Abstract
We have shown that the combination of pentostatin (P), cyclophosphamide (C) and rituximab (R) achieves an overall response (OR) rate >90% with more than 40% complete responses (CR) in patients with untreated CLL. To evaluate if the tolerability of this regimen could be enhanced without sacrificing efficacy, we conducted a phase II trial of P and R without cyclophosphamide, using a higher P dose (4 mg/m2). Among the 33 patients enrolled, 82% were male, median age was 65 (9 patients ≥70 years) and 64% were Rai stage III-IV. The OR rate was 76% with 9 CR (27%), 5 nPR, and 11 PRs. At the time of this analysis, 29/33 patients are still alive and the median follow up for patients still alive is 14 months (range: 1-34.8 months). Four (12%) patients experienced grade 3 or higher hematologic toxicity and 5 (15%) experienced grade 3 or higher non-hematologic toxicity. Comparison of this trial to our previous PCR trial showed that patients treated with PCR had a higher OR rate (91% vs. 76%) and CR rate (41% vs. 27%) compared to patients treated with PR. Median treatment-free survival for all accrued patients was notably longer in PCR treated patients compared to PR (30 vs. 16 months). These findings suggest that increasing the dose of the purine nucleoside analogue does not eliminate the need for cyclophosphamide in chemoimmunotherapy for treatment of CLL.
Keywords: pentostatin, rituximab, cyclophosphamide, chemoimmunotherapy, response rates, B-CLL
INTRODUCTION
The treatment of B-cell chronic lymphocytic leukemia (CLL) has had significant advances over the last decade. In particular, the improvement in both overall response (OR) rate and complete response (CR) rates with chemoimmunotherapy (CIT) for patients with previously untreated CLL has been most impressive. CLL CIT regimens typically consist of a purine nucleoside (fludarabine or pentostatin), an alkylating agent (cyclophosphamide), and a monoclonal antibody (rituximab). The most widely tested regimens in phase II trials include fludarabine and rituximab (FR); fludarabine, cyclophosphamide and rituximab (FCR); and pentostatin, cyclophosphamide and rituximab (PCR) (1-3). Although the OR rates for these regimens are all ≥90%, the CR rates observed in phase II trials with these regimens were quite varied, with a CR rate of 72% for FCR, 33% for FR (prior to rituximab consolidation), and 41% for PCR (2-4).
Despite their virtues, these CIT regimens have substantial toxicity, particularly among patients with compromised performance status or comorbid health problems. Treatment related toxicity limits use of CIT in many CLL patients, since the median age at the time of diagnosis of CLL is approximately 70 years. In general, there are three solutions to this dilemna: 1) limit CIT administration to younger patients; 2) select only elderly patients with good performance status and preserved organ function to receive CIT; 3) develop CIT regimens with a high degree of efficacy that can be tolerated by elderly patients.
Condensed Abstract.
Pentostatin based combination treatments for previously untreated CLL are most optimal when cyclophosphamide is added to pentostatin and rituximab. While pentostatin and rituximab is effective in previously untreated CLL, the level of response is reduced compared to PCR but does benefit from reduced toxicity.
As part of our effort to pursue the last of these strategies, we developed the PCR regimen utilizing pentostatin (P, 2 mg/m2) ,cyclophosphamide (C, 600 mg/m2) and rituximab (R, 375 mg/m2) (3). Unlike FCR,(1, 5, 6) the PCR regimen using the 2 mg/m2 pentostatin dose is well tolerated by older patients >70 years as well as those with mildly reduced creatinine clearance (7). Nonetheless, PCR remains an aggressive treatment regimen that cannot be tolerated by all elderly CLL patients in need of treatment. In an effort to improve tolerability while preserving efficacy, we conducted a follow-up trial testing the combination of pentostatin and rituximab without cyclophosphamide and employing a higher pentostatin dose (4 mg/m2).
PATIENTS AND METHODS
Patient Eligibility
This phase II study enrolled patients at Mayo Clinic, Rochester, MN, and The Ohio State University, Columbus, OH. The clinical trial included correlative laboratory analysis designed to assess risk-stratification parameters and estimation of minimal residual disease (MRD). Patients were required to have previously untreated CLL and to meet the National Cancer Institute (NCI) Working Group 1996 criteria (8) to initiate treatment. Patients had relatively preserved renal (Cr ≤ 1.5 × upper limit of normal [ULN]) and hepatic function (bilirubin ≤3.0 × ULN), an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 3, and no other neoplasms with the exception of squamous- or basal-cell skin cancers or carcinoma in situ of the cervix. The protocol was reviewed and approved by the Institutional Review Board at each participating institution. All patients were required to provide written informed consent prior to entry on this study, in accordance with the Declaration of Helsinki.
