Abstract
Sexual behavior is a natural reward for many rodent species, and it often includes chemosensory-directed components. Chemosensory stimuli themselves may also be rewarding. Conditioned place preference (CPP) is one paradigm frequently used to test the rewarding properties of a range of stimuli. Males and females of several rodent species show a CPP for sexual behavior, however, it is currently unknown whether sexual behavior can induce a CPP in male Syrian hamsters. As male Syrian hamsters are an animal model commonly used for investigation of the neurobiology of sexual behavior, understanding the rewarding components of sexual stimuli will better direct future research on brain regions and neurotransmitters involved in these behaviors. Experiment 1 tested the prediction that male hamsters show a CPP for sexual behavior. Female chemosensory stimuli are essential for the display of sexual behavior in male hamsters, however, the rewarding properties of female chemosensory stimuli contained in vaginal secretions (VS) are uncertain. Therefore, Experiment 2 tested the prediction that male hamsters show a CPP for VS. This study is the first demonstration that both sexual behavior and VS induce a CPP in male hamsters. Thus, female chemosensory stimuli are a natural reward in a species that is dependent on these stimuli for reproductive fitness.
Keywords: Conditioned place preference, reproductive behavior, sexual, social, reward, pheromone, chemosensory, olfaction
Introduction
Sexual activity is an effective natural reward in rodents; male rodents approach and prefer opposite sex conspecifics and perform operant behavior tasks for sexual stimuli (Agmo, 2003; Crawford et al., 1993; Everitt, 1990; Murphy, 1973; Paredes, 2009). Another paradigm used extensively to assess the rewarding properties of a range of stimuli is conditioned place preference (CPP) (Schechter and Calcagnetti, 1993; Tzschentke, 1998; Tzschentke, 2007). CPP is a form of classical conditioning in which animals develop a preference for a distinctive environment associated with a rewarding stimulus such as an addictive drug, food, water, copulation, and other social behaviors (Kelley and Berridge, 2002; Pfaus and Phillips, 1991; Robbins and Everitt, 1996; Schechter and Calcagnetti, 1993; Tzschentke, 1998; Tzschentke, 2007). CPP procedures have demonstrated the rewarding value of sexual behavior in male and female rats and mice, and in female hamsters (Agmo and Berenfield, 1990; Agmo and Picker, 1990; Hughes et al., 1990; Mehrara and Baum, 1990; Miller and Baum, 1987; Popik et al., 2003; Harding and McGinnis, 2004; Kippin and van der Kooy, 2003). Specific components of male sexual behavior, including somatosensory stimuli resulting from intromission and ejaculation, are rewarding in and of themselves (Agmo and Gomez, 1993; Kudwa et al., 2005; Tenk et al., 2009). Chemosensory stimuli encountered during sexual behavior may also be rewarding, but this component of sexual interactions has not been as thoroughly investigated. Of the several rodents species for which sexual behavior induces a CPP, only mice are known to show a CPP for volatile (but not non-volatile) chemosensory stimuli derived from the opposite sex (Agustin-Pavon et al., 2007; Martínez-Ricós et al., 2007; Pankevich et al., 2006; Pierman et al., 2006).
Male Syrian hamsters are ideal for investigating the rewarding properties of chemosensory stimuli, because hamsters are unique from rats and mice in their utter reliance on female chemosensory stimuli for the initiation of sexual behavior (Coppola and O’Connell, 1988; Johnston, 1986; Murphy and Schneider, 1970). For example, olfactory bulb removal eliminates odor preferences and copulatory behavior in sexually experienced male hamsters (Murphy and Schneider, 1970; Petrulis, 2009; Petrulis and Johnston, 1995; Powers and Winans, 1975). Vaginal secretions (VS) are the primary source of female chemosensory stimuli (Murphy and Schneider, 1970). Sexually-naïve male hamsters show an unconditioned attraction to VS (Johnston, 1974; Landauer et al., 1977), and a preference for VS over male odors (Maras and Petrulis, 2006). In fact, VS are such powerful stimuli that male hamsters will mount another castrated or anesthetized male scented with VS (Johnston, 1986; Murphy, 1973). The data showing that female chemosensory stimuli are strongly attractive to male hamsters suggest that neural processing of female chemosensory stimuli encountered during anogenital investigation may be a specific component of sexual behavior that is rewarding for male hamsters (Wood, 2004). Surprisingly, however, male hamsters fail to bar press for female hamster VS, but operant conditioning may not have been well-suited to detect responses for VS (Coppola and O’Connell, 1988). Whether sexual behavior or its components can induce a CPP in male Syrian hamsters is unknown.
Syrian hamsters are an animal model that has yielded important insights into the mechanisms underlying the integration of sensory stimuli and hormones that are crucial to the expression of appropriate behaviors during sexual and social interactions (Petrulis, 2009; Sato et al., 2008; Wood, 2004). As we continue to investigate the neurobiology underlying sexual behavior using this model, we must determine whether sexual behavior and related chemosensory stimuli are rewarding to better direct research on brain regions and neurotransmitters involved in these behaviors. To assess the rewarding components of sexual behavior in the male Syrian hamster model, Experiment 1 tested the prediction that male hamsters demonstrate a CPP for sexual behavior, and Experiment 2 tested the prediction that male hamsters demonstrate a CPP for VS. The present study is the first to report that both sexual behavior and VS alone induce a CPP in male hamsters.
