Abstract
Background
Aging results in changes in immune cell function which have been described for T-cells, macrophage, neutrophils, and dendritic cells, but not yet for eosinophils. We sought to define age-related changes in eosinophil function and their potential implications for asthma. Methods. We recruited human subjects with asthma in two age groups, a younger group (20–40 years old) and older group (55–80 years old). Lung function, induced sputum, and peripheral blood were obtained from each subject. Eosinophils isolated from the peripheral blood were examined for in vitro functional activities including degranulation, superoxide anion production, adhesion, and chemotaxis.
Results
Eosinophil degranulation in response to IL-5 stimulation was significantly decreased in the older group (p=0.025). Eosinophil production of superoxide anions in response to phorbol myristate acetate was lower in the older group, but did not achieve statistical significance (p=0.097). Eosinophil adhesion, eosinophil chemotaxis, lung function, and the percentage of sputum eosinophils were similar in the two groups.
Conclusion
Airway eosinophilia is comparable in younger and older asthma subjects. However, there are age-related changes in peripheral blood eosinophil “effector” functions. Diseases such as asthma, in which eosinophils are thought to play a pathophysiological role, may exhibit important clinical differences in the elderly due to age-related changes in inflammatory cell function that affect the manifestations of the disease and/or responsiveness to specific classes of medications.
Keywords: aging, immunosenescence, eosinophil, asthma
Introduction
It has been shown that there are many immune cell functions that decline with aging. The thymus involutes with aging, such that the production of naïve T-cells diminishes with age.1 There is also an increase in memory T-cells with a reduction in overall T-cell diversity.2;3 Furthermore, cytokine secretion and proliferation of T-cells decrease with aging.4;5 These T-cell changes contribute to the overall decline in the function of the adaptive immune system.6 In addition, it has been reported that the function of the innate immune system also declines with aging, such as the functional activities of macrophage, neutrophils and dendritic cells.7
Much of the knowledge of “immunosenescence”, or changes in immune function with aging, is based on studies in animal models and tissue culture systems. Whether immunosenescence has any clinical consequences in humans is not yet fully established. It is thought that increased susceptibility to infections, increase in autoimmune processes and increase in malignancy in the elderly can, in part, be attributed to immunosenescence.8–11 However, the impact of immunosenescence on other inflammatory disorders such as asthma is not known.
The management of allergic diseases in the elderly, such as asthma, is presumed to be identical to younger patients; however, this has not been well studied.12 There is a recent report suggesting that older asthma patients are less responsive to bronchodilators in an emergency room setting.13 Furthermore, a landmark study by Malmstrom et al. introduced the concept that for any therapeutic intervention, there are distinct groups of responders and non-responders.14 It is now thought that there are multiple phenotypes of asthma with differences in inflammatory pathophysiology as the basis for differential responses to distinct classes of medications. This concept has been validated in a study of children with asthma in which specific asthma biomarkers were associated with greater clinical efficacy of either a leukotriene receptor inhibitor, inhaled corticosteroid or both.15 It is also recognized that there are many challenges in the treatment of asthma in an older age group, including potential drug interactions with medications used for co-morbidities and a greater propensity to experience adverse events from medication use.16;17 Therefore, we ultimately seek to determine whether immunosenescence or functional changes of inflammatory cells found in the airway of older asthma patients constitute a distinct asthma phenotype with implications for most appropriate therapy in this age group.
In order to begin characterizing age-related changes in the biology of allergic airway inflammation in asthma, our initial focus was the eosinophil. The eosinophil is abundantly recruited into the airway, thought to be involved in the pathophysiology of asthma exacerbations and its levels in the airway correlate with disease severity.18;19 We examined the differences in peripheral blood eosinophil functional activities from subjects with mild to moderate asthma in two age groups, from 20–40 years of age and 55–80 years of age. The in vitro functional activities examined were representative of in vivo activities important for both the recruitment of eosinophils to the lung and the release of mediators involved in asthma pathophysiology.
Methods
Human Subjects
The studies were approved by the University of Wisconsin Health Sciences Institutional Review Board and informed consent was obtained from each participant. We recruited 15 subjects in each of two groups, 20–40 years of age and 55–80 years of age. Age ranges were selected to represent extreme ages of adult asthma subjects while maintaining an adequate potential for subject recruitment. Inclusion criteria included a physician diagnosis of asthma with a methacholine PC20 < 8mg/mL or albuterol reversibility on spirometry of ≥ 12%. Exclusion criteria included history of tobacco use > 5 pack years, upper respiratory infection within 6 weeks, prednisone use within 1 month, diabetes, pregnancy, chronic lung disease other than asthma, or an active cardiovascular disease other than controlled hypertension. One subject in the older group was excluded due to lack of reversibility on spirometry and a methacholine PC20 > 8mg/mL. Subjects were seen twice within 2 weeks. At visit 1, spirometry with reversibility, sputum induction, and allergy skin-prick testing were done. At visit 2, a methacholine challenge and phlebotomy were performed. On the day of a study visit, the morning doses of asthma medications were held until completion of the study visit. Prior to visit 1, antihistamines were held for 5 days.