Study Design
Study participants received 6 cycles of pentostatin (4 mg/m2) and rituximab administered intravenously every 21 days. Pentostatin was given on the first day of each cycle following infusion of rituximab. Rituximab was given at 100 mg IV on day 1, then 375 mg/m2 IV on days 3 and 5 of the first treatment cycle. During cycles 2 to 6, rituximab was given at 375 mg/m2 as a single IV infusion on day 1. Treatment was discontinued in cases of progressive disease (PD) or excessive toxicity. Prophylactic sulfamethoxazole-trimethoprim and acyclovir were given to all patients for 1 year starting with the first cycle of therapy. All patients were also given filgrastim on day 3 of each treatment cycle for 5 consecutive days or until the total neutrophil count was >1.0 × 109/L (1000/μL) for 2 consecutive days.
Dose Modifications
The first cycle was administered at full dose regardless of pre-existing cytopenias. At the time of retreatment for cycles 2-6, pentostatin was delayed for one week for absolute neutrophil count (ANC) < 1,000 or platelets (PLT) < 50,000. If counts recovered to these levels after the delay, P was given at full dose. If counts partially recovered (ANC: 500 – 1,000 or PLT: 30,000 - 50,000), P was resumed at 1 mg/m2. If counts did not recover to at least ANC > 500 and/or PLT > 30,000 after one week delay or patients experienced ≥ grade 3 organ toxicity or grade 4 gastrointestinal toxicity, treatment was discontinued. PLT and hemoglobin adverse events were graded according to the CLL Working Group grading scale for hematologic toxicity (8). All other adverse events were graded according to the NCI Common Toxicity Criteria (v2.0).
Risk-Stratification Parameters
The following prognostic factors were evaluated on pretreatment specimens: CD38 expression, fluorescence in situ hybridization (FISH)-detectable cytogenetic defects, immunoglobulin heavy-chain variable region (IgVH) mutation status, and ZAP-70 expression. These risk-stratification parameters were performed on peripheral blood cells from all patients as they enrolled in the clinical trial and were evaluated using previously published methods (9).
Criteria for Response Level
Response was evaluated using the NCI Working Group 1996 criteria (8). Response bone marrow biopsies were collected 2 months after day 1 of cycle 6 and at the time of first relapse. After completion of therapy, patients were followed every 3 months for 2 years, then every 6 months for 3 years, or until progression.
Estimation of minimal residual disease (MRD)
Patients with CR, nodular PR, or PR also had peripheral blood MRD evaluation for CLL B-cells using 2-color and 3-color flow cytometry at the time of the 2-month post treatment bone marrow collection. Peripheral blood mononuclear cells (PBMCs) were separated from heparinized venous blood by density gradient centrifugation. To remove adherent cells, PBMCs were suspended in RPMI 1640 supplemented with 10% fetal calf serum and incubated in plastic dishes at 37°C for 1 hour prior to collection of nonadherent cells. These isolated PBMCs were then used for flow cytometry analysis. The 2-color flow involved determination of the percentage of cells positive for CD5 and CD19. The PBMCs were stained by a 2-color (fluorescein isothiocyanate [FITC]/phycoerythrin [PE]) flow cytometric assay. FITC- and PE-conjugated anti-CD19 or anti-CD5 (BD Biosciences, San Diego, CA) was used to determine the presence of CD5+/CD19+ B cells. Mouse IgG1 and IgG2 (BD Biosciences) were included as isotype controls. A 2-color flow cytometry negative status was defined as patients with ≤ 1% positive CD5+/CD19+ cells. The 3-color flow cytometry was a combination of the anti-CD5 and CD19 reagents as described above with addition of an anti-CD79b monoclonal antibody (10). Our laboratory has determined that normal individuals have ≤ 1% positive CD5+/CD19+/CD79b-or dim cells with 3-color flow cytometry. Thus, to be considered negative for the 3-color MRD assay, patients had to have ≤1% CD5+/CD19+/CD79b-or dim cells in their blood.
