Abstract
In the developing nervous system, neurotrophin 3 (NT3) and brain-derived neurotrophic factor (BDNF) have been shown to interact with each other and with different parts of a neuron or glia and over considerable distances in time and space. The auditory system provides a useful model for analyzing these events, insofar as it is subdivided into well-defined groups of specific neuronal types that are readily related to each other at each stage of development. Previous work in our laboratory suggested that NT3 and its receptor TrkC in the mouse cochlear nucleus (CN) may be involved in directing neuronal migration and initial targeting of inputs from cochlear nerve axons in the embryo. NT3 is hard to detect soon after birth, but TrkC lingers longer. Here we found NT3 and TrkC around P8 and the peak around P30. Prominent in ventral CN, associated with globular bushy cells and stellate cells, they were localized to different subcellular sites. The TrkC immunostain was cytoplasmic, and that of NT3 was axonal and perisomatic. TrkC may be made by CN neurons, whereas NT3 has a cochlear origin. The temporal pattern of their development and the likelihood of activity-dependent release of NT3 from cochlear axons suggest that it may not be critical in early synaptogenesis; it may provide long-term trophic effects, including stabilization of synapses once established. Activity-related regulation could coordinate the supply of NT3 with inner ear activity. This may require interaction with other neurotrophins, such as BDNF.
Keywords: ventral cochlear nucleus (VCN), NT3, TrkC, postnatal development
In the developing nervous system, growth factors and their receptors direct and regulate proliferation, migration, differentiation, and cell death by acting at different stages in the same or different ways (Ernfors et al., 1990, 1992, 1994a, 1995a,b; Bianchi et al., 1996; Hossain et al., 1996, 1997; Brumwell et al., 1999; Hossain and Morest, 2000; Agerman et al., 2003). These factors have been shown to interact with each other directly and indirectly. They may influence different parts of a neuron or glia, through which they may travel considerable distances in time and space.
In many ways the cochlear nucleus (CN) provides an ideal preparation with which to relate these factors to specific types of neurons and their synapses. Unlike any other region in the brain, the CN consists of more than a dozen discretely demarcated regions, which contain only a small number of specific neuronal types. These regions have been identified from the earliest stages of development (see, e.g., Brumwell et al., 2005). As a result, one can relate the findings from many different methods to the development of these cells, and their input–output relationships can be related to hearing function (Morest, 1993; Young and Oertel, 2003). Thus it is important to have a clear definition of the subdivisions of the CN (Fig. 1, see list of abbreviations and CN divisions in legend).
Fig. 1.

Left: TrkC staining of a transverse section through the middle one-third of CN in a montage. Staining is most intense in and around PV. Right: A comparable level of the mouse atlas identifies the different regions (Trettel and Morest, 2001). A, anterior part of posteroventral CN (PVCN); AD, anterodorsal part of PVCN; AS, acoustic striae; AV, anteroventral part of PVCN; C, cerebellum; CN, cochlear nucleus; F, fusiform layer of DCN; M, molecular layer of dorsal (D) CN; P, polymorphic layer of DCN; PV, ventral part of posterior anteroventral CN (AVCN); S, small cell shell; TC, taenia choroidea. Scale bars = 200 μm.
A role for the neurotrophins neurotrophin 3 (NT3) and brain-derived neurotrophic factor (BDNF) in the normal development and function of the auditory and vestibular systems has long been sought (Pirvola et al., 1992, 1994; Schecterson and Bothwell, 1994; Wheeler et al., 1994; Zhou and Rush, 1994; Ernfors et al., 1994b, 1995b; Hafidi et al., 1996; Burette et al., 1997, 1998; Fritzsch et al., 1997a,b; Hafidi, 1999; Farinas et al., 2001; Hossain et al., 2002, 2006; Sugawara et al., 2007). NT3 may be more important for the auditory system, BDNF for the vestibular system (Fritzsch et al., 1997a). However, mice knocked out for NT3 (and not for BDNF) have shown a delay in vestibular compensation after unilateral labyrinthectomy (Gacek and Khetarpal, 1998). The scientific and clinical significance of this issue is apparent.
Much of the recent research has been focused on the inner ear, the primary target for therapeutic treatment. We know less about the role of these factors in the CN. Studies on rat (Burette et al., 1997, 1998; Hafidi, 1999) and gerbil (Tierney et al., 2001) have described the immunoreactivity of NT3 and its cognate receptor TrkC in the auditory brain, including the CN. Work in our laboratory showed the interaction of NT3 and other growth factors in the embryonic mouse CN (Hossain et al., 2006), and here we continue the study of NT3 and TrkC in the postnatal CN, from neonatal to adult stages. We are especially interested in the VCN, because its neurons provide the main pathway to higher centers. Our current studies focus on regenerative changes and plasticity of these cells.
