Abstract
Persistent infection of the central nervous system (CNS) of mice with the neuroadapted JHM strain of mouse hepatitis (MHV) is characterized by ongoing demyelination mediated by inflammatory T cells and macrophages that is similar both clinically and histologically with the human demyelinating disease multiple sclerosis (MS).
Although extensive demyelination occurs in mice persistently-infected with MHV there is only limited remyelination. Therefore, the MHV model of demyelination is a relevant model for studying disease and evaluating therapeutic approaches to protect cells of the oligodendrocyte lineage and promote remyelination. This concept is further highlighted as the etiology of MS remains enigmatic, but viruses have long been considered as potential triggering agents in initiating and/or maintaining MS symptoms. As such, understanding mechanisms associated with promoting repair within the CNS in the context of a persistent viral infection is critical given the possible viral eitiology of MS. This review focuses on recent studies using either mouse neural stem cells (NSCs) or human oligodendrocyte progenitor cells (OPCs) derived from human embryonic stem cell (hESCs) to promote remyelination in mice persistently infected with MHV. In addition, the potential role for chemokines in positional migration of transplanted cells is addressed.
1. Introduction
Multiple sclerosis (MS) is a chronic disease of the central nervous system (CNS) characterized by multifocal regions of inflammation and myelin destruction (Steinman, 1996). Typically, MS runs a protracted clinical course lasting over several decades with episodes of exacerbation followed by variable periods of remission. Available evidence indicates that the cause of MS is multifactorial and includes the genetic background of the individual as well as environmental influences (Oksenberg et al., 1993; Poser, 1994; Raine, 1994; Steinman, 1996). However, the fact that a 70% discordance rate among monozygotic twins has been reported suggests that other environmental factors such as microbial infections e.g. viral must be considered as a contributing cause of MS (Ebers et al., 1995). Viral infection has long been considered a potential triggering mechanism involved in demyelination and numerous human viral pathogens have been suggested to be involved in eliciting myelin-reactive lymphocytes and/or antibodies that subsequently infiltrate the CNS and damage the myelin sheath (Ascherio and Munger, 2007a; Ascherio and Munger, 2007b; Ebers et al., 1995; Sospedra and Martin, 2005). Therefore, viral models of demyelination are clearly relevant and have provided important insights into mechanisms associated with disease initiation, neuroinflammation and demyelination. MHV infection of mice results in an acute encephalomyelitis followed by chronic demyelination. Similar to MS, components of the immune system e.g. T cells and macrophages are considered important contributors to white matter destruction (Glass et al., 2004; Glass et al., 2001; Lane et al., 2000; Liu et al., 2001; Wu et al., 2000; Wu and Perlman, 1999). Further, MHV-infected mice undergoing chronic demyelination exhibit similar clinical and histologic disease profiles compared to MS patients (Herndon et al., 1975; Lane and Buchmeier, 1997; Weiner, 1973). Combined with the fact that an environmental agent such as a virus is considered to be a contributing cause of MS, the MHV system offers an excellent model in which to study both the underlying immunopathological mechanisms that may drive demyelination in MS patients as well as novel therapeutic methods for promoting remyelination.
An important clinical aspect related to the pathogenesis of the human demyelinating disease MS is the eventual remyelination failure by endogenous neural stem cells (NSCs). Among the various experimental approaches undertaken to promote remyelination, cell replacement therapies using both mouse and human NSCs have emerged as a clinically relevant and increasingly practical method for promoting remyelination (Pluchino et al., 2009b; Sher et al., 2008). However, the vast majority of these studies have utilized either autoimmune models of neuroinflammatory-mediated demyelination or chemical–induced demyelination to assess the remyelination potential of NSCs. Given the possibility of viral infection in initiating demyelination and the fact that numerous neurotropic viruses exist that are capable of persisting within the CNS, it is imperative to evaluate the remyelination potential of stem cells in the presence of a persistent viral infection that is correlative with chronic neuroinflammation and demyelination.
2. Viral Persistence and immune-mediated demyelination
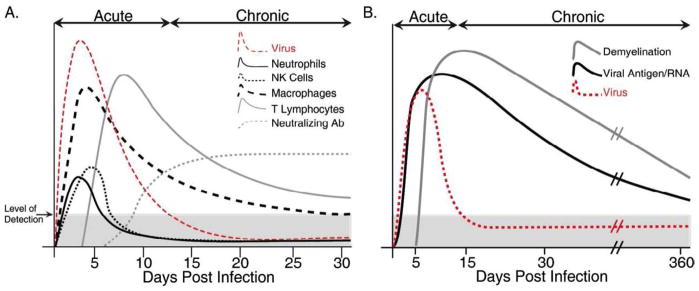
Intracranial (i.c.) infection of susceptible strains of mice such as C57BL/6 with the neuradapted JHM strain of mouse hepatitis virus (JHMV) results in chronic neuroinflammation and demyelination within the CNS (Fleming et al., 1986). A robust inflammatory response characterized by CD4+ T cells, CD8+ T cells, natural killer (NK) cells, B cells, and macrophages occurs during the acute stage of infection (Williamson and Stohlman, 1990; Williamson et al., 1991). Studies have shown that clearance of infectious virus is mediated by both CD4+ and CD8+ T cells (Lane et al., 2000; Marten et al., 2001; Pearce et al., 1994; Williamson and Stohlman, 1990). CD4+ T cells are crucial in allowing for maintenance of CD8+ T cells through an undefined mechanism(s) (Stohlman et al., 1998). CD8+ T cells help eliminate virus by two distinct mechanisms that are in part dictated by the host cell. Clearance from infected astrocytes and microglia is mediated by a perforin-dependent mechanism whereas IFN-γ is responsible for controlling infection in oligodendrocytes (Lin et al., 1997; Parra et al., 1999). The kinetics of immune cell infiltration into the CNS in response to MHV infection is presented in Figure 1A. A detailed description of host defense mechanisms is provided in Bergmann et al. (Bergmann et al., 2006). MHV persistence in white matter tracts results in a chronic demyelinating disease in which foci of demyelination are associated with areas of viral RNA/antigen lasting up to one year post-infection (p.i.) (Figure 1B) (Bergmann et al., 2006; Hosking and Lane, 2009; Lane and Buchmeier, 1997; Perlman et al., 1999). Histopathoglical analysis of mice persistently infected with virus reveals numerous demyelinated axons with very few axons undergoing remyelination (Figure 2). Clinically, mice develop loss of tail tone and a partial-to-complete hind-limb paralysis. As a result of the clinical and histologic similarities between MHV-induced demyelination and the human demyelinating disease multiple sclerosis (MS), the MHV system is considered a relevant model for studying the underlying immunopathological mechanisms contributing to immune-mediated demyelinating diseases (Lane and Buchmeier, 1997).
