineries 1. miRNAs originate from nuclear hairpin RNAs that are sequentially processed by two different RNaseIII/dsRBP complexes: Drosha/Pasha and Dicer-1/Loqs isoform PB (Dcr-1/Loqs-PB), respectively. The resulting mature miRNA is then loaded into the Argonaute protein Ago1. siRNAs originating from exogenous dsRNAs require a different Dicer-dsRBP complex, Dcr-2/R2D2, and are loaded into the Ago2 protein. Exogenous, in this case, refers to dsRNAs that do not undergo nuclear synthesis but originate from cytoplasmic or extra-cellular locales such as experimentally introduced dsRNAs. R2D2 and Loqs perform different biochemical functions with their cognate Dicer partners. Loqs-PB is required for processing of miRNA precursors by Dcr-1 but not for loading of mature miRNAs into Ago1 2. R2D2 is required for Dcr-2 dependent loading of siRNAs into Ago2 but appears dispensable for processing of exogenous dsRNAs 3,4.
Recent studies reported the existence of siRNAs derived from endogenous dsRNAs in C. elegans, Drosophila and mice 5–14. In Drosophila and mammals, endogenous siRNAs (endo-siRNAs) are produced from nuclear dsRNAs originating from transposons, 3′ regions of overlapping transcripts, and structured loci with the capacity to form long hairpin dsRNA 15,16. Endo-siRNA biogenesis and action rely upon Dcr-2, Loqs isoform PD (Loqs-PD), and Ago2 in Drosophila 6,9,10,17. Knockdown experiments performed in Drosophila S2 cells suggested that R2D2 is not involved in the siRNA pathway triggered by endogenous dsRNA 6,10. This contrasts with the known role R2D2 plays in silencing genes by exogenous dsRNA in whole animals 3,18–20. On the basis of these observations, a model was proposed, in which exogenous and endogenous dsRNAs utilize distinct biochemical pathways using Loqs-PD and R2D2 dsRBPs to discriminate between RNA sources 15,16. However, the model relies upon evidence from S2 cells where R2D2 appears dispensable for silencing triggered even by exogenous dsRNA 21–24. We sought to better understand the roles of R2D2 and Loqs in the siRNA pathway within whole animals. Our results suggest a model where Loqs and R2D2 are required in a common silencing pathway triggered by either endogenous and exogenous dsRNAs.
Results
R2D2 and Loqs are required for silencing by exogenous and endogenous dsRNA
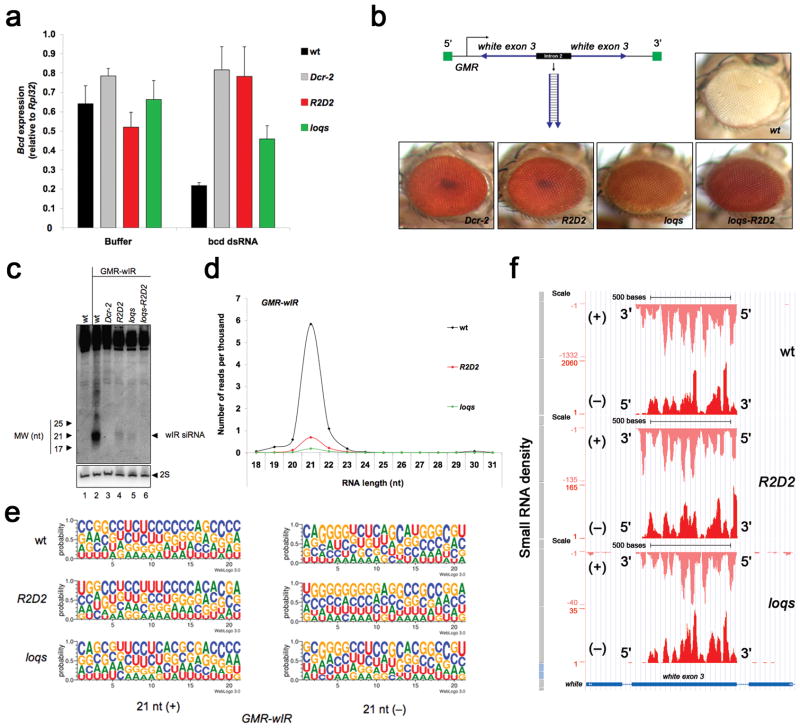
RNAi studies in Drosophila have been greatly aided by the existence of mutants for various critical genes 3,25–28. For this study, we generated loqs R2D2 single- and double-mutant flies using a combination of available alleles (see Methods). We initially used these mutants to test whether dsRBPs discriminate between dsRNAs based on their site of synthesis. Injection of in vitro synthesized dsRNA into the cytoplasm of single-cell embryos is a potent means to silence expression of embryonic genes such as bicoid (bcd) 29. This mode of silencing by exogenous dsRNA completely depends upon Dcr-2 26. Injection of bcd dsRNA into wildtype embryos led to an 80% reduction in bcd mRNA levels (Fig. 1a). As expected, R2D2 and Dcr-2 mutant embryos failed to silence bcd mRNA. However, loqs mutant embryos also showed partially impaired bcd silencing (Fig. 1a). This result suggested that silencing triggered by exogenous dsRNA introduced directly into the cytoplasm of embryos requires both Loqs and R2D2.
Figure 1. R2D2 and Loqs are required for silencing triggered by dsRNA from endogenous or exogenous sources.
(a) Silencing of the bcd gene triggered by injection of dsRNA into embryos, as assessed by RT-qPCR analysis of bcd mRNA. RNA was extracted 1.5 h post-injection, and buffer injected embryos were used as controls. The graph displays the mean and standard deviation (n=3). (b) Silencing of the white gene triggered by the GMR-wIR transgene (detail in the figure), as assessed by eye color. (c) Detection of GMR-wIR-derived siRNA by Northern blotting of small RNA fractions from heads. 2S rRNA blotting was used as a loading control. (d) Size distribution and normalized number of sequenced RNAs derived from GMR-wIR. (e) Sequence logo plots of 21-nt sense (+) and antisense (−) RNAs sequenced from GMR-wIR. The height of symbols within the stack indicates the relative frequency of each nucleotide at that position in all GMR-wIR RNAs. The RNAs are oriented such that the 5′ end is to the left. (f) Small RNA density along the region of the white gene that matches GMR-wIR, as provided by the UCSC Genome Browser. Note the different scales used for plotting the small RNA density in wildtype, R2D2 and loqs mutants to allow for comparison of the relative cover-age. The density of sense (+) and antisense (−) small RNAs, and the 5′ and 3′ ends of the two strands are shown.
