Abstract
Objectives
To determine which lower-limb joint moments and powers characterize the level of gait performance of older adults with symptomatic knee osteoarthritis (OA).
Design
Cross-sectional observational study.
Setting
University motion analysis laboratory.
Participants
Community-dwelling adults (N=60; 27 men, 33 women; age 50–79y) with symptomatic knee OA.
Interventions
Not applicable.
Main Outcome Measures
Physical function was measured using the long-distance corridor walk, the Short Physical Performance Battery, and the Late Life Function and Disability Instrument (LLFDI Function). Joint moments and power were estimated using an inverse dynamics solution after 3-dimensional computerized motion analysis.
Results
Subjects aged 64.2±7.4 years were recruited. Ranges (mean ± SD) for the 400-m walk time and the LLFDI Advanced Lower-Limb Function score were 215.3 to 536.8 (304.1±62.3) seconds and 31.5 to 100 (57.0±14.9) points, respectively. In women, hip abductor moment (loading response), hip abductor power (midstance), eccentric hamstring moment (terminal stance), and power (terminal swing) accounted for 41%, 31%, 14%, and 48% of the variance in the 400-m walk time, respectively (model R2=.61, P<.003). In men, plantar flexor and hip flexor power (preswing) accounted for 19% and 24% of the variance in the 400-m walk time, respectively (model R2=.32, P=.025).
Conclusions
There is evidence that men and women with higher mobility function tend to rely more on an ankle strategy rather than a hip strategy for gait. In higher functioning men, higher knee extensor and flexor strength may contribute to an ankle strategy, whereas hip abductor weakness may bias women with lower mobility function to minimize loading across the knee via use of a hip strategy. These parameters may serve as foci for rehabilitation interventions aimed at reducing mobility limitations.
Keywords: Aging, Knee, Osteoarthritis, Rehabilitation
Symptomatic osteoarthritis is associated with disability resulting from mobility limitations.1-3 Locomotor disability predicts future dependency,4 falls, and reduced quality of life.5 The prospective evaluation of preclinical disability by Fried et al6 revealed that incident difficulty with community ambulation (0.5 mile) and stair ascent at 18-month follow-up was strongly predicted by the need for task modification or slow ambulation at baseline. Therefore, prevention of physical disability through improving walking is one of the most important areas of aging research.2,5-7
The etiology of OA likely is both biomechanical and biochemical, and there is no known cure, making effective early intervention particularly important. Numerous cross-sectional studies have found lower knee extensor strength in subjects with knee OA in comparison with those without knee OA.8,9 Lower strength is associated with greater functional limitations,10 and has been reported as the best independent predictor of age-related decline in the performance of the 10-m walk, the stair climb, the chair-stand time, and home mobility.11,12 However, the presence of a relation between impaired strength and mobility limitation does not necessarily mean that correction of the impairment will be accompanied by improved mobility, as there could be concomitant alterations in gait mechanics at other joints. For example, mobility limitations caused by knee OA may relate to greater mechanical energy expenditure or moments at the ankle and hip.13,14
Assessment of concurrent biomechanical events that may contribute to mobility limitations may inform rehabilitation strategies to improve mobility as well as enable measurement of functional outcomes after interventions. Modifiable risk factors for these mobility limitations include maladaptive gait compensations. Greater mechanical energy expenditure or moments at the ankle and hip in older adults with knee OA may underlie functional limitations in these activities.13,14 Computerized motion analysis enables assessment of compensatory patterns that otherwise may not be detected.
To reduce functional limitations in older adults, there is a strong rationale for the use of motion analysis to characterize movement strategies and eventually inform rehabilitation interventions.15 Therefore, the aim of this study was to assess whether patterns of movement assessed by multisegment motion analysis can distinguish older adults with symptomatic knee OA with more severe mobility limitations (lower function) from those without severe mobility limitations (higher function). A secondary aim was to determine targets for rehabilitation to address mobility limitations.
METHODS
Participants
Sixty subjects with symptomatic knee OA were recruited from one clinical site of the Multicenter Osteoarthritis (MOST Study), a longitudinal study of 3026 men and women aged 50 to 79 years with risk factors for knee OA—obesity, knee injury, surgery, or pain. Recruitment was stratified by decade, sex, and 20-m walk test time (completed as part of the MOST study) to ensure a range of age and mobility level among men and women. All subjects completed an informed consent process and signed a consent form approved by the investigators’ institutional review board.
Knee OA was determined through the examination of radiographs completed as part of the MOST study protocol and was defined by a Kellgren-Lawrence grade of 2 or greater on standardized fixed-flexion anterior-posterior radiographs.16 Frequent knee symptoms were assessed by trained and certified interviewers who asked participants: “During the past 30 days, have you had pain, aching, or stiffness in or around your knee on most days?” Symptomatic knee OA was defined as the combination of radiographic tibiofemoral OA and frequent knee symptoms.
Subjects were ineligible if they limited their activities because of back pain during the preceding 30 days before enrollment in the study; had neuromuscular disease; were not able to walk by themselves without the help of another person or an assistive device; were legally blind; had an injury or illness other than knee OA that affected their walking ability; had surgery in the past year requiring a recuperation period of more than 1 week; reported fainting spells; or in the past year had fallen, resulting in mobility limitation.
Measures
Functional limitations and physical activity
Self-reported difficulty with physical activity was measured using the LLFDI: Function Component (Boston University, Boston, MA).17-19 The advanced lower-limb subscore was the primary measure of mobility limitation. To measure knee pain and stiffness, and physical difficulty, the modified WOMAC (University of Western Ontario, London, Canada) was used.20-23 To measure physical activity level, the Physical Activity Scale for the Elderly (New England Research Institute Inc, Watertown, MA) was used.24-26
Mobility was assessed with the long-distance corridor walk.27,28 For this 2-stage walking test, subjects completed a 2-minute walk followed by a 400-m walk. If subjects’ heart rate exceeded 135 beats/min or if they experienced excessive knee pain, shortness of breath, or chest pain during the 2-minute walk, then they did not complete the 400-m walk and their 400-m walk time instead was predicted from their performance during the 2-minute walk.