Figure 1. Pathogenesis of MHV infection of the CNS of C57BL/6 mice.
(A) Schematic representation of leukocyte infiltration into the CNS of MHV infected mice over time. Early infiltration is characterized by migration of cells of the innate immune response e.g. NK cells and macrophages, while infiltration of virus-specific T cells is critical in controlling viral replication. Neutralizing antibodies, not detectable until chronic disease, are necessary in preventing viral recrudescence. (B) Representation of time course of demyelination in MHV-infected mice. Although viral titers are cleared below detectable levels (via plaque assay) between days 10–15, viral RNA and antigen remains detectable out to a year post-infection (p.i.). As animals reduce viral titers within the CNS, demyelinating lesions and clinical disease is initiated and can also last up to 1 year p.i. Both T cells and macrophages contribute to demyelination in mice persistently infected with MHV. Epitope spreading i.e. generation of autoreactive T cells is not thought to contribute to white matter damage in the MHV model system. Shaded areas in A and B represent the limit of detection for CNS virus as determined by plaque assay.
Figure 2. Extensive demyelination at 5 weeks post-infection within white matter tracts of MHV-infected animals.
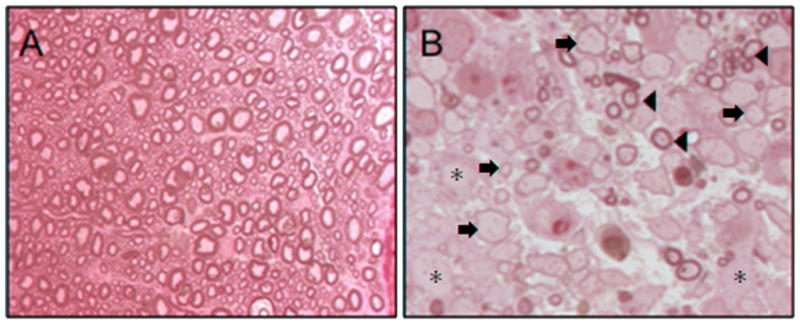
(A) Resin embedded cords from uninfected (sham) animals stained with toluidine blue show normally myelinated axons throughout the white matter. Note the thick, tight, multilayered myelin on all axons (1000×). (B) High magnification shows MHV-infected animals with extensive demyelinated axons (asterisks) throughout the section (1000×). These animals also have some remyelinated axons (arrows) as well as areas of normally myelinated axons (arrowheads).
3. Progenitor cell transplantation and remyelination
Many MS treatments are geared towards reducing neuroinflammation with ultimate goal of attenuating progression of disease (Rizvi and Agius, 2004). However, these treatments often do not address the neurodegenerative phase of disease i.e. demyelination and axonal loss. Indeed, remyelination can occur spontaneously in the adult human brain as evidenced by shadow plaques, in which large regions of white matter undergo remyelination with characteristically thin myelin sheaths (Halfpenny et al., 2002). However remyelination does not occur uniformly within a lesion or across many lesions. Two major events have been proposed to explain the failure of remyelination in MS: (i) difficulties in recruiting oligodendrocyte progenitor cells (OPCs) to areas of active demyelination and (ii) inhibition in differentiation of OPCs into myelin-competent oligodendrocytes capable of promoting remyelination (Franklin, 2002). In each of these two possibilities, signaling by growth factors, cytokines/chemokines, and extracellular matrix molecules expressed during neuroinflammation are thought to contribute to an environment restrictive to remyelination by endogenous oligodendrocytes.
Stem cells and neural precursors represent attractive sources for the generation of remyelination-competent cells, as they can be readily amplified and differentiated to the oligodendrocyte lineage (Ben-Hur et al., 1998; Brustle et al., 1999b). Studies in animal models have proven invaluable for identifying new methods for inducing remyelination in animals with established demyelination. Transplant of rodent embryonic stem cells into myelin-deficient shiverer mice resulted in cellular migration in the spinal cord, differentiation into oligodendrocytes and astrocytes, and myelination of axons (Brustle et al., 1999a). Similarly, transplant of human embryonic stem cell (hESC) derived oligodendrocyte progenitor cells into myelin-deficient shiverer mice resulted in oligodendrocyte differentiation and remyelination (Nistor et al., 2005). Animal models of demyelination have also reported reduced demyelination following transplantation of stem cells. For example, transplantation of stem cells into animal models of acute demyelination resulted in remyelination (Cao et al., 2002). Injection of adult neuronal precursors into mice with EAE induced recovery from disease and a significant decrease in the level of demyelination (Pluchino et al., 2003; Pluchino et al., 2005). Similarly, transplantation of stromal bone marrow cells into demyelinated rat spinal cord yielded remyelination (Akiyama et al., 2002).
Transplantation of human OPCs (hOPCs) generated from hESCs into spinal cord injured rats results in successful engraftment associated with cell survival and functional recovery (Keirstead et al., 2005). In addition, studies have also supported that neural progenitor cells inherently are not immunogenic as they do not elicit an immune response and resist destruction as allografts over relatively short periods of time (Hori et al., 2003; Hori et al., 2007; Modo et al., 2002). Moreover, xenogeneic transplantation of hNSCs derived from hESCs resulted in suppression of the host immune response (Akesson et al., 2009) and these observations have been supported in additional studies involving transplantation of hNSCs into non-human primate models of demyelination (Pluchino et al., 2009a). Nonetheless, not all studies support that stem cells or their derivatives are immunosuppressive and/or resist rejection following transplantation. This was recognized by studies over 20 years ago that demonstrated that transplantation of allogeneic neural cells into the CNS of recipient mice resulted in immune cell infiltration and rejection (Mason et al., 1986; Nicholas et al., 1987a; Nicholas et al., 1987b). Transplantation of allogeneic mixed glial cell cultures resulted in remyelination failure and graft rejection by one month in rats with demyelinating lesions induced by spinal cord injection of ethidium bromide (Tepavcevic and Blakemore, 2006). More recently, elegant tracking methodologies employed by Swijnenburg et al. (Swijnenburg et al., 2008) demonstrated hESCs are rapidly rejected following injection into immune-competent mice. Indeed, we have also determined that hOPCs derived from hESCs transplanted into MHV-infected mice do not survive and only focal remyelination occurs that is most likely mediated by endogenous OPCs (Hatch et al., 2009). Grinnemo et al. (Grinnemo et al., 2006) showed that hESCs expressed HLA class I and II genes and elicited robust infiltration of T cells and macrophages that surrounded hESCs soon after transplantation. Collectively, these findings emphasize the potential clinical hurdles facing transplantation of stem cells for therapeutic use. Even though stem cells reportedly express low levels of MHC antigens, even single differences between donor ESC and recipient minor histocompatibility antigens may facilitate graft rejection (Robertson et al., 2007). Therefore, it is critical to investigate novel strategies to induce sustained “life-long” tolerance to ensure long-term survival of stem cells.