We next examined the effect of R2D2 and Loqs on silencing triggered by an artificial endogenous dsRNA. A nuclear transgene, called GMR-wIR, encodes a long hairpin dsRNA corresponding to one exon of the white gene 26. Flies containing two copies of GMR-wIR have a white eye due to complete silencing of the white gene (Fig. 1b). Dcr-2 or ago2 mutant flies failed to silence the white gene and exhibited a red eye phenotype (Fig. 1b and data not shown). We also observed that both loqs and R2D2 single mutants had impaired white silencing, consistent with a previous report 28. The loqs-R2D2 double mutant also failed to silence the white gene (Fig. 1b). These results suggest that Loqs and R2D2 are non-redundant components of silencing triggered by either endogenous or exogenous dsRNAs.
R2D2 and Loqs are required at different steps of the siRNA pathway
The abundance of GMR-wIR derived siRNAs was severely reduced in R2D2 and loqs single mutants (Fig. 1c). GMR-wIR derived siRNAs were even further reduced in the loqs-R2D2 double mutant, comparable to depletion observed in the Dcr-2 mutant (Fig. 1c). These results were confirmed by deep sequencing small RNAs from wildtype, R2D2 and loqs single mutant flies (see sequence summary in Supplementary Table 1). We observed an ~8- and ~31-fold reduction in the normalized number of 21-nucleotide (nt) RNAs matching GMR-wIR in R2D2 and loqs mutants, respectively (Fig. 1d).
The genetic non-redundancy we observed with GMR-wIR silencing suggests that Loqs and R2D2 perform discrete functions despite similar effects on GMR-wIR siRNA levels. R2D2 qualitatively affected the pool of small RNAs derived from GMR-wIR, consistent with the role for R2D2 in strand selection during loading of Ago2 19. There was a C-bias at the 5′ terminal nucleotide of 21-nt GMR-wIR RNAs that was altered to a U-bias in the R2D2 mutant (Fig. 1e). The 3′ terminal nucleotide and middle 9–11 nucleotides also showed different biases in the R2D2 mutant. Differences in relative nucleotide bias were also apparent in 20- and 22-nt GMR-wIR RNAs from R2D2 when compared to wildtype (Supplementary Fig. 1). Density of siRNA coverage over GMR-wIR showed a relative increase in RNAs that mapped to the 3′ ends of both strands in the R2D2 mutant (Fig. 1f). In contrast, the loqs mutant showed few differences from wildtype in nucleotide bias and coverage of GMR-wIR siRNAs (Fig. 1e,f and Supplementary Fig. 1). Rather, loqs quantitatively reduced the abundance of siRNAs, suggesting no role for Loqs in strand selection and Ago2 loading.
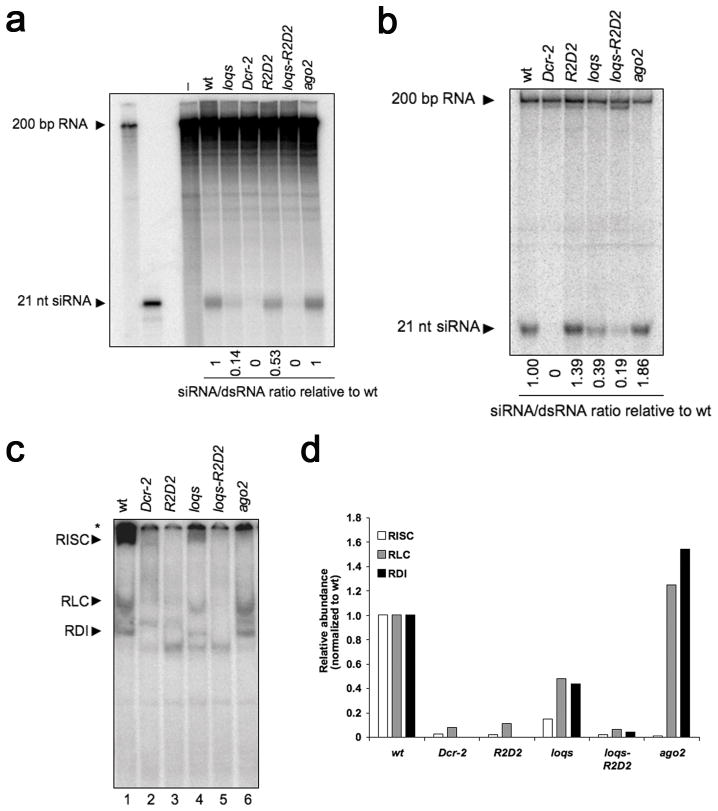
Abundance of siRNAs could be regulated by the rate of processing or by siRNA turnover, as affected by siRNA loading into Ago2. To analyze the production of siRNAs from dsRNA in different mutants, we performed in vitro dsRNA processing reactions. When labelled dsRNA was incubated with embryo extract, Dcr-2 dependent production of siR-NAs was observed (Fig. 2a). Production of siRNAs was severely reduced in loqs mutant extract (14% of wildtype), and slightly reduced in R2D2 mutant extract (53%). Extract from the loqs-R2D2 double mutant showed no detectable siRNA production, comparable to Dcr-2 mutant extract (Fig. 2a). To validate these in vitro observations, we injected embryos with labelled dsRNA and measured production of siRNAs 2 hours post-injection. We observed no detectable processing in Dcr-2 mutant embryos, and a 60% and 80% reduction in processing in loqs single and loqs-R2D2 double mutant embryos, respectively (Fig. 2b). In contrast, R2D2 and ago2 mutant embryos exhibited a 40% and 86% increase in dsRNA processing, respectively (Fig. 2b). Our data indicate that Dcr-2 collaborates primarily with Loqs to process dsRNA into siRNAs, although Dcr-2 might also perform processing with R2D2, particularly when Loqs is absent. To monitor loading of siRNAs into Ago2, we performed in vitro RISC loading reactions 4,30. Addition of labelled siRNA to embryo extract results in rapid association with R2D2/Dcr-2 to form RDI. This is converted into the RISC-loading complex (RLC) and subsequently, the siRNA is loaded into Ago2 to form RISC. Dcr-2 and R2D2 mutant extracts failed to form RDI, RLC, and Ago2-RISC complexes, while ago2 mutants only lacked the Ago2-RISC complex (Fig. 2c,d) 4,18,25. loqs mutant extract was capable of forming all three complexes although the levels were reduced by ~80% (Fig. 2c,d). Interestingly, UV crosslinking of Dcr-2 to labelled siRNA was lost in R2D2 mutant extract but unaffected in loqs mutant extract (Supplementary Fig. 2a).
Figure 2. R2D2 and Loqs are required at different steps of the siRNA pathway.
(a) In vitro processing of labelled 200 bp dsRNA into siRNAs in embryo extracts. (b) In vivo processing of labelled 200 bp dsRNA into siRNAs 2 h post-injection into embryos. (c) Native gel analysis of complexes formed on the pathway to loaded Ago2-RISC, after labelled siRNA incubation with embryo extract. Complexes are indicated by arrowheads. The asterisk marks the complexes that failed to enter the gel. (d) Quantification of the RDI, RLC and Ago2-RISC complexes as shown in c.