Methods
Animals
Forty sexually naïve adult male Syrian hamsters (Mesocricetus auratus) obtained from Harlan Sprague-Dawley laboratories (Madison, WI) were experimental subjects. All testing began when hamsters were 61–70 days old. Twenty-eight ovariectomized adult female hamsters, approximately 12 months old, were primed with estradiol benzoate and progesterone, 52 h and 4 h respectively, prior to use as stimulus females. Before use in Experiment 1, the females were tested for receptivity by placing a non-experimental, sexually experienced male from our colony in the females’ home cage until the female showed lordosis but before the male achieved an intromission. All hamsters were singly housed in clear polycarbonate cages (30.5 × 10.2 × 20.3 cm) with ad libitum access to food (Teklad Rodent diet no. 8640, Harlan, Madison, WI) and water. Male and female hamsters were housed in separate temperature- and humidity-controlled vivaria with a reversed light:dark cycle (14:10; lights off at 1400 h). Hamsters were treated in accordance with the National Institute of Health Guide for Care and Use of Laboratory Animals, and protocols were approved by the Michigan State University Institutional Animal Care and Use Committee.
Experimental Design
Place preference conditioning occurred in an apparatus with three distinct compartments (Med Associates, St. Albans, VT). The middle compartment (12 × 21 × 21 cm) was gray with a smooth Plexiglas floor and was connected to the two outer compartments (28 × 21 × 21 cm) by manually controlled sliding guillotine doors. One outer compartment was white, with metal grid flooring. Fresh pine pellets were placed in the waste pan beneath the floor before each conditioning session. The other outer compartment was black, with black scalloped solid Plexiglas flooring, and scented with a 2% glacial acetic acid solution swabbed along the top of the walls and ceiling before each conditioning session. These contextual cues were used in both Experiment 1 and 2, as we felt that that an olfactory contextual cue was important for this olfactory-oriented species and would not interfere with conditioning for VS. Pilot experiments determined that these conditions reduced initial preferences for one or the other compartment. Time spent in each compartment was recorded using MED-PC software connected to infrared photobeams spaced at five cm intervals along the bottom of the apparatus. All conditioning and tests were conducted under dim red light at least one hour into the dark phase.
An initial place preference test, here called the pretest, was used to determine each hamster’s initial compartment preference. Following a 5-min habituation period in the middle gray compartment, the doors were raised and the hamster could move freely throughout the apparatus for 15 min. The outer compartment in which the hamster spent the most time was defined as the initially preferred compartment. Experimental and control groups, n = 10/group, were matched such that average initial preferences were similar across the groups. Following the pretest, the hamsters received a series of 30 min conditioning sessions, one session per day on consecutive days. Stimulus-paired (sex behavior, SB or vaginal secretions, VS) or no-stimulus conditioning sessions occurred on alternating days, beginning with the no-stimulus conditioning session. Although three stimulus-paired sessions were sufficient to induce a CPP for SB (Experiment 1), in a pilot study using similar procedures, three stimulus-paired sessions were insufficient to induce a CPP for VS. Therefore, in Experiment 2 we increased the number of stimulus-paired sessions to five with the idea that more exposure to VS would lead to a CPP. During the no-stimulus conditioning sessions, hamsters in both the experimental and control groups were taken directly from their homecages and placed into their initially preferred compartments, where they remained alone for 30 min. During stimulus-paired conditioning sessions, hamsters in the experimental group were taken from their homecages, placed in the initially non-preferred compartments and given access to SB or VS. The hamsters in the control group were also placed in their initially non-preferred compartments, but were not given the stimulus so that their experiences in the two compartments were comparable. The CPP apparatus was cleaned thoroughly with 25% ethanol following each conditioning session and test. Twenty-four hours after the last conditioning session, hamsters were tested for their place preference following the same procedure used for the pretest.
Experiment 1: CPP for Sexual Behavior
In Experiment 1, the stimulus tested for its rewarding value was sexual behavior with a receptive female hamster. In the SB-paired conditioning sessions, a female was placed into the compartment immediately before the male. Hamsters were observed for the duration of these sessions to verify that each male performed sexual behavior. They engaged in bouts of sexual behavior, including ejaculations, throughout the 30-min session, although behavior was not quantified. Hamsters in the control group were alone in compartments for both conditioning sessions, while hamsters in the SB group were alone in the compartment during the no-stimulus sessions. Six total conditioning sessions occurred, including three no-stimulus and three stimulus-paired sessions. The experiment took place over eight consecutive days including the pretest and test days.