Statistical Methods
A single-stage phase II trial with an interim analysis was conducted in patients with previously untreated B-cell CLL to assess the toxicity and efficacy of a combination of pentostatin and rituximab (PR). The primary endpoint was the CR rate. A total of 30 evaluable patients were required to test the null hypothesis that the true CR rate is at most 25% versus the alternative that it is at least 50%. Assuming that the number of CRs is binomially distributed, the study had 87% power, with a 5% Type I error rate. A patient was considered evaluable for response if they were eligible and received treatment regardless of the number of cycles they received. The proportion of CR was estimated by the number of patients who achieved a CR divided by the total number of evaluable patients. Ninety-five-percent confidence intervals were calculated for the true CR rate using the Duffy-Santner approach. Duration of response was defined as the time from the date a response was first achieved until the date of progression. Treatment-free survival (TFS) was defined as the time from the date of completion of treatment until the date of initiation of subsequent treatment or death. The distributions of duration of response and treatment-free survival were estimated using the method of Kaplan-Meier (11). Differences between groups were evaluated using the Fisher exact test, Wilcoxon rank sum test, and Kruskal-Wallis test where appropriate. Overall significance was declared for p < 0.05.
RESULTS
Patient demographics
Thirty-three patients were enrolled at the Mayo Clinic and the Ohio State University between July 2005 and February 2008. All 33 patients were eligible. The characteristics of the 33 patients relative to our previous PCR cohort are presented in Table 1. Twenty-seven (82%) patients were male, and the median age was 65 years (range: 45-81 years). Nine patients (27%) were 70 years or older. The majority of patients (21 of 33, 64%) had Rai stage III or IV disease at study entry. Prognostic testing revealed that 36% of patients were CD38 positive, 50% of patients were ZAP-70 positive, and 39% of patients were IgVH unmutated. Chromosomal analysis by FISH found that 61%, 27%, and 3% of patients had 1, 2, or 3 FISH defects, respectively. FISH risk category using the Dohner classification (12) is shown in Table 1. Overall, patient characteristics were similar to the prior PCR cohort (9) with the exception that PR treated patients were less likely to be IgVH unmutated (39% vs. 71%; p=0.006) and had higher leukocyte counts (median 127 × 109/L vs. 79 × 109/L; p=0.04). Bone marrow biopsy at study entry revealed that 36% of patients had a diffuse pattern of marrow involvement while 3%, 6% and 55% had a nodular, interstitial, or nodular and interstitial pattern, respectively
Table 1.
Patient Characteristics
| |
PR Trial N=33 |
PCR Trial N=64 |
P value |
|---|---|---|---|
| Age, median(range) | 65 years (45-81) | 63 years (38-80) | 0.34 |
| ≥70 years (%) |
27% |
28% |
|
| Male |
82% |
77% |
0.61 |
| ECOG PS | |||
| 0 | 76% | 53% | 0.09 |
| 1 | 24% | 37% | |
| 2 | 0 | 8% | |
| 3 |
0 |
2% |
|
| Rai stage 0 | 0 | 5% | 0.46 |
| Rai stage I-II | 36% | 42% | |
| Rai stage III-IV |
64% |
53% |
|
| White Cell Count, median(range) | 127 × 109/L (8-430) | 79 × 109/L (11-519) | 0.04 |
| <50 × 109/L | 28% | 36% | |
| 50-149 × 109/L | 25% | 44% | |
| >150 × 109/L |
47% |
20% |
|
| Serum B2-microglobulin, median(range) | 3.80 (2.0-8.2) | 3.97 (1.8-13.5) | 0.81 |
| >2 × Upper Limit Normal(%) |
58% |
57% |
|
| CD38 Positive |
36% |
35% |
1.00 |
| ZAP-70 Positive |
50% |
35% |
0.26 |
| IgVH Unmutated |
39% |
70% |
0.006 |
| FISH | NA* | ||
| Normal | 9% | 11% | |
| 13q- | 43% | 35% | |
| +12 | 24% | 21% | |
| 6q- | 0 | 2% | |
| 11q- | 18% | 22% | |
| 17p- | 3% | 6% | |
| Other |
3% |
3% |
|
| Number of FISH defects | |||
| 0 | 9% | 11% | 0.93 |
| 1 | 61% | 54% | |
| 2 | 27% | 29% | |
| 3 |
3% |
6% |
|
| Bone Marrow Pattern | |||
| Diffuse | 36% | 50% | 0.30 |
| Nodular | 3% | 7% | |
| Interstitial | 6% | 9% | |
| Nodular + Interstitial | 55% | 33% |
1 The p-value is not given due to small subgroup sizes
Toxicity and tolerability
At the time of this report, all patients have completed active treatment. Patients received a median of 6 cycles (range: 1-6) of treatment with 28 of 33 patients (85%) completing the intended 6 cycles of therapy. Five patients discontinued therapy early due to pleural effusions (n=1), grade 4 neutropenia (n=1), CMV reactivation (n=1), refusal (n=1), and other unspecified medical problems (n=1). While on treatment, 6 of 33 patients (18%) experienced a dose delay due to hematologic toxicity (4 patients), bronchitis (1 patient), or urinary retention (1 patient). One patient (3%) had P held for one cycle due to a rash, and a second patient (3%) had a P dose reduction for one cycle due to a hematologic adverse event.