NT3 may be involved in determining the localization of neurons and initial targeting of inputs from cochlear nerve axons in the embryonic CN (Hossain et al., 2006). The same study also reported that NT3 was not detected immediately after birth, although TrkC lingers on. This is consistent with observations in rat (Hafidi, 1999) and gerbil (Tierney et al., 2001). In the gerbil, NT3 reappeared several days after birth and then became abundant in the adult (Hafidi et al., 1996; Tierney et al., 2001).
In the current study, we compared NT3 and TrkC in mice to see whether their postnatal development follows the same trend as in gerbils and rats. This information will be instrumental for further studies using mice as a model to understand the normal and pathological development of the auditory system. We asked whether the presence of NT3 and TrkC, if parallel, will be found in the same or different populations of neurons. Are they localized to the same or different subcellular sites? We hypothesize that, after the completion of their role in cellular migration and the initial targeting of cochlear axons in the embryo, NT3 probably will assume a different role in the adult nervous system. If so, then the regional distribution and discrete subcellular localization of this molecule and its receptor might be different from the embryonic pattern. Another question of interest is whether there is any correlation between the presence of these two molecules and synaptogenesis in the CN. The answers to these questions may shed some light on their function in the postnatal CN.
MATERIALS AND METHODS
Preparation of Histological Sections
Normal F1 offspring of female C57BL and male CBA/J mice (Jackson Laboiratory, Bar Harbor, ME) at different ages were used. The total number of animals in this study was 25 (seven at P8, eight at P14, and ten adults, including P30 and P60). It should be noted that a P30 mouse has reached maturity in the development of its CN. In this context, P30 is comparable to P60.
Animals were anesthetized by intraperitoneal pentobarbital and fixed by cardiovascular perfusion with 2 ml of 1% sodium nitrite, followed by 20 ml of 4% paraformaldehyde in 0.1 M phosphate buffer at pH 7.4 at room temperature. The head with the calvarium removed was immersed for 1 hr in the same fixative at 4°C. After the brain was removed, cryoprotected, and blocked, transverse sections were cut at 15 μm on a cryostat.
Immunostaining
Sections were treated with 0.3% Triton X-100 for 20 min at room temperature. After blocking with 10% normal goat serum, the sections were incubated with primary antibodies (polyclonal anti-NT3, monoclonal anti-TrkC, or monoclonal anti-SV2 to identify the terminal and preterminal endings) at 4°C overnight and then with appropriate biotinylated secondary antibodies and processed by Vectastain Elite ABC Kit (Vector Laboratories, Burlingame, CA). Sections were then incubated with diaminobenzidine (DAB-Peroxidase Substrate Kit; Vector Laboratories). Signals were intensified using nickel-sulfate. All rinses were done with PBS. The DAB exposure times for all sets of experiments were kept similar. Finally, sections were covered with Crystalmount (Biomeda, Foster City, CA). Negative controls were performed routinely by the omission of primary antibodies in adjacent sections. The specificity of the antibodies was confirmed by Western blots (Suneja and Potashner, 1998).
Anti-NT3 antibody was from Alomone Laboratories (Jerusalem, Israel), anti-TrkC was from R&D Systems (Min-neapolis, MN), and anti-SV2 was from the Developmental Studies Hybridoma Bank (Department of Biological Sciences, Iowa City, IA). For immunofluorescence double labeling of NT3 and SV2, the sections were incubated for 1 hr with Alexa Fluor 488- and 594-conjugated secondary antibodies, respectively (Molecular Probes, Eugene, OR).
Imaging
The imaging system consisted of a Dage MTI model 300 cooled CCD camera mounted on a Zeiss Axiophot microscope (Carl Zeiss, Thornwood, NY). Northern Eclipse imaging software (Olympus Corp, Tokyo, Japan) was used for image capture. For the double-labeling image in Figure 4, two stacks of images were Z-projected in NIH ImageJ and merged for evidence of colocalization. High-magnification images were taken with a ×40 water-immersion lens or a ×63 oil-immersion lens. The figures were constructed in Adobe Photoshop 7.0 (Adobe Systems Inc, San Jose, CA). For Figure 6, images were also captured with Z stacks to increase resolution. Each panel represents an image at a single focal plane.
Fig. 4.

Fluoresecent double labeling for NT3 (green) and SV2 (red) to show colocalization (yellow) in the PV region (P60). Left arrow, axons abundant with NT3; right arrow, NT3 in the perisomatic region. SV2 labels nerve terminals (double arrows); many of them are also labeled for NT3 (arrowheads). Asterisk, example of a globular bushy cell. Scale bar = 20 μm.
Fig. 6.

Synaptogenesis in PV as shown by SV2 staining. Single focal planes are shown from through-focus stacks of images (see Materials and Methods). At P30, the mature synaptic endings from the cochlear nerve, the endbulbs of Held, thickly enclose a typical globular bushy cell soma (thick arrow). The jagged line indicates the position of the eccentric nucleus, which could be visualized at another focal plane (compare with the inset in Fig. 2). At P14, an immature endbulb of Held (thick arrow) partially encloses a globular bushy cell (asterisk). Single processes of cochlear nerve fibers (thin arrow) and clusters of cochlear nerve fiber endings (circle) end freely in the neuropil, short of their targets. At P8, a single branching cochlear nerve fiber forms a cluster of endings in the neuropil (thin arrow). Scale bar = 20 μm.