Total IgE
Approximately 5mL of peripheral blood from each subject was placed into a serum separator tube and allowed to clot at room temperature. The sample was then centrifuged for 10 minutes at 1500RPM and 4°C. The supernatant was collected as serum and stored at −20°C. Sera from the subjects were analyzed per manufacturer protocol with a human total IgE ELISA kit (Bethyl Laboratories, Montgomery, TX).
Sputum analysis
Sputum induction and processing were performed as described previously.20 Briefly, subjects were given two puffs of albuterol followed by inhalation of a 3% saline solution. Sputum collection was performed at regular timed intervals. The sputum was incubated with Sputolysin (Calbiochem, La Jolla, CA) for 20 minutes and centrifuged. The sample cell pellet was washed with HBSS and filtered through a nylon mesh. Cells were stained with the HEMA 3 Stain Kit (Fisher Scientific, Kalamazoo, MI).
Eosinophil purification
Peripheral blood eosinophils were isolated using magnetic bead negative selection as described previously.21 Briefly, venous blood was separated by density centrifugation to obtain a granulocyte pellet, which was then incubated with magnetic beads coated with anti-CD16, anti-CD14, and anti-CD3 (Miltenyi Biotechnology, Auburn, CA). Negative selection was performed using an AutoMACS separator (Miltenyi Biotechnology). The eosinophils were typically >99% pure and >98% viable at the time of purification. Viability was reassessed by Trypan blue exclusion at 24 and 48 hours and were comparable in both age groups (54% vs 51% at 24 hours and 21% vs. 18% at 48 hours for the younger and older groups, respectively). Due to subject variability in their peripheral blood eosinophil counts and yields from the purification, not each eosinophil functional assay was performed for each subject.
Eosinophil superoxide anion production
Eosinophils at 0.5×106 cells/mL in HEPES-buffered saline solution (HBSS) with 0.1% gelatin were incubated at 37°C with 1.2mg/mL ferro-cytochrome C (Sigma, St. Louis, MO) and stimulated with or without 1ng/mL phorbol myristate acetate (PMA, Sigma), as previously described.22 The addition of superoxide dismutase at 20μg/mL served as a blank for each sample. Superoxide anion production was monitored for 1 hour as a colorimetric change at 550nm. The superoxide anion production was reported as nmol of cytochrome C reduced per 1×106 cells, calculated as previously described.22
Eosinophil adhesion
Eosinophils at 0.1×106 cells/mL in HBSS with 0.3% gelatin were cultured either alone, on vascular cell adhesion molecule (VCAM)-1 (R&D Systems, Minneapolis, MN) coated wells, or with 1ng/mL PMA (Sigma) for 30 minutes at 37°C. The tissue culture wells were washed and the remaining adherent eosinophils were cultured in 55mM Tris buffer, pH8.0, with 1mM H2O2 (Sigma), 1mM o-phenylenediamine HCl (Sigma), and 0.1% Triton X-100 (Sigma) for 30 minutes at room temperature, as described previously.23 Eosinophil peroxidase activity was measured as a colorimetric change at 490nm. Eosinophil adherence was measured as a percentage of eosinophil peroxidase activity after rigorous washes compared to the total activity from the number of cells initially added to the wells.23;24
Eosinophil chemotaxis
Eosinophils at 3–5×106 cells/mL in HBSS with 0.1% gelatin were cultured in the upper compartment of 24-well 5.0μm Transwell plates (Costar, Cambridge, MA), as previously described.25 The bottom compartment contained HBSS with 0.1% gelatin alone or supplemented with either 100ng/mL eotaxin (Biosource, Carlsbad, CA) or 100nM formyl-met-leu-phe (fMLP, Sigma). After incubation at 37°C and 5% CO2 for 1 hour, cells were counted from the bottom chamber and the percent migration calculated as the number of cells in the bottom chamber divided by the initial number added to the upper chamber.
Eosinophil degranulation
Eosinophils were cultured at 1×106 cells/mL and stimulated with 100nM fMLP (Sigma) or 1ng/mL IL-5 (BD Biosciences) for 4 hours at 37°C and 5% CO2, as previously described.26 Eosinophils were lysed with 50mM HCl and 0.5% Triton X-100 to release total granule contents. Cell-free supernatants were collected and stored at −20°C. Eosinophil derived neurotoxin (EDN) protein levels were measured by ELISA per manufacturer protocol (MBL International, Nagoya, Japan). Percent degranulation was defined as the EDN release in the culture supernatant divided by the total EDN.
Statistics
Statistical analysis was performed using SigmaStat software, version 3.1 (Systat, San Jose, CA). The comparisons between the younger and older groups were performed with t-test analysis, Mann-Whitney rank sum analysis, or Fisher-exact test. Statistical significance was defined as a p value < 0.05.
Results
Subject characteristics
Table 1 shows a comparison of subject lung function and peripheral blood characteristics for the donors utilized in these studies. The FEV1 and FVC were significantly less in the older group, which is consistent with lung volumes changes with aging.27 However, the percent predicted for both FEV1 and FVC were comparable between the younger and older groups, suggesting comparable disease severity in the two age groups. Additional measures to assess asthma severity were similar in the younger and older groups, specifically the methacholine PC20 and FEV1 reversibility with albuterol. The total serum IgE levels was lower in the older group, but this difference did not achieve statistical significance (p=0.190). However. previously published studies with larger sample sizes have reported significantly lower levels of total serum IgE in the elderly.28–30 The difference in inhaled corticosteroid (ICS) use in the two groups also did not achieve statistical significance (p=0.066 by the Fisher Exact Test). Table 2 shows the respective lists of all medications used by the individual subjects in each group.