This brief review will summarize recent studies from our group that have investigated the therapeutic potential of both mouse and human stem cells to promote repair following transplantation into the CNS of mice persistently infected with MHV. Focus points will include a comparison of the therapeutic benefits of cell replacement therapies using these two distinct stem cell populations and discussion about the potential role for chemokines in contributing to the positional migration of these cells following engraftment.
4. Transplantation of mouse NSCs into MHV-infected mice
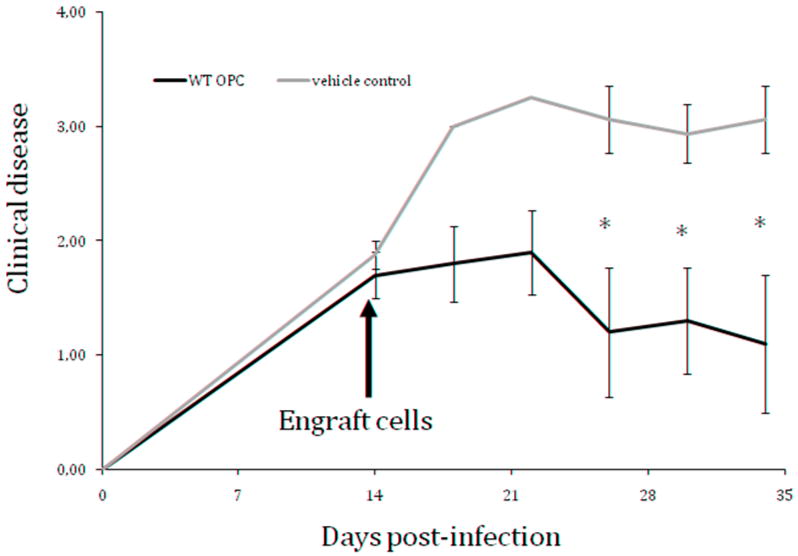
In order to determine whether transplantation represents a viable strategy for treating demyelination, it is necessary to better understand the range of environmental conditions that support transplantation-mediated remyelination. Intracranial injection of MHV provides an environment of ongoing demyelinating pathogenesis, and is thus distinct from gliotoxin lesions as well as autoimmune-mediated demyelination e.g. EAE. We have recently demonstrated that intraspinal transplantation of mouse NSCs into mice persistently infected with JHMV resulted in survival and extensive migration of engrafted cells both rostral and caudal to the site of implantation (Totou 2004). Importantly, our experimental approach was to transplant syngeneic NSCs derived from C57BL/6 mice (H-2b background) into MHV-infected C57BL/6 mice to eliminate the possibility of rejection. Transplantation resulted in successful engraftment and the majority of transplanted cells differentiated into either OPCs or mature oligodendrocytes (Totoiu et al., 2004). Importantly, transplanted cells selectively accumulated within demyelinated white matter tracts accompanied by axonal sparing and extensive remyelination. Remyelinated axons were identified by their characteristically thin myelin sheaths (Figures 3) and appeared in aggregates distributed throughout the dorsal, ventral and lateral columns amongst demyelinated axons. In contrast, non-transplanted animals displayed demyelinated axons that were wide-spread throughout lateral and ventral white matter columns. Remyelination extended 8mm cranial and 6mm caudal to the implantation site and contained 54% to 67% remyelinated axons. With regards to clinical disease, n on-transplanted animals developed clinical disease typically characterized by a waddling gait and partial hind-limb weakness by 8–9 days post-infection, which progressed to complete hind-limb paralysis by 10 days post-infection, persisting for the duration of the experiment. In contrast, transplantation of NSCs resulted in a significant improvement in locomotor abilities (Figure 4).
Figure 3. Remyelination following successful engraftment of mouse NSCs.
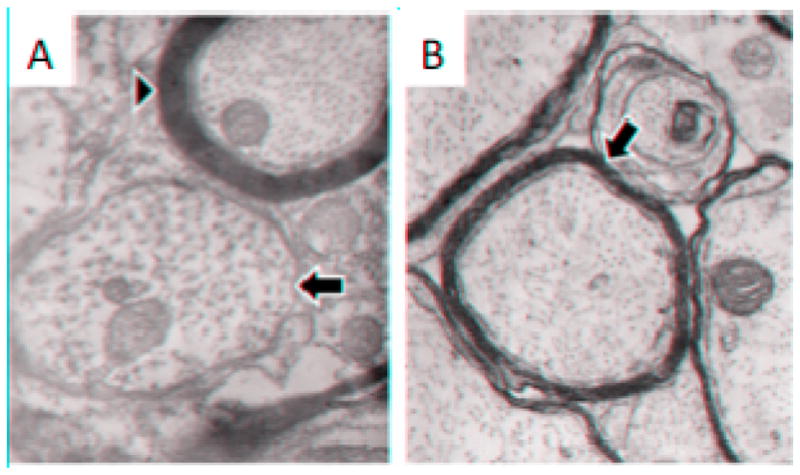
A. Electron photo-micrograph of spinal cord white matter from an MHV-infected mouse 33 days after induction of disease illustrating a demyelinated axon (arrow) and a normally-myelinated axon (arrowhead). B. Electron photomicrograph of spinal cord white matter from an MHV-infected mouse 33 days after induction of disease and 21 days after transplantation of glial-committed progenitor cells. Note the thin myelin sheaths (arrow) characteristic of remyelination (for comparison, note the thickness of the normal myelin sheath in A (arrowhead). Magnification for A and B, 39000X.
Figure 4. Significant clinical improvement following intraspinal injection of myelinogenic neural stem cells to mice with established demyelination.
Average clinical score of mice that received intraspinal injection of neural stem cells (250,000 cells, n=4) or vehicle control (HBSS, n=5). Cells were transplanted into mice with established demyelination, 14 days post-infection with a neuroadapted strain of JHMV. Scores are based on a four point scale: 0 - no abnormality; 1 - limp tail; 2 - waddling gait and partial hind limb weakness; 3- complete hind limb paralysis; 4 - dead. *p < 0.05.