Experiment 2: CPP for Vaginal Secretions
Experiment 2 was similar to Experiment 1, except the experimental stimuli tested for rewarding value were VS. VS were collected by vaginally palpating 14 hormone-primed female hamsters approximately two hours before conditioning sessions began. Because both volatile and non-volatile components of VS have important, and potentially different, roles in male hamster sexual behavior, the paradigm was designed to ensure that both were present throughout the conditioning session. Several pilot studies were performed to determine the optimal method for VS delivery, including cotton swabs and Eppendorf tubes. The latter proved to be the most effective at keeping VS moist and allowing for continued exposure to volatile cues throughout the conditioning session. Approximately 20 μl of VS were applied to water-moistened cotton gauze packed into a 2 ml Eppendorf tube. The tube was out of reach of the male, taped to the top of the back wall of the initially non-preferred compartment in VS-paired conditioning sessions for the VS group. Empty Eppendorf tubes were used for the control group in all conditioning sessions and for the VS group in the no-stimulus conditioning sessions. To ensure exposure to non-volatile components of VS, the remaining VS were mixed with 1.5 ml of mineral oil (see Woodley and Baum, 2004), and approximately 50 μl of this mixture was applied with a metal spatula directly onto the nose of the hamsters in the VS group immediately before being placed in the VS-paired compartment. The concentration of VS used in this experiment far exceeds that which is attractive to male hamsters, but mimics the amount available to a male hamster upon anogenital investigation of an estrous female (Macrides et al., 1984). Clean oil was applied to the nose of hamsters in the control group for all conditioning sessions and in the VS group for no-stimulus conditioning sessions. Ten total conditioning sessions occurred, including five no-stimulus and five stimulus-paired sessions. The experiment took place over 12 consecutive days including the pretest and test days.
Data Analysis
To assess whether the stimuli (Experiment 1 SB; Experiment 2 VS) induced a CPP, data from the pretests and final tests were used to calculate a preference score, defined as time in the stimulus-paired compartment/(time in stimulus-paired compartment + time in no-stimulus compartment), and a difference score, defined as the time in the no-stimulus compartment − time in the stimulus-paired compartment (Dominguez and Hull, 2005; Martínez and Paredes, 2001; Meerts et al., 2010; Meerts and Clark, 2007, 2009a, b, c; Meisel and Joppa, 1994; Parada et al., 2010; Paredes and Alonso, 1997; Tenk et al., 2009). Paired t-tests were used to evaluate the change in preference score and difference score pre- and post-conditioning, and the alpha level was set at p < 0.05 (Martínez and Paredes, 2001; Meerts et al., 2010; Meerts and Clark, 2007, 2009a, b, c; Meisel and Joppa, 1994; Parada et al., 2010; Paredes and Alonso, 1997; Tenk et al., 2009). One methodological concern when using CPP is that the change in time spent in the compartments may be due to habituation across the conditioning sessions. This concern was addressed in these experiments by the use of the control group that received the same experience in the testing apparatus without any stimulus pairings (Meisel and Joppa, 1994).
Results
Experiment 1: CPP for Sexual Behavior
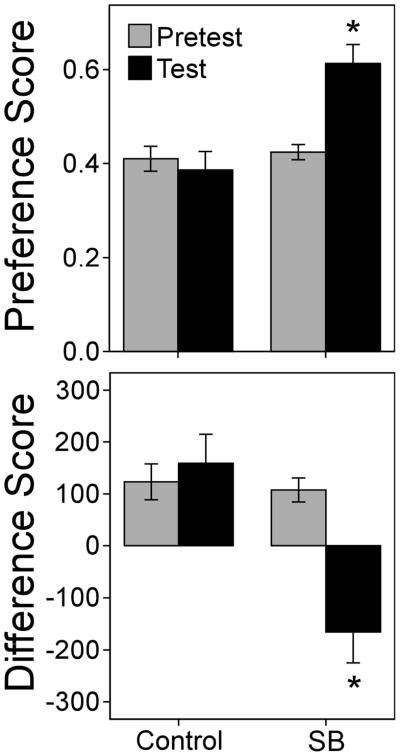
Male hamsters in the sexual behavior group showed a CPP for the SB-paired compartment, whereas control hamsters did not (Figure 1). Paired t-tests showed that the preference score increased significantly for the SB group, t(9)=−4.26, p<0.01, but not the controls, t(9)=.49, p=0.63. Likewise, the difference score decreased significantly for the SB group, t(9)=4.11, p<0.01, but not the controls, t(9)=−.54, p=0.60.
Fig. 1.
Mean±SEM preference score (top) and difference score (bottom) on pretest (gray bars) and test (black bars) for control and SB groups, n=10 per group. Asterisks indicate that the SB group showed a CPP for the compartment associated with sexual behavior; there was a significant change in preference score and difference score for the SB group but not control group, p<0.05.
Experiment 2: CPP for Vaginal Secretions
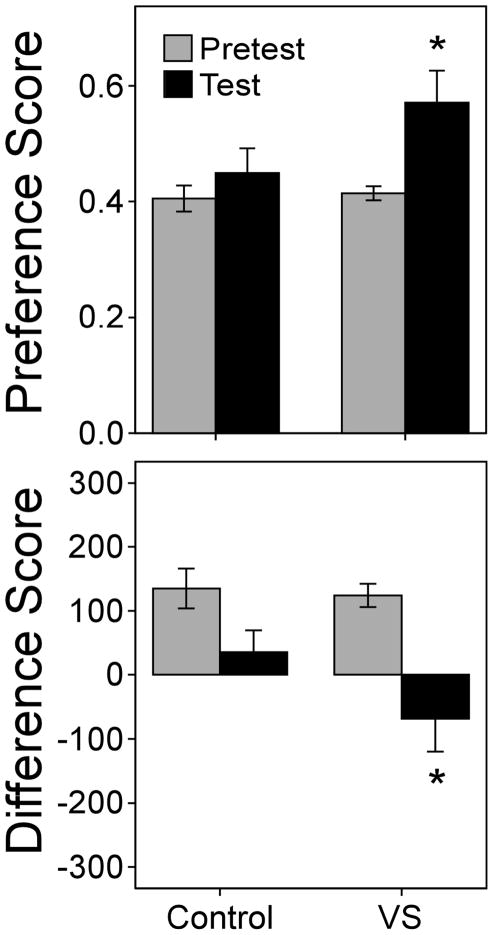
Male hamsters that were given VS showed a CPP for the VS-paired compartment, whereas control hamsters did not (Figure 2). Paired t-tests showed that the preference score increased significantly for the VS group, t(9)=−2.772, p<0.05, but not the controls, t(9)=−0.89, p=0.40. Likewise, the difference score decreased significantly for the VS group, t(9)=0.35, p<0.01, but not the controls, t(9)=2.07, p=0.07.