Summary performance score
Lastly, as a composite mobility and balance assessment, subjects performed the SPPB (National Institutes of Health, Bethesda, MD), including side-by-side, semitandem, and tandem balance tests, as well as timed chair-stand and 4-m walk tests. The summary performance score for the SPPB was calculated using the method of scaling described previously.29 For the enrolled subjects, this was (1–[4-m walk time/5.368])+(1–[chair-stand time/16.22])+(balance time/30), with all times expressed in seconds.
Isokinetic strength testing
Isokinetic strength testing was measured with a Cybex 350 dynamometer.a Knee flexion and extension were evaluated at 60° per second and a chair back angle of 85° from 90° of flexion to each subject's full extension. A standardized protocol for measuring quadriceps strength was followed.30 Subjects were provided instructions using a standardized script for subject testing and 3 practice trials using 50% effort. After the practice trials, 4 repetitions were completed for flexor and extensor torque. Subjects’ concentric knee extensor and flexor strength (Nm) were considered the peak torque obtained over 4 trials. Examiners calibrated the isokinetic dynamometer position, angular velocity, and torque (at 25 and 245Nm) monthly. In a reliability study conducted with the isokinetic dynamometer used, conducted concurrent with the MOST study, the strength testing protocol had an intraclass correlation coefficient of .94 (.82–.99), a coefficient of variation of 8% (6%–12%), and a within-subject variation of 6.3Nm (4.71–9.63Nm).
Isometric strength measurements
Isometric strength testing was completed for hip extension, flexion, and abduction using a Spark Handheld Dynamometer Model 160.31,b Hip abduction was measured with subjects in a seated position, and extension and flexion were measured in the prone and supine positions, repectively.32 Each isometric contraction was held for 3 seconds and repeated for 3 total trials. The highest of the 3 isometric values was considered the peak force.33
Gait evaluation
Three-dimensional kinematic data and ground reaction force data were collected as subjects walked along a 9-m walkway at self-selected and controlled speeds.c,d Three noncollinear infrared markers were used to track each of 8 segments: feet, legs, thighs, pelvis, and trunk. Marker coordinate data and force plate data were collected at 60 and 300Hz, and filtered at 6 and 10Hz, respectively.
Data captured with the subject standing (subject calibration) were used to define the transformation matrix between the external marker reference system and the principal axes of each of 8 body segments used to represent the skeletal system. Segment principal axes were defined based on digitized bony landmarks used to represent the bilateral skeletal system: anterior and posterior superior iliac spines, glenohumeral joints, lateral and medial epicondyles, lateral and medial malleoli, posterior heel, and second toe. Marker position data were combined with anthropometric data and ground reaction forces in an 8-segment model.e A 3-dimensional inverse dynamic solution was obtained to calculate internal net joint moments of force and powers at the ankle, knee, and hip. Net joint power is the product of torque and angular velocity. Power reflects the rate at which work is done at each of the joints (area under the power curve is energy). In general, peak power represents energy from the muscles that is either being added to the body (positive power) to sustain the upright posture and forward progression or taken out of the body (negative power) to slow segment motion or cushion directional changes during walking. Power differences across the lower-limb joints reflect differences in energy demands that are placed on muscles crossing these joints.
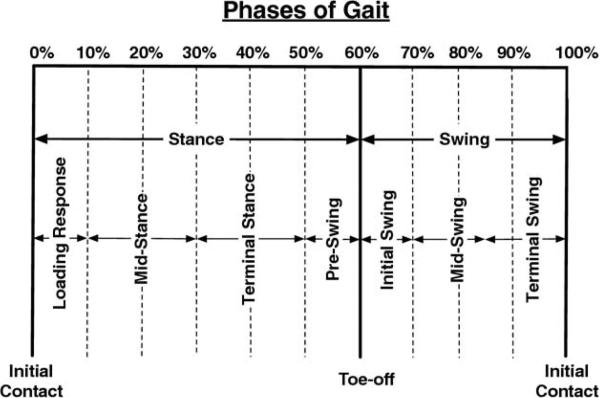
Five walking trials, with the speed closest to .89m/s (2mph), were used to assess the kinematic and kinetic data. Data for all trials were time-normalized, and moments and powers were normalized to body mass. Peak values were determined for the kinematic and kinetic data at every 2% of the gait cycle during each of the phases and events depicted in figure 1.
Fig 1.
Phases and key events of gait by percent of gait cycle.
Statistical Analysis
Univariate statistics were completed to check for normality of continuous variables. For the 5 subjects unable to complete the 400-m walk, their 400-m walk time was imputed by using the equation obtained from linearly regressing 2-minute walk distance on 400-m walk time for same-sex subjects (for men: 672.64780–2.23789 [2-minute walk distance]; for women: 706.83286–2.45140 [2-minute walk distance]). Pearson correlation coefficients were used to compare anthropometric (age, body mass index), activity level, strength, and functional outcome (LLFDI, SPPB/summary performance score, and WOMAC) data with 400-m walk times to confirm the adequacy of the sample to represent higher and lower mobility function.