Overall, 5 patients (15%) had a grade 3 or 4 hematologic adverse event, and 8 patients (24%) had a grade 3 or 4 non-hematologic adverse event. Focusing only on adverse events deemed at least possibly related to treatment, 4 patients (12%) had grade 3 or 4 hematologic toxicity and 5 patients (15%) had grade 3 or 4 non-hematologic toxicity. All grade 3 or 4 toxicities are summarized in Table 2. The most common grade 3 or 4 toxicities were hematologic, where 2 patients had grade 3 thrombocytopenia and 2 patients had grade 3 or 4 neutropenia. One patient developed grade 2 hemolysis during cycle 2 that was felt to be possibly related to study treatment. This patient remained on treatment and completed 5 cycles before going off study for CMV reactivation. Despite these hematologic toxicities, no patients required red blood cell or platelet transfusion during therapy.
Table 2.
Grade 3 or 4 toxicities (Adverse events at least possibly related to treatment)
| Toxicity | Grade 3 N (%) | Grade 4 N (%) |
|---|---|---|
| Dyspnea | 0 | 1 (3%) |
| Headache | 1 (3%) | 0 |
| Effusion-Pleural | 1 (3%) | 0 |
| Hypotension | 1 (3%) | 0 |
| Neutropenia | 1 (3%) | 1 (3%) |
| Pneumonitis | 1 (3%) | 0 |
| Hypersensitivity | 1 (3%) | 0 |
| Tumor Lysis Syndrome | 1 (3%) | 0 |
| Infection with ANC | 1 (3%) | 0 |
| Thrombocytopenia | 2 (6%) | 0 |
| Platelets | 2 (6%) | 0 |
Response to therapy
All 33 patients were evaluable for response. Nine patients achieved a complete response (CR, 27%, 95% CI: 17-55%) by the NCI Working Group 1996 criteria (8). In addition, 5 patients (15%) achieved a nodular partial response (nPR) and 11 patients (33%) achieved a partial response (PR). Thus, the overall response rate (CR + nPR + PR) was 76% (95% CI: 59-88%). Of the 25 patients who responded to treatment, the median time to first documentation of any response (i.e., CR or PR) was 2 cycles (range: 1-6 cycles).
At the time of this analysis, 4 patients (12%) have died and 17 patients (52%) have experienced disease progression. Deaths were due to disease progression (2 patients), stroke (1 patient), and bilobar pneumonia with sepsis and acute renal failure (1 patient). Median follow-up time for patients still alive is 14 months (range: 1-34.8 months). Median duration of response is 10.8 months (95% CI: 9.7 – 19.6 months), and 12 of the 25 responders have progressed. Thirteen of 33 patients have been retreated after completing PR therapy. The median time to subsequent treatment for these thirteen patients was 13.6 months (range: 2.1 – 25.5 months). Median treatment-free survival is 15.8 months (95% CI: 13.6 – 20.6 months).
Assessment of MRD following treatment
Twenty-five patients were assessed for MRD using both 2-color and 3-color flow cytometry (see Methods). The 2-color and 3-color flow cytometry gave similar results, with only 3 patients (2 CR, 1 PR) achieving a MRD negative status in the peripheral blood on both the 2-color and 3-color flow cytometry.