Fig. 2.
Mean±SEM preference score (top) and difference score (bottom) on pretest (gray bars) and test (black bars) for control and VS groups, n=10 per group. Asterisks indicate that the VS group showed a CPP for the compartment associated with VS; there was a significant change in preference score and difference score for the VS group but not control group, p<0.05.
Discussion
The present study is the first to demonstrate that male Syrian hamsters show a CPP for both sexual behavior with a receptive female hamster and VS, reinforcing the concept that sexual activity is a strong natural reward for male rodents (Pfaus and Phillips, 1991). Moreover, the CPP for VS indicates that female chemosensory stimuli are an unconditioned reward to male hamsters because the males were sexually naïve and VS had not been previously associated with sexual activity. Fewer conditioning sessions may be needed to induce a CPP for sexual behavior than for VS because the additional somatosensory, auditory and visual aspects likely contribute to the rewarding properties of sexual behavior. However, differences in VS stimulus intensity when presented by an experimenter versus by a female hamster during a sexual encounter may also be a factor. The present data agree with those from mice showing that chemosensory stimuli are rewarding (Agustin-Pavon et al., 2007; Martínez-Ricós et al., 2007; Pankevich et al., 2006). Mice are also sensitive to chemosensory stimuli in certain reproductive contexts (e.g., pregnancy block and puberty acceleration (Bruce, 1959; Vandenbergh, 1973). Together, the findings from hamsters and mice suggest that the behavioral salience and rewarding value of a chemosensory stimulus may be positively correlated.
Rewarding Components of VS
The specific compounds in VS that are rewarding remain to be identified. The chemosensory stimulus used in the present study contained both volatile and non-volatile components. The volatile component of VS, dimethyl disulfide, is thought to promote anogenital investigation of the female (Macrides et al., 1977; Pfeiffer and Johnston, 1994; Singer et al., 1976), whereas the non-volatile component, aphrodisin, is thought to stimulate sexual arousal and copulatory behavior (Macrides et al., 1977; Singer et al., 1987; Singer et al., 1984). Generally speaking, the different components bind different receptors; volatile olfactants bind receptors located in the main olfactory epithelium (MOE), whereas non-volatile molecules bind those in the vomeronasal organ (VNO) (Keller et al., 2009; Keverne, 2004; Meredith, 1991; Rowe and Edwards, 1972). In male hamsters, the VNO is important for the display of sexual behavior and plasma testosterone responses to VS (Meredith, 1986). MOE lesions have little effect on sexual behavior whereas VNO lesions alone reduce sexual behavior in sexually naïve male hamsters; combined lesions completely eliminate male courtship and sexual activity (Powers and Winans, 1975). The accessory olfactory system also appears to be critical for chemosensory reward in mice (Agustin-Pavon et al., 2007; Martínez-Ricós et al., 2007; Pankevich et al., 2006). Although additional studies are needed to pinpoint the specific components of VS and their site of action that lead to a CPP in male hamsters, the literature suggests that the accessory olfactory system is important to motivate sexual behavior in male hamsters.
Neural Mechanisms of Sexual Reward
Dopamine (DA) and opioids have both been implicated in the rewarding aspects of rodent sexual behavior. DA is released in the medial preoptic area (MPOA) and nucleus accumbens following copulation and exposure to chemosensory stimuli in male rodents (Asmus, 1994; Balfour et al., 2004; Damsma et al., 1992; Mas et al., 1990; Meisel et al., 1993; Mitchell and Gratton, 1992; Pfaus et al., 1990; Sato et al., 1995; Schulz et al., 2003; Wenkstern et al., 1993). In fact, the MPOA DA release in hamsters requires exposure to female chemosensory stimuli (Triemstra et al., 2005), further reinforcing the obligatory nature of chemosensory stimuli for sexual responses in this species. DA is implicated in CPP for sexual behavior in hamsters, as CPP induced by sexual behavior in female hamsters is blocked by administration of a D2 receptor antagonist (Meisel et al., 1996, but see Agmo and Berenfeld, 1990; Agustin-Pavon et al., 2007 for different findings in other rodent species). Opioid receptor activation has also been observed after male rat copulation (Balfour et al., 2004). Studies on the role of opioids in sexual reward have consistently shown that opiate receptor antagonists block CPP for sexual behavior in male rats (Agmo and Berenfeld, 1990; Agmo and Gomez, 1993; Mehrara and Baum, 1990; Miller and Baum, 1987). Although opioids may modulate DA activity in reward-related responses in male rats (Balfour et al., 2004), more research is needed to determine the role of dopamine and opioids in mediating the rewarding value of female hamster VS to male hamsters.