Moment and power data were plotted against the percent of the gait cycle for visual assessment of outlier data. Moment and power peak and trough data in each axis of motion during each phase of the gait cycle were analyzed by generating sexspecific Pearson correlation coefficients between these data and the 400-m walk times, as well as the LLFDI Advanced Lower-Limb scores. Sex-specific analyses were conducted because of the known differences in gait in men and women with knee OA.34-37 Gait parameters with a suggestive independent correlation with these functional measures (r≥.25) were then entered into sex-specific stepwise regression models with 400-m walk time as the continuous dependent variable. Partial correlation coefficients (variable added last) were calculated to assess the independent contribution of each in explaining the functional outcome measure (eg, 400-m walk time).
Those gait parameters that significantly explained 400-m walk time were entered into a second set of stepwise linear regression models with lower-limb strength data to assess whether the relationship between gait parameters and walk time could be accounted for by a specific impairment in strength. Data analysis was performed using SAS softwaref with a study-wide alpha level of .05, meaning that when multiple comparisons were made, significance was determined using the Bonferroni method of adjustment to ensure that the probability of making a type I error remained below .05 for the group of comparisons (tables 1–3).
Table 1.
Recruitment Results
| Subject Characteristics | Correlation With 400-m Walk Time (r) | P | |
|---|---|---|---|
| Age (y) | All | .227 | .081 |
| Men | .427 | .026 | |
| Women | .042 | .816 | |
| WOMAC pain score | All | .212 | .104 |
| Men | .069 | .734 | |
| Women | .348 | .048 | |
| Physical Activity Scale for the Elderly | All | –.171 | .192 |
| Men | –.218 | .275 | |
| Women | –.050 | .782 | |
Table 3.
Strength in More Symptomatic Lower Limb*
| Strength Variables | Correlation With 400-m Walk Time (r) | P | |
|---|---|---|---|
| Isokinetic knee extensor strength | All | –.587 | <.001 |
| Men | –.729 | <.003 | |
| Women | –.408 | .048 | |
| Isokinetic knee flexor strength | All | –.587 | <.001 |
| Men | –.779 | <.001 | |
| Women | –.234 | .271 | |
| Isometric hip extension | All | –.456 | .005 |
| Men | –.305 | .219 | |
| Women | –.500 | .029 | |
| Isometric hip flexion | All | –.367 | .020 |
| Men | –.256 | .290 | |
| Women | –.393 | .078 | |
| Isometric hip abduction | All | –.327 | .045 |
| Men | –.253 | .327 | |
| Women | –.272 | .233 | |
Strength for limb with most symptomatic knee OA.
An a priori power calculation was conducted for a 3-way analysis of variance, with factors being decade (3 levels: 50s, 60s, 70s), sex (2 levels), and function (2 levels: high/low, with low based on the slowest quartile of 20-m walk time in the MOST study). Based on estimates from prior reports, we estimated that a random effects model with .39 SD within and .32 SD contrast between groups would require 5 subjects in each of the 12 sex-decade-function groups to provide 87.6% power at an alpha level of .05. Review of more recent publications during the study period led us to examine the differences between sexes and functional levels, but not between decades.
RESULTS
Subjects
The 60 subjects (33 women) had a mean age ± SD of 64.2±7.4 years, and the more symptomatic knee had a Kellgren-Lawrence grade of 2 for 21 subjects, 3 for 25 subjects, and 4 for 14 subjects. Leg dominance was on the right for 96.5% of the subjects.
Of the 60 subjects recruited, motion analysis data were lost for 4 subjects because of computer malfunction at the time of data collection, and motion analysis data damage led to an inability to process complete data, leaving partial datasets for an additional 7 subjects. Therefore, although data were available for 60 subjects for demographic and physical functional measures, partial or complete motion analysis data were available for 54 subjects.
Validation of 400-m Walk Time as a Measure of Mobility Limitation
In the full cohort, as well as in men and women separately, the 400-m walk time did not significantly differ by age, knee pain severity, or activity level (see table 1). The 400-m walk time also was concordant with other self-reported and objective functional measures (see table 2) despite no difference in knee pain among those with higher and lower gait function.
Table 2.
Functional Outcome Measures
| Physical Functional Measures | Correlation With 400-m Walk Time (r) | P | |
|---|---|---|---|
| LLFDI: Advanced Lower-Limb Function | All | –.561 | <.001 |
| Men | –.529 | .005 | |
| Women | –.591 | <.001 | |
| Summary performance score | All | –.750 | <.001 |
| Men | –.845 | <.001 | |
| Women | –.648 | <.001 | |
| Two-minute walk distance (m) | All | –.921 | <.001 |
| Men | –.953 | <.001 | |
| Women | –.889 | <.001 | |
Long-Distance Corridor Walk
Fifty-five subjects completed the 400-m walk portion of the long-distance corridor walk (mean ± SD: 304.1±62.3s; range, 215.3–536.8s). Three subjects were excluded because of hospitalization for 3 or more days within the preceding 3 months, and 2 were excluded because their heart rate exceeded 135 beats/min during the 2-minute walk portion of the test. After combining the 400-m walk times that were interpolated from the 2-minute walk data for these 5 subjects (mean ± SD, 318.6±50.0s; range, 233.6–359.0s), the group of 60 subjects had a mean ± SD 400-m walk time of 305.3±61.2 seconds with a range of 215.3 to 536.8 seconds. This did not significantly differ from the mean for the 55 subjects completing the full test.
Short Physical Performance Battery
The raw SPPB score was relatively insensitive to detecting mobility limitation in our subjects, with 97% of subjects scoring 10 or more points (12 points, 46 subjects; 11 points, 9 subjects; 10 points, 3 subjects; 9 and 8 points, 1 subject each). However, scaling subjects’ performance using the summary performance score was much more sensitive to detecting mobility limitation (see table 2) and correlated well with the LLFDI Advanced Lower-Limb Function (r=.64, P<.001), 400-m walk time (r=–.77, P<.001), and chair-stand time (r=–.77, P<.001).