Table 1.
Subject characteristics.
| Younger | Older | |
|---|---|---|
| Age (years, range given)* | 26.7 (20–38) | 62.9 (57–75) |
| FEV1 (L)* | 3.22 (2.89–3.38) | 2.18 (1.97–2.33) |
| FEV1 % Predicted | 80.0 (68.0–91.5) | 76.0 (66.3–86.7) |
| FVC (L)* | 4.37 (+/− 0.79) | 3.22 (+/− 0.85) |
| FVC % Predicted | 92.6 (+/− 9.6) | 86.2 (+/− 10.5) |
| Methacholine PC20 (mg/mL)# | 0.98 (0.30–2.57) | 0.42 (0.35–1.16) |
| % Reversibility with Albuterol | 14.8 (+/− 12) | 11.5 (+/− 8) |
| WBC (cells/nL) | 5.2 (4.5–5.7) | 4.8 (4.0–6.3) |
| Absolute Eosinophil Count (cells/μL) | 158 (123–299) | 123 (84–273) |
| Total IgE (ng/mL)≠ | 226 (85–323) | 99 (47–254) |
| Positive Skin Prick Allergy Testing§ | 15/15 | 13/14 |
| Inhaled Corticosteroid Use | 4/15 | 9/14 |
Mean values (+/− standard deviation), median values (25%–75% interval),
statistically significant difference in the two groups (p < 0.05),
values from three subjects were excluded only from the analysis of the methacholine PC20 due to values > 25mg/mL (1 in the younger group, 2 in the older group),
median total IgE levels expressed in international units were 94 IU/mL and 41 IU/mL for the younger and older groups, respectively,
indicates at least one positive test for grass, mold, ragweed, tree, cat, dog, or dust mite.
Table 2.
Subject medication use.
| Subject # | Age | Medication Use |
|---|---|---|
| Younger | ||
| 1 | 20 | Advair 250/50 1 puff BID, albuterol PRN, Yasmin (OCP) qD |
| 2 | 31 | albuterol PRN, sertraline 50mg qD |
| 3 | 38 | albuterol PRN, fexofenadine 60mg PRN, Aviane (OCP) qD |
| 4 | 35 | albuterol PRN, Exedrine qD, Depo-Provera, morphine tablet PRN |
| 5 | 21 | ibuprofen 200mg PRN, Ortho Tri-cyclen (OCP) qD |
| 6 | 27 | Advair 250/50 1 puff BID, albuterol PRN, loratidine 10mg PRN, ibuprofen 400mg PRN, ranitidine 75mg PRN, multivitamin qD, flaxseed oil qD, fish oil qD |
| 7 | 33 | albuterol PRN |
| 8 | 22 | albuterol PRN |
| 9 | 22 | albuterol PRN, Ortho Tri-cyclen (OCP) qD, multivitamin qD |
| 10 | 24 | albuterol PRN, minocycline qD, multivitamin qD, levothyroxine qD |
| 11 | 22 | Flovent 110mcg 2 puffs qD, albuterol PRN, diphenhydramine 25mg PRN |
| 12* | 21 | Advair 250/50 1 puff BID, albuterol PRN, fexofenadine 180mg PRN, ibuprofen 400mg PRN |
| 13 | 22 | albuterol PRN, ibuprofen 400mg PRN |
| 14 | 30 | albuterol PRN, loratidine 10mg PRN, naproxen PRN, Aviane (OCP) qD, mesalamine 1600mg qD |
| 15 | 28 | Flovent 110mcg 2 puffs qD, albuterol PRN, diphenhydramine PRN, Necon (OCP) qD, sumatriptan PRN |
| Older | ||
| 1 | 60 | Advair 100/50 1 puff BID, albuterol PRN, calcium qD, multivitamin qD |
| 2 | 60 | Advair 500/50 1 puff BID, albuterol PRN, aspirin 81mg qD, multivitamin qD |
| 3 | 66 | Advair 250/50 1 puff BID, Rhinocort 2sp BID, folic acid qD, ibuprofen 400mg PRN |
| 4 | 65 | albuterol PRN, fexofenadine 180mg PRN, aspirin 81mg qD, simvastatin 80mg qD, alendronate 70mg qWk, ibuprofen 200mg PRN, multivitamin qD, calcium qD, vitamin C qD, glucosamine qD, fish oil qD |
| 5 | 75 | Advair 250/50 1 puff BID, albuterol PRN, atenolol 25mg qD, hydrochlorothiazide 25mg qD, aspirin 81mg qD, alendronate 25mg qD |
| 6 | 67 | Flovent 110mcg 1 puff BID, albuterol PRN, Flonase 1sp PRN, fexofenadine 60mg PRN, aspirin 162mg qD, ibandronate qMonth |
| 7 | 69 | Flovent 110mcg 2 puffs BID, Lotrel qD, bupropion qD, glucosamine qD, naproxen PRN |
| 8 | 74 | Pulmicort respule 0.5mg qD, albuterol PRN, multivitamin qD |
| 9 | 57 | albuterol PRN, chlorpheniramine PRN, levothyroxine 112mcg qD, bupropion 150mg qD |
| 10 | 57 | Flovent 100mcg 2 puffs BID, salmeterol 50mcg 1 puff BID, albuterol PRN, cetirizine 10mg PRN, omeprazole 20mg qD, methylphenidate 36mg qD, citalopram 10mg qD, calcium qD, beta-carotene qD, vitamin E qD |
| 11 | 64 | Flovent 220mcg 2 puffs BID, albuterol PRN, coumadin 5mg qD, fenofibrate 145mg qD, triamterene/hydrochlorothiazide 75/50 qD |
| 12 | 59 | monteleukast 10mg qD, albuterol PRN, loratidine PRN, bupropion qD, alendronate 70mg qWeek, nortriptyline 10mg PRN |
| 13 | 58 | pirbuterol PRN, lisinopril 20mg qD, hydrochlorothiazide 25mg qD, acetaminophen 1000mg BID, calcium qD, multivitamin qD, TUMS PRN |
| 14 | 59 | albuterol PRN |
OCP indicates the medication is an oral contraceptive pill.
