Summary
Neuroendocrine (NE) phenotype, seen in >30% of prostate adenocarcinomas (PCa), and NE prostate tumors are implicated in aggressive prostate cancer. Formation of NE prostate tumors in the TRAMP mouse model was suppressed in mice lacking the ubiquitin ligase Siah2, which regulates HIF-1α availability. Cooperation between HIF-1α and FoxA2, a transcription factor expressed in NE tissue, promotes recruitment of p300 to transactivate select HIF-regulated genes, Hes6, Sox9 and Jmjd1a. These HIF-regulated genes are highly expressed in metastatic PCa and required for hypoxia-mediated NE phenotype, metastasis in PCa and the formation of NE tumors. Tissue-specific expression of FoxA2 combined with Siah2-dependent HIF-1α availability enables a transcriptional program required for NE prostate tumor development and NE phenotype in PCa.
Significance
Prostate adenocarcinomas (PCa) with neuroendocrine (NE) phenotype and NE prostate tumors are associated with poor prognosis and androgen independence. Here we demonstrate that formation of NE tumors and metastasis of PCa require the ubiquitin ligase Siah2. Through its role in the control of HIF-1α availability, Siah2 enables cooperation between HIF-1α and the NE-specific transcription factor FoxA2. Genes induced by HIF-1α/FoxA2 cooperation are expressed in NE lesions and in metastatic human PCa and required for formation of the NE phenotype, for metastasis of human PCa, and for the development of NE tumors. Tissue-specific cooperation between transcription factors that promote NE phenotype and prostate tumor development offers a paradigm for the development, progression and potential targeting of aggressive prostate tumors.
Introduction
Prostate cancer is the second leading cause of cancer deaths among men in Western nations. Among the metastatic forms of prostate adenocarcinoma (PCa) are those that express neuroendocrine (NE) markers, often referred to as neuroendocrine differentiation (NED) or NE phenotype. NED is seen in over 30% of PCa and is associated with poor prognosis and androgen independence (Cindolo et al., 2007). A small percentage (0.5–2%) of human prostate tumors develops as highly aggressive NE tumors, which have a 35% survival rate in 2 years (Cindolo et al., 2007; Sella et al., 2000). Factors implicated in NED of LNCaP prostate cancer cells in vitro include IL-6 treatment (Deeble et al., 2001), androgen removal (Yuan et al., 2006) and ionizing radiation (Deng et al., 2008). In transgenic animals, T antigen expression or inactivation of p53 and Rb has been associated with prostate NE tumors (Huss et al., 2007; Zhou et al., 2006).
FoxA2, a member of the FoxA subfamily of forkhead box transcription factor, is expressed in mouse prostate NE carcinomas (Chiaverotti et al., 2008; Mirosevich et al., 2006) and NE foci of human PCa (Mirosevich et al., 2006). HIF-1α, the master regulator of the hypoxia response, is also expressed in NE tumors (Monsef et al., 2007). HIF-1α is regulated under normoxia by the E3 ligase pVHL and is also regulated under mild hypoxia (2–5% O2) by the ubiquitin ligase Siah2. Siah2 controls prolyl hydroxylase 1/3 (PHD) stability (Nakayama et al., 2004), thereby affecting PHD availability to modify HIF-1α, which is essential for HIF-1α’s association with and ubiquitination by pVHL (Ivan et al. 2001). Given that PHD function as cellular oxygen sensors (Aragones et al., 2009; Nakayama et al., 2009), Siah2 is expected to play a central role in controling hypoxia and related biological outcomes, including tumorigenesis and metastasis (Nakayama et al., 2009). Indeed, inhibition of Siah2 activity blocks formation of tumors (Ahmed et al., 2008; Moller et al., 2009; Qi et al., 2008; Schmidt et al., 2007). Further, Siah2’s contribution to melanoma metastasis is HIF-dependent (Qi et al., 2008).
Once stabilized, HIF-1α translocates to the nucleus and dimerizes with HIF-1β, the heterodimers then bind to hypoxia responsive elements (HREs) to regulate transcription of hypoxia-responsive genes (Semenza, 2003). Several transcription factors cooperate with HIF to regulate its transcriptional activity, HIF activity is enhanced by β-catenin (Kaidi et al., 2007) and repressed by FOXO3a (Emerling et al., 2008). HIF can also modulate activity of other transcriptional regulators: HIF-1α potentiates Notch signaling (Gustafsson et al., 2005) and represses c-Myc activity (Gordan et al., 2007).
Given the role of Siah2 in regulation of HIF-1α, we set to determine its role in prostate cancer development and metastasis.
Results
Attenuated formation of prostate NE carcinoma in Siah2-null TRAMP mice
We employed the TRAMP mouse model, in which prostate-specific expression of SV40 T antigen results in prostate tumors that metastasize to lymph nodes, lung and liver (Gingrich et al., 1996), to assess the possible role of Siah in tumor growth and metastasis. Analysis of 8-month-old TRAMP mice with different Siah2 background (TRAMP/Siah2−/−, TRAMP/Siah2+/−, and TRAMP/Siah2+/+) revealed that the majority developed prostate masses (Figure 1A). Most primary masses were composed of benign proliferations of stroma and epithelium with atypical epithelial hyperplasia (AH) in TRAMP/Siah2−/− mice, compared with a preponderance of NE carcinoma in the TRAMP/Siah2+/− and TRAMP/Siah2+/+ mice (Figure 1B, 1C). Although TRAMP AH have been referred to as adenocarcinoma in some literature (e.g., Gingrich et al., 1996), we refer them as TRAMP AH in lieu of clear discrimination, detailed in Chiaverotti et al., (2008). NE carcinomas were identified by morphology (Figure 1B) and expression of the wellestablished NE markers synaptophysin, neuron-specific enolase (NSE) and FoxA2 (Figure 1D), consistent with the notion that the TRAMP tumors are primarily of NE origin (Chiaverotti et al., 2008; Mirosevich et al., 2006). In clear contrast, FoxA2, synaptophysin and NSE were undetectable in normal prostate glands (Figure 1D, arrows) or in AH (data not shown).
Figure 1. Tumorigenesis in TRAMPTg/Siah2 mice.
A. Primary tumor incidences of TRAMP mice with indicated Siah2 genotype are shown as percentages.
B. Typical H&E staining of neuroendocrine carcinoma (NE) derived from TRAMP/Siah2+/− and AH derived from TRAMP/Siah2−/− mice.
C. Incidence of NE and AH in TRAMP mice with indicated Siah genotypes. Number of mice for each genotype is indicated. Dark bars indicate NE+AH. p<0.005 for NE tumor incidence between control and Siah-deficient TRAMP mice.
D. IHC analyses of primary NE carcinoma with indicated genotypes using the indicated antibodies. Arrows indicate normal prostate epithelial tissues.
E. NSE and FoxA2 staining of the NE tumor foci of 5-month-old TRAMP mice.
F. IHC staining of CD31 in NE carcinomas from mice of indicated genotypes.
G. Re-expression of HIF-1α in PHYL-expressing TRAMP-C cells. Cells were stably transfected with indicated expression vectors. PHYL-expressing cells were further stably transfected with HIF-1α and maintained in normoxia (N) or hypoxia (H) for 6 h before analyses by western blot for HIF-1α.
H. and I. TRAMP-C cells (3×106) expressing control pKH3 vector, PHYL or PHYL+HIF-1α were injected subcutaneously into the flanks of nude mice. The frequency of tumor formation (H) and size of xenograft tumor (I) 8-weeks post injection are shown. In panel I, each column represents mean±SD (Standard Deviation) for 5 mice, p<0.05 for pKH3 vs. PHYL+HIF-1α. See also Figure S1 and Table S1.
Since Siah1 also contributes to the regulation of PHD and consequently HIF availability (Nakayama et al., 2004; Qi et al., 2008), we also evaluated Siah1a function in prostate tumor formation. Siah2 and Siah1a doubly homozygous mutant mice are non-viable (Frew et al., 2003). We therefore established a TRAMP/Siah1a+/−Siah2−/− mouse line. TRAMP/Siah1a+/−Siah2−/− mice showed a prostate tumor incidence similar to that seen in TRAMP/Siah2−/− mice (Figure 1A) but lacked NE tumors (Figure 1C).
In the TRAMP mice, the early NE tumor lesions develop in the ventral prostate after 3 months and are recognized as foci that express NE markers. To examine the effect of Siah2 on the development of early stage NE tumors, we analyzed 5-month-old mice. NE foci were identified in 3/6 control mice but in none of the 10 TRAMP/Siah2−/− mice (Figure 1E), the difference is statisticaly significant. These data reveal that in the TRAMP model Siah is required for development of NE carcinomas.
AH predominantly develops in the dorsal lateral lobe of prostate and is identifiable in 1-month-old TRAMP mice (Chiaverotti et al., 2008). To evaluate whether Siah also involved in the progression of AH, we analyzed the dorsal prostate lobes from 1- and 3- month-old mice and found that lack of Siah2 delayed the progression from a normal prostate gland to the early and medium stages AH in 1-month-old mice and into the late stage AH in the 3-month-old mice (Figure S1A, S1B).