These results indicate that Loqs acts primarily to process exogenous dsRNAs whereas R2D2 is essential for the loading of exogenous siRNAs. Both steps are carried out in collaboration with Dcr-2, but it is unclear how Dcr-2 interacts with each dsRBP. To analyze the interaction of Dcr-2 with R2D2 or Loqs, we performed immunoprecipitation experiments using a HA-tagged Dcr-2 transgene that fully rescues lack of endogenous Dcr-2 (data not shown). We observed strong association between R2D2 and Dcr-2 proteins in embryos and adult flies (Supplementary Fig. 2b,c). We estimate that ~50% of R2D2 protein present in embryos is in a complex with Dcr-2 protein. In contrast, Loqs was not detected in the same Dcr-2 immunoprecipitates. These data are consistent with fractionation experiments where most Dcr-2 and R2D2 are present in common fractions but separate from Loqs 3,28,31. However, other studies found weak interaction between Loqs-PD and Dcr-2 such that a small percentage of endogenous Loqs-PD was detected in Dcr-2 immunoprecipitates 6,17. We interpret these results to suggest that R2D2 exists in a stable complex with Dcr-2 while the association between Loqs and Dcr-2 is either unstable or occurs transiently. It remains unclear how Loqs and Dcr-2 might interact to perform dsRNA processing in vivo. Purified recombinant Dcr-2 is capable of dsRNA processing without Loqs and is unaffected by the presence of R2D2 3.
R2D2 and Loqs are required for silencing triggered by endogenous structured loci
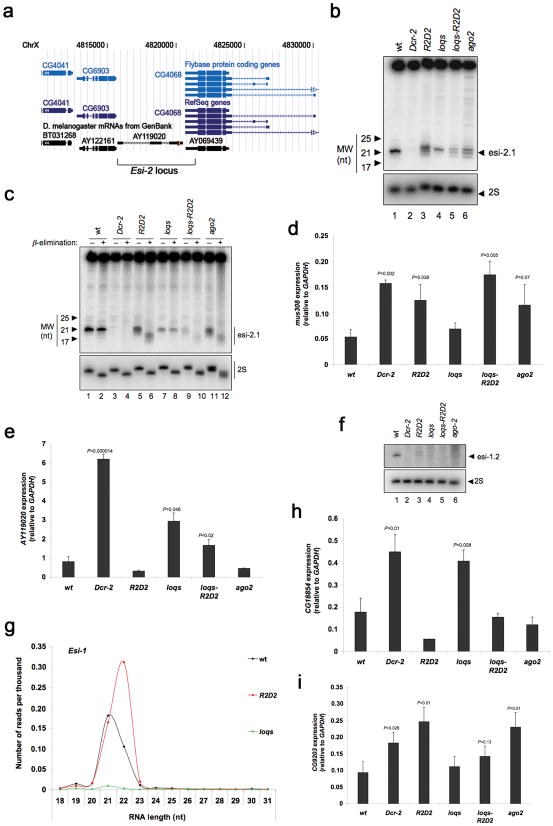
The GMR-wIR hairpin RNA resembles endogenous structured loci that are precursors of natural endo-siRNAs. This prompted us to explore the functions of R2D2 and Loqs in endo-siRNA biogenesis and silencing. A cluster of endo-siRNAs maps to a genomic region referred to as the esi-2 locus that is predicted to form extensive secondary structure 6,10. The esi-2 locus (also hp-CG4068) is located between CG6903 and CG4068 with some overlap between the end of the cluster and the 5′ end of CG4068 (Fig. 3a) 10.
Figure 3. R2D2 and Loqs are required in the endogenous siRNA pathway.
(a) Graphic representation of the genomic region corresponding to the esi-2 locus on the X chromosome, provided by the UCSC Genome Browser. (b) Levels of esi-2.1 RNA as determined by Northern blotting of small RNA fractions from whole males. 2S rRNA blotting was used as a loading control. (c) Sensitivity of esi-2.1 RNA to β-elimination reactions was visualized by Northern blotting of small RNA fractions. 2S rRNA blotting was used to control for loading and elimination. (d,e) mus308 (d) and AY119020 (e) transcript levels as determined by RT-qPCR of total RNA from testes. The graphs display the mean and standard deviation (n=3). (f) Levels of esi-1.2 RNA as determined by Northern blotting of RNA extracted from whole males. 2S rRNA blotting was used as a loading control. (g) Size distribution and normalized number of sequenced RNAs mapping to the esi-1 locus. (h, i) CG18854 (h) and CG9203 (i) transcript levels as determined by RT-qPCR of total RNA from testes. The graphs display the mean and standard deviation (n=3). Statistically significant differences are indicated (Student t test compared to wildtype).
The endo-siRNAs were detected in testes 6 and we therefore used RNA isolated from this tissue or from male flies for analysis. The most abundant endo-siRNA derived from the esi-2 locus is called esi-2.1 6,10. Levels of esi-2.1 were reduced in loqs and Dcr-2 mutants as reported 10 (Fig. 3b). In contrast, levels of esi-2.1 were mildly reduced in R2D2 and ago2 mutants. However, while other strains had one major esi-2.1 RNA species, R2D2 and ago2 mutants had additional esi-2.1 RNA species (Fig. 3b). Interestingly, the loqs-R2D2 double mutant displayed both reduction and heterogeneity of esi-2.1 RNAs, indicating a composite phenotype between loqs and R2D2 single mutants (Fig. 3b).
The presence of additional species of esi-2.1 RNA in R2D2 and ago2 mutants prompted us to investigate the cause of this heterogeneity. Endo-siRNAs that are successfully loaded into Ago2 are methylated by DmHen1 on the 3′ terminal nucleotide 10,32. It was possible that some of the heterogeneity observed with esi-2.1 RNA was due to lack of 3′ end methylation since this modification in plants prevents non-templated addition of uridyl residues at the 3′ end of siRNAs 33. To test this hypothesis, we performed β-elimination reactions on total small RNAs from various mutant flies and analyzed esi-2.1 endo-siRNA by Northern blot. When the RNA is not methylated, the 3′-most nucleotide is eliminated by treatment with NaIO4, resulting in an RNA species that is smaller in size. Esi-2.1 RNA was fully resistant to β-elimination in wildtype and fully sensitive in an ago2 mutant (Fig. 3c). Esi-2.1 RNA from the R2D2 mutant was also fully sensitive to β-elimination, indicating that the RNA is not methylated in the absence of R2D2 (Fig. 3c). Although esi-2.1 RNA was reduced in abundance from the loqs mutant, it was completely resistant to β-elimination (Fig. 3c). As expected, 2S rRNA and mir-8 miRNA, which are unmodified at their 3′ termini, were sensitive regardless of the source of RNAs (Fig. 3c and Supplementary Fig. 3). These result are consistent with a role for Loqs in esi-2.1 RNA biogenesis and a role for R2D2 in esi-2.1 RNA loading into Ago2.