Motion Analysis: Women
Correlation coefficients between functional measures (either long-distance corridor walk or LLFDI) and the following gait parameters in women (n=24) were significant (r≥.25): hip sagittal power during terminal stance/preswing, hip frontal power and moments during loading response and power during midstance, knee sagittal power during terminal swing and moment during terminal stance, and ankle sagittal moment at toe-off and initial swing.
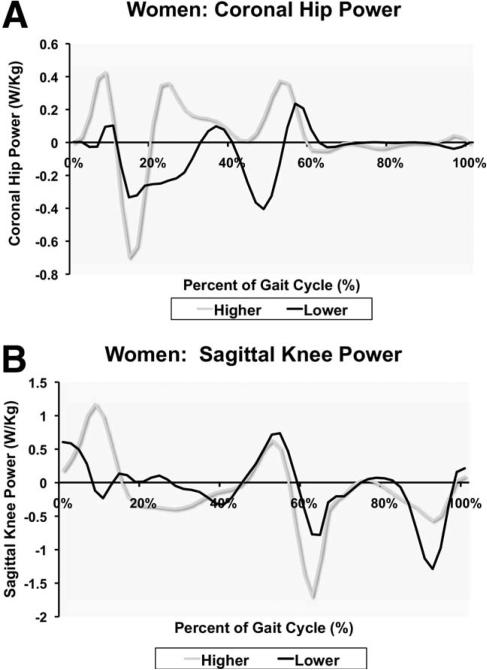
In stepwise regression analysis, hip frontal moment during loading response and hip frontal power during midstance (fig 2A), respectively, explained 41% and 31% of the variability in the 400-m walk time. In this model, knee sagittal power during terminal swing (fig 2B) and knee sagittal moment during the interval between preswing and initial swing accounted for 48% and 14% of the variability in the 400-m walk time, respectively (full model R2=.61, P=.003).
Fig 2.
Example gait data for women. (A) High-functioning person with greater negative power at the knee during preswing and initial swing and less negative power at the end of swing phase. (B) Low-functioning person generating less positive power during midstance (typically associated with moving the pelvis towards a more neutral position).
Motion Analysis: Men
Correlation coefficients between the 400-m walk time and the following gait parameters in men (n=25) were significant (r≥.25): hip sagittal power at push-off, hip frontal power during terminal stance, ankle sagittal power and moment during initial swing, and hip frontal moment during loading response.
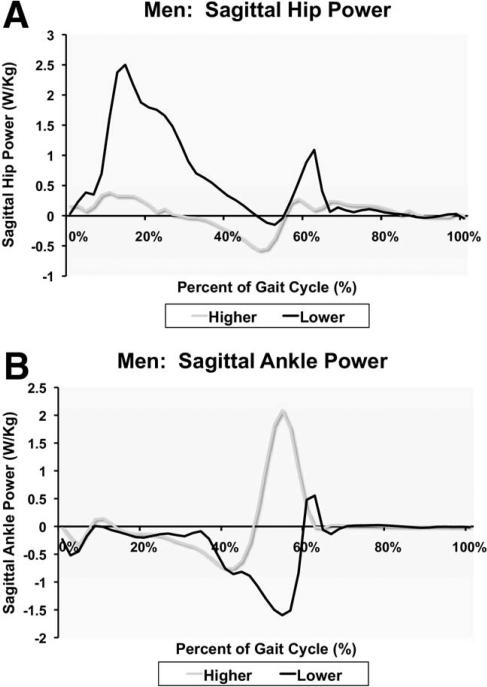
In men, stepwise regression revealed that hip sagittal power at terminal stance (P=.026) and ankle sagittal power during preswing (P=.048), respectively, explained 24% and 19% of the variance in the 400-m walk time (full model R2=.32, P=.025) (fig 3).
Fig 3.
Example gait data for men; same persons in both plates. (A) Low-functioning person demonstrating more of a hip strategy in which greater positive power is generated at the hip to compensate for decreased power generation at the ankle (B).
Lower-Limb Muscle Strength
All strength data significantly differed between women and men (all P≤.002), supporting the decision to stratify analyses by sex. In women, neither knee (flexion and extension) nor hip (abduction, flexion, and extension) strength was associated with gait function (see table 3). However, hip frontal power in terminal stance correlated well with hip extension (r=.49, P=.033), flexion (r=.44, P=.044), and abduction strength (r=.42, P=.050) in women. In men, knee strength (flexion and extension), but not hip strength, was significantly associated with the level of gait function (see table 3). In addition, hip flexion strength in men correlated with both hip sagittal power at push-off (r=.47 P=.038) and ankle sagittal power during initial swing (r=.44, P=.052).
Effect of Unilateral or Bilateral Symptomatic Knee Osteoarthritis
There were 32 subjects with unilateral and 28 subjects with bilateral, symptomatic knee OA. There were no significant differences in age, body mass index, or activity level, nor in SPPB, WOMAC, or LLFDI scores when comparing subjects with unilateral, symptomatic knee OA with subjects with bilateral, symptomatic knee OA. Subjects with bilateral, symptomatic knee OA had greater mean 400-m walk times (315.6±75.8s vs 293.0±44.5s; P=.188) and reduced mean isokinetic knee extensor strength (85.1±37.6Nm vs 104.1±39.5Nm; P=.069), although these differences were not statistically significant.
DISCUSSION
The purpose of this study was to discern which biomechanical factors characterize the gait of older adults with symptomatic knee OA who have a range of mobility function. This characterization is functionally significant and provides potential foci for directed rehabilitation with the goal of advancing mobility in older adults with symptomatic knee OA who have more severe functional limitations. It also is significant that gait characteristics differed between men and women in this study, supporting the need for sex-stratified analyses and suggesting that rehabilitation should target different gait parameters in men and women.