Altered HIF-1α expression correlates with reduced cell proliferation and enhanced cell death in primary NE tumor from TRAMP/Siah2−/− mice
Consistent with Siah2 regulates HIF-1α availability, HIF-1α level is reduced in the very few NE carcinomas observed (Figure 1D) and in AH (Figure S1C) from TRAMP/Siah2−/− mice compared with TRAMP/Siah2+/− mice. HIF-2α staining was also reduced in AH from TRAMP/Siah2−/− mice (Figure S1C), whereas HIF-2α was undetectable in NE carcinoma from any genotype (data not shown). These findings are also consistent with the observation that HIF-1α but not HIF-2α is co-expressed with NE markers in prostate cancers (Monsef et al., 2007).
Potential changes in proliferation, apoptosis and angiogenesis were evaluated using PCNA, TUNEL/active caspase-3 and CD31, respectively. NE tumors, but not AH, from TRAMP/Siah2−/−mice showed reduced cell proliferation and increased apoptosis compared with those seen in TRAMP/Siah2+/− mice (Figure 1C, 1D, Table S1). Vascular density was similar in the two strains in both the NE tumor (Figure 1F, Table S1) and AH (Figure 1C, Table S1). Hence, loss of Siah and consequent downregulation of HIF levels appear to specifically govern proliferation and cell survival of NE tumors.
To directly assess a possible role for Siah2 and HIF-1α in tumorigenesis of TRAMP cells, we analyzed TRAMP-C cells derived from TRAMP tumors (Foster et al., 1997). These cells likely represent NE tumor-derived cells as they express multiple NE specific transcription factors (Figure 4A, and data not shown). The PHYL peptide binds to Siah’s substrate recognition site (House et al., 2003) and attenuates Siah2’s effect on PHD1/3, thus reduces HIF-1α levels under hypoxia (Moller et al., 2009; Qi et al., 2008). Expression of the PHYL peptide in TRAMP-C cells effectively abolished their ability to form tumors (Figure 1H, 1I), consistent with the finding that NE tumors do not form in TRAMP/Siah1a+/−Siah2−/− mice (Figure 1C). Forced HIF-1α expression in TRAMP-C cells expressing PHYL peptide by transfection (Figure 1G) partially recovered their ability to form tumors (Figure 1H, 1I). These data support the role of Siah2, in part through its regulation of HIF-1α levels, in formation of NE prostate tumors.
Figure 4. A subset of HIF target genes are regulated by cooperation with FoxA2.
A. TRAMP-C cells were transfected with FoxA2 siRNAs. 48-h post transfection, cells were maintained in 1% oxygen for 10 h then RNA was isolated for qRT-PCR analyses of the indicated transcripts. Control (H) vs. FoxA2 (H): p>0.1 for VEGFA and Glut-1, p<0.005 for all others.
B. TRAMP-C cells were transfected with indicated siRNAs. 48-h post transfection, cells were maintained in 1% oxygen for 10 h. RNA was isolated for qRT-PCR analyses of the Hes6 transcript. p<0.05 between control (H) and HIF-1α (H), or FoxA2 (H).
C. TRAMP-C cells were transfected with a 1.25 kb Hes6 promoter-Luc construct containing the wild type or mutated −66 bp HRE. 24-h post transfection, cells were treated with DMOG (1 mM, 16 h) before analysis of luciferase activity. p<0.005 for WT Hes6 −DMOG vs. +DMOG, p>0.1 for mutant Hes6 −DMOG vs. +DMOG.
D. TRAMP-C cells were transfected with indicated plasmids. 24-h post transfection, cells were treated with DMOG (1 mM, 16 h) before analysis of luciferase activity. WT Hes6: p<0.01 pcDNA−DMGO vs. pcDNA+DMOG, p<0.05 pcDNA+DMGO vs. Foxa2+DMOG.
E. TRAMP-C cells were transfected with control or FoxA2 siRNA. 48-h post transfection, cells were grown in 1% oxygen for 5 h before ChIP assays of the HRE-containing region of Hes6 promoter were performed using indicated antibodies. PCR products were analyzed on 2% agarose gel electrophoresis. A representative reversed gel image of triplicate experiments is shown.
F. FoxA2 shRNA-expressing cells were treated with 1% oxygen for 6 h and subjected to ChIP assays with p300 antibodies. The immunoprecipitated materials were used for QPCR analyses of the HRE-containing regions of VEGFA, Jmjd1a and Hes6. The results of ChIP QPCR were normalized to those of the input. p<0.01 for all genes between control and shFoxA2 for both cell lines.
G Cells were transfected with control or p300 siRNA for 48 h then analyzed by qRT-PCR for the indicated transcripts. Both TRAMP-C and Rv1: p>0.1 control vs. p300 for VEGFA and p<0.05 for all others.
H. TRAMP-C cells were co-transfected with Hes6 or VEGFA promoter-Luc vector, HIF-1α, and increasing amounts of p300. 24-h post transfection, cell lysates were collected for a luciferase assay. Hes6: p<0.001 0 µg vs. all three; VEGFA: p<0.005 0 µg vs. 0.5 µg, p>0.1 0 µg vs. the other two.
I and J. TRAMP-C cells were transfected with Hes6 promoter-Luc (H) or VEGFA promoter-Luc (I) together with the plasmids indicated. 24-h post transfection, cell lysates were collected for analysis of luciferase activity. In panels A–D, F–J, each column represents mean±SD of 3 experiments. Hes6: p<0.01 HIF vs. HIF+FoxA2, HIF vs. HIF+p300, and HIF+FoxA2 vs. HIF+FoxA2+p300; VEGFA: p>0.1 for these comparisons.
K. Flag-p300 was translated in vitro and coupled to M2 beads. HIF-1α or FoxA2 was translated in vitro and labeled with 35S. Equal amounts of 35S-HIF-1α and 35S-FoxA2 were incubated with M2 bead-bound Flag-p300. Bound proteins were monitored as indicated in Figure 3H.
L. Flag-p300 (wt or ΔCH1) was translated in vitro and bound to M2 beads. HIF-1α and FoxA2 were translated in vitro and labeled with 35S. Equal amount of 35S-HIF-1α and 35S-FoxA2 was mixed and incubated with M2 bead-bound Flag-p300 or Flag-p300ΔCH1. Bound proteins were monitored as indicated in Figure 3H. See also Figure S3, Table S2, S3.
Reduced metastasis in TRAMP/Siah2−/− mice
Metastatic lesions in liver, lung and lymph nodes of TRAMP mice were identified as NE carcinomas, based on morphology (Figure 2A) and FoxA2/synaptophysin expression (Figure 2B). However, both the frequency and size of metastatic lesions were significantly reduced (6 fold) in the lung and were not found in liver and lymph nodes of TRAMP/Siah2−/− mice, compared with TRAMP/Siah2+/− or TRAMP/Siah2+/+ animals (Figure 2A, 2C). Furthermore, we found no metastases in TRAMP/Siah1a+/−Siah2−/− mice (Figure 2C). The very few lung metastases observed in TRAMP/Siah2−/− mice were smaller, showed reduced cell proliferation and enhanced cell death (Figure 2D, 2E). These findings point to the role of Siah2 in TRAMP tumor metastasis.
Figure 2. Metastasis in TRAMPTg/Siah2 mice.
A. H&E staining of indicated tissues from TRAMP mice of indicated Siah2 genotypes. Metastatic lesions are indicated by arrows.
B. Expression of NE markers in metastatic lesions of TRAMP/Siah2+/− mice revealed by IHC staining with indicated antibodies.
C. Percentage of metastases in TRAMP mice with indicated Siah genotypes. Number of mice and tissues examined: N=32 (S2+/+ and S2+/−), 16 (S2−/−), or 9 (S1a+/−:S2−/−) for liver and lung, and N=26 (S2+/+ and S2+/−), 11 (S2−/−), or 6 (S1a+/−:S2+/−) for lymph nodes. For each case, 5 serial sections of lung or liver were examined by H&E staining.