this subject ran out of Advair two days prior to visit 1 and had not resumed its use by visit 2; therefore, this subject was not designated as an ICS user in Table 1.
Eosinophil Degranulation and Superoxide Anion Production
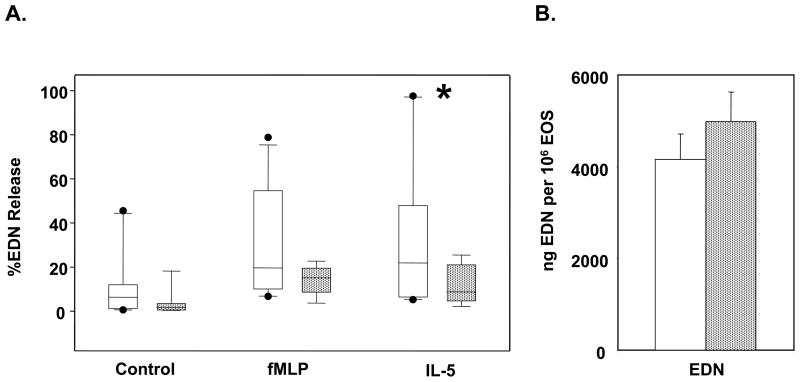
One of the eosinophil “effector” functions in allergic inflammation is degranulation. The fMLP and IL-5 stimulated degranulation of eosinophil derived neurotoxin (EDN), which is an eosinophil specific granule proteins, was compared between eosinophils isolated from younger and older subjects (Figure 1A). Upon stimulation with IL-5, the release of EDN was significantly decreased from the eosinophils isolated from the older group (p=0.025). Furthermore, there was less spontaneous EDN release and less fMLP stimulated release from eosinophils isolated from the older group that did not achieve statistical significance (p=0.132 and 0.073, respectively). In order to determine whether the decreased release of EDN was due to less EDN content in eosinophils from older subjects, the total EDN in the eosinophils was also compared (Figure 1B) and was found to be comparable in the younger and older groups.
Figure 1.
Eosinophil EDN degranulation. Eosinophils from younger (open bars) and older (stippled bars) asthma subjects were cultured for 4 hours at 37°C. A, Eosinophils were either not stimulated, stimulated with fMLP or IL-5. Cells were centrifuged and supernatants analyzed by ELISA for EDN shown as box plots. *p=0.025, by Mann-Whitney rank sum test. B, Total EDN was measured by ELISA from the complete lysis of a known number of eosinophils with Triton X-100 and HCl. Error bars indicate standard error of the mean.
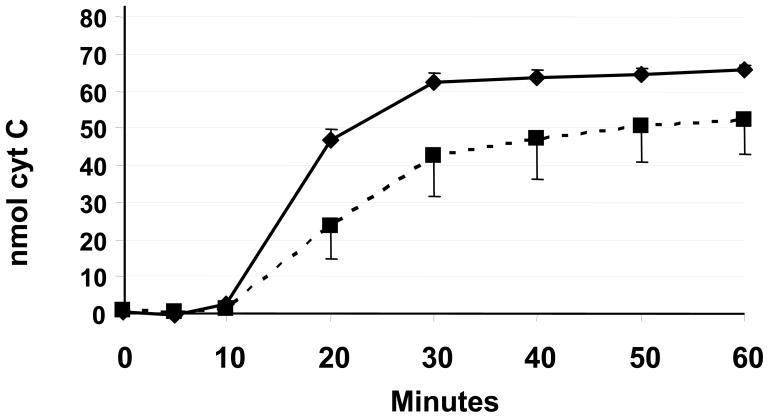
Another eosinophil “effector” function in allergic inflammation is the production of superoxide anions. The kinetics and extent of superoxide anion production following PMA stimulation were compared between the eosinophils isolated from younger and older subjects (Figure 2). At 20 minutes, the PMA stimulation suggested decreased superoxide anion production from eosinophils isolated from the older group; however, this did not reach statistical significance (p=0.097).
Figure 2.