There were several unique characteristics of the design and methodology used in this study. First, we examined kinematic and kinetic data in the frontal and transverse as well as the sagittal plane, extending prior findings as described below.38 Second, the stratified recruitment led to a sample representative of men and women with symptomatic knee OA, distributed in age between 50 and 80 years, with higher and lower gait function. These features enabled the study to provide useful information regarding which impairments in gait might be targeted with physical therapy and gait training, as well as whether there are potentially modifiable impairments that are associated with mobility limitations. In our subjects, the biomechanical factors that were associated with functional measures were confined to sagittal plane kinetics at the ankle, knee, and hip, and frontal plane kinetics at the hip.
Despite similar knee OA radiographic and symptomatic severity, we found a range of both 400-m walking ability and magnitude of key gait parameters. Because the present study represented a spectrum of lower-limb functional abilities that were disassociated with knee OA severity, our analyses differed from previous studies where OA severity was an independent variable and results were contrasted with gait parameters of subjects without knee OA. These basic differences are compounded by other design and methodological differences. In prior studies, sex, walking speed, and footwear conditions were not considered in the analyses, and the biomechanical models and strategy for identifying the variable of interest were not consistent between studies.15,36,37,39,40 However, comparisons with these studies do demonstrate general agreement.
The peak frontal plane moment during midstance in the current study was comparable to that reported for sex-differentiated groups37 (mean difference, 9%) and undifferentiated groups (mean difference, 6%)39,41 of subjects with symptomatic knee OA. The peak sagittal plane knee extensor moments during midstance for men and women were comparable to the data of Kaufman et al37 (mean difference, 9%). At the hip, the frontal plane moment was similar to that reported by Chang et al42 for subjects with progressive medial compartment knee OA (mean difference, 3%). Additionally, the peak negative hip power during terminal stance and peak positive ankle power during preswing in our study also were very similar to values reported by McGibbon et al15 for subjects with knee OA and those reported by Graf et al43 for elderly subjects with low gait function (mean differences, 5% and 9%, respectively). Our findings for the peak plantar flexor moment during terminal stance also were very similar to peak values reported by Astephen et al39 (mean difference, 2%).
Previous research has identified the sagittal plane knee moment as being sensitive to the presence of knee OA in women.36,37 The direction of change in this moment, however, was not consistent between studies during terminal stance and early swing. For women in our study, the ability to place greater demands on the knee extensor muscles during the preswing and early swing phases of the gait cycle was associated with higher function. It is possible that the ability of the knee to control and transfer energy from the ankle to the hip and pelvis was tied to the increased functional abilities of the women, because such ankle strategies have been identified in healthy and higher functioning patients with knee OA.44
This possibility is supported by post hoc analysis that demonstrated significant associations between the sagittal knee moment during preswing and initial swing and the ankle power during preswing (r=.60), as well as between the 400-m walk time and the sagittal ankle (r=.43) and knee (r=.40) moments during preswing and initial swing. In addition, the strategy of transferring energy from the foot to the pelvis comes at the cost of increased knee joint reaction forces,45 which may not be tolerated by people who are functioning at a lower level. This is supported by the fact that ankle strategies have been identified in healthy and higher functioning elderly subjects.
Our model also identified an association between decreased knee power at terminal swing and higher mobility function in women. This finding may also be linked to the use of an ankle strategy by the higher functioning subjects. Decreasing the control offered by the knee extensors during preswing results in the shank-foot segment acting more like a free-swinging pendulum, requiring greater knee power at the end of the swing phase to control the motion of the knee and prevent knee hyperextension. This possibility is indirectly supported by post hoc analyses that showed associations between hip and ankle power and the magnitude of the negative knee power burst in terminal swing. Specifically, we found that higher ankle power during preswing was negatively associated with knee power during terminal swing (r=–.52), while hip power generation was positively associated with terminal knee power (r=.53).
Chang et al42,46 identified the potential importance of the frontal plane moment in affecting the progression of medial knee OA by controlling the varus thrust that can occur during weight acceptance. Our model identified the frontal plane moment and power as helping to distinguish between high- and low-functioning women. While the frontal plane moment is fairly substantial during weight acceptance, the power value is typically small because of the limited displacement that occurs at the hip. The inclusion of hip frontal plane power may therefore be a sensitive measure that is linked to pelvic control.
Our finding that higher functioning women exhibit greater pelvic control is associated with the finding that these subjects also had stronger hip abductors. The relation between pelvic control and mobility function may be viewed in terms of the frontal plane control of the knee. The stress that the knee experiences with each step may be greater in persons with less pelvic control. The importance of frontal plane pelvic kinetics has been documented by others who found that the magnitude of the frontal plane hip moment was inversely related to OA severity.41,42 In our study, it was the hip power rather than the moments that was associated with lower-limb functional ability.
The finding that, in men, knee kinetics during walking did not differ with level of functional mobility is concordant with previous research that demonstrated no kinetic differences between men with or without knee OA.36,37 However, previous studies suggested a trend towards hip and ankle sagittal plane kinetic differences that distinguished men with versus without knee OA.36 Our model for men identified sagittal hip energy absorption during terminal stance and ankle energy generation during preswing as distinguishing between high- and low-functioning persons. These differences in sagittal plane powers between our low- and high-functioning subjects are concordant with the differences in power data reported for adults with and without OA, respectively.47 Whereas knee strength had a moderate association with function, the association between knee extensor strength and the extensor moment during preswing (r=.32), while not part of the final model, may be part of the background that helps to define the strategy used during propulsion.