D. TUNEL staining (dark brown signals indicated by arrows) of lung sections from TRAMP/Siah2+/− and TRAMP/Siah2−/− mice.
E. IHC staining for PCNA on lung sections derived from TRAMP/Siah2+/− and TRAMP/Siah2−/− mice.
FoxA2 stimulates HIF transcriptional activity
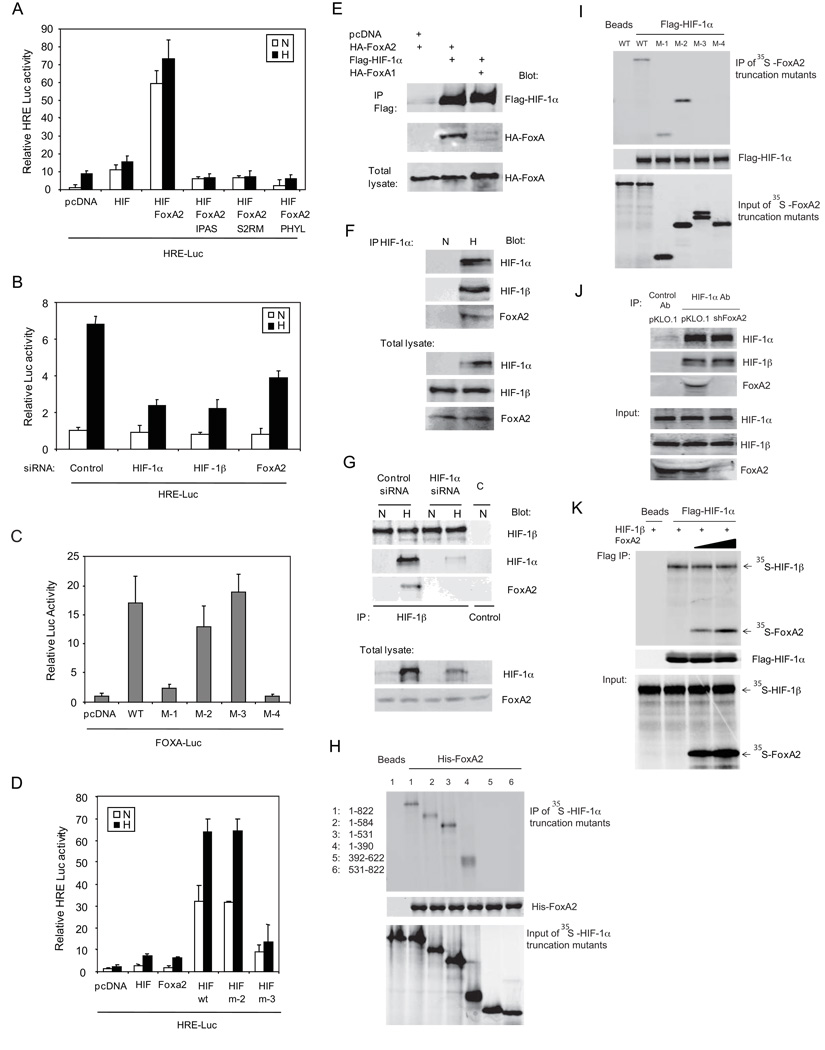
Since the NE-specific transcription factor FoxA2 is co-expressed with HIF-1α protein in nuclei of NE carcinoma cells (Figure 1D), we tested the possibility that these transcription factors cooperate with each other. While expression of exogenous FoxA2 in TRAMP-C cells did not alter the expression of an HRE-linked luciferase construct (HRE-Luc, data not shown), expression of exogenous HIF-1α alone elicited a modest increase in HRE-Luc activity as expected (Figure 3A). Significantly, co-expression of HIF-1α and FoxA2 led to a 6-fold increase in luciferase activity over expression of HIF-1α alone (Figure 3A). Co-expression of IPAS, a spliced form of HIF-3α with potent dominant-negative activity towards all HIFs (Makino et al., 2002) or inhibiting HIF-1α expression by expressing S2RM (a dominant-negative form of Siah2) or PHYL abolished FoxA2-potentiated HRE-Luc activity (Figure 3A). Importantly, knockdown of FoxA2 caused an approximately 40% reduction in HRE-Luc activity under hypoxia, although the degree of inhibition was lower than that seen using HIF-1α or HIF-1β siRNA (Figure 3B, Figure S2A). These data establish a role for FoxA2 in enhancing HIF-mediated transcriptional activity. In contrast, HIF-1α did not stimulate FoxA2 transcription activity (data not shown), indicating that HIF/FoxA2 transcriptional synergy may be restricted to the context of an HRE. Notably, FoxA1 did not increase HRE-Luc activity in the presence of HIF-1α (data not shown).
Figure 3. FoxA2 enhances HIF transcriptional activity.
A. TRAMP-C cells were transfected with an HRE-Luc construct and the indicated plasmids. 24-h post transfection, cells were maintained in 1% oxygen for 10 h, and cell lysates were collected to measure luciferase activity. β-Gal plasmid was used to normalize for transfection efficiency. N and H denote normoxia and hypoxia, respectively. p<0.01 pcDNA (N) vs. HIF (N), p<0.0005 HIF (N) vs. HIF+FoxA2 (N).
B. TRAMP-C cells were first transfected with indicated siRNAs then 48-h later with an HRE-luc construct. 24 h after the second transfection, cells were maintained in 1% oxygen for 10 h before analysis of luciferase activity. p<0.005 for Control (H) vs. HIF-1α (H), HIF-1β (H), or FoxA2 (H).
C. TRAMP-C cells were transfected with the FOXA-Luc construct and indicated FoxA2 deletion mutants. 24-h post transfection, cells were treated with 1% oxygen for 10 h before analysis of luciferase activity. p<0.01 WT vs. M-1, p>0.1 WT vs. M-2 or M-3.
D. TRAMP-C cells were transfected with an HRE-Luc construct and the indicated plasmids. 24-h post transfection, cells were maintained in 1% oxygen for 10 h before analysis of luciferase activity. p<0.001 HIF+WT (N) vs. HIF+m3 (N). Each column in panels A–D represents mean±SD of 3 replicates.
E. 293T cells were transfected with indicated plasmids. HIF-1α was precipitated with Flag antibody-conjugated beads (M2) 48-h post transfection and the precipitated proteins were analyzed by immunoblot.
F. TRAMP-C cells were maintained in 1% O2 for 5 h. Endogenous HIF-1α was precipitated and co-precipitated proteins were analyzed by immunoblot.
G. TRAMP-C cells were transfected with HIF-1α siRNA for 48 h then maintained in hypoxia for 5 h. Endogenous HIF-1β was precipitated and co-precipitated proteins were analyzed by immunoblot.
H. HIF-1α and its truncation mutants were translated in vitro, labeled with 35S, and incubated with Nickel beads coated with His-FoxA2. After 3 washes, proteins on the beads were separated by SDS-PAGE and transferred to nitrocellulose membrane. 35S-labeled HIF-1α was detected by phosphor-imager, followed by immunoblot with a His antibody to detect His-FoxA2. 4% of in vitro translated 35S-HIF-1α was used as input.
I. Flag-HIF-1α was translated in vitro and bound to the M2 beads then incubated with 35S-labeled in vitro translated FoxA2 or its truncation mutants. Bound proteins were monitored as indicated in panel H.
J. TRAMP-C cells were stably transfected with indicated vectors and then grown in 1% O2 for 6 h before immunoprecipitation of HIF-1α. The co-precipitated proteins were analyzed by western blot.
K. Flag-HIF-1α was translated in vitro and bound to the M2 beads then incubated with 35S-labeled in vitro translated FoxA2 and HIF-1β. Bound proteins were monitored as indicated in panel H. See also Figure S2.
We then mapped the FoxA2 domains required for cooperation with HIF-1α. FoxA2 fragments consisting of the N-terminal transactivation domain (N-TAD), the central forkhead domain, or the C-terminal transactivation domain (C-TAD) were generated and evaluated for their effect on FOXA- and HIF-1α-dependent transcription activity (Figure S2B). FoxA2 mutants lacking either the N-TAD or C-TAD exhibited FOXA-dependent transcriptional activity similar to that of the wild-type FoxA2 (Figure 3C), indicating that one transactivation domain is sufficient for FoxA2 transcriptional activity. FoxA2 mutants lacking the C-TAD promoted HIF-dependent transcriptional activity to a level similar to wild-type FoxA2, whereas the FoxA2 mutant lacking the N-TAD was much less effective (Figure 3D). The FoxA2 C-terminus, which contains intrinsic chromatin remodeling activity (Cirillo et al., 2002), was dispensable for HRE activation (construct m-2 in Figure 3D, Figure S2B) thereby excluding the role of chromatin remodeling activity in FoxA2 cooperation with HIF. These data suggest that the N-TAD and forkhead domains of FoxA2 are required to stimulate HIF-mediated transcriptional activity.
HIF interacts with FoxA2
Co-expression of HA-FoxA2 or HA-FoxA1 with Flag-HIF-1α in 293T cells followed by immunoprecipitation of Flag-HIF-1α identified HA-FoxA2,but not HA-FoxA1, as an HIFassociated protein (Figure 3E), consistent with the effect of FoxA2 but not FoxA1 on HIF-dependent transcriptional activity (data not shown). Importantly, endogenous FoxA2 was co-precipitated with endogenous HIF-1α (Figure 3F) or HIF-1β (Figure 3G) in TRAMP-C cells maintained under hypoxia. The association of HIF-1β with FoxA2 was abolished following HIF-1α knockdown (Figure 3G), indicating that HIF-1α recruits FoxA2 to the HIF complex under hypoxia. Furthermore, in vitro binding between purified His-FoxA2 and in vitro translated Flag-HIF-1α confirmed their direct interaction (Figure 3H).
In vitro binding using truncation mutants of HIF-1α (Figure S2C) and FoxA2 (Figure S2B) identified the bHLH-PAS domain of HIF-1α (Figure 3H) and the N-TAD of FoxA2 (Figure 3I) as the minimal regions mediating the HIF-1α-FoxA2 interaction. Although the bHLH-PAS domain of HIF-1α was found to interact with FoxA2 and the PAS domain of HIF-1α is known to interact with HIF-1β (Erbel et al., 2003), alternation of the FoxA2 level in vitro or in TRAMP-C cells, had no apparent effect on the interaction of HIF-1β with HIF-1α (Figure 3J and 3K).