Eosinophil superoxide anion production. Eosinophil superoxide anion production from younger (solid line) and older (dashed line) asthma subjects is shown. Superoxide anion production was measured as a reduction of cytochrome C (cyt C), specifically nmol of cytochrome C reduced per 1×105 eosinophils. Error bars indicate standard error of the mean.
Eosinophil Adhesion and Chemotaxis
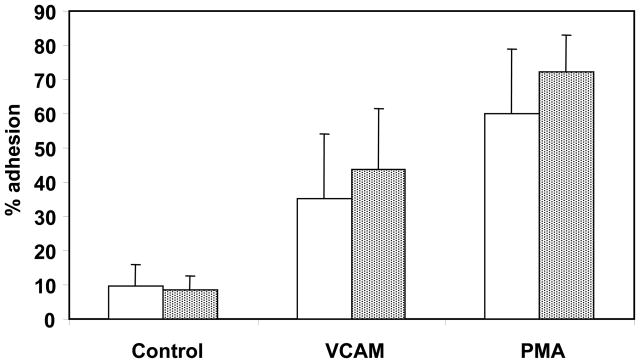
Eosinophil adhesion to vascular endothelium represents an initial step in the process of migration from the peripheral circulation into target tissues such as the lung. An in vitro assessment of this activity is the ability of eosinophils to adhere to vascular cell adhesion molecule (VCAM)-1 or adherence following PMA stimulation. Adhesion was compared in eosinophils isolated from younger and older subjects (Figure 3). The extent of eosinophil adhesion did not differ significantly between the younger and older groups in control untreated cells, in VCAM-1 coated wells, or for PMA stimulated cells.
Figure 3.
Eosinophil adhesion. Eosinophils from younger (open bars) and older (stippled bars) asthma subjects were cultured unstimulated, with VCAM-1 coated wells, or with 1ng/mL PMA stimulation for 30 minutes. Percent adhesion was calculated as described in the methods. Error bars indicate standard deviation.
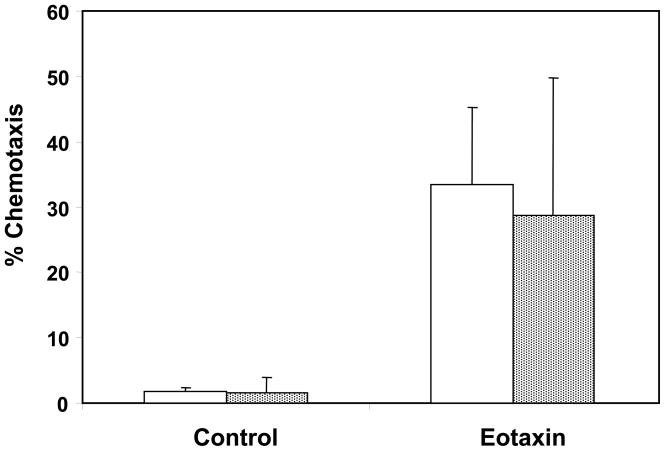
Eosinophil migration into target tissue is facilitated by chemokine signaling, which can be assessed in vitro with a chemotaxis assay. Therefore, the ability of eosinophils to cross a semi-permeable membrane in response to a chemotactic signal was examined with eotaxin stimulation (Figure 4). There was no significant difference in chemotaxis upon eotaxin stimulation in the younger and older groups.
Figure 4.
Eosinophil chemotaxis. Eosinophils from younger (open bars) and older (stippled bars) asthma subjects were cultured in the top compartment of a 5.0μm Transwell semi-permeable membrane. Migration into the bottom chamber after 1 hour was measured in response to no stimulus or 100ng/mL eotaxin. The percent migration was calculated as the number of eosinophils in the bottom chamber divided by the total eosinophils initially cultured multiplied by 100. Error bars indicate standard deviation.
Sputum Cell Differentials
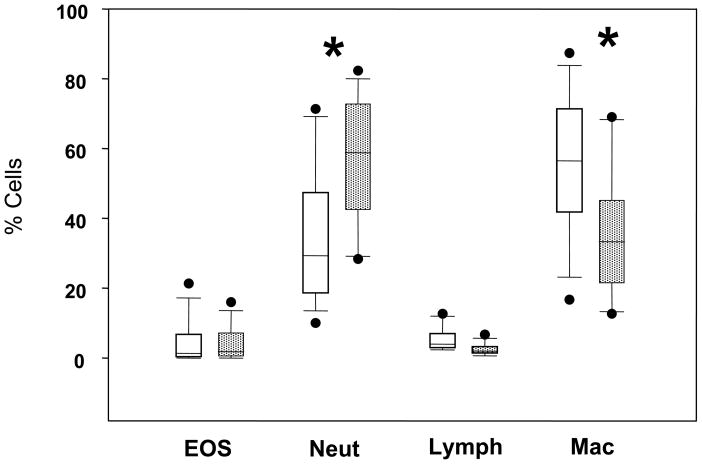
The in vitro assays of eosinophil activities suggest that eosinophil immunosenescence involves a decrease in “effector” functions with intact tissue localizing functions. We examined sputum cell differentials to determine whether aging affected the in vivo localization of eosinophils to the airways of allergic asthma subjects. There was a significant increase in the percentage of neutrophils (p=0.008) and a decrease in the percentage of macrophage (p=0.031) in the older group (Figure 5), which are consistent with previously published observations with normal aging.31 Notably, the percentage of eosinophils was similar in the two groups. The absolute numbers of eosinophils were also similar in the two age groups (data not shown).