RESULTS
In the adult mouse brain, both NT3 and TrkC were more prominent in the ventral CN compared with its dorsal counterpart. This is illustrated for TrkC in Figure 1 (left panel), an immunostained transverse section through the middle one-third of CN at postnatal day 30 (P30). Many large stained neurons congregated in PV and AV. In the more dorsal AD and D, smaller neurons were less intensely stained. The congregation of larger TrkC-positive neurons gave the appearance of more intense staining in the nerve root region. This appeared to have functional implications, insofar as it was echoed by the same pattern of staining in NT3 sections, although for a different reason (shown in later figures).
The major area examined in this study is the nerve root region, where we found most of the labeled large neurons and axons. To help identify the different areas of CN, a diagram of a comparable level of mouse CN is also shown in Figure 1 (right panel; Trettel and Morest, 2001). The CN has three divisions: the dorsal CN (DCN), the ventral CN (VCN), and the small cell shell (S), which surrounds and separates them. The anterior (AVCN) and posterior (PVCN) divisions of VCN are separated by the cochlear nerve root projecting from the cochlea. PVCN is divided into anterior (A), anterodorsal (AD), and anteroventral (AV) parts. DCN consists of three layers, molecular (M), fusiform (F), and polymorphic (P). Individual cochlear nerve axons branch within the CN to provide synaptic endings to all subdivisions, thus supplying essentially the same input to the different groups of cells.
The defining characteristics of TrkC expression in the nerve root region appeared most clearly in the mature animal, and its presence only increased gradually after birth. For this reason, we shall present the mature state first and trace its features through the earlier ages. We report here the features observed throughout the present material. We will use the figures to illustrate the general pattern. The general pattern shows granules intensely stained by antibody to TrkC filling the cytoplasm of older cells, with the nucleus clearly unstained. The younger cells have fewer stained granules in their cytoplasm. In the P30 panel, two globular bushy cells (Fig. 2) (single arrows and inset) and one stellate cell (double arrows) are shown. The neuron indicated by the large arrow is displayed in the inset to show more details. In this typical globular-shaped bushy cell, an unstained nucleus occupies an eccentric position. A clear space surrounds the stained soma (arrowhead), presumably occupied by incoming nerve terminals, as seen in Figures 3–6. As a typical example, the cytoplasm of this cell is filled with heavily stained granular particles. One process extending from the soma is also stained. Bordering this cell are an astrocyte with stained processes (thin arrows) and two stained oligodendrocytes (asterisks).
Fig. 2.
TrkC staining of PV. The perikaryal cytoplasm is stained at all ages, albeit to different degrees. Single arrows, globular bushy cells; double arrows, stellate cell; large arrows, cells in the insets. The P30 inset shows a typical globular bushy cell with unstained eccentric nucleus. The large arrowhead points to the nerve terminal region around the soma, which is unstained, next to a stained astrocyte (thin arrows point to the cell body and astrocytic processes). Next to the astrocyte are two oligodendrocytes (asterisks). The cytoplasmic stain in both P30 and P14 insets is granular, unlike the cell in P8 (lower inset). In the P8 main panel and the upper inset, astrocytic processes (thin arrow) between two neurons are stained. Scale bar = 20 μm; 5 μm for insets.
Fig. 3.
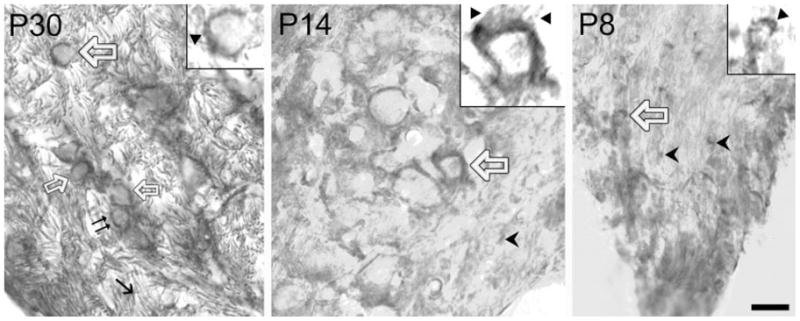
NT3 staining of PV. Perisomatic endings are stained at all ages. Same symbols for cell types as in Figure 2. Cochlear axons at P30 are heavily stained (thin arrow) along with clusters of nerve terminals at P30 and P8 and the incoming terminals at P14 (triangles in the insets). The arrowheads in P14 and P8 point to nerve terminals approaching their target sites. Scale bar = 20 μm; 5 μm for insets.
At P14, there is the same pattern of perikaryal staining, albeit weaker (Fig. 2). At P8, we could still discern the morphology of some of the weakly stained globular bushy cells (Fig. 2, inset). The presence of astrocytic processes also can be seen at P8 (thin arrow and the upper inset). In sum, the TrkC staining is cytoplasmic, with some glial processes also stained. The pattern of cytoplasmic staining of TrkC at all ages suggests the hypothesis that these stained neurons make this protein and transport it to the cell surface to act as receptors.