Testosterone is another potential mediator of chemosensory reward because sexual stimuli elicit an acute increase in plasma testosterone (Romeo et al., 1998; Pfeiffer and Johnson, 1994), and testosterone is intrinsically rewarding. For example, hamsters self-administer intra-cerebroventricularly-delivered testosterone (Wood, 2004) and rats and mice show a CPP for systemic testosterone injections (Alexander et al., 1994; De Beun et al., 1992; Arnedo et al., 2000). In addition, castration prevents formation of CPP for an estrous female rat (Harding and McGinnis, 2004). Thus, the sex- and VS-induced increase in testosterone in hamsters (Romeo et al., 1998; Pfeiffer and Johnson, 1994) may be a neuroendocrine response that contributes to the rewarding aspects of these stimuli.
In summary, adult male hamsters show a CPP for sexual behavior and VS, indicating that the chemosensory stimuli encountered during anogenital investigation is one specific component of sexual behavior in male hamsters that is rewarding. It is noteworthy that the chemosensory stimuli are rewarding in both hamsters and mice, as the animals had never before associated the chemosensory stimuli with sexual behavior. Both these species are heavily influenced by chemosensory stimuli in different aspects of social behavior, suggesting that there may be an evolutionary connection between the dependence on these stimuli for social behaviors and their intrinsically rewarding properties.
Acknowledgments
This research was supported by MH068764. Many thanks to Dr. Heather Molenda-Figueira, Kayla De Lorme, Rayson Figueira, Jane Venier, Margaret Mohr, Josh Olszewicz, Christine Azizkhan, Bradley Lawrence, Chrisanthi Karanikas, Ashley Pratt, Allison Melkonian, Dana Gradl, and Nichole Griep for their assistance in behavior testing and paradigm design. We also thank Dr. Ann S. Clark for her thoughtful comments and Dr. Michael J. Baum for his suggestion on delivering VS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnedo MT, Salvador A, Martinez-Sanchez S, Gonzalez-Bono E. Rewarding properties of testosterone in intact male mice: a pilot study. Pharmacol Biochem Behav. 2000;65:327–32. doi: 10.1016/s0091-3057(99)00189-6. [DOI] [PubMed] [Google Scholar]
- Agmo A. Unconditioned sexual incentive motivation in the male norway rat (rattus norvegicus) J Comp Psych. 2003;117:3–14. doi: 10.1037/0735-7036.117.1.3. [DOI] [PubMed] [Google Scholar]
- Agmo A, Berenfeld R. Reinforcing properties of ejaculation in the male rat: Role of opioids and dopamine. Behav Neurosci. 1990;104:177–182. doi: 10.1037//0735-7044.104.1.177. [DOI] [PubMed] [Google Scholar]
- Agmo A, Gomez M. Sexual reinforcement is blocked by infusion of naloxone in to the medial preoptic area. Behav Neurosci. 1993;107:812–818. doi: 10.1037//0735-7044.107.5.812. [DOI] [PubMed] [Google Scholar]
- Agmo A, Picker Z. Catecholamines and the initiation of sexual behavior in male rats without sexual experience. Pharm, bio, and beh. 1990;35:327–334. doi: 10.1016/0091-3057(90)90164-d. [DOI] [PubMed] [Google Scholar]
- Agustin-Pavon C, Martinez-Ricos J, Martinez-Garcia F, Lanuza E. Effects of dopaminergic drugs on innate pheromone-mediated reward in female mice: A new case of dopamine-independent ‘liking’. Behav Neurosci. 2007;121:920–932. doi: 10.1037/0735-7044.121.5.920. [DOI] [PubMed] [Google Scholar]
- Alexander GM, Packard MG, Hines M. Testosterone has rewarding affective properties in male rats: Implications for the biological basis of sexual motivation. Behav Neurosci. 1994;108:424–428. doi: 10.1037//0735-7044.108.2.424. [DOI] [PubMed] [Google Scholar]
- Asmus SE, Newman Sarah Winans. Colocalization of tyrosine hydroxylase and fos in the male syrian hamster brain following different states of arousal. J Neurobiol. 1994;25:156–168. doi: 10.1002/neu.480250207. [DOI] [PubMed] [Google Scholar]
- Balfour ME, Yu L, Coolen LM. Sexual behavior and sex-associated environmental cues activate the mesolimbic system in male rats. Neuropsychopharmacology. 2004;29:718–730. doi: 10.1038/sj.npp.1300350. [DOI] [PubMed] [Google Scholar]
- Bruce HM. An exteroceptive block to pregnancy in the mouse. Nature. 1959;184:105. doi: 10.1038/184105a0. [DOI] [PubMed] [Google Scholar]
- Coppola DM, O’Connell RJ. Are pheromones their own reward? Physiol Behav. 1988;44:811–816. doi: 10.1016/0031-9384(88)90067-4. [DOI] [PubMed] [Google Scholar]
- Crawford LL, Holloway KS, Domjan M. The nature of sexual reinforcement. J Exp Anal Behav. 1993;60:55–66. doi: 10.1901/jeab.1993.60-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsma G, Pfaus JG, Wenkstern D, Phillips AG, Fibiger HC. Sexual behavior increases dopamine transmission in the nucleus accumbens and striatum of male rats: Comparison with novelty and locomotion. Behav Neurosci. 1992;106:181–191. doi: 10.1037//0735-7044.106.1.181. [DOI] [PubMed] [Google Scholar]