Correlative prognostic factors for predicting treatment outcome
To evaluate whether PR was equally effective in older patients (≥ 70 years) and individuals with various prognostic risk markers, we analyzed response by patient's pretreatment characteristics. Comparison of response and TFS were performed for the following groups: IgVH mutational status (≤ 98% vs. > 98% from germline sequence where ≤ 98% was designated as unmutated), CD38+ vs. CD38- patients (30% cutoff), ZAP-70+ vs. ZAP-70- patients (20% cutoff), and comparison of high risk [del(17p13.1) or del(11q22.3)] vs. low/intermediate risk [del(13q14), trisomy (+) 12, or normal] FISH groups. Similar to our prior results with PCR (3), no relationship between attaining a CR or nPR was observed for any prognostic factors. The estimated median TFS was longer in patients who had low/intermediate risk FISH as compared to patients who had 11q22.3 deletion (median 15.9 vs. 7.9 months, p=0.001) or patients with high risk FISH (median 15.9 months vs 10.8 months, p=0.01). No differences in TFS have yet been seen between risk groups for IgVH mutation, CD38, ZAP-70, or age (Table 3).
Table 3.
Risk Factors by Response Group
| |
CR/nPR N (%) |
Not CR/nPR N (%) |
P value |
TFS Estimate months (95% CI) |
Log rank p value |
|---|---|---|---|---|---|
| Age | |||||
| < 70 | 13 (54%) | 11 (46%) | 0.05 | 15.8 (11.2-23.7) | 0.13 |
| ≥ 70 |
1 (11%) |
8 (89%) |
|
14.8 (6.5-18.9) |
|
| CD38 | |||||
| Negative (≤ 30) | 10 (48%) | 11 (52%) | 0.49 | 15.8 (11.2-23.7) | 0.43 |
| Positive (>30) |
4 (33%) |
8 (67%) |
|
20.6 (5.0- 20.6) |
|
| Zap 70 * | |||||
| Negative (≤ 20) | 5 (31%) | 11 (69%) | 0.47 | 15.7 (13.6-15.9) | 0.26 |
| Positive (>20) |
8 (50%) |
8 (50%) |
|
20.6 (10.8-25.5) |
|
| Ig VH Mutation ** | |||||
| Unmutated | 5 (42%) | 7 (58%) | 1.0 | 15.9 (6.5-23.7) | 0.66 |
| Mutated |
9 (47%) |
10 (53%) |
|
15.7 (10.8 - 18.9) |
|
| FISH*** | |||||
| Low/Intermediate Risk | 13 (50%) | 13 (50%) | 15.9 (15.7-23.7) | ||
| High Risk | 1 (14%) | 6 (86%) | 0.20a | 10.8 (3.2-18.9) | 0.01a |
| Del(11q22.3) | 1(17%) | 5(83%) | 0.20b | 7.9 (3.2-13.6) | 0.001b |
Zap missing one observation
Mutation missing two observations
FISH low/intermediate risk: del (13q14), trisomy (+) 12, or normal; FISH high risk: del(17p13.1) or del(11q22.3).