The presence of NT3-positive neural elements in the nerve root region follows the same temporal trend as for TrkC. It was at its peak at P30, less evident at P14, and weak at P8. However, the staining pattern is very different, as is illustrated in Figure 3.
At P30, the regions in and around PV are intensely stained, more strongly than the small neurons in AD and D (data not shown). This resulted in the more intense staining in the ventral CN, primarily because of the packed, thick bundles of the parallel cochlear axons in and adjacent to PV (thin arrow).
When neurons were stained by antibody to NT3, the staining was around the soma, and the cytoplasm was only lightly stained. Three globular bushy cells (thick arrows) and one stellate cell (double arrows) buried in the axons are shown as examples. The perisomatic pattern of staining is further shown in the inset, where a triangle points to the dark rim surrounding the soma of this cell (indicated by the large arrow in the main panel). This rim actually consists of several clusters, resembling the endbulbs of Held, the synaptic terminals from the cochlear axons terminating on the globular bushy cells.
In the P14 panel, we show an example of another globular bushy cell, with the same perisomatic pattern of staining. In the inset in which this cell is displayed, the incoming nerve terminals are framed by two triangles, and the dark rim also resembles clusters of endbulbs of Held. A slight difference between this neuron and those at P30 lies in the weaker staining of its cytoplasm as distinguished from the perisomatic staining.
At P8, two neurons (thick arrow) and some other scattered elements were weakly stained. The types of neurons are hard to define at this stage (see inset). However, the dark perisomatic rim (triangle) is obvious. The scattered elements (arrowheads) resemble nerve terminals approaching their target sites.
In sum, the NT3 staining pattern is primarily perisomatic, suggesting its presence in the nerve terminals. The abundance of NT3 in the incoming cochlear axons raises the question of its source (see Discussion).
Is NT3 in the cochlear axons being transported to the perisomatic terminal, as suggested above? To investigate this question further, we used fluorescent markers to double label neurons. The result in a P60 CN is shown in Figure 4. The perisomatic nerve terminals were stained red by an antibody to SV2, and axons filled with NT3 were green. Colocalization would appear as yellow.
In Figure 4, an arrow at left indicates the robust axons in green running in the nerve root region, and the arrow at right points to the perisomatic endings, also in green. The cytoplasm is not stained above the background level. Red dots, representing the punctae of nerve terminals (double arrow), hugged the soma. Around globular bushy cells one could often discern yellow (e.g., Fig. 4, two arrowheads), indicating the presence of NT3 in perisomatic endings.
The association of NT3 and TrkC with globular bushy cells and stellate cells raises the question of whether NT3 and TrkC are made by these cells. Do one or both neuronal cell types make NT3 and TrkC, or is there another source for these molecules? If the latter is so, then their staining patterns might show some differences. Further analysis of the material in different sections of the same group of neurons provides some insight into these questions. Figure 5 shows sections of the nerve root region from P30. NT3 is shown as a dark rim around the soma, with light staining of cytoplasm in some of the neurons (upper panel). This is in sharp contrast to the neurons darkly stained for TrkC, where no distinction between the cytoplasmic and the cell membrane domains was found (lower panel). This difference in staining patterns applies to both globular bushy cells (arrows) and stellate cells (data not shown). This finding strengthens the possibility that there are different sources for NT3 and TrkC (see Discussion). There are TrkC-positive elements scattered between neurons (thin arrows), reminiscent of the astrocytic processes shown in Figure 2.
Fig. 5.

Comparison of perisomatic staining of NT3 and perikaryal location of TrkC in globular bushy cells (thick arrows; P30). Images taken at high magnification (×63 oil). Astrocytes are also stained for TrkC (thin arrows). Inset: A cell with granular cytoplasm from the same section in another image. Scale bar = 20 μm.
NT3 and TrkC appear a few days after birth and are detected at low levels until P30 and later. At P14, there is very little NT3 associated with the cochlear nerve root (e.g, Fig. 3). By P30, the cochlear axons are filled with NT3. What could this mean in terms of their function? Perhaps NT3 does not start to play a critical role in the CN until P30 or later. If so, then this trophic factor may not be critical during synaptogenesis, a process taking place in the CN before P30.
To explore this possibility, we examined the temporal pattern of synaptogenesis in our material using SV2 as a marker for synaptic endings and their preterminal axons. Figure 6 exemplifies the intensity of SV2 staining across different postnatal ages.
At P30, strongly stained perisomatic rings define the well-formed synapses, with some of them resembling the endbulbs of Held (e.g., Fig. 6, arrow). The target neurons of the cochlear nerve are wrapped in a plexus of nerve terminals. These terminals are filled with NT3 at this stage (Figs. 3–5), but not TrkC (Fig. 2).