Given the importance of the inflammatory response in initiating and maintaining disease in MHV-infected mice (Glass et al., 2004; Glass et al., 2001; Lane et al., 2000; Liu et al., 2001; Stiles et al., 2006; Wu et al., 2000; Wu and Perlman, 1999), we sought to determine if one mechanism by which transplanted progenitor cells promoted remyelination was through modifying the severity of inflammation. To address this question, we first evaluated leukocyte infiltration into the CNS by flow cytometry at 10 and 21 days post transplantation of NSCs or vehicle as a control. The overall frequency of infiltrating of CD4+ T cells, CD8+ T cells, and macrophages (F4/80+CD45high) was not altered in mice receiving progenitor cells as compared to control mice. Further, the frequency and number of virus-specific CD4+ and CD8+ T cells was not affected in transplanted mice (Hardison et al., 2006). Analysis of proinflammatory chemokine and cytokine gene expression revealed no change in the abundance of specific mRNA transcripts in transplanted versus non-transplanted mice. Our findings are in marked contrast to earlier studies by Pluchino and colleagues (Pluchino et al., 2005) demonstrating that neuroprotective effects of NSCs is mediated through predominantly immunomodulatory effects using a mouse model of EAE. Long-term surviving undifferentiated NSCs persist within perivascular areas whereby they participate in protection by promoting apoptosis of infiltrating inflammatory T cells (Pluchino et al., 2005). Similarly, Einstein et al. (Einstein et al., 2006) also observed muted infiltration of T cells into the CNS following NSC transplantation into the CNS of mice with EAE that coincided with an increase in T regulatory cells. Dampened neuroinflammation was associated with diminished tissue injury accompanied by improved clinical outcome (Einstein et al., 2006). Mechanisms associated with NSC-mediated immune regulation indicate that upon subcutaneous injection of these cells into mice with established EAE, NSCs are able to preferentially accumulate within draining lymph nodes (Pluchino et al., 2009a). Residing in defined anatomical locations, NSCs were found to dampen activation of myeloid dendritic cells resulting in restrained expansion of antigen-specific encephalitongenic T cells. Differences between the therapeutic effects of NSCs observed in the MHV model of disease compared to those observed in EAE models may be explained by several potentially important reasons: 1) persistent viral infection of the CNS may create a vastly different environment within the CNS compared to disease induced by injection of encephalitogenic myelin peptides (in combination with adjuvant) and this influences the biology of transplanted NSCs through as-of-yet undefined mechanisms; 2) the site of NSC implantation as well as timing of injection may be critical with regards to how these cells function. We only injected NSCs into the spinal cords of mice persistently infected with virus well after neuroinflammation and demyelination were evident. Moreover, we do not observe migration of engrafted cells to peripheral organs e.g. lymph nodes or spleens where they may have immunodulatory effects. In contrast, introduction of NSCs via intravenous, intraventricular, or subcutaneous injection early following immunization with myelin epitopes clearly resulted in differential outcome within the context of where cells accumulate, function, and therapeutic outcome. These factors must be considered when evaluating the translational benefits of cell replacement therapies in treating chronic inflammatory neurologic diseases such as MS.
5. Transplantation of OPCs derived from hESCs into JHMV-infected mice
Our laboratory has been interested in determining whether transplantation of clinically applicable hESC-derived OPCs promote remyelination using the MHV-model of demyelination. The ability of clinically applicable cells to facilitate remyelination in a model of MS would be an essential step towards developing novel therapies. Perhaps the most alluring source of OPCs are from hESCs as they can be easily amplified, appear to harbor limitless potential for propagation and can be readily differentiated. Moreover, embryonic derivatives may be more proficient in migration and myelination when compared to their adult counterparts, as recently suggested by others (Blakemore and Irvine, 2008; Sim et al., 2002; Wolswijk and Noble, 1992). It is important to note, however, that the broad plasticity of hESCs or undifferentiated cells (Amariglio et al., 2009) may also be detrimental, as spurious propagation of unwanted and potentially harmful cell types could result in tumors or heterologous tissue formation within the recipient. Therefore, obtaining a high-purity population of pre-differentiated OPCs is essential for safe and effective clinical use. Indeed, hESCs can be differentiated into high-purity OPCs (~90%) that are able to integrate, mature and form compact myelin in dysmyelinated shiverer mice (Nistor et al., 2005) and spinal cord injured rats (Keirstead et al., 2005). However, we recently determined that transplantation of high-purity OPCs in MHV-infected mice with established demyelination did not result in either improved clinical recovery or promote extensive remyelination (Hatch et al., 2009). Importantly, implanted cells failed to survive longer than 2 weeks post-transplantation in spite of administration of immunosuppressants that dampened T cell function. Transplanted cells did not migrate from the site of injection and only focal remyelination was observed. In contrast, human NSCs have recently been shown to survive and exert neuroprotective effects in both mouse and non-human primate models of EAE (Aharonowiz et al., 2008; Pluchino et al., 2009a). Similar to results observed with mouse NSCs, the therapeutic effects of human NSCs were associated with immune regulation. Collectively, these findings emphasize the complexities of cell replacement therapies with regards to understanding local environments that allow for transplanted cells to survive. Moreover, these studies highlight the importance of the model systems utilized, maturation state of transplanted cells, as well as the route of injection with regards to understanding the nature of the clinical relevance of these approaches to treat human neurological diseases. With regards to differences in survival and repair following transplantation of hESC-derived OPCs into MHV-infected mice with the studies discussed above as well as our work on mouse NSC-mediated repair in MHV-infected mice, there are several possible explanations. First, it is unlikely that differentiated OPCs share the same capacity for migration as NSCs (either mouse or human) and this restricts the ability of transplanted cells to extensively remyelinate axons following injection into MHV-infected mice. Secondly, we have never observed engrafted cells – mouse or human – migrating to secondary lymphatic tissue such as cervical lymph nodes following transplantation into MHV-infected mice and this limits the possibility of immune modulation. These findings emphasize the importance of route of delivery e.g. i.v. delivery versus injection into the spinal cord with effector function of the transplanted cell population and further highlights that the maturation stage of the cell – OPC versus NSC – may also be critical not only in trafficking to lymph nodes but also in affecting T cell responses directly within the CNS.