p-values for low/intermediate risk vs. high risk ((del(17p13.1) or del(11q22.3))
p-values for low/intermediate risk vs. del(11q22.3) alone
Comparison to PCR
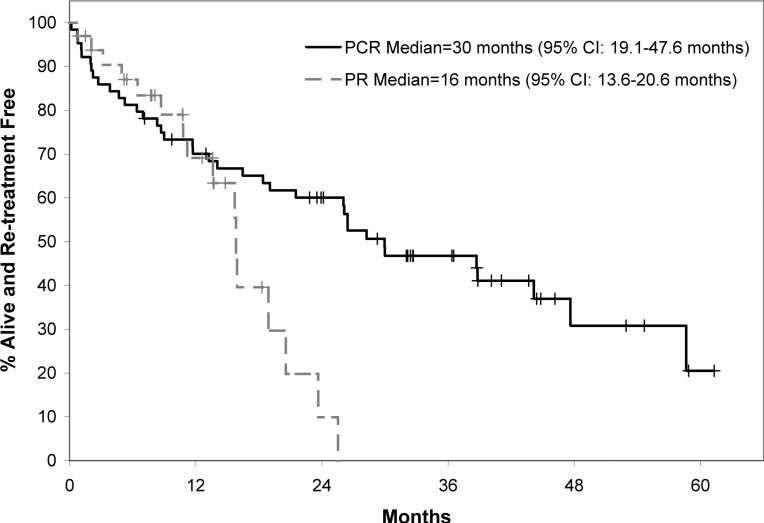
It should be emphasized that analysis of outcomes among PR treated patients relative to PCR represent comparison of two sequential phase II trials rather than a randomized study. However, the eligibility criteria were nearly identical between PR and PCR and enrollment accrued at the same two academic centers over a very short time interval. Patients in the two studies were generally similar with respect to all demographic and prognostic characteristics, although patients in the PR study were less likely to be IgVH unmutated. Based on these similarities, we conducted a comparison of the response rates and treatment-free survival (TFS) of all evaluable patients on the two studies in an exploratory manner. Patients treated with PCR had a higher overall response rate (91% vs. 76%) and CR rate (41% vs. 27%) compared to patients treated with PR. Similarly, the median TFS was notably longer in patients treated with PCR as compared to PR (30 months vs. 16 months; Figure 1). For all responders, the median duration of response on PCR was 34 months (95% CI: 29- not reached) and the median duration of response for PR was 10.8 months (95% CI: 9.1-19.2). Since indirect evidence from other trials suggests that cyclophosphamide may be particularly important in patients with 11q-, we also compared PR to PCR among those without this cytogenetic defect. The differences in OR, CR, and TFS were of similar magnitude to those of the overall cohort when analysis was limited to patients without 11q-. The grade 3+ hematologic toxicity rate was 52% in the PCR patients and 12% in the PR patients. The grade 3+ non-hematologic toxicity rate was 34% in the PCR patients and 15% in the PR patients.
Fig. 1.
Figure shows the treatment free survival of all patients treated on the phase II trial of PCR (n=64) and the phase II trial of PR (n=33) regardless of response to treatment.
DISCUSSION
The development of chemoimmunotherapy has been a major treatment advance for patients with chronic lymphocytic leukemia. The present trial of pentostatin and rituximab was designed to enhance the tolerability of pentostatin-based chemoimmunotherapy in the hopes of broadening the availability of CIT to elderly patients with chronic lymphocytic leukemia. The trial design eliminated cyclophosphamide while doubling the dose of pentostatin from 2 mg to 4 mg/m2 but retained a similar schedule and length. Indeed the PR regimen was generally well tolerated with limited grade 3-4 toxicities. As expected, toxicity was primarily hematologic. However in this current trial with PR as with the PCR trial there was no need for blood product transfusion support during induction. Despite these virtues, the complete remission rate and treatment-free survival of the PR approach appear inferior to that of our prior reported experience with PCR. These differences were independent of the presence of 11q- on FISH or the presence of bulky nodal disease suggesting that cyclophosphamide remains an important component of pentostatin-based chemoimmunotherapy (CIT) platforms and that increasing the purine nucleoside analog dose does not eliminate the need for cyclophosphamide in CIT.
Although the comparisons between PR and PCR are not a randomized trial, the nearly identical eligibility criteria and patient characteristics of the patients enrolled on the two studies lend credibility to the comparison. The findings are also consistent with the results of several randomized phase III trials that indicate the combination of cyclophosphamide and fludarabine leads to superior overall and complete response rates as well as progression-free survival as compared to fludarabine monotherapy (13-15). The recently completed German CLL study group CLL 8 trial suggests that FCR appears superior to FC combination therapy (17). The complete remission rate in the FCR arm of the CLL 8 trial was 44.5%, which is substantially lower than the 72% CR rate in the phase II experience of the MD Anderson Cancer Center (1). In the CLL8 trial while FCR was superior to FC for CR the overall survival was not (17). Of note only the FCR regimen reported by M.D Anderson and the FCR-lite trials have reported CRs over 70 percent (1,17) Importantly the ongoing North American Intergroup Trial directly compares the FCR and FR regimens and will help provide definitive evidence regarding the need of cyclophosphamide in fludarabine/rituximab based chemoimmunotherapy
One potential issue for the F-based CIT regimens is that there are often significant cytopenias both during and after the use of FCR for CLL patients resulting in over half of the patients over 65 not being able to receive 6 complete cycles (4). In contrast, we have found that the PCR regimen is well tolerated by CLL patients over the age of 70 (7). In addition, we have recently found that as many as 25% of all CLL patients will not fit eligibility criteria for entry into CLL clinical trials and this group does not subsequently fare well clinically (19). Clearly while CIT approaches have significant clinical impact for previously untreated CLL patients in terms of OR and CR there is a need to have a continued evaluation via randomized clinical trials of the efficacy of each CIT regimen for CLL population where age and co-morbidity are taken into consideration.