At P14, some bushy cells have a less well-developed endbulb of Held wrapping around their soma. The weaker staining by antibody to SV2 suggests the presence of fewer synaptic vesicles (e.g., Fig. 6, thick arrow). Clusters of preterminal endings and single processes stained by antibody to SV2 suggest that these terminals are approaching their targets. The single processes were identified as cochlear nerve fibers by their location in the cochlear nerve root and their large size. Likewise the clusters are comparable in diameter to cochlear nerve fibers, but they end freely in the neuropil and are not yet in contact with their targets. This is the time when cochlear nerve axons begin to form synapses with neurons in the nerve root region. Such preterminal endings could also be seen at P8, the earlier stage of synaptogenesis. Our previous studies have validated the identification of such preterminal endings in synapse formation (Feng and Morest, 2006; for review see Rubel and Fritzsch, 2002).
In summary, when synapses are beginning to form in the CN, very little NT3 or TrkC was observed. NT3 and TrkC reached their peak only after synapses in this nucleus became fully established. Thus NT3 must have a major functional role in the adult CN.
DISCUSSION
A previous study from our laboratory showed the temporal expression of NT3 and its cognate receptor TrkC in the developing mouse CN (Hossain et al., 2006). In this follow-up study, we continued the examination of NT3 and TrkC in postnatal CN. We showed a regional difference in their distribution. At P30, the adult stage, VCN is more intensely stained compared with DCN (Fig. 1). Our observation is consistent with a previous report on the weaker staining for NT3 in the DCN compared with VCN (Burette et al., 1998). However, the aggregation of these two molecules is determined by different factors. For TrkC, it is the prominent expression in the cytoplasm of globular bushy cells and stellate cells, the principal cells of VCN in and around PV. For NT3, it is the abundant presence in the cochlear axons arranged in thick bundles in the same area. This observation highlights the importance of NT3 and TrkC in the development of VCN (AVCN and PVCN), but probably less so for DCN. Both NT3 and TrkC seemed to follow the same trend in becoming more prominent from neonatal to adult, by and after P30. We did not see the decline in TrkC after P21 (Hafidi et al., 1996).
The destination of NT3 transported by cochlear axons into VCN appeared to be the same type of cells that express TrkC (Figs. 2, 3); specifically, they are the globular bushy cells and stellate cells, the principal cell types in the VCN. This, and the same temporal sequence of their expression, highlights their functional significance in the VCN.
Even though NT3 and TrkC were associated with the same group of cells, they do not seem to be made by these same cells. Our supporting evidence is the staining patterns, which indicate their subcellular locations. One major source of abundant NT3 appears to be the cochlea. The double-labeling experiment showed that NT3 overlaps with synaptic vesicle protein SV2, suggesting their presence in the nerve terminals (Fig. 4). TrkC, on the other hand, was observed in the perikaryal granules (Figs. 2, 5). These observations are consistent with the notion that TrkC is made by neurons in the CN, whereas NT3 has a cochlear origin.
Can spiral ganglion neurons make NT3? Although there are some data for immature animals (Pirvola et al., 1992, 1994; Wheeler et al., 1994; Schecterson and Bothwell, 1994; Fritzsch et al., 1997a,b; Farinas et al., 2001), there has been no report on the production of NT3 by these neurons in adult animals. A recent study using a reporter gene in postnatal mice found NT3 gene transcription associated with inner hair cells and supporting cells but not with neuronal structures in the cochlea (Sugawara et al., 2007). In older mice (P135), labeled cells were restricted to the supporting cells. However, it is possible that NT3 is released from the supporting cells and inner hair cells, taken up by cochlear nerve endings, and transported to the cochlear ganglion cells and their central axons in the CN, where sufficient levels may accumulate to demonstrate by immunostaining. The transport of NT3 from the sensory epithelium to the cochlear ganglion cell bodies in prenatal mice has been suggested previously (Hossain et al., 2008). In postnatal mice, after arriving at its target sites, NT3 presumably could leave the nerve terminals to bind to its membrane receptor TrkC on neighboring cells, including CN neurons. In a similar way (Ferguson et al., 1990), basic fibroblast growth factor (bFGF) could be internalized by its high-affinity receptors on the retinal ganglion cells. It may then be transported anterogradely to the lateral geniculate body and the superior colliculus with limited proteolytic cleavage or even intact.
Although NT3 staining in the cytoplasm of VCN neurons has been reported for gerbils (Tierney et al., 2001), in the present material the staining appears in the region of the perisomatic nerve terminals hugging the soma. On some of the globular bushy cells, one can even discern clusters of the endbulbs of Held in our high-magnification images (Fig. 3). This interpretation was further supported by the double-labeling experiment (Fig. 4). Burette et al. (1998) reported cytoplasmic punctate staining of NT3 in the adult rat, including spherical and globular neurons, but they could not exclude the possibility that some of the punctate labeling was in pre-synaptic terminals.