6. Chemokine/chemokine receptors and stem cell transplantation
Although the mechanisms by which transplanted NSCs protect against tissue injury and promote clinical recovery may differ depending upon the model system employed, what is clear is surviving engrafted cells are mobile and able to specifically accumulate to target tissue and positionally migrate to defined anatomic locations. Numerous studies have demonstrated that NSCs express chemokine receptors supporting an important role in allowing cells to proliferate and/or migrate upon injection (Beech et al., 2007; Dziembowska et al., 2005; Gordon et al., 2009; Guan et al., 2008; Imitola et al., 2004; Itoh et al., 2009; Tran et al., 2007; van der Meulen et al., 2009). We have determined that glial-enriched cultures derived from mouse NSCs secrete chemokines in response to cytokine treatment (Whitman et al., 2009). In addition, we have found that NSCs as well as differentiated oligodendrocyte progenitor cells (OPCs) derived from NSCs express an array of chemokine receptors including CXCR2, CXCR3, CXCR4, and CXCR7 (unpublished observations) (Figure 5). Moreover, treatment of hOPCs with either IFN-γ or TNF-α alone or in combination also results in increased expression of mRNA transcripts specific for defined chemokines including CXCL9 and CXCL10 as well as chemokine receptors CXCR2 and CXCR4. Transplanted cells were also found to express chemokine receptors. Immunostaining revealed that engrafted mouse NSCs expressed both CXCR4 and CXCR7 and these cells were enriched within white matter tracts undergoing demyelination (Figure 6). Further, CXCL12, the ligand for both CXCR4 and CXCR7, is also strongly expressed within demyelinating white matter tracts suggesting that implanted NSCs preferentially migrate and accumulate within areas of demyelination by responding to CXCL12 (unpublished observations). We are currently pursuing the functional relevance of chemokine receptor expression on transplanted NSCs. CXCR4 is expressed on hESC-derived OPCs at 2 weeks post-transplantation yet we do not believe that this chemokine receptor, nor others, were functional as transplanted cells displayed only minimal migration from site of injection (Hatch et al., 2009) (unpublished observations). It also bears mentioning that we have not observed a dramatic increase in transcripts encoding for members of the CCR binding chemokine receptors on cultured NSCs treated with cytokines. While this does not eliminate a potentially important role for CC chemokines in participating in transplanted NSC-mediated repair in the MHV model system, we interpret these findings that CXC binding receptors may be more important in mediating trafficking as well as maturation following engraftment.
Figure 5. CXCR2 and CXCR3 expression in OPC-enriched cultures.
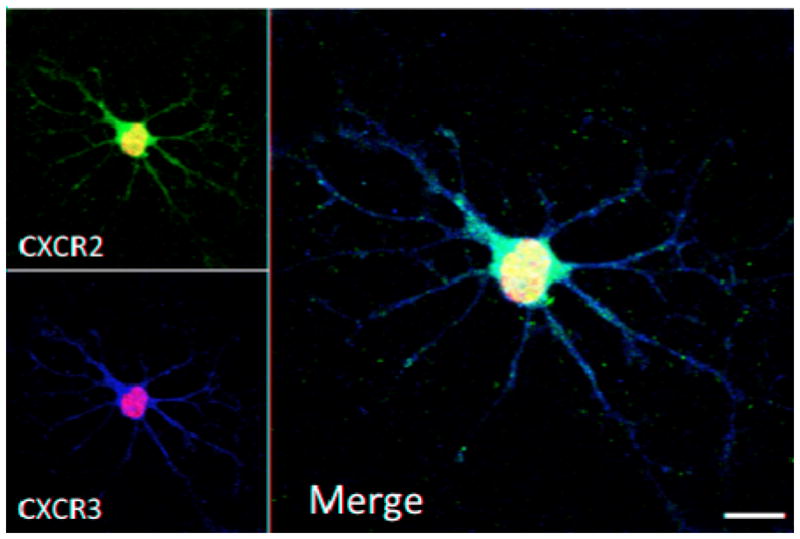
NSCs were isolated from striatal tissue of postnatal day 1 mice and differentiated in high purity cultures of OPCs. Immunostaining using specific antibodies directed against CXCR2 and CXCR3 was detected with secondary fluorescent conjugated antibodies and analyzed using confocal microscopy. Figure is the merge of 8 μm Z-stack originated from projection of 0.5 μm thickness planes. CXCR2 and CXCR3 staining are shown as red and green respectively, blue represents nuclear DAPI staining; scale bar 10 μm.
Figure 6. NSCs express chemokine receptors 3 weeks post-transplant.
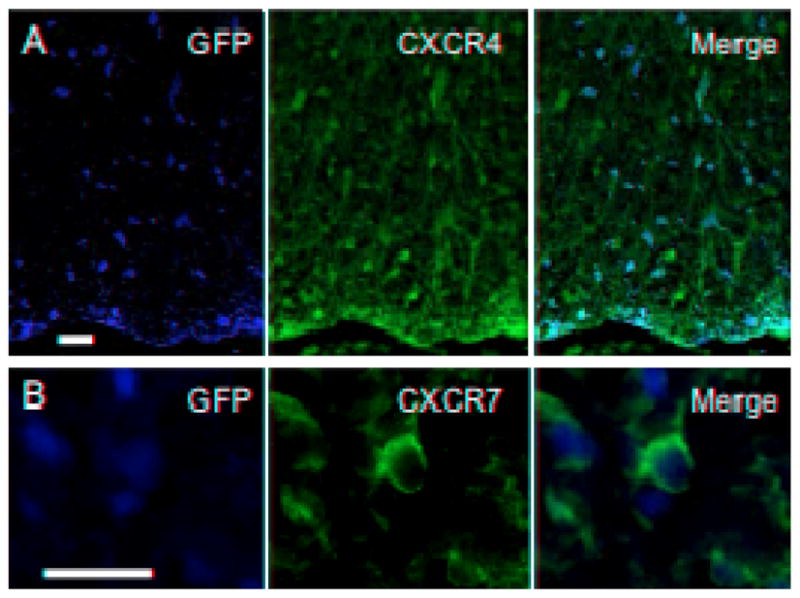
NSCs transplanted into the spinal cords of MHV-infected mice with widespread demyelination migrate extensively and selectively accumulate within damaged white matter tracts. Merging of fluorescence images illustrates CXCR4+ (A) and CXCR7+ (B) GFP+ NSCs 3 weeks post-transplantation (bar s= 20 μm).
7. Perspectives
Standard treatment with immunomodulators and/or immunosuppressants have yielded a ranging degree of success in patients with immune-mediated demyelinating diseases (Rizvi and Agius, 2004) or injury (Sayer et al., 2006), and it is clear that the complex nature of demyelination warrants novel approaches that focus not only on limiting inflammation and neurodegeneration, but also on promoting repair. Potentially novel therapies may be related to i) discovering specific targets that exploit the dual nature of neuroinflammation in order to suppress the degenerative components, while simultaneously enhancing neuroprotection through remyelination by endogenous oligodendrocytes, ii) providing support for remyelination-competent endogenous OPCs and oligodendrocytes and iii) implanting myelinogenic cells into sites of neuroinflammation that are directly capable of inducing remyelination of the damaged axons. Individual application of these methods has been met with success in murine models of demyelination, but it is important to remember that success in these systems may not necessarily translate to the human disease. Moreover, understanding how transplanted cells undergo migration in order to accumulate within areas of pathology will also be critical when considering how to potentially enhance trafficking. In this regard, evaluating the functional contributions of chemokine receptors expressed on NSCs in allowing engrafted cells to positionally migrate represents an important hurdle in our understanding of cell replacement therapies for treating chronic neurologic diseases.