Silencing of the coding gene mus308 by esi-2 endo-siRNAs has been reported 6. mus308 levels are significantly elevated in testes of Dcr-2 and ago2 mutant flies 6 (Fig. 3d). We observed that mus308 levels were also significantly elevated in testes of R2D2 and loqs-R2D2 mutants, and to a lesser extent in loqs mutant testes (Fig. 3d). This result indicates that silencing by endo-siRNAs requires R2D2 in addition to Dcr-2 and Ago2.
The esi-2 locus is located within an intergenic region from which a cDNA called AY119020 has been cloned 34 (therefore we refer to this intergenic region as AY119020) (Fig. 3a). Expression of RNA arising from the AY119020 region was detected by qPCR in testes where the esi-2 endo-siRNAs are abundant (Fig. 3e). AY119020 RNA levels were significantly elevated in Dcr-2 and loqs mutants relative to wildtype, and slightly reduced in R2D2 and ago2 mutants (Fig. 3e). Levels of the two surrounding genes, CG6903 and CG4068, were unchanged in mutants (Supplementary Fig. 4a,b). These results suggest that AY119020 RNA levels are down-regulated by processing carried out by Dcr-2 and Loqs. The lower levels of AY119020 RNA in ago2 and R2D2 mutants suggest enhanced processing in the absence of Ago2 loading, similar to the observed effects on exogenous dsRNA processing (Fig. 3b).
We performed similar analysis on a cluster of endo-siRNAs mapping to another structured locus called the esi-1 locus (also hp-CG18854) 6,10. Levels of esi-1.2 RNA, an abundant endo-siRNA from the esi-1 locus, were reduced in ago2, loqs and R2D2 mutants, and were undetectable in loqs-R2D2 and Dcr-2 mutants (Fig. 3f). There was heterogeneity of esi-1.2 RNA species in R2D2 mutant males and females (Fig. 3f,g). Deep sequencing analysis showed most esi-1 siRNAs originated from two major sites on the hairpin precursor, around positions 609 and 1553 of the CG18854-RC mRNA (indicated as 1 and 2, respectively in Supplementary Fig. 5). Site 1 (corresponding to the esi-1.2 siRNA) generated roughly equal amounts of 21- and 22-nt RNA species whereas site 2 generated primarily 21-nt RNA species. In the R2D2 mutant, esi-1 siRNAs were skewed towards 22-nt species and mapped almost exclusively to the first site on the CG18854-RC mRNA (Fig. 3g and Supplementary Fig. 5). No evidence of non-templated addition of nucleotides to the 3′ or 5′ ends was observed (data not shown). In contrast, the size distribution of esi-1 derived RNAs was unchanged in the loqs mutant (Fig. 3g). This suggests that the siRNA pool from the esi-1 locus is affected qualitatively in R2D2 and quantitatively in loqs. Together, our data suggest impaired loading of siRNAs into Ago2 causes the size heterogeneity of both esi-1.2 and esi-2.1 RNAs, and heterogeneity is not simply due to imprecise processing of dsRNA precursors.
The CG18854 transcript is predicted to be the precursor of esi-1 endo-siRNAs. We observed that CG18854 RNA levels were significantly elevated in Dcr-2 and loqs mutants but were slightly reduced in R2D2 and ago2 mutants (Fig. 3h). The esi-1 precursor RNA level in the loqs-R2D2 mutant was intermediate between loqs and R2D2 single mutants. The esi-1 locus bears some homology to CG8289, and upon ectopic expression of esi-1, it was shown to be able to repress this transcript 10. However, we could not confidently detect CG8289 expression in testes (data not shown). Nevertheless, the esi-1 locus has similar genetic requirements to the esi-2 locus, suggesting a general role for Dcr-2/R2D2 in loading Ago2-RISC with endo-siRNAs from hairpin RNAs that are processed by Dcr-2/Loqs in vivo.
R2D2 is required for silencing triggered by overlapping transcripts
A number of endo-siRNAs map to genomic regions that are predicted to generate overlapping transcripts 5,6. Therefore, we analyzed an endo-siRNA cluster in testes derived from CG14033, which is inserted in the opposite orientation to the Thickveins gene (Supplementary Fig. 6a). Although the cluster maps to these two overlapping transcripts (CG14033 and Thickveins), their levels were unaffected in various mutants with the possible exception of Dcr-2 (Supplementary Fig. 6b,c). The siRNAs from this cluster show complementarity and are predicted to target the CG9203 transcript 6. We observed that CG9203 levels were significantly elevated in Dcr-2, R2D2, and ago2 mutants compared to wildtype (Fig. 3i). The loqs-R2D2 mutant had elevated CG9203 levels but it was not statistically significant (p=0.13), whereas the loqs single mutant showed comparable levels to wildtype. Nevertheless, our results indicate that R2D2 is required for silencing triggered by endo-siRNAs derived from both structured loci and overlapping transcripts.
R2D2 and Loqs are required for transposon silencing in adult flies
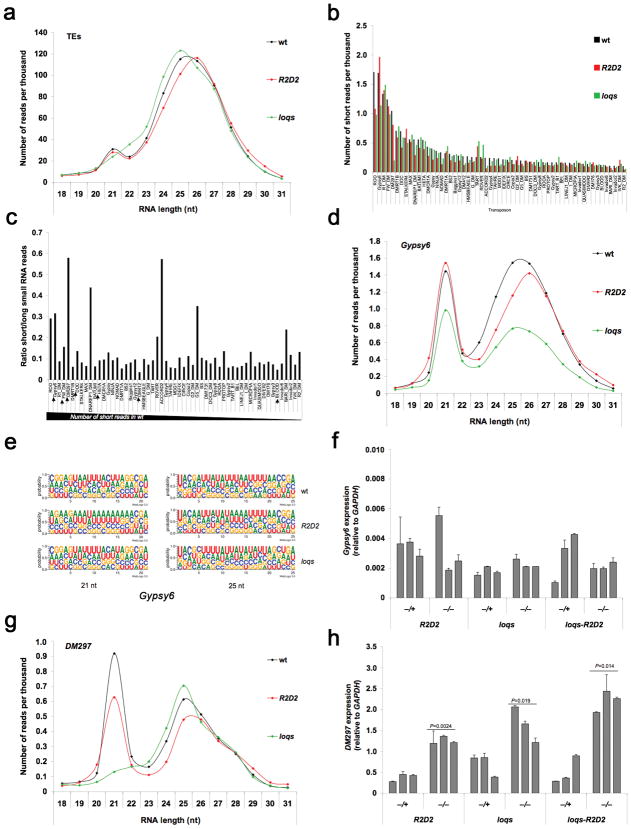
Endo-siRNAs mapping to transposable elements (TEs) comprise the majority of 21-nt RNAs in somatic tissues of adult flies 7. TEs are also a target of 24–27 nt piwi-interacting RNAs (piRNAs) in the germline 35. Approximately 30% of sequenced small RNAs (excluding rRNAs) mapped to TEs in female flies (Supplementary Table 1). The size distribution of RNAs mapping to TEs showed clear peaks at 20–21 nt and 24–27 nt, presumably corresponding to siRNAs and piRNAs, respectively (Fig. 4a). We plotted the top individual TEs generating 20–21 nt small RNAs (Fig. 4b). In addition, we plotted the ratio between short (20–21 nt siRNAs) and long (24–27 nt piRNAs) RNAs for each of individual TE to serve as a proxy for assessing relative silencing by each class of small RNAs (Fig. 4c). The TEs generating the largest numbers of 20–21 nt RNAs did not necessarily have the highest siRNA/piRNA ratios (compare Fig. 4b, c). Distinct groups of TEs could be defined by the siRNA/piRNA ratio, and thus we analyzed select TEs (indicated by arrowheads in Fig. 4c) with high (Gypsy6 and DM297), intermediate (FW and DOC) and low (Het-A, DM412 and Blood) ratios.