Insofar as we also observed light staining of the cytoplasm for NT3 in some of the CN neurons (Figs. 3, 5), it is possible that this NT3 was incorporated from the cochlear nerve endings. Could it be that these CN neurons also make NT3? Two observations are not consistent with this interpretation. First, the staining of cytoplasm for NT3 was faint, much fainter than that of the nerve terminals. Second, the cytoplasmic staining for TrkC in the same group of neurons was consistently much stronger. Thus, the notion that these CN cells make NT3 and release it to the cochlear axon terminals does not receive support from our observations.
Some glial components were also stained for TrkC. These include astrocytic processes and oligodendrocytes (Figs. 2, 5). This staining pattern was observed only in proximity to stained neurons. It is possible that these glial cells express TrkC, but it is equally if not more plausible that TrkC released from neurons was taken up by nearby glia. Exchange of growth factors and their receptors between neurons and adjacent glia is the topic of much recent research (see, e.g., Althaus and Richter-Landsberg, 2000; Stephens et al., 2005). This speculation is consistent with the present analysis of the data and is a suggestion for future research. The same phenomenon was not observed with NT3; one reason might be that the overall staining of the bundles of cochlear axons in PV was too strong and covered up that pattern. In fact NT3-like immunoreactivity has been reported in astrocytes in the adult rat VCN (Burette et al., 1998).
Previous work from our laboratory suggests that NT3 may play a role in directing migration of neurons and targeting of cochlear axons in the embryonic CN (Hossain et al., 2006). Interestingly, there is a pause in the production of NT3 around birth, when it briefly disappears. When it reappears several days later, presumably it assumes a different role. Because the presence of NT3 in the early stages of synaptogenesis is scanty but becomes abundant with the formation of the definitive synapses, we conclude that this trophic factor very probably does not play a critical role in the early stage of synaptogenesis. We suggest that it plays some role in stabilizing the established structures, for example, the fully formed synapses.
What is the functional advantage of having the cochlear axons anterogradely transport NT3 into the CN? The answer may lie in the sorting and releasing mechanisms. NT3 could be released via the constitutive or the regulated secretory pathways. For example, in hippocampal neurons, NT3 can divert its secretory pathway from the constitutive to the regulated pathway when BDNF is coexpressed (Farhadi et al., 2000). There would be an advantage if NT3 were released by the cochlear axons, because the ganglion cells could adopt the regulated pathway, a process controlled by the firing of cochlear nerve impulses. Consequently, activity-related regulation could coordinate the supply of NT3 with the level of activity of the inner ear.
If diverting to the regulated secretory pathway requires the coordination of other neurotrophins, then the interactions of NT3 with other growth factors, such as BDNF and FGF (Hossain et al., 2006; D’sa et al., 2007), may be important in understanding the function of NT3. These interactions may involve other receptors, including the low-affinity p75 receptor, and should be included in future studies.
Acknowledgments
We thank Drs. Waheeda Hossain and Chrystal D’sa-Reed for reading the manuscript and Julie Gross for technical help.
Contract grant sponsor: NIH; Contract grant number: DC006387; Contract grant number: DC000127; Contract grant number: T32DC00025 (to J.B.); Contract grant number: F32DC006120.
References
- Agerman K, Hjerling-Leffler J, Blanchard MP, Scarfone E, Canlon B, Nosrat C, Ernfors P. BDNF gene replacement reveals multiple mechanisms for establishing neurotrophin specificity during sensory nervous system. Development. 2003;130:1479–1491. doi: 10.1242/dev.00378. [DOI] [PubMed] [Google Scholar]
- Althaus HH, Richter-Landberg C. Glial cells as targets and producers of neurotrophins. Int Rev Cytol. 2000;197:203–277. doi: 10.1016/s0074-7696(00)97005-0. [DOI] [PubMed] [Google Scholar]
- Bianchi LM, Conover JC, Fritzsch B, DeChiara T, Lindsay RM, Yancopoulos GD. Degeneration of vestibular neurons in late embryogenesis of both heterozygous and honoaygous BDNF null mutant mice. Development. 1996;122:1965–1973. doi: 10.1242/dev.122.6.1965. [DOI] [PubMed] [Google Scholar]
- Brumwell CL, Hossain WA, Morest DK, Bernd P. Role for basic fibroblast growth factor (FGF-2) and TrkB expression in the development of the inner ear: in vitro and in situ studies. Exp Neurol. 1999;162:121–145. doi: 10.1006/exnr.2000.7317. [DOI] [PubMed] [Google Scholar]