A subset of HIF target genes is cooperatively regulated by FoxA2/HIF-1α
Transcript levels of the HIF targets VEGFA and Glut-1 were not altered following FoxA2 expression or co-expression of HIF-1α and FoxA2 in TRAMP-C, PC3, and HeLa cells (data not shown) suggesting that FoxA2/HIF-1α cooperation selectively affects HIF-regulated genes. We thus compared gene expression profiles of TRAMP-C cells transfected with pcDNA control vector, or expression vectors encoding FoxA2, HIF-1α, or HIF-1α plus FoxA2. Approximately 140 genes in TRAMP-C cells were upregulated by hypoxia (Table S2). Comparison of genes expressed in each condition identified 47 genes upregulated in the HIF-1α + FoxA2 group under hypoxia, in comparison with the other three groups (Table S3). Of these and other hypoxia-induced genes, we further assessed 30 genes for FoxA2-dependent expression, selected based on prostate- and NE tumor-specific expression. To confirm FoxA2-dependent transcription of this gene set we monitored changes in their expression in TRAMP-C cells expressing FoxA2 siRNA. qRT-PCR analysis of selected genes identified Hes6, Sox9, Jmjd1a and Plod2 among those that displayed FoxA2-dependent transcription under hypoxia (Figure 4A). Sox9, Jmjd1a and Plod2 are known HIF target genes (Amarilio et al., 2007; Beyer et al., 2008; Hofbauer et al., 2003). In contrast, transcription of Glut-1 and VEGFA was not altered following FoxA2 knockdown (Figure 4A). Overall, these results suggest that in TRAMP-C cells FoxA2 cooperates with HIF-1α to regulate a subset of HIF targets under hypoxia.
HIF-1α and FoxA2 cooperate to activate Hes6 transcription
The transcription factor Hes6 is reportedly highly upregulated in human metastatic prostate cancers with a NE phenotype (Vias et al., 2008). Upregulation of Hes6 transcripts under hypoxia suggests that Hes6 is a HIF target gene. Indeed, knockdown of HIF-1α or HIF-1β reduced hypoxia-induced Hes6 transcription (Figure 4B). We identified 3 potential HREs and cloned the corresponding 1.25 kb of the mouse Hes6 promoter region upstream of a luciferase reporter. This construct was activated 2.5 fold by the hypoxia mimic DMOG (Figure S3A) and 1.8 fold by hypoxia (data not shown). Deletion analysis of the Hes6 promoter revealed that the HRE at −66bp was required for hypoxia-induced reporter activity (Figure S3A). Mutation of this HRE in the full-length 1.25 kb fragment resulted in loss of the response to DMOG (Figure 4C). FoxA2 knockdown repressed Hes6 transcription (Figure 4A, 4B), while FoxA2 overexpression increased Hes6 promoter activity following addition of DMOG, an effect not seen using the HRE-mutant promoter construct (Figure 4D). These observations strongly suggest that FoxA2-induced Hes6 promoter activity requires an HRE and thus HIF activity. Chromatin immunoprecipitation (ChIP) confirmed that HIF-1α and HIF-1β bound the −66 bp HRE but not the other two putative HREs in the HES6 promoter under hypoxia (Figure S3B). ChIP analysis confirmed the binding of FoxA2 to the same −66 bp HRE, which was impaired following HIF-1α knockdown (Figure S3C), suggesting that FoxA2 is recruited to the promoter through HIF-1α. Similarly, FoxA2 bound to HRE-containing promoter regions of Sox9 (Figure S3C) and Jmdj1a (data not shown) in a HIF-1α-dependent manner. These results indicate that FoxA2 regulates a subset of HIF target genes possibly through direct binding to HIF.
FoxA2-dependent recruitment of p300 to the promoters of HIF target genes
To analyze mechanisms underlying selectivity of FoxA2 cooperation with HIF-1α, we investigated whether FoxA2 expression changed HIF-1α levels, its asparagine hydroxylation, or its binding to the Hes6 promoter but found none (data not shown). To directly assess the contribution of FoxA binding sites to FoxA2/HIF-1α cooperation, we determined possible changes in FoxA2/HIF-1α-mediated transactivation using Hes6 promoter mutants (Figure S3D). While a single FoxA site was sufficient to retain full responsiveness to FoxA2/HIF-1α cooperation, a mutation within this element attenuated such cooperation (Figure S3D). Notably, analysis of the effect of FoxA2 on recruitment of p300, a co-activator of HIF transcriptional activity (Arany et al., 1996), revealed that binding of p300 to the Hes6 promoter was attenuated following FoxA2 knockdown (Figure 4E). FoxA2 knockdown also reduced the binding of p300 to HRE-containing promoters of Jmjd1a and VEGFA in both TRAMP-C cells and Rv1 cells (Figure 4F). However, p300 knockdown only reduced the transcript levels of Hes6 and Jmjd1a but not VEGFA (Figure 4G), which resembled changes seen upon knockdown of FoxA2 (Figure 4A) on the hypoxia-induced transcription of these genes. These results suggest that p300 serves a distinct FoxA2-dependent HIF transcriptional program. Consistent with this possibility, HIFinduced promoter activity of Hes6 was more sensitive to increased p300 levels than that of VEGFA (Figure 4H). Notably, p300, but not p300ΔCH1 (a p300 mutant that cannot interact with HIF-1α), could further increase the degree of HIF/FoxA2 effect on Hes6 promoter activity (Figure 4I). In contrast, FoxA2 and p300 showed no apparent effect on HIF-dependent activation of the VEGFA promoter (Figure 4J). These results suggest that recruitment of p300 is, in part, responsible for the degree and the selectivity of the transcriptional program elicited by HIF/FoxA2 cooperation.
In vitro protein binding showed that FoxA2 enhanced HIF-1α/p300 interaction but had no effect on p300 binding to the HIF-1α 531–822 mutant that cannot associate with FoxA2 (Figures S2C, 3H, 4K). p300 lacking the CH1 domain neither interacted with the HIF/FoxA2 complex (Figure 4L) nor enhanced Hes6 transcription by HIF/FoxA2 (Figure 4I). These results suggest that FoxA2 promotes HIF and p300 interaction unlikely require HIF-1α N-TAD and p300 CH3 domain (Ruas et al., 2010).
Genes regulated by HIF/FoxA2 are important for tumorigenesis of TRAMP-C cells
To determine whether genes regulated by HIF/FoxA2 cooperation are important for tumorigenesis we employed retroviral vectors to re-express Hes6, Sox9 and Jmjd1a individually or in combination in TRAMP-C cells stably expressing PHYL or FoxA2 shRNA. While the endogenous expression of these genes was attenuated upon expression of PHYL peptide or FoxA2 shRNA, their transcript levels were restored following ectopic expression (Figure S4A, S4B). TRAMP-C cells expressing control (pBabe vector) or NxN, a mutant PHYL that cannot interact with Siah, but not those expressing PHYL or shFoxA2 could form colonies on soft agar (Figure 5A, 5B). Significantly, co-expression of Hes6, Sox9 and Jmjd1a effectively rescued the ability of TRAMP-C cells expressing either PHYL or shFoxA2 to form colonies in soft agar but re-expression of each individually was insufficient (Figure 5A, 5B). Consistent with the effect of PHYL being Siah-dependent, reduction of Siah1a and Siah2 (Figure S4C) reduced levels of HIF-1α protein (Figure S4D) and Hes6, Sox9 and Jmjd1a transcripts (Figure S4E), and attenuated the ability of these cells to form colonies in soft agar under hypoxia (Figure S4F). The reduced ability to form colonies in soft agar could be partially rescued upon re-expression of Hes6, Sox9 and Jmjd1a (Figure S5F).
Figure 5. Hes6, Sox9 and Jmjd1a are required for tumorigenesis of TRAMP cells.
A. TRAMP-C cells were stably transfected with indicated vectors. Inset shows western blot of PHYL and NxN. PHYL-expressing cells were then infected with retroviral constructs encoding Hes6, Sox9 or Jmjd1a individually or all together (HSJ). 1×105 cells were monitored for growth on soft agar under 1% O2 for 3 weeks. Shown is the number of colonies per well of 6-well plate. p=0.4 (pBabe vs N×N), p=0.053 (N×N vs. PHYL+HSJ), p<0.0005 (N×N vs. PHYL, PHYL+Hes6, PHYL+Sox9, or PHYL+Jmjd1a), p<0.05 (PHYL+HSJ vs. PHYL, PHYL+Hes6, PHYL+Sox9, or PHYL+Jmjd1a).
B. TRAMP-C cells were transfected with indicated shRNA. Inset shows western blot of FoxA2. shFoxA2-expressing cells were then infected with retroviral constructs of Hes6, Sox9 or Jmjd1a individually or all together (HSJ). 1×105 cells were monitored for their growth on soft agar under 1% O2 for 3 weeks. Shown is the number of colonies per well of 6-well plate. In panel A and B, each column represents mean±SD for 3 replicates. p=0.06 (pKLO.1 vs. shFoxA2+HSJ), p<0.005 (pKLO.1 vs. shFoxA2, shFoxA2+Hes6, shFoxA2+Sox9, or shFoxA2+Jmjd1a), p<0.005 (shFoxA2+HSJ vs. shFoxA2, shFoxA2+Hes6, shFoxA2+Sox9, or shFoxA2+Jmjd1a).
C, D, E, F. G. 1×106 of TRAMP-C transfectants as described in Figure 5A and 5B were injected into the prostate of nude mice. Two months after injection, genitourinary tracts of mice were dissected and prostate tumor formation was quantified. Panel C shows the representative images of prostate tumors, which were indicated by arrows. Panels D and F depict the frequency of tumor formation. Panels E and G show the average size of the tumors formed. In panels E and F, each column represents mean±SD for 5 mice. p<0.05 pBabe vs. PHYL+HSJ and NxN vs. PHYL+HSJ, p<0.001 (pKLO.1 vs. shFoxA2, shFoxA2+Hes6, or shFoxA2+Jmjd1a), p<0.005 (shFoxA2+HSJ vs. shFoxA2, shFoxA2+Hes6, or shFoxA2+Jmjd1a), p<0.01 (pKLO.1 vs. shFoxA2+Sox9), p=0.2 (pKLO.1 vs. shFoxA2+HSJ). See also Figure S4.