Figure 4. Requirements of R2D2 and Loqs for silencing of TEs.
(a) Size distribution and normalized number of sequenced RNAs mapping to TEs. (b) Number of short (20–21 nt) RNAs mapping to individual TEs. (c) Ratio of short (20–21 nt)/long (24–27 nt) RNAs in wildtype for the TEs listed in b. Arrowheads mark TE selected for further analysis. (d) Size distribution and normalized number of sequenced RNAs derived from Gyp-sy6. (e) Sequence logo plots of 21-nt and 25-nt RNAs mapping to Gypsy6. (f) Gypsy6 RNA levels as determined by RT-qPCR of total RNA from whole females. The graphs show the mean and standard deviation results of three independent experiments. (g) Size distribution and normalized numbers of sequenced RNAs derived from DM297. (h) DM297 RNA levels as determined by RT-qPCR of total RNA from whole females. The graphs show the mean and standard deviation results of three independent experiments. Statistically significant differences are indicated (Student t test compared to each heterozygous control).
Abundance of small RNAs mapping to Gypsy6 was slightly reduced in the R2D2 mutant and was more appreciably reduced in the loqs mutant, although there was a preferential loss of longer RNAs (Fig. 4b–d). The siRNA/piRNA ratio increased from ~0.3 in wildtype to ~0.43 in both mutants. The normal C-bias at the 5′ terminal nucleotide of 21-nt Gyp-sy6 RNAs was absent in the R2D2 mutant but present in the loqs mutant (Fig. 4e). Importantly, R2D2 and loqs mutants showed no differences in nucleotide biases at the termini of 25-nt piRNAs. This illustrates the qualitative impact of R2D2 on the pool of Gyp-sy6 siRNAs without affecting piRNAs. Despite an abundance of Gypsy6 siRNAs, Gypsy6 transcript levels showed no significant changes in any of the mutants tested, including R2D2, loqs, loqs-R2D2, Dcr-2 and ago2 (Fig. 4f and Supplementary Fig. 7a).
We analyzed another TE with a high siRNA/piRNA ratio: DM297. The siRNA peak was virtually absent in the loqs mutant whereas the R2D2 mutant showed only a small decrease (Fig. 4g). This was reflected in the siRNA/piRNA ratio (0.57, 0.54 and 0.10 in wildtype, R2D2 and loqs). The C-bias at the 5′ end of Dm297-derived siRNAs was absent in both R2D2 and loqs mutants (Supplementary Fig. 7b). These changes in siRNAs were accompanied by impaired silencing of DM297, as indicated by a significant increase in transcript levels in all mutants (Fig. 4h).
Small RNAs mapping to Het-A, DM412 and Blood exhibited low siRNA/piRNA ratios (0.09, 0.03 and 0.046) whereas FW and DOC had intermediate ratios (0.15 and 0.13) (Supplementary Fig. 7c–g). Thus, these TEs might be primarily regulated by piRNAs in the germline. Nevertheless, siRNAs mapping to these TEs were consistently reduced or absent in loqs and R2D2 mutants. We therefore measured the transcript levels for each TE in different mutants (Supplementary Fig. 7). DOC transcript levels were elevated in R2D2, loqs-R2D2, Dcr-2 and ago2 mutants. FW and Het-A transcript levels did not significantly change in any of the mutants tested, with the possible exception of FW in ago2. Blood transcript levels were elevated in loqs and loqs-R2D2 mutants but not R2D2, Dcr-2 or ago2. The reason for the strict dependence on Loqs is unclear, but it could suggest an involvement of miRNAs instead of siRNAs. DM412 transcript levels were consistently elevated in all mutants (Supplementary Fig. 7a,l). An enrichment for U at the 5′ end of 21-nt DM412 RNAs was observed (Supplementary Fig. 7m). This bias was exacerbated in loqs and R2D2 mutants. Possibly some of the 21-nt DM412 RNAs represent short piRNAs, which become enriched when siRNAs are lost in the mutants. Overall, the changes in levels of transposon transcripts in mutants did not correlate with the absolute number of siRNAs or the siRNA/piRNA ratio. This suggests the involvement of additional mechanisms and illustrates the complexity of the regulation of TEs.
Biogenesis of endo-siRNAs derived from TEs in vivo was not observed previously, 6 but the study used a loqs hypomorphic allele. Despite the well-described role of Loqs in the miRNA pathway, no changes in the total number of miRNAs were observed in the loqs hypomorph 6.
Loqs is required for biogenesis of miRNAs
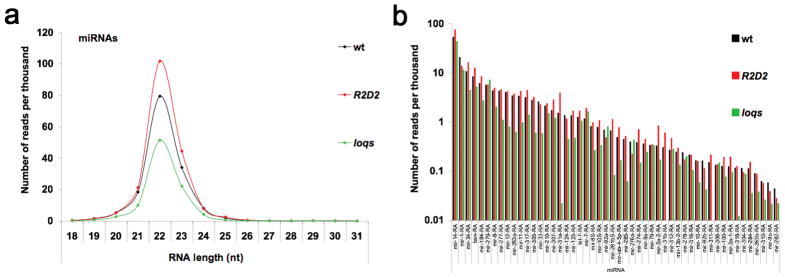
We predicted that the stronger loqs mutant used in the present study would have an impact on miRNA populations. There was a 50% decrease in total numbers of mature miRNAs in the loqs mutant compared to wildtype (Fig. 5a). We also observed a specific decrease in the abundance of most miRNAs in the loqs mutant with a few exceptions such as mir-279 (Fig. 5b). This differential effect on mature miRNA levels observed in loqs was described earlier 2. In the R2D2 mutant, there was an increase in abundance of some individual miRNAs (Fig. 5). Although this could suggest some cross-talk between small RNA pathways, we cannot rule out that it was caused by the strategy we used to normalize deep sequencing data based on the size of the libraries (see Methods).
Figure 5. The impact of Loqs and R2D2 on miRNAs.
(a) Size distribution and normalized numbers of sequenced RNAs derived from miRNAs (miRNA sequences obtained from miRBase-v12). (b) Normalized numbers of sequenced RNAs mapping to the top 50 most abundant miRNAs. Note that log scale was used to allow for comparison of miR-NAs throughout a wide range of expression.