- Brumwell CL, Hossain WA, Morest DK, Wolf B. Biotinidase reveals the morphogenetic sequence in cochlea and cochlear nucleus of mice. Hear Res. 2005;209:104–121. doi: 10.1016/j.heares.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Burette A, Jalenques I, Romand R. Neurotrophin receptor immunostaining in the rat ventral cochlear nucleus. Brain Res. 1997;776:10–23. doi: 10.1016/s0006-8993(97)00934-7. [DOI] [PubMed] [Google Scholar]
- Burette A, Belliot G, Albuisson E, Romand R. Localization of neurotrophin-3-like immunoreactivity in the rat cochlear nucleus. Microsc Res Techniq. 1998;41:224–233. doi: 10.1002/(SICI)1097-0029(19980501)41:3<224::AID-JEMT6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- D’sa C, Gross J, Francone VP, Morest DK. Plasticity of synaptic endings in the cochlear nucleus following noise-induced hearing loss is facilitated in the adult FGF2 overexpressor mouse. Eur J Neursci. 2007;26:666–680. doi: 10.1111/j.1460-9568.2007.05695.x. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Wetmore C, Olson L, Persson H. Identification of cells in rat brain and peripheral tissues expressing mRNA for members of the nerve growth factor family. Neuron. 1990;5:511–526. doi: 10.1016/0896-6273(90)90090-3. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Merlio JP, Persson H. Cells expressing mRNA for neurotrophins and their receptors during embryonic rat development. Eur J Neurosci. 1992;4:1140–1158. doi: 10.1111/j.1460-9568.1992.tb00141.x. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Lee KF, Kucera J, Jaenisch R. Lack of neurotrophin-3 leads to deficiencies in the peripheral nervous system and loss of limb proprioceptive afferents. Cell. 1994a;77:503–512. doi: 10.1016/0092-8674(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Lee KF, Jaenisch R. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 1994b;368:147–150. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Kucera J, Lee KF, Loring J, Jaenisch R. Studies on the physiological role of brain-derived neurotrophic factor and neurotrophin-3 in knockout mice. Int J Dev Biol. 1995a;39:799–807. [PubMed] [Google Scholar]
- Ernfors P, Van De Water T, Loring J, Jaenisch R. Complementary roles of BDNF and NT-3 in vestibular and auditory development. Neuron. 1995b;14:1153–1164. doi: 10.1016/0896-6273(95)90263-5. [DOI] [PubMed] [Google Scholar]
- Farhadi HF, Mowla SJ, Petrecca K, Morris NG, Seidah NG, Murphy RA. Neurotrophin-3 sorts to the constitutive secretory pathway of hippocampal neurons and is diverted to the regulated secretory pathway by coexpression with brain-derived neurotrophic factor. J Neurosci. 2000;20:4059–4068. doi: 10.1523/JNEUROSCI.20-11-04059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinas I, Jones KR, Tessarollo l, Vigers AJ, Huang E, Kirstein M, de Caprona DC, Coppola V, Backus C, Reichardt LF, Fritzsch B. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. J Neurosci. 2001;21:6170–6180. doi: 10.1523/JNEUROSCI.21-16-06170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng JJ, Morest DK. Development of synapses and expression of a voltage-gated potassium channel in chick embryonic auditory nuclei. Hear Res. 2006;216–217:116–126. doi: 10.1016/j.heares.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Ferguson IA, Schweitzer JB, Johnson EM., Jr Basic fibroblast growth factor: receptor-mediated internalization, metabolism, and anterograde axonal transport in retinal ganglion cells. J Neurosci. 1990;10:2176–2189. doi: 10.1523/JNEUROSCI.10-07-02176.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Farinas I, Reichardt LF. Lack of neurotrophin 3 causes losses of both classes of spiral ganglion neurons in the cochlea in a region-specific fashion. J Neurosci. 1997a;17:6213–6226. doi: 10.1523/JNEUROSCI.17-16-06213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Silos-Santiago I, Bianchi LM, Farinas I. The role of neurotrophic factors in regulating the development of inner ear innervation. TINS. 1997b;20:159–164. doi: 10.1016/s0166-2236(96)01007-7. [DOI] [PubMed] [Google Scholar]
- Gacek RR, Khetarpal U. Neurotrophin 3, not brain-derived neurotrophic factors or neurotrophin 4, knockout mice have delay in vestibular compensation after unilateral labyrinthectomy. Laryngoscope. 1998;108:671–678. doi: 10.1097/00005537-199805000-00009. [DOI] [PubMed] [Google Scholar]
- Hafidi A. Distribution of BDNF, NT-3 and NT-4 in the developing auditory brainstem. Int J Dev Neurosci. 1999;17:285–294. doi: 10.1016/s0736-5748(99)00043-x. [DOI] [PubMed] [Google Scholar]