- De Beun R, Jansen E, Slangen JL, van de Poll Ne. Testosterone as appetitive and discriminative stimulus in rats: Sex- and dose-dependent effects. Physiol Behav. 1992;52:629–634. doi: 10.1016/0031-9384(92)90389-j. [DOI] [PubMed] [Google Scholar]
- Dominguez JM, Hull EM. Dopamine, the medial preoptic area, and male sexual behavior. Physiol Behav. 2005;86:356–368. doi: 10.1016/j.physbeh.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Everitt BJ. Sexual motivation: A neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci Biobehav Rev. 1990;14:217–232. doi: 10.1016/s0149-7634(05)80222-2. [DOI] [PubMed] [Google Scholar]
- Harding SM, McGinnis MY. Androgen receptor blockage in the MPOA or VMN: Effects on male sociosexual behaviors. Physiol Behav. 2004;81:671–680. doi: 10.1016/j.physbeh.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Hughes AM, Everitt BJ, Herbert J. Comparative effects of preoptic area infusions of opioid peptides, lesions and castration on sexual behaviour in male rats: Studies of instrumental behavior, conditioned place preference and partner preference. Psychopharmacology. 1990;102:243–256. doi: 10.1007/BF02245929. [DOI] [PubMed] [Google Scholar]
- Johnston RE. Sexual attraction function of golden hamster vaginal secretion. Behavioral Biology. 1974;12:111–117. doi: 10.1016/s0091-6773(74)91101-8. [DOI] [PubMed] [Google Scholar]
- Johnston RE. Effects of female odors on the sexual behavior of male hamsters. Beh and Neural Bio. 1986;46:168–188. doi: 10.1016/s0163-1047(86)90654-0. [DOI] [PubMed] [Google Scholar]
- Keller M, Baum MJ, Brock O, Brennan PA, Bakker J. The main and the accessory olfactory systems interact in the control of mate recognition and sexual behavior. Beh Brain Res. 2009;200:268–276. doi: 10.1016/j.bbr.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: Relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keverne EB. Importance of olfactory and vomeronasal systems for male sexual function. Physiol Behav. 2004;83:177–187. doi: 10.1016/j.physbeh.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Kippin TE, van der Kooy D. Excitotoxic lesions of the tegmental pedunculopontine nucleus impair copulation in naive male rats and block the rewarding effects of copulation in experienced male rats. Eur J Neurosci. 2003;18:2581–2591. doi: 10.1046/j.1460-9568.2003.02918.x. [DOI] [PubMed] [Google Scholar]
- Kudwa AE, Dominguez-Salazar E, Cabrera DM, Sibley DR, Rissman EF. Dopamine d5 receptor modulates male and female sexual behavior in mice. Psychopharmacology. 2005;180:206–214. doi: 10.1007/s00213-005-2150-5. [DOI] [PubMed] [Google Scholar]
- Landauer MR, Banks EM, Carter CS. Sexual preferences of male hamsters (mesocricetus auratus) for conspecifics in different endocrine conditions. Horm Behav. 1977;9:193–202. doi: 10.1016/0018-506x(77)90055-1. [DOI] [PubMed] [Google Scholar]
- Macrides F, Johnson PA, Schneider SP. Responses of the male golden hamster to vaginal secretion and dimethyl disulfide: Attraction versus sexual behavior. Behav Bio. 1977;20:377–386. doi: 10.1016/s0091-6773(77)90931-2. [DOI] [PubMed] [Google Scholar]
- Macrides F, Singer AG, Clancy AN, Goldman BD, Agosta WC. Male hamster investigatory and copulatory responses to vaginal discharge: Relationship to the endocrine status of females. Physiol Behav. 1984;33:633–637. doi: 10.1016/0031-9384(84)90383-4. [DOI] [PubMed] [Google Scholar]
- Maras PM, Petrulis A. Chemosensory and steroid-responsive regions of the medial amygdala regulate distinct aspects of opposite-sex odor preference in male syrian hamsters. Eur J Neurosci. 2006;24:3541–3552. doi: 10.1111/j.1460-9568.2006.05216.x. [DOI] [PubMed] [Google Scholar]
- Martínez-Ricós J, Agustín-Pavón C, Lanuza E, Martínez-García F. Intraspecific communication through chemical signals in female mice: Reinforcing properties of involatile male sexual pheromones. Chem Senses. 2007;32:139–148. doi: 10.1093/chemse/bjl039. [DOI] [PubMed] [Google Scholar]
- Martínez I, Paredes RG. Only self-paced mating is rewarding in rats of both sexes. Horm Behav. 2001;40:510–517. doi: 10.1006/hbeh.2001.1712. [DOI] [PubMed] [Google Scholar]
- Mas M, Gonzalez-Mora JL, Louilot A, Sole C, Guadalupe T. Increased dopamine release in the nucleus accumbens of copulating male rats as evidenced by in vivo voltammetry. Neurosci Lett. 1990;110:303–308. doi: 10.1016/0304-3940(90)90864-6. [DOI] [PubMed] [Google Scholar]