Similar to the findings in culture, control but not the PHYL-expressing TRAMP-C cells were able to form tumors upon injection into the prostate of mice (Figure 5C, 5D, 5E). Whereas re-expression of Hes6, Sox9 or Jmjd1a individually in PHYL-expressing TRAMP-C cells failed to rescue tumorigenesis, expression of all three was able to restore (4/5 mice) tumorigenicity and partially rescue tumor growth (30% of tumor size) (Figure 5C, 5D, 5E). Orthotopic injection of shFoxA2-expressing TRAMP-C cells into the prostate also showed significant reduction in tumor formation, which was almost fully rescued by co-expression of Hes6, Sox9 and Jmjd1a (Figure 5F, 5G). These results further establish the importance of HIF/FoxA2 target genes, Hes6, Sox9 and Jmjd1a, in the development of NE prostate tumor.
Genes co-regulated by HIF and FoxA2 are important for hypoxia-associated NE phenotype and metastasis of human prostate adenocarcinoma cells
We next assessed the importance of the pathway discovered using the TRAMP model in human PCa. To this end we selected CWR22Rv1 cells (Rv1), which were derived from a human prostate adenocarcinoma xenograft displaying an NE phenotype (Huss et al., 2004; Sramkoski et al., 1999). Rv1 cells grown under hypoxia showed upregulation of NE markers NSE and chromogranin B (ChgB) (Figure 6A, 6B) and demonstrated protrusion of neurite-like structures from cells located at the periphery of colonies (Figure 6C). Concomitant with the induction of NE phenotype was the upregulation of Hes6, Sox9 and Jmjd1a transcripts (Figure 6D). Significantly, inhibition of Siah or knockdown of FoxA2 attenuated hypoxia-induced upregulation of Hes6, Sox9 and Jmjd1a (Figure S5A, S5B). To determine whether Hes6, Sox9 and Jmjd1a are important for hypoxia-induced NE phenotype, we re-expressed these genes individually or in combination in PHYL- or shFoxA-expressing Rv1 cells (Figure S5A, S5B) and found only co-expression of all three could partially restore hypoxia-induced NSE upregulation and formation of neurite-like structures (Figure 6E, 6F, 6G). These results point to a requirement for Siah-HIF/FoxA2 regulated genes in the hypoxia-induced NE phenotype of human prostate adenocarcinoma cells.
Figure 6. Hypoxia-induced NE phenotype in human prostate cancer cells.
A. CWR22Rv1 cells were cultured under normoxia or hypoxia (1% O2) for indicated times before qRT-PCR analysis of NSE and ChgB. Transcript levels under hypoxia were normalized to those under normoxia. p<0.05, p<0.005, p<0.0001 for NSE hypoxia vs. normoxia at days 1, 3 and 5, respectively. p=0.19, p<0.005, p<0.0001 for ChgB hypoxia vs. normoxia at days 1, 3 and 5, respectively.
B. Rv1 cells were grown under 1% O2 for indicated times. The total lysates were used for western blot analyses of NSE and ChgB. Hes6 and Sox9 were immunoprecipitated followed by western blot analysis.
C. Rv1 cells were seeded onto the tissue culture plate at low density and cultured in 1% O2 for 6 days. Cells were fixed and immunostaind for NSE or ChgB. Note the neurite-like protrusions from cells cultured under hypoxia.
D. Rv1 cells were cultured under hypoxia for indicated times before qRT-PCR analysis. The transcript level under hypoxia was normalized to that of corresponding normoxia samples. P<0.005 for all three transcripts at all three time points.
E. Rv1 cells were stably transfected with control pBabe vector or PHYL. Inset: western blot shows expression of PHYL. PHYL-expressing Rv1 cells were further infected with viral constructs of Hes6, Sox9 or Jmjd1a either individually or in combination (HSJ). Cells were cultured under 1% O2 for 5 days before qRT-PCR analysis of NSE. p<0.01 pBabe vs. PHYL+HSJ, P<0.0001 pBabe vs. all others, p<0.005 PHYL+HSJ vs. other PHYL-expressing cells.
F. Rv1 cells were transfected with control pKLO.1 vector or FoxA2 shRNA. Inset: western blot shows the knockdown of FoxA2. shFoxA2-expressing cells were further infected with viral constructs of Hes6, Sox9 or Jmjd1a either individually or in combination (HSJ). Cells were cultured under 1% O2 for 5 days before qRT-PCR analysis of NSE. p<0.05 pKLO.1 vs. shFoxA2+HSJ, p<0.0001 pKLO.1 vs. all others, p<0.001 shFoxA2+HSJ vs. other shFoxA2-expressing cells.
G. Rv1 transfectants were seeded on tissue culture plates at low density and maintained at 1% O2 for 6 days. The morphology of cells was examined under phase-contrast microscopy. The number of colonies with neurite-like structures (criteria: >1/3 of cells in the periphery of colonies have neurite-like structure that is over 20 µm long) were scored at triplicate of 6-well plates. p<0.01 pBabe vs. PHYL or PHYL+HSJ, p<0.001 PHYL+HSJ vs. PHYL, p<0.05 pKLO.1 vs. shFoxA2 or shFoxA2+HSJ, p<0.005 shFoxA2 vs. shFoxA2+HSJ. In panel A, D, E, F, G, each column represents mean±SD for 3 replicates.
H. 1×106 Rv1 transfectants as described in E and F were injected into the prostates of nude mice. 4-week-post injection, the orthotopic tumors were collected and size measured. p<0.05 pBabe vs. PHYL or PHYL+HSJ, p>0.1 PHYL vs. PHYL+HSJ and pKLO.1 vs. shFoxA2 or shFoxA2+HSJ.
I. Blood was collected from the heart of mice described in H before the sacrifice, cultured in the selection medium for 2 weeks. The number of colonies on the plates was scored and normalized to the volume of blood. p<0.01 pBabe vs. PHYL or PHYL+HSJ, p<0.001 PHYL vs. PHYL+HSJ, p<0.05 pKLO.1 vs. shFoxA2 or shFoxA2+HSJ, p<0.005 shFoxA2 vs. shFoxA2+HSJ. In panel H and I, each column represents mean±SD for 5 mice.
J. Lymph nodes (LN) were collected from the mice described in H, and stained with H & E to determine the metastases. p<0.05 (pBabe vs. PHYL), p=0.17 (PHYL vs. PHYL+HSJ), p<0.01 (pKLO.l vs. shFoxA2), p<0.05 (shFoxA2 vs. shFoxA2+HSJ).
K. Orthotopic tumor sections as described in panel H were subjected to IHC staining of NSE, HIF-1α and FoxA2.
L. The LN or liver metastasis from Rv1 Orthotopic model was subjected to IHC staining of NSE. See also Figure S5, Table S4.
To evaluate the biological significance of NE phenotype for human prostate cancer in vivo, we injected the above Rv1 transfectants into the prostate of nude mice. Surprisingly, unlike TRAMP-C cells, Rv1 cells expressing PHYL only showed about 30% reduction in the tumor size which could not be rescued by co-expression of Hes6, Sox9 and Jmjd1a (Figure 6H). Similarly, Rv1 cells expressing shFoxA2 did not exhibit any notable decrease in tumor formation (Figure 6H). These results indicate that Hes6, Sox9 and Jmjd1a are not involved in tumorigenesis of Rv1 cells. The 30% reduction of tumor size by PHYL was correlated with a 35% reduction in the tumor vessel density (Figure S5C, Table S4) and 40% reduction of VEGFA transcript in PHYL-expressing Rv1 cells in vitro (Figure S5D). Similarly, colony formation in the soft agar assay was comparable for PHYL-expressing Rv1 cells and control cells (Figure S5E). Significantly, the circulating PHYL- or shFoxA2-expressing Rv1 cells in the blood were reduced, indicating impaired intravasation of these cells, which was partially rescued by co-expression of Hes6, Sox9 and Jmjd1a (Figure 6I). Although Rv1 cells exhibited limited ability to form liver metastasis (2 out of 10 mice injected with Rv1 cells), they were very efficient in periaortic lymph node metastases. Expression of PHYL or shFoxA2 abolished lymph node metastases of Rv1 cells, which could be largely restored by co-expression of Hes6, Sox9 and Jmjd1a (Figure 6J). These findings suggest that genes co-regulated by HIF and FoxA2 play a key role in metastasis of prostate adenocarcinoma cells.
IHC analyses of Rv1 orthotopic tumors revealed that HIF-1α and NSE staining were concentrated around the necrotic regions, which are known to be highly hypoxic, whereas FoxA2 staining was evenly distributed (Figure 6K). As expected, expression of PHYL resulted in reduced HIF-1α staining and loss of NSE staining in the hypoxic regions (Figure 6K). FoxA2 shRNA reduced the NSE staining in the hypoxic regions without affecting HIF-1α levels (Figure 6K), consistent with our in vitro data (Figure 6E, 6F, 6G). Importantly, consistent with our in vitro results, co-expression of Hes6, Sox9 and Jmjd1a in PHYL- or shFoxA2-expressing Rv1 cells restored the NSE staining in the hypoxic regions (Figure 6A to 6G). Strong NSE staining was primarily seen within the more hypoxic regions, proximal to the necrosis, of the primary tumor (Figure 6K) and metastatic lesions in liver and lymph nodes (Figure 6L), implying that the NE-differentiated Rv1 cells may be responsible for the metastasis. These results establish that genes co-regulated by HIF and FoxA2 play a key role in hypoxia-induced NE phenotype of PCa in vitro and in vivo, and that NE phenotype is tightly associated with PCa metastasis.