- Hafidi A, Moore T, Sanes DH. Regional distribution of neurotrophin receptors in the developing auditory brainstem. J Comp Neurol. 1996;367:454–464. doi: 10.1002/(SICI)1096-9861(19960408)367:3<454::AID-CNE10>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Hossain WA, Morest DK. Fibroblast growth factors (FGF-1, FGF-2) promote migration and neurite growth of mouse cochlear ganglion cells in vitro: immunohistochemistry and antibody perturbation. J Neurosci Res. 2000;62:40–55. doi: 10.1002/1097-4547(20001001)62:1<40::AID-JNR5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Hossain WA, Zhou X, Rutledge A, Baier C, Morest DK. Basic fibroblast growth factor affects neuronal migration and differentiation. Exp Neurol. 1996;138:121–143. doi: 10.1006/exnr.1996.0052. [DOI] [PubMed] [Google Scholar]
- Hossain WA, Rutledge A, Morest DK. Critical periods of basic fibroblast growth factor (FGF-2) and brain-derived neurotrophic factor in the development of the chicken cochleovestibular ganglion in vitro. Exp Neurol. 1997;147:437–451. doi: 10.1006/exnr.1997.6623. [DOI] [PubMed] [Google Scholar]
- Hossain WA, Brumwell CL, Morest DK. Sequential interactions of fibroblast growth factor-2, brain-derived neurotrophic factor, neurotrophin-3, and their receptors define critical periods in the development of cochlear ganglion cells. Exp Neurol. 2002;175:138–151. doi: 10.1006/exnr.2002.7872. [DOI] [PubMed] [Google Scholar]
- Hossain WA, D’sa C, Morest DK. Site-specific interactions of neurotrophin-3 and fibroblast growth factor (FGF2) in the embryonic development of the mouse cochlear nucleus. J Neurobiol. 2006;66:897–915. doi: 10.1002/neu.20264. [DOI] [PubMed] [Google Scholar]
- Hossain WA, D’sa C, Morest DK. Interactive roles of fibroblast growth factor 2 and neurotrophin 3 in the sequence of migration, process outgrowth, and axonal differentiation of mouse cochlear ganglion cells. J Neurosci Res. 2008;86:2376–2391. doi: 10.1002/jnr.21685. [DOI] [PubMed] [Google Scholar]
- Morest DK. The cellular basis for signal processing in the mammalian cochlear nuclei. In: Merchan MD, Juiz JM, Godfrey DA, Mugnaini E, editors. The mammalian cochlear nuclei: organization and function. New York: Plenum; 1993. pp. 1–18. [Google Scholar]
- Pirvola U, Ylikoski J, Palgi J, Lehtonen E, Arumae U. Brain-derived neurotrophic factor and neurotrophin 3 mRNAs in the peripheral target fields of developing inner ear ganglia. Proc Natl Acad Sci U S A. 1992;89:9915–9919. doi: 10.1073/pnas.89.20.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirvola U, Arumae U, Moshnyakov M, Palgi J, Saarma M, Ylikoski J. Coordinated expression and function of neurotrophins and their receptors in the rat inner ear during target innervation. Hear Res. 1994;75:131–144. doi: 10.1016/0378-5955(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Schecterson LC, Bothwell M. Neurotrophin and neurotrophin receptor mRNA expression in developing inner ear. Hear Res. 1994;73:92–100. doi: 10.1016/0378-5955(94)90286-0. [DOI] [PubMed] [Google Scholar]
- Stephens HE, Belliveau AC, Gupta JS, Mirkovic S, Kablar B. The role of neurotrophins in the maintenance of the spinal cord motor neurons and the dorsal root ganglia proprioceptive sensory neurons. Int J Dev Neurosci. 2005;23:613–620. doi: 10.1016/j.ijdevneu.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Sugawara M, Murtie JC, Stankovic KM, Liberman MC, Corfas G. Dynamic patterns of neurotrophin 3 expression in the postnatal mouse inner ear. J Comp Neurol. 2007;501:30–37. doi: 10.1002/cne.21227. [DOI] [PubMed] [Google Scholar]
- Suneja SK, Potashner SJ. Quantification of a neurotrophin receptor from submilligram quantities of brain tissue using Western blotting. Brain Res Protoc. 1998;3:88–93. doi: 10.1016/s1385-299x(98)00028-2. [DOI] [PubMed] [Google Scholar]
- Tierney TS, Doubell TP, Xia G, Moore DR. Development of brain-derived neurotrophic factor and neurotrophin-3 immunoreactivity in the lower auditory brainstem of the postnatal gerbil. Eur J Neurosci. 2001;14:785–793. doi: 10.1046/j.0953-816x.2001.01690.x. [DOI] [PubMed] [Google Scholar]
- Trettel J, Morest DK. Atlas of the cochlear nucleus of the mouse. In: Willot J, editor. Handbook of the mouse auditory system: from behavior to molecular biology. Boca Raton, FL: CRC Press; 2001. pp. 279–296. [Google Scholar]
- Wheeler EF, Bothwell M, Schecterson LC, von Bartheld CS. Expression of BDNF and NT3 mRNA in hair cells of the organ of Corti: quantitative analysis in developing rats. Hear Res. 1994;73:46–56. doi: 10.1016/0378-5955(94)90281-x. [DOI] [PubMed] [Google Scholar]
- Young ED, Oertel D. The cochlear nucleus. In: Shepherd GM, editor. Synaptic organization of the brain. New York: Oxford University Press; 2003. pp. 125–163. [Google Scholar]
- Zhou XF, Rush RA. Localization of neurotrophin-3 like immunoreactivity in the rat central nervous system. Brain Res. 1994;643:162–172. doi: 10.1016/0006-8993(94)90022-1. [DOI] [PubMed] [Google Scholar]