Men with higher gait function in our study used a motor pattern that was similar to that used by the group without knee OA in McGibbon and Krebs’ study47—they appeared to rely more on generating ankle power and absorbed less energy at the hip. McGibbon and Krebs47 speculated that the increased energy absorption at the hip seen in their OA group and our lower functioning subjects was associated with limitation in the ability of subjects to extend their hips.
Post hoc analysis, however, showed that for men in our study, there was a positive association between hip extension range of motion and negative hip power (r=.72). Furthermore, speculation that the additional negative energy, caused by tight hip flexors, might result in storage and recovery of energy, as the power in the hip flexors becomes positive, was not evident in our data, where the association between negative and positive power was minimal (r=.12). Thus, it appears that the higher functioning men in our study had a bias towards using an ankle strategy to generate energy for propulsion. However, as in prior studies, kinetic differences did not distinguish between men to the extent that was evident in women.
Although the sample was representative of functional ability in both sexes and 3 decades of age, the sample size in each of these strata was not large enough to also stratify by direction or degree of malalignment or other variables. In addition, this was a cross-sectional study, so causality cannot be determined. Therefore, it is unclear whether differences relate to compensations by the higher functioning subjects or excess impairments in the lower functioning subjects.
Future research could advance on the findings of this study in several ways. First, longitudinal gait studies may reveal whether particular gait patterns precede or follow the development of symptomatic knee OA. Alternatively, interventional studies could use the differences detected in gait parameters in this study to design gait training protocols aimed at moving people with symptomatic knee OA from a lower to a higher gait function status. In addition, this initial study focused on level gait. However, future research could also quantify differences in kinetics and kinematic parameters in higher and lower gait function subjects doing other functional activities, such as stair ascent/descent and rising from a chair.
CONCLUSIONS
There is evidence that men and women with higher mobility function tend to rely more on an ankle strategy rather than a hip strategy to progress the lower limb. This finding is consistent with previous work that identified ankle strategies in normal or higher functioning groups. While the model for low functioning men shows a direct association with decreased ankle power, the model for low-functioning women shows secondary evidence linked to a hip strategy. The differences between model associations for men and women may be tied to overall strength differences. In men, knee strength was linked to functional performance, which indirectly may help to account for the bias to an ankle strategy selected by the higher functioning persons. In women, the frontal plane weakness at the hip may place additional stress on the knee that biases women with low mobility function to minimize loading across the knee via use of a hip strategy.
Acknowledgments
We thank Natalie Johnston, PT, for her assistance with data collection, and the American Geriatrics Society 2006 Dennis W. Jahnigen Career Development Scholars Award and the Multicenter Osteoarthritis Study Group for supporting this work. The authors have no conflict of interest with respect to any aspect of the study described in this article. Segal and Yack were involved in the study design, application for funding, data collection, data analysis, and manuscript preparation. Brubaker was involved in the data collection and manuscript preparation. Torner and Wallace were involved in the study design, application for funding and manuscript preparation. The sponsors for the study provided funding for the study, but were not involved in the design, data collection, analysis, interpretation or decision to publish this work.
Supported by a 2006 Dennis W. Jahnigen Career Development Scholars Award through the American Geriatrics Society and by the following NIH/NIA grants: U01-AG-18832, U01-AG-18820, U01-AG-18947, and U01-AG-19069.
List of Abbreviations
- LLFDI
Late Life Function and Disability Instrument
- MOST
Multicenter Osteoarthritis Study
- OA
osteoarthritis
- SPPB
Short Physical Performance Battery
- WOMAC
Western Ontario McMasters Knee Osteoarthritis Index
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier's archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
Presented to the Association of Academic Physiatrists, 2008, Anaheim, CA, and the American Geriatrics Society, Washington, DC.
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit on the authors or on any organization with which the authors are associated.
Suppliers
Cybex International, Inc, 10 Trotter Dr, Medway, MA 02053.
Spark Instruments and Academics Inc, Iowa City, IA.
Optotrak; Northern Digital Inc, 103 Randall Dr, Waterloo, ON, Canada N2V 1C5.
Model 9286; Kistler, 75 John Glenn Dr, Amherst, NY 14228-2171.
Visual 3D; C-Motion, 20030 Century Blvd, Ste 104, Germantown, MD 20874.
SAS Inc, 100 SAS Campus Dr, Cary, NC 27513-2414.