Figure 5.
Sputum cell type differentials. Box plots of sputum cell percentages are shown for younger (open boxes) and older (stippled boxes) asthma subjects. EOS, eosinophils; Neut, neutrophils; Lymph, lymphocytes; Mac, macrophage. *p<0.05, by student’s t-test.
Discussion
Peripheral blood eosinophils from younger and older subjects were analyzed in vitro for several activities representative of in vivo functions to determine whether there were age-related differences. Eosinophils perform activities categorized as “effector” functions that are thought to have a role in the pathophysiology of asthma including degranulation and superoxide production.32 The eosinophil degranulation of EDN was examined upon stimulation with fMLP and IL-5 and eosinophil production of superoxide anion was examined upon stimulation with PMA. The IL-5 stimulated degranulation was significantly decreased in the eosinophils from older subjects in comparison to younger subjects. This demonstrates an age-related change in eosinophil functional activity. The declines in fMLP stimulated EDN degranulation and superoxide anion production from the eosinophils from the older group did not achieve statistical significance.
There are other eosinophil activities that are important for localization to tissues such as the lung. Eosinophils express many integrin molecules that, upon activation, facilitate cell adhesion to the vascular endothelium.33 This activity can be examined in vitro by analyzing adhesion to substrates such as VCAM-1. Furthermore, eosinophil migration into target tissues is facilitated by signaling from inflammatory mediators such as the chemokine eotaxin.34 The peripheral blood eosinophil adhesion and chemotactic activities were both comparable in the younger and older subjects. Therefore, one would expect that eosinophil recruitment into the airway should be the same in the younger and older groups. As predicted, there were comparable levels of sputum eosinophils in younger and older asthma subjects.
Although only the IL-5 stimulated eosinophil degranulation exhibited a statistically significant decline in the older subjects, we speculate that additional eosinophil “effector” functions are also diminished. Given the trends that we observed, our study was likely underpowered to achieve statistical significance for other age-related changes in eosinophil function.
A recent study described the use of a sensitized aged mice to study airway inflammation.35 The aged mice had greater airway eosinophilia in response to ovalbumin challenge compared to the young mice; however, the airway hyperresponsiveness was less. Thus, in aged mice, despite the increased airway eosinophilia, the airway hyperresponsiveness attributable to eosinophil “effector” function was less than observed in younger mice. Since our study did not examine airway eosinophilia during an asthma exacerbation, we cannot address whether older human asthma subjects also recruit greater numbers of eosinophils. However, the diminished eosinophil effector function in older mice is consistent with our observations of diminished functional activity of eosinophils with aging. In another study utilizing aged rats, the process of sensitization and airway challenge to ovalbumin resulted in a lack of eosinophil accumulation in the airway.36 This was attributed to decreased T-cell derived chemotactic signaling for the recruitment of eosinophils. Furthermore, the baseline levels of eosinophils prior to challenge were also lower in the aged rats. Our demonstration of comparable levels of eosinophils in the induced sputum of older and younger subjects is in contrast to this finding in the rat model.
One limitation of our study is that the eosinophil functional analyses were performed on peripheral blood eosinophils rather than eosinophils from the airway. It is known that there are differences in the cell surface markers and activation state of airway eosinophils compared to peripheral blood eosinophils.23 This raises the question of whether airway eosinophils also exhibit age-related decreases in “effector” functions, such as degranulation, production of superoxide or production of leukotrienes. Furthermore, another limitation of our study is that the study subjects were asymptomatic with respect to asthma symptoms. Therefore, whether the eosinophil functional activities are diminished during an exacerbation in older asthma subjects is not known. We are currently pursuing a followup study to address these questions.
The older group of subjects appeared to have greater inhaled corticosteroid (ICS) use than the younger group, though the difference was not statistically significant. Notably, the functional assays were all performed using peripheral blood eosinophils, which have significantly less corticosteroid exposure than airway eosinophils from ICS use. We also performed subgroup statistical analyses within each age group to compare eosinophil EDN degranulation in the ICS users versus non-users and found no difference (data not shown), although these analyses were underpowered in each age group. Furthermore, since eosinophil survival is known to be markedly reduced by corticosteroids,37 we compared peripheral blood eosinophil survival between the two groups by Trypan blue exclusion with no detectable difference at 24 or 48 hours of culture (data not shown). In Table 2, other medications are also shown to be used disproportionately in the two age groups such as oral contraceptives and bisphosphonates. This raises the question of whether our observations may have been due to the use of medications rather than aging. As with ICS use above, we performed subgroup statistical analyses to compare eosinophil EDN degranulation in the users versus non-users of oral contraceptives, bisphosphonates, nonsteroidal anti-inflammatory drugs, antihypertensives, antidepressants, and vitamins and found no significant differences (data not shown). Although these subgroup analyses were also underpowered, the lack of a significant finding suggests that age is the predominant association with decreased EDN degranulation with a potential for smaller effects of medication use or the co-morbid condition being treated.