Expression of FoxA2-HIF-1α target genes in prostate tumors
We next asked whether FoxA2/HIF-1α transcriptional targets were expressed in NE tumors. The very few NE tumors identified in a TRAMP/Siah2−/− mice had lower transcript levels of Hes6, Sox9, Jmjd1a and Plod2 compared with TRAMP/Siah2+/−-derived NE tumors (Figure 7A), consistent with HIF-1α-dependent expression.
Figure 7. Changes in HIF/FoxA2 targets in prostate tumors.
A. Laser capture microdissection was performed to collect NE carcinoma cells from tumors of TRAMP/Siah2+/− or TRAMP/Siah2−/− mice. RNA was isolated for qRT-PCR analyses of indicated transcripts. Each column represents mean±SD for 2 replicates. Siah2+/− vs. Siah2−/− p<0.05 for Hes6, Sox9 and Jmjd1a, p=0.78 and 0.46 for VEGFA and Glut-1, respectively.
B. IHC of FoxA2, Hes6, and HIF-1α was performed on a human prostate adenocarcinoma with NED foci. Shown are representative images with a corresponding NE control marker NSE.
C. IHC staining using the indicated antibodies were performed on serial sections of 15 human PCa specimens, among which 10 cases have NE phenotype (NSE positive) and 5 cases have no NE phenotype (NSE negative). Co-expression of Hes6, Sox9 and Jmjd1a in human PCa with NE phenotype is statistically significant compared with that in human PCa without NE phenotype (p<0.05).
D. IHC staining of the indicated proteins was performed on a human prostate TMA consisting PINs and prostate cancers of various Gleason scores, the numbers of each tumor group are shown on the figure. The staining intensity is scored according to 4 scales by 2 pathologists: 0 (no staining), 1 (weak staining), 2 (medium staining) and 3 (strong staining). Scale 0 and 1 are defined as negative staining, while scale 2 and 3 are defined as positive staining. Shown is the percentage of cores that are positively stained for indicated antibodies.
E. Shown are clustered patterns of gene expression taken from GSE3325. Columns represent tumor samples (Benign, B1–B6; Primary, P1–P7; Metastatic, M1–M6), and rows represent genes. The heatmap represents higher expression levels in red and lower levels in blue. Expression data for each gene was row normalized. Transcript levels of Siah2, FoxA2, Hes6, Jmjd1a, Plod2, DDC, ChgB, and ENO2 in metastatic PCa are statistically significantly higher than those in primary PCa. See also Figure S6.
The NE phenotype seen in human PCa can be classified to three types based on IHC staining of NE markers (Cindolo et al., 2007; Hirano et al., 2005; Shimizu et al., 2007). Focal -where NE markers distinguish clusters of cells - found in low-grade and moderately differentiated PCa; general staining -where larger tumor areas are positive for NE markers - found in high-grade and poorly differentiated PCa; and single cells that are stained positively for NE markers (Hirano et al., 2005). We examined 15 cases of human PCa and found 10 to exhibit NE phenotype. Two of the 10 samples displayed focal staining of NE marker NSE, where FoxA2, Hes6 and Sox9 were concentrated in the NE foci (Figure 7B, Figure S6A). Eight of the 10 specimens showed a general staining of NSE, with co-staining of FoxA2, Hes6 and Sox9 in 5/8 cases. Co-expression of FoxA2, Hes6 and Sox9 found in 70% of PCa specimens with NE phenotype was statistically significant (p<0.05; Figure 7C).
We next performed IHC staining of a human prostate TMA consisting of 79 cases representing prostatic intraepithelial neoplasia (PIN) and different Gleason stages of PCa. A higher NSE staining was found in high-grade PCa (G4 and G5), which also showed increased staining of Siah2, FoxA2, Hes6 and Sox9, compared with low-grade tumors (PIN and G3) (Figure 7D). The difference in the expression of NSE, Siah2, FoxA2, Hes6 and Sox9 between high-grade and low-grade tumors is statistically significant and correlated with pathoclinical staging. In addition, IHC staining of 3 human prostate NE tumors revealed co-expression of synaptophysin, FoxA2, HIF-1α, Hes6 and Sox9 in all cases (Figure S6B). These findings suggest that NE-positive tumors are associated with the more malignant human prostate cancers, in which the HIF/FoxA2 target genes are expected to play a role in the development of the NE phenotype. In agreement, gene expression data from human prostate adenocarcinoma identified a marked increase in Hes6, Plod2, Jmjd1a and FoxA2 expression in metastatic prostate tumors, compared with primary prostate tumors and normal prostate tissues (Figure 7E). The expression of these genes correlates with the expression of NE markers, further illustrating the link between their expression, NED and metastatic prostate tumors (Figure 7E).
Whereas Siah2 staining was higher in high-grade PCa, HIF-1α staining was found in both low-grade and high-grade tumors (Figure 7D). Despite high level of HIF-1α expression in all grades of tumors, the level of Glut-1, a common readout for HIF transcriptional activity, was high only in high-grade PCa, pointing to a correlation between Siah2 expression and HIF-1α activity (Figure 7D). In agreement, gene expression analyses revealed an increased Siah2 transcript and enhanced HIF activity in metastatic PCa as reflected by increased transcript of HIF target genes such as CA9, VEGFA and Glut-1, compared with primary PCa (Figure 7E). These results substantiate the correlation between Siah2 expression and HIF activity in human PCa, consistent with the role of Siah in regulation of PHD3 and factor-inhibiting HIF-1 (FIH) stability, which control HIF-1α availability and activity (Nakayama et al., 2004; Fukuba et al., 2008). The cooperation between HIF and FoxA2 in determining NE phenotype can be attributed to a higher level of Siah2 which increases HIF stability and activity, and availability of FoxA2 in the high-grade PCa.
Discussion
Our results provide insight into regulation and function of the FoxA2/HIF-1α complex in determining NE prostate tumor formation and NE phenotype, an important component of metastatic prostate adenocarcinomas. These results also point to a role for Siah2 in determining tumor differentiation. Siah2 loss has little effect on development and growth of the prostate luminal epithelium but decreases initiation of NE carcinomas and, consequently, the metastatic burden in the TRAMP model. We show that partial deletion of Siah1a on a Siah2-null background fully ablated NE tumor formation, suggesting that both Siah2 and Siah1 are required to enable the development of prostate NE tumors.
As HIF-1α is stabilized under hypoxia and FoxA2 is expressed in NE tissues, our findings suggest conditional and spatial cooperation between these two factors under specific tissue and oxygen requirements. Siah2-dependent regulation of HIF coupled with NE-specific expression of FoxA2 provides a framework for a specific tumor differentiation program associated with a highly metastatic phenotype. Among the four FoxA2/HIF targets identified in this study, Hes6 is reportedly highly upregulated in metastatic prostate cancers displaying NE markers (Vias et al., 2008) and Plod2 is overexpressed in metastatic prostate NE tumors (Shah et al., 2004). Sox9 expression is increased in relapsed androgen-refractory prostate cancers and is associated with enhanced growth, invasion and angiogenesis (Wang et al., 2008). It is noteworthy that Hes6, Sox9 and Jmjd1a have been shown to regulate differentiation of stem/progenitor cells (Eun et al., 2008; Loh et al., 2007; Nowak et al., 2008), raising the possibility that FoxA2/HIF cooperation initiates a transcriptional program that regulates neuroendocrine differentiation of normal and/or prostate cancer stem cells. In agreement, the NE phenotype of PCa is also associated with expression of the stem/progenitor markers, supporting the notion that the NE-like cells may harbor prostate cancer stem cells (Bonkhoff, 1998; Helpap et al., 1999; Sotomayor et al., 2008).
Several plausible mechanisms could underlie HIF-1α/FoxA2 transcriptional synergy, we ruled out that the intrinsic chromatin remodeling activity of FoxA2 is important (Cirillo et al., 2002) and that FoxA2 displaces HIF-1β. Our data implicate FoxA2 in enhanced recruitment of p300 for the selective activation of a subset of HIF-target genes. Consistent with our findings, MEFs from mice expressing a p300/CBP mutant that cannot interact with HIF exhibited attenuated HIF activity in luciferase reporter assays but they showed attenuated expression of only a small subset of HIF target genes, not including VEGFA and Glut-1 (Kasper et al., 2005).
The importance of Hes6, Sox9 and Jmjd1a for NE phenotype is demonstrated in human PCa cells that exhibit NE phenotype under hypoxia. Inhibition of these genes attenuates the NE phenotype and PCa metastasis, whereas their co-expression rescues the NE phenotype and metastasis even upon knockdown of FoxA2 or Siah2 - their upstream regulators. Notably, the requirement of hypoxia for NE phenotype was confirmed by IHC analyses of PCa in vivo where level of NSE coincided with that of HIF, FoxA2 and their regulated genes. Of equal significance, inhibition of mouse prostate tumor growth by attenuating Siah2 activity could be overcome upon co-expression of Hes6, Sox9 and Jmjd1a, further illustrating the importance of their cooperation for prostate tumor development.