Discussion
Previous studies led to a proposed model where Dcr-2/Loqs and Dcr-2/R2D2 act in two parallel pathways: the former in an endo-siRNA pathway and the latter in an exo-siRNA pathway 15,16. Our results instead indicate that R2D2 and Loqs have largely non-redundant roles in a common silencing pathway triggered by either exogenous or endogenous dsRNA (Fig. 6).
Figure 6. The siRNA pathway in Drosophila.
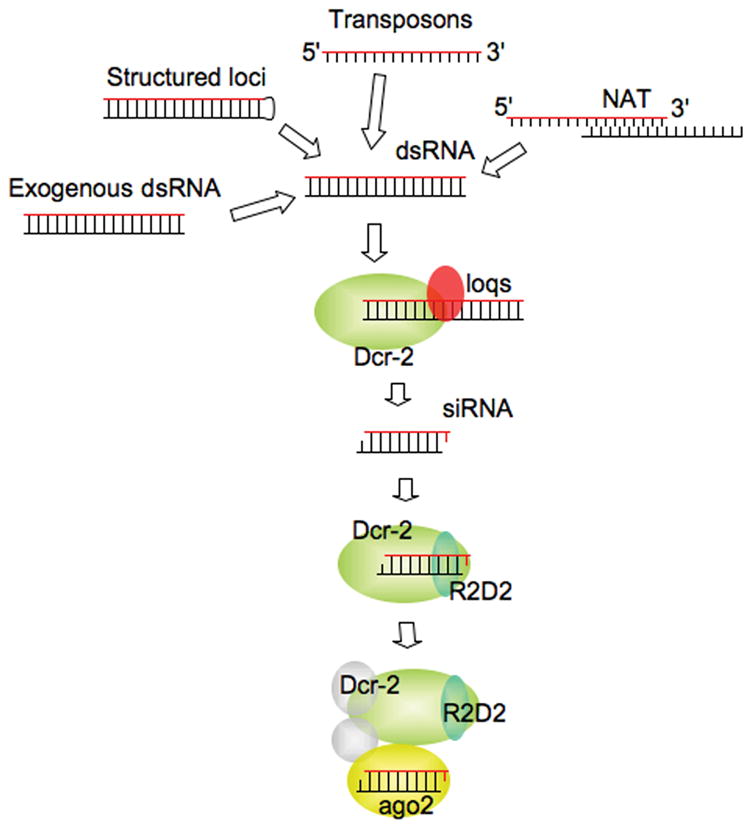
Endogenous and exogenous sources of dsRNA are processed by Dcr-2 primarily in collaboration with Loqs and the resulting siRNAs are sorted and loaded onto Ago2-RISC by Dcr-2/R2D2.
In our model, Loqs is necessary for efficient processing of dsRNA into mature siRNA duplexes by Dcr-2. Loqs could provide Dcr-2 with the ability to process dsRNAs of different structural conformations or increase the affinity of Dcr-2 for dsRNA substrate. There was residual processing activity by Dcr-2 detected in the loqs mutant, and this was at least partially dependent on R2D2. Therefore, Dcr-2/R2D2 likely carries out a small amount of processing independent of Loqs. Our results do not exclude the possibility that Dcr-2 can process some dsRNA without any dsRBP in vivo. Indeed, recombinant Dicer proteins from diverse organisms, including Drosophila Dcr-2, can process dsRNA without their dsRBP partners in vitro 3,36. We detected a small level of processing in loqs-R2D2 double mutant extracts, suggesting that Dcr-2 alone might have some processing activity. However, this interpretation is tempered by the fact that the loqs mutant we used did not completely eliminate Loqs 27, and so residual Loqs could have provided some processing capability to Dcr-2.
R2D2 is essential for siRNA-mediated silencing at a step downstream of processing. It is required for Dcr-2 to load either exo-siRNAs or endo-siRNAs into Ago2. R2D2 and Dcr-2 appear to carry out loading as a stable heterodimer. Our model would logically dictate that the processing complex releases its siRNA product before the loading complex can deliver it to Ago2. This prediction has been validated by earlier observations 37. Indeed, previously it was unclear why a processing complex with Dcr-2 and R2D2 would have to release the siRNA before loading. Our model provides a coherent framework to explain the separation between biogenesis and loading of siRNAs.
Finally, our model could also be relevant in the context of mammalian RNAi as Dicer dependent endogenous siRNAs have also been described in mammals 11,12. In addition, two different dsRNA binding proteins, PACT and TRBP, interact with Dicer 38 although their roles in the endogenous siRNA pathway have not yet been characterized.
Methods
Fly stocks and reagents
For our experiments, we placed a loqs null allele (loqsKO) 27 in combination with a partial loss-of-function allele (loqsf00791) 28. We also used a combination of two different null R2D2 alleles, R2D21 and R2D2S165fsX 3,20. We created two distinct recombined chromosomes, each containing a different loqs and R2D2 allele (loqsKO-R2D2S165fsX and loqsf00791-R2D21) that were used to generate loqs-R2D2 double mutant flies. Other Drosophila mutants analyzed were Dcr-2 (Dcr-2L812bsX)26 and ago2 (ago2414) 25. A genomic rescue of Dcr-2, HA-tagged in the C-terminus, was used in a Dcr-2 (Dcr-2L812bsX) background 26. Anti-Loqs serum 17 was kindly provided by Greg Hannon (Cold Spring Harbor). Anti-HA was purchased from Sigma and anti-R2D2 from Abcam.
RT-qPCR
Total RNA was extracted using Trizol reagent according to the manufacturer’s protocol (Invitrogen). 100 ng to 1 μg of DNase-treated RNA was reverse transcribed using random primers. cDNA was used as template for qPCR reaction using Sybr Green (Invitrogen). The relative expression of indicated RNAs was normalized to an internal control using the delta Ct method (GAPDH, Rpl32 or Actin 5C). qPCR primers are described in Supplementary Table 2. A two-tailed student t test was used to statistically analyze differences in RNA transcript levels.
Northern Blot
Northern blots were performed using probes with LNA modifications (Ex-iqon). Probes for 2S rRNA, esi-2.1 and esi-1.2 were previously described 6. Northern blots for small RNAs were performed on low molecular weight (LMW) RNA fractions separated from total RNA as described 39. 10 μg of LMW RNA was electrophoresed in denaturing 15% polyacrylamide. RNA was transferred in a semi-dry apparatus, and chemical crosslinking was performed as described 40. Hybridizations were performed in Ultra-Hyb oligo-hybridization buffer (Ambion) at 37° C for 16 h.
β-elimination
β-elimination was performed essentially as described 32. Briefly, 5 μg of LMW RNA was incubated in Borax/Boric Acid buffer (60 mM Borax, 60 mM Boric acid, pH 8.6) and 25 mM NaIO4 for 30 minutes at 22°C. NaOH was added to a final concentration of 45 mM and the samples were incubated for 90 minutes at 45° C. 300 mM of NaCl and 3 volumes of ethanol were added and the RNA was incubated at −20° C for 3 hours and precipitated by centrifugation for 15 minutes at 16,000 g.