Our data demonstrate an age-related change in the function of eosinophils. We speculate this finding is representative of additional age-related changes in the function of cells involved in airway inflammation. Further investigation into the biological changes of airway inflammation in the elderly are warranted to provide guidance for more effective therapies.
Acknowledgments
We thank Robyn Brandau for assistance with laboratory procedures. We thank the research coordinators for this study, Paula Smailes, Jennifer Bernstein, and Sara Zimmerman. We thank Mary Anne Kennedy, Tina Palas and Cheri Swenson for administrative assistance. We thank Michael Evans for assistance with statistical analysis. We thank Dr. Julie Sedgwick for providing expertise on eosinophils and review of this manuscript. We thank Dr. Keith Meyer for review of this manuscript.
Grant Support:
ASP-American Academy of Allergy, Asthma and Immunology Geriatric Development Initiative Junior Faculty Development Award co-sponsored by Atlantic Philanthropies, American Academy of Allergy, Asthma and Immunology, the John A. Hartford Foundation, and the Association of Subspecialty Professors (SKM)
University of Wisconsin Clinical Research Scholar (NIH K12, PI: Dr. Frank Graziano) (SKM)
Abbreviations
- EDN
eosinophil-derived neurotoxin
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- fMLP
formyl-methionine, leucine, phenylalanine
- OCP
oral contraceptive pill
- PMA
phorbol myristate acetate
- PRN
as needed
- VCAM
vascular cell adhesion molecule
Footnotes
Author S.K. Mathur has no conflicts to disclose.
Author E.A. Schwantes has no conflicts to disclose.
Author N.N. Jarjour has no conflicts to disclose.
Author W.W. Busse has no conflicts to disclose.
Reference List
- 1.Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120(4):435–446. doi: 10.1111/j.1365-2567.2007.02555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nature Immunology. 2004;5(2):133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 3.Goronzy JJ, Weyand CM. T cell development and receptor diversity during aging. Current Opinion in Immunology. 2005;17(5):468–475. doi: 10.1016/j.coi.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 4.Haynes L, Eaton SM, Burns EM, et al. CD4 T cell memory derived from young naive cells functions well into old age, but memory generated from aged naive cells functions poorly. PNAS. 2003;100(25):15053–15058. doi: 10.1073/pnas.2433717100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linton PJ, Haynes L, Klinman NR, et al. Antigen-independent changes in naive CD4 T cells with aging. J Exp Med. 1996;184(5):1891–1900. doi: 10.1084/jem.184.5.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weng Np. Aging of the Immune System: How Much Can the Adaptive Immune System Adapt? Immunity. 2006;24(5):495–499. doi: 10.1016/j.immuni.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plackett TP, Boehmer ED, Faunce DE, et al. Aging and innate immune cells. J Leukoc Biol. 2004;76(2):291–299. doi: 10.1189/jlb.1103592. [DOI] [PubMed] [Google Scholar]
- 8.Weyand CM, Fulbright JW, Goronzy JJ. Immunosenescence, autoimmunity, and rheumatoid arthritis. Experimental Gerontology. 2003;38(8):833–841. doi: 10.1016/s0531-5565(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 9.Meyer KC. Aging. Proc Am Thorac Soc. 2005;2(5):433–439. doi: 10.1513/pats.200508-081JS. [DOI] [PubMed] [Google Scholar]
- 10.Gavazzi G, Krause KH. Ageing and infection. Lancet Infectious Diseases. 2002;2(11):659–666. doi: 10.1016/s1473-3099(02)00437-1. [DOI] [PubMed] [Google Scholar]
- 11.Hakim FT, Flomerfelt FA, Boyiadzis M, et al. Aging, immunity and cancer. Current Opinion in Immunology. 2004;16(2):151–156. doi: 10.1016/j.coi.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 12.NIH Publication No. 96-3662 ed. National Institutes of Health: National Heart, Lung, and Blood Institute; 1996. Considerations for Diagnosing and Managing Asthma in the Elderly. [Google Scholar]
- 13.Banerji A, Clark S, Afilalo M, et al. Prospective multicenter study of acute asthma in younger versus older adults presenting to the emergency department. Journal of the American Geriatrics Society. 2006;54(1):48–55. doi: 10.1111/j.1532-5415.2005.00563.x. [DOI] [PubMed] [Google Scholar]
- 14.Malmstrom K, Rodriguez-Gomez G, Guerra J, et al. Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma - A randomized, controlled trial. Ann Intern Med. 1999;130(6):487. doi: 10.7326/0003-4819-130-6-199903160-00005. [DOI] [PubMed] [Google Scholar]
- 15.Szefler SJ, Phillips BR, Martinez FD, et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol. 2005;115(2):233–242. doi: 10.1016/j.jaci.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 16.McLean AJ, Le Couteur DG. Aging Biology and Geriatric Clinical Pharmacology. Pharmacol Rev. 2004;56(2):163–184. doi: 10.1124/pr.56.2.4. [DOI] [PubMed] [Google Scholar]