Despite the apparently inferior CR rate and TFS of PR relative to PCR, the favorable toxicity profile of PR suggests that PR may be a treatment option for elderly patients who would not be candidates for three drug combination chemoimmunotherapy. In this regard, it should be noted that the OR and CR rates observed with first-line PR appear to be substantially better than those observed with first line chlorambucil (20-22) or lenalidomide (23, 24) and similar to those of first-line alemtuzumab (20) or bendamustine (22).
In summary, the pentostatin/rituximab combination is a well tolerated and highly active treatment regimen, certainly in comparison to other more traditional regimens such as Chlorambucil, for patients with previously untreated CLL. Nonetheless, the regimen appears to have lower overall and complete remission rates as well as a shorter treatment-free survival when compared to the PCR regimen. Continued efforts to develop new chemoimmunotherapy platforms that can be tolerated by elderly CLL patients are needed.
Acknowledgements
This work was supported in part by the following grants from the NIH/National Cancer Institute, CA95241, NEK; CA113408 TDS. We also thank Mr. Edson Spencer and the Donner Family Foundation for their philanthropic support.
Financial disclosures: Research support is received according to the following notations: Authors NE Kay and TD Shanafelt and CS Zent receive support from Genentech; CS Zent receives support from Bayer; NE Kay and TD Shanafelt receive support from Hospira.
Footnotes
Informed consent: All patients were required to provide written informed consent prior to entry on this study, in accordance with the Declaration of Helsinki.
REFERENCES
- 1.Keating MJ, O'Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–88. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 2.Byrd JC, Peterson BL, Morrison VA, et al. Randomized phase 2 study of fludarabine with concurrent versus sequential treatment with rituximab in symptomatic, untreated patients with B-cell chronic lymphocytic leukemia: results from Cancer and Leukemia Group B 9712 (CALGB 9712). Blood. 2003;101:6–14. doi: 10.1182/blood-2002-04-1258. [DOI] [PubMed] [Google Scholar]
- 3.Kay NE, Shanafelt TD, Byrd JC, Grever MR. Community-based phase II trial of PCR for CLL/SLL patients. Cancer Biother Radiopharm. 2007;22:713–4. doi: 10.1089/cbr.2007.0424. author reply 5-7. [DOI] [PubMed] [Google Scholar]
- 4.Tam CS, Keating MJ. Chemoimmunotherapy of chronic lymphocytic leukemia. Best Pract Res Clin Haematol. 2007;20:479–98. doi: 10.1016/j.beha.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Ferrajoli A, O'Brien S, Wierda W. Treatment of Patients with CLL 70 Years Old and Older: A Single Center Experience of 142 Patients. Leukemia and Lymphoma. 2005;46:S86. al. e. [Google Scholar]
- 6.Tam CS, O'Brien S, Wierda W, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112:975–80. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shanafelt TD, Lin T, Geyer SM, et al. Pentostatin, cyclophosphamide, and rituximab regimen in older patients with chronic lymphocytic leukemia. Cancer. 2007;109:2291–8. doi: 10.1002/cncr.22662. [DOI] [PubMed] [Google Scholar]
- 8.Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–7. [PubMed] [Google Scholar]
- 9.Kay NE, O'Brien SM, Pettitt AR, Stilgenbauer S. The role of prognostic factors in assessing ‘high-risk’ subgroups of patients with chronic lymphocytic leukemia. Leukemia. 2007;21:1885–91. doi: 10.1038/sj.leu.2404802. [DOI] [PubMed] [Google Scholar]