- Meerts SH, Boisvert EM, Spjut KA, Clark AS. Paced mating behavior persists in rats with vaginocervical lidocaine. Physiol Behav. 2010;99:139–141. doi: 10.1016/j.physbeh.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerts SH, Clark AS. Female rats exhibit a conditioned place preference for nonpaced mating. Horm Behav. 2007;51:89–94. doi: 10.1016/j.yhbeh.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Meerts SH, Clark AS. Artificial vaginocervical stimulation induces a conditioned place preference in female rats. Horm Behav. 2009a;55:128–132. doi: 10.1016/j.yhbeh.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerts SH, Clark AS. Conditioned place preference for mating is preserved in rats with pelvic nerve transection. Behav Neurosci. 2009b;123:539–546. doi: 10.1037/a0015267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerts SH, Clark AS. Lesions of the medial preoptic area interfere with the display of a conditioned place preference for vaginocervical stimulation in rats. Behav Neurosci. 2009c;123:752–757. doi: 10.1037/a0016077. [DOI] [PubMed] [Google Scholar]
- Mehrara BJ, Baum MJ. Naloxone disrupts the expression but not the acquisition by male rats of a conditioned place preference response for an oestrous female. Psychopharmacology. 1990;101:118–125. doi: 10.1007/BF02253728. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Camp DM, Robinson TE. A microdialysis study of ventral striatal dopamine during sexual behavior in female syrian hamsters. Beh Brain Res. 1993;55:151–157. doi: 10.1016/0166-4328(93)90111-3. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Joppa MA. Conditioned place preference in female hamsters following aggressive or sexual encounters. Physiol Behav. 1994;56:1115–1118. doi: 10.1016/0031-9384(94)90352-2. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Joppa MA, Rowe RK. Dopamine receptor antagonists attenuate conditioned place preference following sexual behavior in female syrian hamsters. Eur J Pharmacol. 1996;309:21–24. doi: 10.1016/0014-2999(96)00389-5. [DOI] [PubMed] [Google Scholar]
- Meredith M. Vomeronasal organ removal before sexual experience impairs male hamster mating behavior. Physiol Behav. 1986;36:737–743. doi: 10.1016/0031-9384(86)90362-8. [DOI] [PubMed] [Google Scholar]
- Meredith M. Sensory processing in the main and accessory olfactory systems: Comparisons and contrasts. J of Ster Biochem and Mol Bio. 1991;39:601–614. doi: 10.1016/0960-0760(91)90258-7. [DOI] [PubMed] [Google Scholar]
- Miller RL, Baum MJ. Naloxone inhibits mating and conditioned place preference for an estrous female in male rats soon after castration. Pharmacol Biochem Behav. 1987;26:781–789. doi: 10.1016/0091-3057(87)90611-3. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, Gratton A. Mesolimbic dopamine release elicited by activation of the accessory olfactory system: A high speed chronoamperometric study. Neurosci Lett. 1992;140:81–84. doi: 10.1016/0304-3940(92)90687-3. [DOI] [PubMed] [Google Scholar]
- Murphy MR. Effects of female hamster vaginal discharge on the behavior of male hamsters. Behav Bio. 1973;9:367–375. doi: 10.1016/s0091-6773(73)80185-3. [DOI] [PubMed] [Google Scholar]
- Murphy MR, Schneider GE. Olfactory bulb removal eliminates mating behavior in the male golden hamster. Science. 1970;167:302–304. doi: 10.1126/science.167.3916.302. [DOI] [PubMed] [Google Scholar]
- Pankevich DE, Cherry JA, Baum MJ. Accessory olfactory neural fos responses to a conditioned environment area blocked in male mice by vomeronasal organ removal. Physiol Behav. 2006;87:781–788. doi: 10.1016/j.physbeh.2006.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada M, Chamas L, Censi S, Coria-Avila G, Pfaus JG. Clitoral stimulation induces conditioned place preference and fos activation in the rat. Horm Behav. 2010;57:112–118. doi: 10.1016/j.yhbeh.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Paredes RG. Evaluating the neurobiology of sexual reward. Institute for Laboratory Animals Research Journal. 2009;50:15–27. doi: 10.1093/ilar.50.1.15. [DOI] [PubMed] [Google Scholar]
- Paredes RG, Alonso A. Sexual behavior regulated (paced) by the female induces conditioned place preference. Behav Neurosci. 1997;111:123–128. doi: 10.1037//0735-7044.111.1.123. [DOI] [PubMed] [Google Scholar]
- Petrulis A. Neural mechanisms of individual and sexual recognition in syrian hamsters (mesocricetus auratus) Behav Brain Res. 2009;200:260–267. doi: 10.1016/j.bbr.2008.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrulis A, Johnston RE. A reevaluation of dimethyl disulfide as a sex attractant in golden hamsters. Physiol Behav. 1995;57:779–784. doi: 10.1016/0031-9384(94)00332-7. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Damsma G, Nomikos GG, Wenkstern D, Blaha CD, Phillips AG, Fibiger HC. Sexual behavior enhances central dopamine transmission in the male rat. Brain Res. 1990;530:345–348. doi: 10.1016/0006-8993(90)91309-5. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Phillips AG. Role of dopamine in anticipatory and consummatory aspects of sexual behavior in the male rat. Behav Neurosci. 1991;105:727–743. doi: 10.1037//0735-7044.105.5.727. [DOI] [PubMed] [Google Scholar]