Overall, our study offers a paradigm underlying formation of NE phenotype and NE prostate tumor development. This pathway requires the ubiquitin ligase Siah2, which determines the level and activity of HIF-1α, which then cooperates with the NE-specific transcription factor FoxA2. It is the conditional and spatial regulation of these factors that transactivates a subset of genes critical for NE phenotype and metastasis of PCa as well as for development of NE prostate tumors. Common to NE prostate tumors and PCa harboring the NE phenotype is their strong propensity to metastasize, and the poor outcome associated with these more aggressive forms of prostate cancer. Our findings unveil mechanisms underlying their development and progression, respectively, while identifying possible targets for therapy and markers for improved detection and monitoring of these tumors.
Experimental Procedures
Prostate tumor samples
Prostate tumor samples representing NE tumors and/or prostate adenocarcinomas were obtained as part of approved clinical studies from the University of California Davis (IRB # 200312072), University of California Irvine (SPECS project, IRB # 20005-4806) and Northwestern University (SPORE tissue banking protocol, IRB # NCI01×2: STU00009126). In all cases informed consent was obtained from all subjects.
Animal studies
All animals were housed in the Sanford-Burnham Institute’s animal facility and the experiments with live animals were approved by our institute animal committee (IACUC # 04–135, 04–141, 07–132) and conducted following the Institute’s animal policy in accordance with NIH guidelines.
Cell Lines
TRAMP-C cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5% Nu-serum IV, 5% fetal bovine serum (FBS), 5 µg/ml insulin and antibiotics. CWR22 Rv1 cells were maintained in RPMI1640 medium with 5% FBS and antibiotics.
Generation of TRAMP mice in a Siah2 knockout background
Siah2+/− mice (129 SVJ strain) were crossed with TRAMP transgenic mice (C57/Bl6 strain) to obtain Siah2 heterozygotes carrying the TRAMP transgene (C57/Bl6 and 129 SVJ mixed strain). Female Siah2+/−/TRAMP mice were crossed with male Siah2+/− mice to generate male TRAMP mice of three genotypes (Siah2 +/+, +/−, −/−), which were predominantly of the 129 strain. Female Siah2+/−/TRAMP mice were also crossed with male Siah1a+/−Siah2+/− mice to generate male TRAMP mice with a Siah1a+/−Siah2−/− genotype. Siah/TRAMP mice were analyzed at 8 months of age.
Antibodies and reagents
Antibodies to HIF-1α, HIF-2α and Sox9 (NOVUS), to Hes6, Jmjd1a, p300 and NSE (Abcam), to synaptophysin (BD Bioscience), to FoxA2, HIF-1β and CD31, Chromogranin B (Santa Cruz), to active caspase-3 (Chemicon), to PCNA (Cell Signaling), to FLAG, HA, α-tubulin and β-actin (Sigma) were used according to manufacturers’ recommendations. An ApopTag peroxidase in situ apoptosis kit was obtained from Chemicon
Statistical analysis
Student’s t-test or Fisher’s exact test was used for the statistical analyses.
Highlights.
Ubiquitin ligase Siah2 is required for NE prostate tumor development
HIF1α & FoxA2 cooperation identifies tissue-specific HIF transcriptional synergy
HIF1α/ FoxA2 target genes are required for NE phenotype of prostate tumors
HIF1α/ FoxA2 target genes are highly expressed in metastatic prostate tumors
Supplementary Material
Acknowledgements
We thank members of the Ronai Lab for helpful discussions. We thank Drs. Lorenz Poellinger, Hueng-Sik Choi, Wei Gu, Andreas Möller, Collin House, Robert Abraham, Gary Chiang, Norman Greenberg, James Jacobberger, Marja Nevalainen, for reagents, Jeremy Mathews for preparation of the prostate tumor TMA, Ling Wang for help with intraprostatic injection, Joan Massague for protocol of retroviral infections. Support by NCI grant CA111515 (to Z.R.), P50CA090386 (K.K.), and U01CA114810 (to D.M.) is gratefully acknowledged. J.Q. was supported by a CIHR fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession number. Microarray data was deposited in the GEO database (GSE18478).
Contributor Information
Jianfei Qi, Signal Transduction Program, Sanford-Burnham Medical Research Institute, La Jolla, CA, 92037, USA.
Koh Nakayama, MTT program, Medical Research Institute, Tokyo Medical and Dental University, Tokyo 113-8510, Japan.
Robert D. Cardiff, Center for Comparative Medicine and Department of Pathology, School of Medicine, University of California, Davis, CA 95616
Alexander D. Borowsky, Center for Comparative Medicine and Department of Pathology, School of Medicine, University of California, Davis, CA 95616
Karen Kaul, NorthShore University Health System, Evanston Hospital, Evanston, IL 60201 and Pritzker School of Medicine, University of Chicago, Chicago, IL 60637.
Roy Williams, Signal Transduction Program, Sanford-Burnham Medical Research Institute, La Jolla, CA, 92037, USA.
Stan Krajewski, Signal Transduction Program, Sanford-Burnham Medical Research Institute, La Jolla, CA, 92037, USA.
Dan Mercola, Translational Cancer Biology, University of California, Irvine, CA 92697.
Philip M. Carpenter, Translational Cancer Biology, University of California, Irvine, CA 92697
David Bowtell, Research Division, Peter McCallum Cancer Centre, Melbourne 8006, VIC, Australia.
Ze’ev A. Ronai, Signal Transduction Program, Sanford-Burnham Medical Research Institute, La Jolla, CA, 92037, USA
References
- Ahmed AU, Schmidt RL, Park CH, Reed NR, Hesse SE, Thomas CF, Molina JR, Deschamps C, Yang P, Aubry MC, Tang AH. Effect of disrupting seven-inabsentia homolog 2 function on lung cancer cell growth. J Natl Cancer Inst. 2008;100:1606–1629. doi: 10.1093/jnci/djn365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarilio R, Viukov SV, Sharir A, Eshkar-Oren I, Johnson RS, Zelzer E. HIF1alpha regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development. 2007;134:3917–3928. doi: 10.1242/dev.008441. [DOI] [PubMed] [Google Scholar]
- Aragones J, Fraisl P, Baes M, Carmeliet P. Oxygen sensors at the crossroad of metabolism. Cell Metab. 2009;9:11–22. doi: 10.1016/j.cmet.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Arany Z, Huang LE, Eckner R, Bhattacharya S, Jiang C, Goldberg MA, Bunn HF, Livingston DM. An essential role for p300/CBP in the cellular response to hypoxia. Proc Natl Acad Sci U S A. 1996;93:12969–12973. doi: 10.1073/pnas.93.23.12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer S, Kristensen MM, Jensen KS, Johansen JV, Staller P. The Histone Demethylases JMJD1A and JMJD2B Are Transcriptional Targets of Hypoxia-inducible Factor HIF. J Biol Chem. 2008;283:36542–36552. doi: 10.1074/jbc.M804578200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkhoff H. Neuroendocrine cells in benign and malignant prostate tissue: morphogenesis, proliferation, and androgen receptor status. Prostate. 1998 Suppl 8:18–22. [PubMed] [Google Scholar]
- Chiaverotti T, Couto SS, Donjacour A, Mao JH, Nagase H, Cardiff RD, Cunha GR, Balmain A. Dissociation of epithelial and neuroendocrine carcinoma lineages in the transgenic adenocarcinoma of mouse prostate model of prostate cancer. Am J Pathol. 2008;172:236–246. doi: 10.2353/ajpath.2008.070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cindolo L, Cantile M, Vacherot F, Terry S, de la Taille A. Neuroendocrine differentiation in prostate cancer: from lab to bedside. Urol Int. 2007;79:287–296. doi: 10.1159/000109711. [DOI] [PubMed] [Google Scholar]
- Cirillo LA, Lin FR, Cuesta I, Friedman D, Jarnik M, Zaret KS. Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Mol Cell. 2002;9:279–289. doi: 10.1016/s1097-2765(02)00459-8. [DOI] [PubMed] [Google Scholar]
- Deeble PD, Murphy DJ, Parsons SJ, Cox ME. Interleukin-6- and cyclic AMP-mediated signaling potentiates neuroendocrine differentiation of LNCaP prostate tumor cells. Mol Cell Biol. 2001;21:8471–8482. doi: 10.1128/MCB.21.24.8471-8482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Liu H, Huang J, Cheng L, Keller ET, Parsons SJ, Hu CD. Ionizing radiation induces prostate cancer neuroendocrine differentiation through interplay of CREB and ATF2: implications for disease progression. Cancer Res. 2008;68:9663–9670. doi: 10.1158/0008-5472.CAN-08-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerling BM, Weinberg F, Liu JL, Mak TW, Chandel NS. PTEN regulates p300-dependent hypoxia-inducible factor 1 transcriptional activity through Forkhead transcription factor 3a (FOXO3a) Proc Natl Acad Sci U S A. 2008;105:2622–2627. doi: 10.1073/pnas.0706790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbel PJ, Card PB, Karakuzu O, Bruick RK, Gardner KH. Structural basis for PAS domain heterodimerization in the basic helix--loop--helix-PAS transcription factor hypoxia-inducible factor. Proc Natl Acad Sci U S A. 2003;100:15504–15509. doi: 10.1073/pnas.2533374100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eun B, Lee Y, Hong S, Kim J, Lee HW, Kim K, Sun W, Kim H. Hes6 controls cell proliferation via interaction with cAMP-response element-binding protein-binding protein in the promyelocytic leukemia nuclear body. J Biol Chem. 2008;283:5939–5949. doi: 10.1074/jbc.M707683200. [DOI] [PubMed] [Google Scholar]