- 10.Rawstron AC, Kennedy B, Evans PA, et al. Quantitation of minimal disease levels in chronic lymphocytic leukemia using a sensitive flow cytometric assay improves the prediction of outcome and can be used to optimize therapy. Blood. 2001;98:29–35. doi: 10.1182/blood.v98.1.29. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan E, Meier P. Nonparametric estimation for incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 12.Stilgenbauer S, Bullinger L, Lichter P, Dohner H. Genetics of chronic lymphocytic leukemia: genomic aberrations and V(H) gene mutation status in pathogenesis and clinical course. Leukemia. 2002;16:993–1007. doi: 10.1038/sj.leu.2402537. [DOI] [PubMed] [Google Scholar]
- 13.Flinn IW, Neuberg DS, Grever MR, et al. Phase III trial of fludarabine plus cyclophosphamide compared with fludarabine for patients with previously untreated chronic lymphocytic leukemia: US Intergroup Trial E2997. J Clin Oncol. 2007;25:793–8. doi: 10.1200/JCO.2006.08.0762. [DOI] [PubMed] [Google Scholar]
- 14.Hallek M, Schmitt B, Wilhelm M, et al. Fludarabine plus cyclophosphamide is an efficient treatment for advanced chronic lymphocytic leukaemia (CLL): results of a phase II study of the German CLL Study Group. Br J Haematol. 2001;114:342–8. doi: 10.1046/j.1365-2141.2001.02959.x. [DOI] [PubMed] [Google Scholar]
- 15.Catovsky D, Richards S, Matutes E, et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): a randomised controlled trial. Lancet. 2007;370:230–9. doi: 10.1016/S0140-6736(07)61125-8. [DOI] [PubMed] [Google Scholar]
- 16.Byrd JC, Rai K, Peterson BL, et al. Addition of rituximab to fludarabine may prolong progression-free survival and overall survival in patients with previously untreated chronic lymphocytic leukemia: an updated retrospective comparative analysis of CALGB 9712 and CALGB 9011. Blood. 2005;105:49–53. doi: 10.1182/blood-2004-03-0796. [DOI] [PubMed] [Google Scholar]
- 17.Hallek M, Fingerle-Rowson G, Fink AM, et al. Immunochemotherapy with Fludarabine (F), Cyclophosphamide (C), and Rituximab (R) (FCR) Versus Fludarabine and Cyclophosphamide (FC) Improves Response Rates and Progression-Free Survival (PFS) of Previously Untreated Patients (pts) with Advanced Chronic Lymphocytic Leukemia (CLL) Blood. 2008;112 Abst #325. [Google Scholar]
- 18.Foon KA, Boyiadzis M, Land SR, et al. Chemoimmunotherapy with low-dose fludarabine and cyclophosphamide and high dose rituximab in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol. 2009;27:498–503. doi: 10.1200/JCO.2008.17.2619. [DOI] [PubMed] [Google Scholar]
- 19.Thurmes P, Call T, Slager S, et al. Comorbid conditions and survival in unselected, newly diagnosed patients with chronic lymphocytic leukemia. Leukemia & lymphoma. 2008;49:49–56. doi: 10.1080/10428190701724785. [DOI] [PubMed] [Google Scholar]
- 20.Hillmen P, Skotnicki AB, Robak T, et al. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J Clin Oncol. 2007;25:5616–23. doi: 10.1200/JCO.2007.12.9098. [DOI] [PubMed] [Google Scholar]
- 21.Rai KR, Peterson BL, Appelbaum FR, et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. [see comments]. N Engl J Med. 2000;343:1750–7. doi: 10.1056/NEJM200012143432402. [DOI] [PubMed] [Google Scholar]
- 22.Knauf WU, Lissitchkov T, Aldaoud A, et al. Bendamustine Versus Chlorambucil as First-Line Treatment in B Cell Chronic Lymphocytic Leukemia: An Updated Analysis from An International Phase III Study. ASH Annual Meeting Abstracts. 2008;112:2091. [Google Scholar]
- 23.Chen C, Paul H, Xu W, et al. A Phase II Study of Lenalidomide in Previously Untreated, Symptomatic Chronic Lymphocytic Leukemia (CLL). ASH Annual Meeting Abstracts. 2008;112:44. [Google Scholar]
- 24.Ferrajoli A, O'Brien S, Wierda W, et al. Lenalidomide as Initial Treatment of Elderly Patients with Chronic Lymphocytic Leukemia (CLL). ASH Annual Meeting Abstracts. 2008;112:45. [Google Scholar]