- Pfeiffer CA, Johnston RE. Hormonal and behavioral responses of male hamsters to females and female odors: Roles of olfaction, the vomeronasal system, and sexual experience. Physiol Behav. 1994;55:129–138. doi: 10.1016/0031-9384(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Pierman S, Tirelli E, Douhard Q, Baum MJ, Bakker J. Male aromatase knockout mice acquire a conditioned place preference for cocaine but not for contact with an estrous female. Behav Brain Res. 2006;174:64–69. doi: 10.1016/j.bbr.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Popik P, Wrobel M, Rygula R, Bisaga A, Bespalov AY. Effects of memantine, an nmda receptor antagonist, on place preference conditioning with drug and nondrug reinforcers in mice. Behav Pharm. 2003;14:237–244. doi: 10.1097/00008877-200305000-00008. [DOI] [PubMed] [Google Scholar]
- Powers JB, Winans SS. Vomeronasal organ: Critical role in mediating sexual behavior of the male hamster. Science. 1975;187:961–963. doi: 10.1126/science.1145182. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Parfitt DB, Richardson HN, Sisk CL. Pheromones elicit equivalent levels of fos-immunoreactivity in prepubertal and adult male syrian hamsters. Horm Behav. 1998;34:48–55. doi: 10.1006/hbeh.1998.1463. [DOI] [PubMed] [Google Scholar]
- Rowe FA, Edwards DA. Olfactory bulb removal: Influences on the mating behavior of male mice. Physiol Behav. 1972;8:37–41. doi: 10.1016/0031-9384(72)90127-8. [DOI] [PubMed] [Google Scholar]
- Sato SM, Schulz KM, Sisk CL, Wood RI. Adolescents and androgens, receptors and rewards. Horm Behav. 2008;53:647–658. doi: 10.1016/j.yhbeh.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Wada H, Horita H, Suzuki N, Shibuya A, Adachi H, Kato R, Tsukamoto T, Kumamoto Y. Dopamine release in the medial preoptic area during male copulatory behavior in rats. Brain Res. 1995;692:66–70. doi: 10.1016/0006-8993(95)00656-b. [DOI] [PubMed] [Google Scholar]
- Schechter MD, Calcagnetti DJ. Trends in place preference conditioning with a cross-indexed bibliography; 1957–1991. Neurosci Biobehav Rev. 1993;17:21–41. doi: 10.1016/s0149-7634(05)80228-3. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Richardson HN, Romeo RD, Morris JA, Lookingland KJ, Sisk CL. Medial preoptic area dopaminergic responses to female pheromones develop during puberty in the male syrian hamster. Brain Res. 2003;988:139–145. doi: 10.1016/s0006-8993(03)03358-4. [DOI] [PubMed] [Google Scholar]
- Singer AG, Agosta WC, Clancy AN, Macrides F. The chemistry of vomeronasally detected pheromones: Characterization of an aphrodisiac protein. Ann N Y Acad Sci. 1987;519:287–298. doi: 10.1111/j.1749-6632.1987.tb36304.x. [DOI] [PubMed] [Google Scholar]
- Singer AG, Agosta WC, O’Connell RJ, Pfaffmann C, Bowen DV, Field FH. Dimethyl disulfide: An attractant pheromone in hamster vaginal secretion. Science. 1976;191:948–950. doi: 10.1126/science.1251205. [DOI] [PubMed] [Google Scholar]
- Singer AG, Clancy AN, Macrides F, Agosta WC. Chemical studies of hamster vaginal discharge: Male behavioral responses to a high molecular weight fraction require physical contact. Physiol Behav. 1984;33:645–651. doi: 10.1016/0031-9384(84)90385-8. [DOI] [PubMed] [Google Scholar]
- Tenk CM, Wilson H, Zhang Q, Pitchers KK, Coolen LM. Sexual reward in male rats: Effects of sexual experience on conditioned place preferences associated with ejaculation and intromissions. Horm Behav. 2009;55:93–97. doi: 10.1016/j.yhbeh.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triemstra JL, Nagatani S, Wood RI. Chemosensory cues are essential for mating-induced dopamine release in MPOA of male syrian hamsters. Neuropsychopharmacology. 2005;30:1436–1442. doi: 10.1038/sj.npp.1300685. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: A comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (cpp) paradigm: Update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Vandenbergh JG. Acceleration and inhibition of puberty in female mice by pheromones. J of reproduction and fertility Supplement. 1973;19:411–419. [PubMed] [Google Scholar]
- Wenkstern D, Pfaus JG, Fibiger HC. Dopamine transmission increases in the nucleus accumbens of male rats during their first exposure to sexually receptive female rats. Brain Res. 1993;618:41–46. doi: 10.1016/0006-8993(93)90426-n. [DOI] [PubMed] [Google Scholar]
- Wood RI. Reinforcing aspects of androgens. Physiol Behav. 2004;83:279–289. doi: 10.1016/j.physbeh.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Woodley SK, Baum MJ. Differential activation of glomeruli in the ferret’s main olfactory bulb by anal scent gland odours from males and females: An early step in mate identification. Eur J Neurosci. 2004;20:1025–1032. doi: 10.1111/j.1460-9568.2004.03571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]