Acknowledgments
Work described in this article was funded by grants from the California Institute for Regenerative Medicine (CIRM) (RS1-409), The National Multiple Sclerosis Society (RG3847), and National Institutes of Health (NS41249) to T.E.L. CIRM pre-doctoral fellowships funded K.S.C. (T1-00008) and L.W. (TG2-01152). A CIRM postdoctoral fellowship funded C.S. (T1-00008).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aharonowiz M, Einstein O, Fainstein N, Lassmann H, Reubinoff B, Ben-Hur T. Neuroprotective effect of transplanted human embryonic stem cell-derived neural precursors in an animal model of multiple sclerosis. PLoS One. 2008;3:e3145. doi: 10.1371/journal.pone.0003145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akesson E, Wolmer-Solberg N, Cederarv M, Falci S, Odeberg J. Human neural stem cells and astrocytes, but not neurons, suppress an allogeneic lymphocyte response. Stem Cell Res. 2009;2:56–67. doi: 10.1016/j.scr.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Akiyama Y, Radtke C, Kocsis JD. Remyelination of the rat spinal cord by transplantation of identified bone marrow stromal cells. J Neurosci. 2002;22:6623–6630. doi: 10.1523/JNEUROSCI.22-15-06623.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amariglio N, Hirshberg A, Scheithauer BW, Cohen Y, Loewenthal R, Trakhtenbrot L, Paz N, Koren-Michowitz M, Waldman D, Leider-Trejo L, Toren A, Constantini S, Rechavi G. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009;6:e1000029. doi: 10.1371/journal.pmed.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part I: the role of infection. Ann Neurol. 2007a;61:288–299. doi: 10.1002/ana.21117. [DOI] [PubMed] [Google Scholar]
- Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part II: Noninfectious factors. Ann Neurol. 2007b;61:504–513. doi: 10.1002/ana.21141. [DOI] [PubMed] [Google Scholar]
- Beech JS, Wheeler DW, Reckless J, Grant AJ, Price J, Mastroeni P, Grainger DJ, Menon DK. The MHP36 line of murine neural stem cells expresses functional CXCR1 chemokine receptors that initiate chemotaxis in vitro. J Neuroimmunol. 2007;184:198–208. doi: 10.1016/j.jneuroim.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Ben-Hur T, Rogister B, Murray K, Rougon G, Dubois-Dalcq M. Growth and fate of PSA-NCAM+ precursors of the postnatal brain. J Neurosci. 1998;18:5777–5788. doi: 10.1523/JNEUROSCI.18-15-05777.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann CC, Lane TE, Stohlman SA. Coronavirus infection of the central nervous system: host-virus stand-off. Nat Rev Microbiol. 2006;4:121–132. doi: 10.1038/nrmicro1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore WF, Irvine KA. Endogenous or exogenous oligodendrocytes for remyelination. J Neurol Sci. 2008;265:43–46. doi: 10.1016/j.jns.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Brustle O, Jones KN, Learish RD, Karram K, Choudhary K, Wiestler OD, Duncan ID, McKay RD. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 1999a;285:754–756. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- Brustle O, Jones KN, Learish RD, Karram K, Choudhary K, Wiestler OD, Duncan ID, McKay RD. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 1999b;285:754–756. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- Cao Q, Benton RL, Whittemore SR. Stem cell repair of central nervous system injury. J Neurosci Res. 2002;68:501–510. doi: 10.1002/jnr.10240. [DOI] [PubMed] [Google Scholar]
- Dziembowska M, Tham TN, Lau P, Vitry S, Lazarini F, Dubois-Dalcq M. A role for CXCR4 signaling in survival and migration of neural and oligodendrocyte precursors. Glia. 2005;50:258–269. doi: 10.1002/glia.20170. [DOI] [PubMed] [Google Scholar]
- Ebers GC, Sadovnick AD, Risch NJ. A genetic basis for familial aggregation in multiple sclerosis. Canadian Collaborative Study Group. Nature. 1995;377:150–151. doi: 10.1038/377150a0. [DOI] [PubMed] [Google Scholar]
- Einstein O, Grigoriadis N, Mizrachi-Kol R, Reinhartz E, Polyzoidou E, Lavon I, Milonas I, Karussis D, Abramsky O, Ben-Hur T. Transplanted neural precursor cells reduce brain inflammation to attenuate chronic experimental autoimmune encephalomyelitis. Exp Neurol. 2006;198:275–284. doi: 10.1016/j.expneurol.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Fleming JO, Trousdale MD, el-Zaatari FA, Stohlman SA, Weiner LP. Pathogenicity of antigenic variants of murine coronavirus JHM selected with monoclonal antibodies. J Virol. 1986;58:869–875. doi: 10.1128/jvi.58.3.869-875.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJ. Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci. 2002;3:705–714. doi: 10.1038/nrn917. [DOI] [PubMed] [Google Scholar]
- Glass WG, Hickey MJ, Hardison JL, Liu MT, Manning JE, Lane TE. Antibody targeting of the CC chemokine ligand 5 results in diminished leukocyte infiltration into the central nervous system and reduced neurologic disease in a viral model of multiple sclerosis. J Immunol. 2004;172:4018–4025. doi: 10.4049/jimmunol.172.7.4018. [DOI] [PubMed] [Google Scholar]
- Glass WG, Liu MT, Kuziel WA, Lane TE. Reduced macrophage infiltration and demyelination in mice lacking the chemokine receptor CCR5 following infection with a neurotropic coronavirus. Virology. 2001;288:8–17. doi: 10.1006/viro.2001.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon RJ, McGregor AL, Connor B. Chemokines direct neural progenitor cell migration following striatal cell loss. Mol Cell Neurosci. 2009;41:219–232. doi: 10.1016/j.mcn.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Grinnemo KH, Kumagai-Braesch M, Mansson-Broberg A, Skottman H, Hao X, Siddiqui A, Andersson A, Stromberg AM, Lahesmaa R, Hovatta O, Sylven C, Corbascio M, Dellgren G. Human embryonic stem cells are immunogenic in allogeneic and xenogeneic settings. Reprod Biomed Online. 2006;13:712–724. doi: 10.1016/s1472-6483(10)60663-3. [DOI] [PubMed] [Google Scholar]