- 17.Turnheim K. Drug therapy in the elderly. Experimental Gerontology. 2004;39(11–12):1731–1738. doi: 10.1016/j.exger.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Busse WW, Lemanske RF. Advances in immunology - Asthma. New England Journal of Medicine. 2001;344(5):350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 19.Green RH, Brightling CE, McKenna S, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360(9347):1715–1721. doi: 10.1016/S0140-6736(02)11679-5. [DOI] [PubMed] [Google Scholar]
- 20.Gern JE, Vrtis R, Grindle KA, et al. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am J Respir Crit Care Med. 2000;162(6):2226–2231. doi: 10.1164/ajrccm.162.6.2003019. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto H, Sedgwick JB, Busse WW. Differential Regulation of Eosinophil Adhesion and Transmigration by Pulmonary Microvascular Endothelial Cells. J Immunol. 1998;161(2):971–977. [PubMed] [Google Scholar]
- 22.Sedgwick JB, Vrtis RF, Gourley MF, et al. Stimulus-Dependent Differences in Superoxide Anion Generation by Normal Human Eosinophils and Neutrophils. Journal of Allergy and Clinical Immunology. 1988;81(5):876–883. doi: 10.1016/0091-6749(88)90945-1. [DOI] [PubMed] [Google Scholar]
- 23.Sedgwick JB, Calhoun WJ, Vrtis RF, et al. Comparison of Airway and Blood Eosinophil Function After Invivo Antigen Challenge. Journal of Immunology. 1992;149(11):3710–3718. [PubMed] [Google Scholar]
- 24.Nagata M, Sedgwick JB, Bates ME, et al. Eosinophil Adhesion to Vascular Cell-Adhesion Molecule-1 Activates Superoxide Anion Generation. Journal of Immunology. 1995;155(4):2194–2202. [PubMed] [Google Scholar]
- 25.Sedgwick JB, Hwang YS, Gerbyshak HA, et al. Oxidized low-density lipoprotein activates migration and degranulation of human granulocytes. Am J Respir Cell Mol Biol. 2003;29(6):702–709. doi: 10.1165/rcmb.2002-0257OC. [DOI] [PubMed] [Google Scholar]
- 26.Kita H, Weiler DA, Abughazaleh R, et al. Release of Granule Proteins from Eosinophils Cultured with Il-5. Journal of Immunology. 1992;149(2):629–635. [PubMed] [Google Scholar]
- 27.Sears MR, Greene JM, Willan AR, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. New England Journal of Medicine. 2003;349(15):1414–1422. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 28.Delespesse G, De Maubeuge J, Kennes B, et al. IgE Mediated Hypersensitivity in Aging. Clinical Allergy. 1977;7(2):155–160. doi: 10.1111/j.1365-2222.1977.tb01436.x. [DOI] [PubMed] [Google Scholar]
- 29.Hanneuse Y, Delespesse G, Hudson D, et al. Influence of Aging on IgE-Mediated Reactions in Allergic Patients. Clinical Allergy. 1978;8(2):165–174. doi: 10.1111/j.1365-2222.1978.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 30.Wittig HJ, Belloit J, De Fillippi I, et al. Age-Related Serum Immunoglobulin-E Levels in Healthy-Subjects and in Patients with Allergic Disease. Journal of Allergy and Clinical Immunology. 1980;66(4):305–313. doi: 10.1016/0091-6749(80)90026-3. [DOI] [PubMed] [Google Scholar]
- 31.Meyer KC, Rosenthal NS, Soergel P, et al. Neutrophils and low-grade inflammation in the seemingly normal aging human lung. Mechanisms of Ageing and Development. 1998;104(2):169–181. doi: 10.1016/s0047-6374(98)00065-7. [DOI] [PubMed] [Google Scholar]
- 32.Gleich GJ. Mechanisms of eosinophil-associated inflammation. Journal of Allergy and Clinical Immunology. 2000;105(4):651–663. doi: 10.1067/mai.2000.105712. [DOI] [PubMed] [Google Scholar]
- 33.Broide D, Sriramarao P. Eosinophil trafficking to sites of allergic inflammation. Immunological Reviews. 2001;179(1):163–172. doi: 10.1034/j.1600-065x.2001.790116.x. [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Zepeda EA, Rothenberg ME, Ownbey RT, et al. Human eotaxin is a specific chemoattractant for eosinophil cells and provides a new mechanism to explain tissue eosinophilia. Nature Medicine. 1996;2(4):449–456. doi: 10.1038/nm0496-449. [DOI] [PubMed] [Google Scholar]
- 35.Busse PJ, Zhang TF, Srivastava K, et al. Effect of ageing on pulmonary inflammation, airway hyperresponsiveness and T and B cell responses in antigen-sensitized and -challenged mice. Clinical & Experimental Allergy. 2007;37(9):1392–1403. doi: 10.1111/j.1365-2222.2007.02775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yagi T, Sato A, Hayakawa H, et al. Failure of aged rats to accumulate eosinophils in allergic inflammation of the airway. Journal of Allergy and Clinical Immunology. 1997;99(1):38–47. doi: 10.1016/s0091-6749(97)70298-7. [DOI] [PubMed] [Google Scholar]
- 37.Wallen N, Kita H, Weiler D, et al. Glucocorticoids Inhibit Cytokine-Mediated Eosinophil Survival. Journal of Immunology. 1991;147(10):3490–3495. [PubMed] [Google Scholar]