References
- 1.Ettinger WH, Jr, Fried LP, Harris T, Shemanski L, Schulz R, Robbins J. Self-reported causes of physical disability in older people: the Cardiovascular Health Study. CHS Collaborative Research Group. J Am Geriatr Soc. 1994;42:1035–44. doi: 10.1111/j.1532-5415.1994.tb06206.x. [DOI] [PubMed] [Google Scholar]
- 2.Fried LP, Guralnik JM. Disability in older adults: evidence regarding significance, etiology, and risk. J Am Geriatr Soc. 1997;45:92–100. doi: 10.1111/j.1532-5415.1997.tb00986.x. [DOI] [PubMed] [Google Scholar]
- 3.Hochberg MC, Kasper J, Williamson J, Skinner A, Fried LP. The contribution of osteoarthritis to disability: preliminary data from the Women's Health and Aging Study. J Rheumatol Suppl. 1995;43:16–8. [PubMed] [Google Scholar]
- 4.Gill TM, Robison JT, Tinetti ME. Difficulty and dependence: two components of the disability continuum among community-living older persons. Ann Intern Med. 1998;128:96–101. doi: 10.7326/0003-4819-128-2-199801150-00004. [DOI] [PubMed] [Google Scholar]
- 5.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried LP, Bandeen-Roche K, Chaves PH, Johnson BA. Preclinical mobility disability predicts incident mobility disability in older women. J Gerontol A Biol Sci Med Sci. 2000;55:M43–52. doi: 10.1093/gerona/55.1.m43. [DOI] [PubMed] [Google Scholar]
- 7.Chaves PH, Garrett ES, Fried LP. Predicting the risk of mobility difficulty in older women with screening nomograms: the Women's Health and Aging Study II. Arch Intern Med. 2000;160:2525–33. doi: 10.1001/archinte.160.16.2525. [DOI] [PubMed] [Google Scholar]
- 8.Stauffer RN, Chao EY, Gyory AN. Biomechanical gait analysis of the diseased knee joint. Clin Orthop. 1977;(126):246–55. [PubMed] [Google Scholar]
- 9.Fransen M, Crosbie J, Edmonds J. Isometric muscle force measurement for clinicians treating patients with osteoarthritis of the knee. Arthritis Rheum. 2003;49:29–35. doi: 10.1002/art.10923. [DOI] [PubMed] [Google Scholar]
- 10.Ling SM, Fried LP, Garrett ES, Fan MY, Rantanen T, Bathon JM. Knee osteoarthritis compromises early mobility function: the Women's Health and Aging Study II. J Rheumatol. 2003;30:114–20. [PubMed] [Google Scholar]
- 11.Schiller BC, Casas YG, Tracy BL, DeSouza CA, Seals DR. Age-related declines in knee extensor strength and physical performance in healthy Hispanic and Caucasian women. J Gerontol A Biol Sci Med Sci. 2000;55:B563–9. doi: 10.1093/gerona/55.12.b563. [DOI] [PubMed] [Google Scholar]
- 12.Rantanen T, Era P, Heikkinen E. Maximal isometric strength and mobility among 75-year-old men and women. Age Ageing. 1994;23:132–7. doi: 10.1093/ageing/23.2.132. [DOI] [PubMed] [Google Scholar]
- 13.McGibbon CA, Puniello MS, Krebs DE. Mechanical energy transfer during gait in relation to strength impairment and pathology in elderly women. Clin Biomech (Bristol, Avon) 2001;16:324–33. doi: 10.1016/s0268-0033(01)00004-3. [DOI] [PubMed] [Google Scholar]
- 14.DeVita P, Hortobagyi T. Age causes a redistribution of joint torques and powers during gait. J Appl Physiol. 2000;88:1804–11. doi: 10.1152/jappl.2000.88.5.1804. [DOI] [PubMed] [Google Scholar]
- 15.McGibbon CA, Krebs DE, Scarborough DM. Rehabilitation effects on compensatory gait mechanics in people with arthritis and strength impairment. Arthritis Rheum. 2003;49:248–54. doi: 10.1002/art.11005. [DOI] [PubMed] [Google Scholar]
- 16.Kellgren J, Lawrence J. Osteoarthosis and disk degeneration in an urban population. Ann Rheum Dis. 1958;17:388–96. doi: 10.1136/ard.17.4.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jette AM, Haley SM, Coster WJ, et al. Late Life Function and Disability Instrument: I. Development and evaluation of the disability component. J Gerontol A Biol Sci Med Sci. 2002;57:M209–16. doi: 10.1093/gerona/57.4.m209. [DOI] [PubMed] [Google Scholar]
- 18.Sayers SP, Jette AM, Haley SM, Heeren TC, Guralnik JM, Fielding RA. Validation of the Late-Life Function and Disability Instrument. J Am Geriatr Soc. 2004;52:1554–9. doi: 10.1111/j.1532-5415.2004.52422.x. [DOI] [PubMed] [Google Scholar]
- 19.McAuley E, Konopack JF, Motl RW, Rosengren K, Morris KS. Measuring disability and function in older women: psychometric properties of the Late-Life Function and Disability Instrument. J Gerontol A Biol Sci Med Sci. 2005;60:901–9. doi: 10.1093/gerona/60.7.901. [DOI] [PubMed] [Google Scholar]
- 20.Jinks C, Jordan K, Croft P. Measuring the population impact of knee pain and disability with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). Pain. 2002;100:55–64. doi: 10.1016/s0304-3959(02)00239-7. [DOI] [PubMed] [Google Scholar]
- 21.Jones JG, Leighton F. Comparison of WOMAC with SF-36 for OA of the knee or hip. Ann Rheum Dis. 2002;61:182–3. doi: 10.1136/ard.61.2.182-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuptniratsaikul V, Rattanachaiyanont M. Validation of a modified Thai version of the Western Ontario and McMaster (WOMAC) osteoarthritis index for knee osteoarthritis. Clin Rheumatol. 2007;26:1641–5. doi: 10.1007/s10067-007-0560-y. [DOI] [PubMed] [Google Scholar]
- 23.Roos EM, Klassbo M, Lohmander LS. WOMAC osteoarthritis index. Reliability, validity, and responsiveness in patients with arthroscopically assessed osteoarthritis. Western Ontario and MacMaster Universities. Scand J Rheumatol. 1999;28:210–5. doi: 10.1080/03009749950155562. [DOI] [PubMed] [Google Scholar]