- Guan Y, Jiang Z, Ciric B, Rostami AM, Zhang GX. Upregulation of chemokine receptor expression by IL-10/IL-4 in adult neural stem cells. Exp Mol Pathol. 2008;85:232–236. doi: 10.1016/j.yexmp.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Halfpenny C, Benn T, Scolding N. Cell transplantation, myelin repair, and multiple sclerosis. Lancet Neurol. 2002;1:31–40. doi: 10.1016/s1474-4422(02)00004-2. [DOI] [PubMed] [Google Scholar]
- Hardison JL, Nistor G, Gonzalez R, Keirstead HS, Lane TE. Transplantation of glial-committed progenitor cells into a viral model of multiple sclerosis induces remyelination in the absence of an attenuated inflammatory response. Exp Neurol. 2006;197:420–429. doi: 10.1016/j.expneurol.2005.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch MN, Schaumburg CS, Lane TE, Keirstead HS. Endogenous remyelination is induced by transplant rejection in a viral model of multiple sclerosis. J Neuroimmunol. 2009;212:74–81. doi: 10.1016/j.jneuroim.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Herndon RM, Griffin DE, McCormick U, Weiner LP. Mouse hepatitis virus-induced recurrent demyelination. A preliminary report. Arch Neurol. 1975;32:32–35. doi: 10.1001/archneur.1975.00490430054008. [DOI] [PubMed] [Google Scholar]
- Hori J, Ng TF, Shatos M, Klassen H, Streilein JW, Young MJ. Neural progenitor cells lack immunogenicity and resist destruction as allografts. Stem Cells. 2003;21:405–416. doi: 10.1634/stemcells.21-4-405. [DOI] [PubMed] [Google Scholar]
- Hori J, Ng TF, Shatos M, Klassen H, Streilein JW, Young MJ. Neural progenitor cells lack immunogenicity and resist destruction as allografts. 2003. Ocul Immunol Inflamm. 2007;15:261–273. doi: 10.1080/09273940701382242. [DOI] [PubMed] [Google Scholar]
- Hosking MP, Lane TE. The Biology of Persistent Infection: Inflammation and Demyelination following Murine Coronavirus Infection of the Central Nervous System. Curr Immunol Rev. 2009;5:267–276. doi: 10.2174/157339509789504005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Satou T, Ishida H, Nishida S, Tsubaki M, Hashimoto S, Ito H. The relationship between SDF-1alpha/CXCR4 and neural stem cells appearing in damaged area after traumatic brain injury in rats. Neurol Res. 2009;31:90–102. doi: 10.1179/174313208X332995. [DOI] [PubMed] [Google Scholar]
- Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane TE, Buchmeier MJ. Murine coronavirus infection: a paradigm for virus-induced demyelinating disease. Trends Microbiol. 1997;5:9–14. doi: 10.1016/S0966-842X(97)81768-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane TE, Liu MT, Chen BP, Asensio VC, Samawi RM, Paoletti AD, Campbell IL, Kunkel SL, Fox HS, Buchmeier MJ. A central role for CD4(+) T cells and RANTES in virus-induced central nervous system inflammation and demyelination. J Virol. 2000;74:1415–1424. doi: 10.1128/jvi.74.3.1415-1424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Stohlman SA, Hinton DR. Mouse hepatitis virus is cleared from the central nervous systems of mice lacking perforin-mediated cytolysis. J Virol. 1997;71:383–391. doi: 10.1128/jvi.71.1.383-391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MT, Keirstead HS, Lane TE. Neutralization of the chemokine CXCL10 reduces inflammatory cell invasion and demyelination and improves neurological function in a viral model of multiple sclerosis. J Immunol. 2001;167:4091–4097. doi: 10.4049/jimmunol.167.7.4091. [DOI] [PubMed] [Google Scholar]
- Marten NW, Stohlman SA, Bergmann CC. MHV infection of the CNS: mechanisms of immune-mediated control. Viral Immunol. 2001;14:1–18. doi: 10.1089/08828240151061329. [DOI] [PubMed] [Google Scholar]
- Mason DW, Charlton HM, Jones AJ, Lavy CB, Puklavec M, Simmonds SJ. The fate of allogeneic and xenogeneic neuronal tissue transplanted into the third ventricle of rodents. Neuroscience. 1986;19:685–694. doi: 10.1016/0306-4522(86)90292-7. [DOI] [PubMed] [Google Scholar]
- Modo M, Rezaie P, Heuschling P, Patel S, Male DK, Hodges H. Transplantation of neural stem cells in a rat model of stroke: assessment of short-term graft survival and acute host immunological response. Brain Res. 2002;958:70–82. doi: 10.1016/s0006-8993(02)03463-7. [DOI] [PubMed] [Google Scholar]
- Nicholas MK, Antel JP, Stefansson K, Arnason BG. Rejection of fetal neocortical neural transplants by H-2 incompatible mice. J Immunol. 1987a;139:2275–2283. [PubMed] [Google Scholar]
- Nicholas MK, Stefansson K, Antel JP, Arnason BG. An in vivo and in vitro analysis of systemic immune function in mice with histologic evidence of neural transplant rejection. J Neurosci Res. 1987b;18:245–257. doi: 10.1002/jnr.490180135. [DOI] [PubMed] [Google Scholar]
- Nistor GI, Totoiu MO, Haque N, Carpenter MK, Keirstead HS. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005;49:385–396. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- Oksenberg JR, Begovich AB, Erlich HA, Steinman L. Genetic factors in multiple sclerosis. Jama. 1993;270:2362–2369. [PubMed] [Google Scholar]
- Parra B, Hinton DR, Marten NW, Bergmann CC, Lin MT, Yang CS, Stohlman SA. IFN-gamma is required for viral clearance from central nervous system oligodendroglia. J Immunol. 1999;162:1641–1647. [PubMed] [Google Scholar]
- Pearce BD, Hobbs MV, McGraw TS, Buchmeier MJ. Cytokine induction during T-cell-mediated clearance of mouse hepatitis virus from neurons in vivo. J Virol. 1994;68:5483–5495. doi: 10.1128/jvi.68.9.5483-5495.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SR, Lane TE, Buchmeier MJ. Coronaviruses: Hepatitis, peritonitis, and central nervous system disease. In: Cunningham MW, Fujinami RS, editors. Effects of Microbes on the Immune System. Vol. 1. Lippincott Williams & Wilkins; Philadelphia: 1999. pp. 331–348. [Google Scholar]