Native gel electrophoresis of siRNA-protein complexes
Embryo extract preparation was performed as described 4,41. Radiolabelled Ppluc siRNA was incubated with extract prior to native gel electrophoresis, as described 30,4,41.
dsRNA processing assays
RNA was transcribed from 200 bp-PCR templates generated using a MAXIscript kit (Ambion) and internally radiolabelled with α-32P-UTP. The complementary strands were then annealed. For in vitro dsRNA processing, embryo extracts were incubated with radiolabelled dsRNA in 10 μl reactions as described 26 at 25° C for 2 h. RNA was immediately extracted with phenol-chloroform and ethanol precipitated. For in vivo dsRNA processing, dsRNA was injected into embryos that were incubated at 25° C for 2 hrs. Total RNA was extracted using Trizol reagent according to the manufacturer’s protocol (Invitrogen). In both cases, RNA was analyzed on a denaturing 15% polyacrylamide gel.
bcd silencing
bcd dsRNA and embryo injections were described previously 29. After injection, embryos were incubated at 25° C for 90 min, and total RNA isolation and qPCR were performed as described above.
Deep sequencing and informatics
RNA isolated from 2–4 day-old females was used to prepare the libraries. All animals also contained GMR-wIR. Small RNAs in the 18–34 nt size range were PAGE purified from LMW RNA and used to prepare a library using the SOLiD small RNA expression kit according to the manufacturer’s protocol (Ambion). Sequencing of the libraries was performed using the SOLiD platform (ABI).
Reads were aligned to a reference consisting of release 5.2 of the Drosophila genome and the ABI sequencing primer products using the Small RNA Analysis Pipeline Tool (Rna2Map). Rna2Map uses the Mapreads program from Applied Biosystems to simultaneously align reads to a reference and to filter out contamination from sequencing adaptors. Up to three mismatches in the first 18 colors and six mismatches in the overall alignment were allowed during mapping. Only reads aligning to the Drosophila genome were retained.
Reads mapping to Drosophila miRNAs were determined by collecting the coordinates of known miRNAs from Flybase v5.18. The same process was repeated for Drosophila mRNAs and transposons, where mRNA coordinates were taken from Flybase and transposons coordinates were taken from version dm3 of the UCSC RepeatMasker annotation. Reads mapping to rRNA sequences were determined by filtering transposon, miRNA, and mRNA reads from the genome alignment and matching the remaining reads against Drosophila rRNA sequences from Flybase v5.18. Ad hoc perl scripts were used in all steps, including the calculation of read size/strand distributions. The numbers of reads mapping to rRNA, mRNA, miRNA and TEs for each sample are detailed in Supplementary Table 1.
The total number of reads aligning to the Drosophila genome was used to normalize the libraries to allow for comparison between different libraries. Abundance of specific small RNAs was plotted as the number of reads in a thousand reads from the total number of reads in each library as described 42. This normalization strategy has its limitations due to the fact that specific depletion of certain types of small RNAs in different mutants could distort the representation of other types of small RNAs in the same library (such as an apparent increase in numbers of TE derived piRNAs likely caused by the depletion of miRNAs in the loqs mutant).
Data for uniquely mapping reads were generated by extracting reads aligned to only one locus within the Drosophila genome. For reads aligning to more than one location we retained only the longest genomic match of each read. The number of reads overlapping each position along the fly chromosomes (separated by strand) was calculated, converted to wiggle format and used to generate the UCSC Genome Browser (http://genome.ucsc.edu/) plots. Sequence logo plots were generated using Weblogo 3 (http://weblogo.threeplusone.com/create.cgi) to depict the frequency of the four nucleotides as a function of 5′ – 3′ position in a pool of small RNAs of identical length.
Supplementary Material
Acknowledgments
We thank Catherine Reinke, Yanxia Bei and Carthew lab members for valuable discussion. Francesca Valsecchi for help with bioinformatics. Curt Horvath, Erik Sontheirmer and Jason Brickner for sharing equipment and reagents. Phillip Zamore for loqsf00791, Mikiko Siomi for ago2414 stocks, Quinghua Liu for R2D21 and loqsKO stocks and Greg Hannon for anti-loqs antibody. This work was supported by a grant from the NIH (GM68743) to R.W.C.
Footnotes
Author contributions: J.T.M. and K.K. conceived and designed the experiments. J.T.M., K.K., P.H.W., T.A. and N.J. performed the experiments. J.T.M, K.K. and R.W.C. analyzed the data. J.T.M. and R.W.C. wrote the paper.
Accession numbers: The deep sequencing datasets described in this manuscript are available at NCBI GEO under the accession number GSE18871.
References
- 1.Carthew RW, Sontheimer EJ. Origins and Mechanisms of miRNAs and siR-NAs. Cell. 2009;136:642–55. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu X, et al. Dicer-1, but not Loquacious, is critical for assembly of miRNA-induced silencing complexes. RNA. 2007;13:2324–9. doi: 10.1261/rna.723707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Q, et al. R2D2, a bridge between the initiation and effector steps of the Drosophila RNAi pathway. Science. 2003;301:1921–5. doi: 10.1126/science.1088710. [DOI] [PubMed] [Google Scholar]
- 4.Pham JW, Pellino JL, Lee YS, Carthew RW, Sontheimer EJ. A Dicer-2-dependent 80s complex cleaves targeted mRNAs during RNAi in Drosophila. Cell. 2004;117:83–94. doi: 10.1016/s0092-8674(04)00258-2. [DOI] [PubMed] [Google Scholar]
- 5.Chung WJ, Okamura K, Martin R, Lai EC. Endogenous RNA interference provides a somatic defense against Drosophila transposons. Curr Biol. 2008;18:795–802. doi: 10.1016/j.cub.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czech B, et al. An endogenous small interfering RNA pathway in Drosophila. Nature. 2008;453:798–802. doi: 10.1038/nature07007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghildiyal M, et al. Endogenous siRNAs derived from transposons and mRNAs in Drosophila somatic cells. Science. 2008;320:1077–81. doi: 10.1126/science.1157396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawamura Y, et al. Drosophila endogenous small RNAs bind to Argonaute 2 in somatic cells. Nature. 2008;453:793–7. doi: 10.1038/nature06938. [DOI] [PubMed] [Google Scholar]