- 24.Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The Physical Activity Scale for the Elderly (PASE): evidence for validity. J Clin Epidemiol. 1999;52:643–51. doi: 10.1016/s0895-4356(99)00049-9. [DOI] [PubMed] [Google Scholar]
- 25.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 26.Martin KA, Rejeski WJ, Miller ME, James MK, Ettinger WH, Jr, Messier SP. Validation of the PASE in older adults with knee pain and physical disability. Med Sci Sports Exerc. 1999;31:627–33. doi: 10.1097/00005768-199905000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–26. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 28.Simonsick EM, Montgomery PS, Newman AB, Bauer DC, Harris T. Measuring fitness in healthy older adults: the Health ABC Long Distance Corridor Walk. J Am Geriatr Soc. 2001;49:1544–8. doi: 10.1046/j.1532-5415.2001.4911247.x. [DOI] [PubMed] [Google Scholar]
- 29.Onder G, Penninx BW, Ferrucci L, Fried LP, Guralnik JM, Pahor M. Measures of physical performance and risk for progressive and catastrophic disability: results from the Women's Health and Aging Study. J Gerontol A Biol Sci Med Sci. 2005;60:74–9. doi: 10.1093/gerona/60.1.74. [DOI] [PubMed] [Google Scholar]
- 30.Segal NA, Torner JC, Yang M, Curtis JR, Felson DT, Nevitt MC. Muscle mass is more strongly related to hip bone mineral density than is quadriceps strength or lower activity level in adults over age 50 year. J Clin Densitom. 2008;11:503–10. doi: 10.1016/j.jocd.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daniels L, Worthingham C. Techniques of manual examination. 6th ed. WB Saunders; Philadelphia: 1995. pp. 11–26.pp. 169–201. [Google Scholar]
- 32.Andrews AW, Thomas MW, Bohannon RW. Normative values for isometric muscle force measurements obtained with hand-held dynamometers. Phys Ther. 1996;76:248–59. doi: 10.1093/ptj/76.3.248. [DOI] [PubMed] [Google Scholar]
- 33.Docherty CL, Moore JH, Arnold BL. Effects of strength training on strength development and joint position sense in functionally unstable ankles. J Athl Train. 1998;33:310–4. [PMC free article] [PubMed] [Google Scholar]
- 34.Debi R, Elbaz A, Segal O, et al. Do female gait patterns differ from male gait patterns in knee osteoarthritis?. Proceedings of the 2008 World Congress on Osteoarthritis.; Rome, Italy. September 18-21, 2008.p. 125. [Google Scholar]
- 35.Sayers SP, Guralnik JM, Thombs LA, Fielding RA. Effect of leg muscle contraction velocity on functional performance in older men and women. J Am Geriatr Soc. 2005;53:467–71. doi: 10.1111/j.1532-5415.2005.53166.x. [DOI] [PubMed] [Google Scholar]
- 36.McKean KA, Landry SC, Hubley-Kozey CL, Dunbar MJ, Stanish WD, Deluzio KJ. Gender differences exist in osteoarthritic gait. Clin Biomech (Bristol, Avon) 2007;22:400–9. doi: 10.1016/j.clinbiomech.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Kaufman KR, Hughes C, Morrey BF, Morrey M, An KN. Gait characteristics of patients with knee osteoarthritis. J Biomech. 2001;34:907–15. doi: 10.1016/s0021-9290(01)00036-7. [DOI] [PubMed] [Google Scholar]
- 38.McGibbon CA, Krebs DE. Discriminating age and disability effects in locomotion: neuromuscular adaptations in musculoskeletal pathology. J Appl Physiol. 2004;96:149–60. doi: 10.1152/japplphysiol.00422.2003. [DOI] [PubMed] [Google Scholar]
- 39.Astephen JL, Deluzio KJ, Caldwell GE, Dunbar MJ. Biomechanical changes at the hip, knee, and ankle joints during gait are associated with knee osteoarthritis severity. J Orthop Res. 2008;26:332–41. doi: 10.1002/jor.20496. [DOI] [PubMed] [Google Scholar]
- 40.Andriacchi TP, Mundermann A. The role of ambulatory mechanics in the initiation and progression of knee osteoarthritis. Curr Opin Rheumatol. 2006;18:514–8. doi: 10.1097/01.bor.0000240365.16842.4e. [DOI] [PubMed] [Google Scholar]
- 41.Mundermann A, Dyrby CO, Andriacchi TP. Secondary gait changes in patients with medial compartment knee osteoarthritis: increased load at the ankle, knee, and hip during walking. Arthritis Rheum. 2005;52:2835–44. doi: 10.1002/art.21262. [DOI] [PubMed] [Google Scholar]
- 42.Chang A, Hayes K, Dunlop D, et al. Hip abduction moment and protection against medial tibiofemoral osteoarthritis progression. Arthritis Rheum. 2005;52:3515–9. doi: 10.1002/art.21406. [DOI] [PubMed] [Google Scholar]
- 43.Graf A, Judge JO, Ounpuu S, Thelen DG. The effect of walking speed on lower-extremity joint powers among elderly adults who exhibit low physical performance. Arch Phys Med Rehabil. 2005;86:2177–83. doi: 10.1016/j.apmr.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 44.Astephen JL, Deluzio KJ, Caldwell GE, Dunbar MJ, Hubley-Kozey CL. Gait and neuromuscular pattern changes are associated with differences in knee osteoarthritis severity levels. J Biomech. 2008;41:868–76. doi: 10.1016/j.jbiomech.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 45.Robon MJ, Perell KL, Fang M, Guererro E. The relationship between ankle plantar flexor muscle moments and knee compressive forces in subjects with and without pain. Clin Biomech (Bristol, Avon) 2000;15:522–7. doi: 10.1016/s0268-0033(00)00007-3. [DOI] [PubMed] [Google Scholar]
- 46.Chang A, Hayes K, Dunlop D, et al. Thrust during ambulation and the progression of knee osteoarthritis. Arthritis Rheum. 2004;50:3897–903. doi: 10.1002/art.20657. [DOI] [PubMed] [Google Scholar]
- 47.McGibbon CA, Krebs DE. Compensatory gait mechanics in patients with unilateral knee arthritis. J Rheumatol. 2002;29:2410–9. [PubMed] [Google Scholar]