- Pluchino S, Gritti A, Blezer E, Amadio S, Brambilla E, Borsellino G, Cossetti C, Del Carro U, Comi G, t Hart B, Vescovi A, Martino G. Human neural stem cells ameliorate autoimmune encephalomyelitis in non-human primates. Ann Neurol. 2009a;66:343–354. doi: 10.1002/ana.21745. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A, Furlan R, Comi G, Vescovi AL, Martino G. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Zanotti L, Brini E, Ferrari S, Martino G. Regeneration and repair in multiple sclerosis: the role of cell transplantation. Neurosci Lett. 2009b;456:101–106. doi: 10.1016/j.neulet.2008.03.097. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, Martinello M, Cattalini A, Bergami A, Furlan R, Comi G, Constantin G, Martino G. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005;436:266–271. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- Poser CM. The epidemiology of multiple sclerosis: a general overview. Ann Neurol. 1994;36(Suppl 2):S180–193. doi: 10.1002/ana.410360805. [DOI] [PubMed] [Google Scholar]
- Raine CS. The Dale E. McFarlin Memorial Lecture: the immunology of the multiple sclerosis lesion. Ann Neurol. 1994;36(Suppl):S61–72. doi: 10.1002/ana.410360716. [DOI] [PubMed] [Google Scholar]
- Rizvi SA, Agius MA. Current approved options for treating patients with multiple sclerosis. Neurology. 2004;63:S8–14. doi: 10.1212/wnl.63.12_suppl_6.s8. [DOI] [PubMed] [Google Scholar]
- Robertson NJ, Brook FA, Gardner RL, Cobbold SP, Waldmann H, Fairchild PJ. Embryonic stem cell-derived tissues are immunogenic but their inherent immune privilege promotes the induction of tolerance. Proc Natl Acad Sci U S A. 2007;104:20920–20925. doi: 10.1073/pnas.0710265105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayer FT, Kronvall E, Nilsson OG. Methylprednisolone treatment in acute spinal cord injury: the myth challenged through a structured analysis of published literature. Spine J. 2006;6:335–343. doi: 10.1016/j.spinee.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Sher F, Balasubramaniyan V, Boddeke E, Copray S. Oligodendrocyte differentiation and implantation: new insights for remyelinating cell therapy. Curr Opin Neurol. 2008;21:607–614. doi: 10.1097/WCO.0b013e32830f1e50. [DOI] [PubMed] [Google Scholar]
- Sim FJ, Zhao C, Penderis J, Franklin RJ. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci. 2002;22:2451–2459. doi: 10.1523/JNEUROSCI.22-07-02451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- Steinman L. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell. 1996;85:299–302. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- Stiles LN, Hosking MP, Edwards RA, Strieter RM, Lane TE. Differential roles for CXCR3 in CD4+ and CD8+ T cell trafficking following viral infection of the CNS. Eur J Immunol. 2006;36:613–622. doi: 10.1002/eji.200535509. [DOI] [PubMed] [Google Scholar]
- Stohlman SA, Bergmann CC, Lin MT, Cua DJ, Hinton DR. CTL effector function within the central nervous system requires CD4+ T cells. J Immunol. 1998;160:2896–2904. [PubMed] [Google Scholar]
- Swijnenburg RJ, Schrepfer S, Govaert JA, Cao F, Ransohoff K, Sheikh AY, Haddad M, Connolly AJ, Davis MM, Robbins RC, Wu JC. Immunosuppressive therapy mitigates immunological rejection of human embryonic stem cell xenografts. Proc Natl Acad Sci U S A. 2008;105:12991–12996. doi: 10.1073/pnas.0805802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepavcevic V, Blakemore WF. Haplotype matching is not an essential requirement to achieve remyelination of demyelinating CNS lesions. Glia. 2006;54:880–890. doi: 10.1002/glia.20425. [DOI] [PubMed] [Google Scholar]
- Totoiu MO, Nistor GI, Lane TE, Keirstead HS. Remyelination, axonal sparing, and locomotor recovery following transplantation of glial-committed progenitor cells into the MHV model of multiple sclerosis. Exp Neurol. 2004;187:254–265. doi: 10.1016/j.expneurol.2004.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PB, Banisadr G, Ren D, Chenn A, Miller RJ. Chemokine receptor expression by neural progenitor cells in neurogenic regions of mouse brain. J Comp Neurol. 2007;500:1007–1033. doi: 10.1002/cne.21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meulen AA, Biber K, Lukovac S, Balasubramaniyan V, den Dunnen WF, Boddeke HW, Mooij JJ. The role of CXC chemokine ligand (CXCL)12-CXC chemokine receptor (CXCR)4 signalling in the migration of neural stem cells towards a brain tumour. Neuropathol Appl Neurobiol. 2009;35:579–591. doi: 10.1111/j.1365-2990.2009.01036.x. [DOI] [PubMed] [Google Scholar]
- Weiner LP. Pathogenesis of demyelination induced by a mouse hepatitis. Arch Neurol. 1973;28:298–303. doi: 10.1001/archneur.1973.00490230034003. [DOI] [PubMed] [Google Scholar]
- Whitman L, Zhou H, Perlman S, Lane TE. IFN-gamma-mediated suppression of coronavirus replication in glial-committed progenitor cells. Virology. 2009;384:209–215. doi: 10.1016/j.virol.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson JS, Stohlman SA. Effective clearance of mouse hepatitis virus from the central nervous system requires both CD4+ and CD8+ T cells. J Virol. 1990;64:4589–4592. doi: 10.1128/jvi.64.9.4589-4592.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson JS, Sykes KC, Stohlman SA. Characterization of brain-infiltrating mononuclear cells during infection with mouse hepatitis virus strain JHM. J Neuroimmunol. 1991;32:199–207. doi: 10.1016/0165-5728(91)90189-E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolswijk G, Noble M. Cooperation between PDGF and FGF converts slowly dividing O-2Aadult progenitor cells to rapidly dividing cells with characteristics of O-2Aperinatal progenitor cells. J Cell Biol. 1992;118:889–900. doi: 10.1083/jcb.118.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GF, Dandekar AA, Pewe L, Perlman S. CD4 and CD8 T cells have redundant but not identical roles in virus-induced demyelination. J Immunol. 2000;165:2278–2286. doi: 10.4049/jimmunol.165.4.2278. [DOI] [PubMed] [Google Scholar]
- Wu GF, Perlman S. Macrophage infiltration, but not apoptosis, is correlated with immune-mediated demyelination following murine infection with a neurotropic coronavirus. J Virol. 1999;73:8771–8780. doi: 10.1128/jvi.73.10.8771-8780.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]