- Foster BA, Gingrich JR, Kwon ED, Madias C, Greenberg NM. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997;57:3325–3330. [PubMed] [Google Scholar]
- Frew IJ, Hammond VE, Dickins RA, Quinn JM, Walkley CR, Sims NA, Schnall R, Della NG, Holloway AJ, Digby MR, et al. Generation and analysis of Siah2 mutant mice. Mol Cell Biol. 2003;23:9150–9161. doi: 10.1128/MCB.23.24.9150-9161.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuba H, Takahashi T, Jin HG, Kohriyama T, Matsumoto M. Abundance of aspargynyl-hydroxylase FIH is regulated by Siah-1 under normoxic conditions. Neurosci Lett. 2008;433:209–214. doi: 10.1016/j.neulet.2007.12.069. [DOI] [PubMed] [Google Scholar]
- Gingrich JR, Barrios RJ, Morton RA, Boyce BF, DeMayo FJ, Finegold MJ, Angelopoulou R, Rosen JM, Greenberg NM. Metastatic prostate cancer in a transgenic mouse. Cancer Res. 1996;56:4096–4102. [PubMed] [Google Scholar]
- Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. 2007;11:335–347. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Hirano D, Jike T, Okada Y, Minei S, Sugimoto S, Yamaguchi K, Yoshikawa T, Hachiya T, Yoshida T, Takimoto Y. Immunohistochemical and ultrastructural features of neuroendocrine differentiated carcinomas of the prostate: an immunoelectron microscopic study. Ultrastruct Pathol. 2005;29:367–375. doi: 10.1080/019131290945718. [DOI] [PubMed] [Google Scholar]
- House CM, Frew IJ, Huang HL, Wiche G, Traficante N, Nice E, Catimel B, Bowtell DD. A binding motif for Siah ubiquitin ligase. Proc Natl Acad Sci U S A. 2003;100:3101–3106. doi: 10.1073/pnas.0534783100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Ippolito JE, Garabedian EM, Humphrey PA, Gordon JI. Molecular characterization of a metastatic neuroendocrine cell cancer arising in the prostates of transgenic mice. J Biol Chem. 2002;277:44462–44474. doi: 10.1074/jbc.M205784200. [DOI] [PubMed] [Google Scholar]
- Huss WJ, Gray DR, Tavakoli K, Marmillion ME, Durham LE, Johnson MA, Greenberg NM, Smith GJ. Origin of androgen-insensitive poorly differentiated tumors in the transgenic adenocarcinoma of mouse prostate model. Neoplasia. 2007;9:938–950. doi: 10.1593/neo.07562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huss WJ, Gregory CW, Smith GJ. Neuroendocrine cell differentiation in the CWR22 human prostate cancer xenograft: association with tumor cell proliferation prior to recurrence. Prostate. 2004;60:91–97. doi: 10.1002/pros.20032. [DOI] [PubMed] [Google Scholar]
- Kaidi A, Williams AC, Paraskeva C. Interaction between beta-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat Cell Biol. 2007;9:210–217. doi: 10.1038/ncb1534. [DOI] [PubMed] [Google Scholar]
- Kasper LH, Boussouar F, Boyd K, Xu W, Biesen M, Rehg J, Baudino TA, Cleveland JL, Brindle PK. Two transactivation mechanisms cooperate for the bulk of HIF-1-responsive gene expression. EMBO J. 2005;24:3846–3858. doi: 10.1038/sj.emboj.7600846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh YH, Zhang W, Chen X, George J, Ng HH. Jmjd1a and Jmjd2c histone H3 Lys 9 demethylases regulate self-renewal in embryonic stem cells. Genes Dev. 2007;21:2545–2557. doi: 10.1101/gad.1588207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino Y, Kanopka A, Wilson WJ, Tanaka H, Poellinger L. Inhibitory PAS domain protein (IPAS) is a hypoxia-inducible splicing variant of the hypoxia-inducible factor-3alpha locus. J Biol Chem. 2002;277:32405–32408. doi: 10.1074/jbc.C200328200. [DOI] [PubMed] [Google Scholar]
- Moller A, House CM, Wong CS, Scanlon DB, Liu MC, Ronai Z, Bowtell DD. Inhibition of Siah ubiquitin ligase function. Oncogene. 2009;28:289–296. doi: 10.1038/onc.2008.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsef N, Helczynski L, Lundwall A, Pahlman S. Localization of immunoreactive HIF-1αlpha and HIF-2alpha in neuroendocrine cells of both benign and malignant prostate glands. Prostate. 2007;67:1219–1229. doi: 10.1002/pros.20594. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Frew IJ, Hagensen M, Skals M, Habelhah H, Bhoumik A, Kadoya T, Erdjument-Bromage H, Tempst P, Frappell PB, et al. Siah2 regulates stability of prolyl-hydroxylases, controls HIF1alpha abundance, and modulates physiological responses to hypoxia. Cell. 2004;117:941–952. doi: 10.1016/j.cell.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Qi J, Ronai Z. The ubiquitin ligase Siah2 and the hypoxia response. Mol Cancer Res. 2009;7:443–451. doi: 10.1158/1541-7786.MCR-08-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008;3:33–43. doi: 10.1016/j.stem.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Nakayama K, Gaitonde S, Goydos JS, Krajewski S, Eroshkin A, Bar-Sagi D, Bowtell D, Ronai Z. The ubiquitin ligase Siah2 regulates tumorigenesis and metastasis by HIF-dependent and -independent pathways. Proc Natl Acad Sci U S A. 2008;105:16713–16718. doi: 10.1073/pnas.0804063105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruas JL, Berchner-Pfannschmidt U, Malik S, Gradin K, Fandrey J, Roeder RG, Pereira T, Poellinger L. Complex regulation of the transactivation function of hypoxia-inducible factor-1 alpha by direct interaction with two distinct domains of the CREB-binding protein/p300. J Biol Chem. 2010;285:2601–2609. doi: 10.1074/jbc.M109.021824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RL, Park CH, Ahmed AU, Gundelach JH, Reed NR, Cheng S, Knudsen BE, Tang AH. Inhibition of RAS-mediated transformation and tumorigenesis by targeting the downstream E3 ubiquitin ligase seven in absentia homologue. Cancer Res. 2007;67:11798–11810. doi: 10.1158/0008-5472.CAN-06-4471. [DOI] [PubMed] [Google Scholar]
- Sella A, Konichezky M, Flex D, Sulkes A, Baniel J. Low PSA metastatic androgen- independent prostate cancer. Eur Urol. 2000;38:250–254. doi: 10.1159/000020289. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Shah RB, Mehra R, Chinnaiyan AM, Shen R, Ghosh D, Zhou M, Macvicar GR, Varambally S, Harwood J, Bismar TA, et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res. 2004;64:9209–9216. doi: 10.1158/0008-5472.CAN-04-2442. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Kumagai J, Eishi Y, Uehara T, Kawakami S, Takizawa T, Koike M. Frequency and number of neuroendocrine tumor cells in prostate cancer: no difference between radical prostatectomy specimens from patients with and without neoadjuvant hormonal therapy. Prostate. 2007;67:645–652. doi: 10.1002/pros.20493. [DOI] [PubMed] [Google Scholar]
- Sotomayor P, Godoy A, Smith GJ, Huss WJ. Oct4A is expressed by a subpopulation of prostate neuroendocrine cells. Prostate. 2008;69:401–410. doi: 10.1002/pros.20895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sramkoski RM, Pretlow TG, Giaconia JM, 2nd, Pretlow TP, Schwartz S, Sy MS, Marengo SR, Rhim JS, Zhang D, Jacobberger JW. A new human prostate carcinoma cell line, 22Rv1. In Vitro Cell Dev Biol Anim. 1999;35:403–409. doi: 10.1007/s11626-999-0115-4. [DOI] [PubMed] [Google Scholar]
- Vias M, Massie CE, East P, Scott H, Warren A, Zhou Z, Nikitin AY, Neal DE, Mills IG. Pro-neural transcription factors as cancer markers. BMC Med Genomics. 2008;1:17. doi: 10.1186/1755-8794-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Leav I, Ibaragi S, Wegner M, Hu GF, Lu ML, Balk SP, Yuan X. SOX9 is expressed in human fetal prostate epithelium and enhances prostate cancer invasion. Cancer Res. 2008;68:1625–1630. doi: 10.1158/0008-5472.CAN-07-5915. [DOI] [PubMed] [Google Scholar]
- Yuan TC, Veeramani S, Lin FF, Kondrikou D, Zelivianski S, Igawa T, Karan D, Batra SK, Lin MF. Androgen deprivation induces human prostate epithelial neuroendocrine differentiation of androgen-sensitive LNCaP cells. Endocr Relat Cancer. 2006;13:151–167. doi: 10.1677/erc.1.01043. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Flesken-Nikitin A, Corney DC, Wang W, Goodrich DW, Roy-Burman P, Nikitin AY. Synergy of p53 and Rb deficiency in a conditional mouse model for metastatic prostate cancer. Cancer Res. 2006;66:7889–7898. doi: 10.1158/0008-5472.CAN-06-0486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.