- 9.Okamura K, Balla S, Martin R, Liu N, Lai EC. Two distinct mechanisms generate endogenous siRNAs from bidirectional transcription in Drosophila melanogaster. Nat Struct Mol Biol. 2008;15:581–90. doi: 10.1038/nsmb.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okamura K, et al. The Drosophila hairpin RNA pathway generates endogenous short interfering RNAs. Nature. 2008;453:803–6. doi: 10.1038/nature07015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tam OH, et al. Pseudogene-derived small interfering RNAs regulate gene expression in mouse oocytes. Nature. 2008;453:534–8. doi: 10.1038/nature06904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe T, et al. Endogenous siRNAs from naturally formed dsRNAs regulate transcripts in mouse oocytes. Nature. 2008;453:539–43. doi: 10.1038/nature06908. [DOI] [PubMed] [Google Scholar]
- 13.Ambros V, Lee RC, Lavanway A, Williams PT, Jewell D. MicroRNAs and other tiny endogenous RNAs in C. elegans. Curr Biol. 2003;13:807–18. doi: 10.1016/s0960-9822(03)00287-2. [DOI] [PubMed] [Google Scholar]
- 14.Pak J, Fire A. Distinct populations of primary and secondary effectors during RNAi in C. elegans. Science. 2007;315:241–4. doi: 10.1126/science.1132839. [DOI] [PubMed] [Google Scholar]
- 15.Ghildiyal M, Zamore PD. Small silencing RNAs: an expanding universe. Nat Rev Genet. 2009;10:94–108. doi: 10.1038/nrg2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okamura K, Lai EC. Endogenous small interfering RNAs in animals. Nat Rev Mol Cell Biol. 2008;9:673–8. doi: 10.1038/nrm2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou R, et al. Processing of Drosophila endo-siRNAs depends on a specific Loquacious isoform. RNA. 2009;15:1886–95. doi: 10.1261/rna.1611309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Jiang F, Kalidas S, Smith D, Liu Q. Dicer-2 and R2D2 coordinately bind siRNA to promote assembly of the siRISC complexes. RNA. 2006;12:1514–20. doi: 10.1261/rna.101606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomari Y, Matranga C, Haley B, Martinez N, Zamore PD. A protein sensor for siRNA asymmetry. Science. 2004;306:1377–80. doi: 10.1126/science.1102755. [DOI] [PubMed] [Google Scholar]
- 20.Wang XH, et al. RNA interference directs innate immunity against viruses in adult Drosophila. Science. 2006;312:452–4. doi: 10.1126/science.1125694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saleh MC, et al. The endocytic pathway mediates cell entry of dsRNA to induce RNAi silencing. Nat Cell Biol. 2006;8:793–802. doi: 10.1038/ncbl439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ulvila J, et al. Double-stranded RNA is internalized by scavenger receptor-mediated endocytosis in Drosophila S2 cells. J Biol Chem. 2006;281:14370–5. doi: 10.1074/jbc.M513868200. [DOI] [PubMed] [Google Scholar]
- 23.Zhou R, et al. Comparative analysis of Argonaute-dependent small RNA pathways in Drosophila. Mol Cell. 2008;32:592–9. doi: 10.1016/j.molcel.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dorner S, et al. A genomewide screen for components of the RNAi pathway in Drosophila cultured cells. Proc Natl Acad Sci U S A. 2006;103:11880–5. doi: 10.1073/pnas.0605210103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okamura K, Ishizuka A, Siomi H, Siomi MC. Distinct roles for Argonaute proteins in small RNA-directed RNA cleavage pathways. Genes Dev. 2004;18:1655–66. doi: 10.1101/gad.1210204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee YS, et al. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell. 2004;117:69–81. doi: 10.1016/s0092-8674(04)00261-2. [DOI] [PubMed] [Google Scholar]
- 27.Park JK, Liu X, Strauss TJ, McKearin DM, Liu Q. The miRNA pathway intrinsically controls self-renewal of Drosophila germline stem cells. Curr Biol. 2007;17:533–8. doi: 10.1016/j.cub.2007.01.060. [DOI] [PubMed] [Google Scholar]
- 28.Forstemann K, et al. Normal microRNA maturation and germ-line stem cell maintenance requires Loquacious, a double-stranded RNA-binding domain protein. PLoS Biol. 2005;3:e236. doi: 10.1371/journal.pbio.0030236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kennerdell JR, Yamaguchi S, Carthew RW. RNAi is activated during Drosophila oocyte maturation in a manner dependent on aubergine and spindle-E. Genes Dev. 2002;16:1884–9. doi: 10.1101/gad.990802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pham JW, Sontheimer EJ. Molecular requirements for RNA-induced silencing complex assembly in the Drosophila RNA interference pathway. J Biol Chem. 2005;280:39278–83. doi: 10.1074/jbc.M509202200. [DOI] [PubMed] [Google Scholar]
- 31.Jiang F, et al. Dicer-1 and R3D1-L catalyze microRNA maturation in Drosophila. Genes Dev. 2005;19:1674–9. doi: 10.1101/gad.1334005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horwich MD, et al. The Drosophila RNA methyltransferase, DmHen1, modifies germline piRNAs and single-stranded siRNAs in RISC. Curr Biol. 2007;17:1265–72. doi: 10.1016/j.cub.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Yang Z, Yu B, Liu J, Chen X. Methylation protects miRNAs and siRNAs from a 3′-end uridylation activity in Arabidopsis. Curr Biol. 2005;15:1501–7. doi: 10.1016/j.cub.2005.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stapleton M, et al. The Drosophila gene collection: identification of putative full-length cDNAs for 70% of D. melanogaster genes. Genome Res. 2002;12:1294–300. doi: 10.1101/gr.269102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malone CD, Hannon GJ. Small RNAs as guardians of the genome. Cell. 2009;136:656–68. doi: 10.1016/j.cell.2009.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Provost P, et al. Ribonuclease activity and RNA binding of recombinant human Dicer. EMBO J. 2002;21:5864–74. doi: 10.1093/emboj/cdf578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomari Y, Du T, Zamore PD. Sorting of Drosophila small silencing RNAs. Cell. 2007;130:299–308. doi: 10.1016/j.cell.2007.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kok KH, Ng MH, Ching YP, Jin DY. Human TRBP and PACT directly interact with each other and associate with dicer to facilitate the production of small interfering RNA. J Biol Chem. 2007;282:17649–57. doi: 10.1074/jbc.M611768200. [DOI] [PubMed] [Google Scholar]
- 39.Lu C, Meyers BC, Green PJ. Construction of small RNA cDNA libraries for deep sequencing. Methods. 2007;43:110–7. doi: 10.1016/j.ymeth.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Pall GS, Codony-Servat C, Byrne J, Ritchie L, Hamilton A. Carbodiimide-mediated cross-linking of RNA to nylon membranes improves the detection of siRNA, miRNA and piRNA by northern blot. Nucleic Acids Res. 2007;35:e60. doi: 10.1093/nar/gkm112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tuschl T, Zamore PD, Lehmann R, Bartel DP, Sharp PA. Targeted mRNA degradation by double-stranded RNA in vitro. Genes Dev. 1999;13:3191–7. doi: 10.1101/gad.13.24.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fahlgren N, et al. Computational and analytical framework for small RNA profiling by high-throughput sequencing. RNA. 2009;15:992–1002. doi: 10.1261/rna.1473809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.