Abstract
Objective
Several neurologic disorders are treated with deep brain stimulation; however, the mechanism underlying its ability to abolish oscillatory phenomena associated with diseases as diverse as Parkinson's and epilepsy remain largely unknown. In this study we sought to investigate the role of specific neurotransmitters in deep brain stimulation (DBS) and determine the role of non-neuronal cells in its mechanism of action.
Methods
We used the ferret thalamic slice preparation in vitro, which exhibits spontaneous spindle oscillations, in order to determine the effect of high-frequency stimulation on neurotransmitter release. We then performed experiments using an in vitro astrocyte culture to investigate the role of glial transmitter release in HFS-mediated abolishment of spindle oscillations.
Results
In this series of experiments we demonstrated that glutamate and adenosine release in ferret slices was able to abolish spontaneous spindle oscillations. The glutamate release was still evoked in the presence of the Na+ channel blocker tetrodotoxin (TTX), but was eliminated with the vesicular H+-ATPase inhibitor, bafilomycin, and the calcium chelator, BAPTA-AM. Furthermore, electrical stimulation of purified primary astrocytic cultures was able to evoke intracellular calcium transients and glutamate release, and bath application of BAPTA-AM inhibited glutamate release in this setting.
Conclusion
These results suggest that vesicular astrocytic neurotransmitter release may be an important mechanism by which DBS is able to achieve clinical benefits.
Keywords: astrocytes, adenosine, deep brain stimulation, glia, glutamate, high frequency stimulation
Introduction
Deep brain stimulation (DBS) is an effective and increasingly popular treatment for a variety of disorders including Parkinson's Disease,1 dystonia,2-4 tremor,5,6 epilepsy7,8 and chronic pain,9 as well as psychiatric disorders, like Tourette syndrome,10 obsessive–compulsive disorder,11-13 and depression.14-16 For the treatment of tremor and epilepsy, specific thalamic nuclei have been the preferential target for implanted electrodes, while the subthalamic nucleus (STN) is the favored target for the treatment of Parkinson's disease.17 Intra-operative recordings from patients have shown that “tremor” neurons in the thalamus discharge rhythmically, either prior to or in synchrony with the 3-6 Hz oscillatory muscular tremor.18 High frequency stimulation (HFS) applied to the area of the thalamus containing tremor cells leads to immediate tremor arrest, and the tremor rapidly returns when stimulation ceases.19 Absence seizures are also associated with oscillatory phenomena in the thalamus. They are associated with 3 Hz spike and wave oscillations in the EEG and are likely to represent a perverse form of thalamo-cortical activity that is related to the normal generation of spindle waves.20 Spontaneous normal spindle activity and abnormal absence-seizure-like activity can be recorded even in reduced preparations such as ferret thalamic brain slices.21 Thus, the ferret thalamic slice is an ideal model for testing the functional consequences of HFS-mediated neurotransmitter release.
Previously, it has been demonstrated that HFS of the STN22 or the thalamus23,24 results in neurotransmitter release. Indeed, it has been shown in vitro that the depolarizing effects of HFS are eliminated by glutamate receptor antagonist drugs,25 supporting the hypothesis that glutamate release is crucial to HFS function. Furthermore, Bekar et al.26 have also shown that thalamic DBS is associated with a marked increase in extracellular adenosine and that adenosinergic mechanisms are important in tremor control.26 Here, we employed an enzyme-linked glutamate sensor system and fast scan cyclic voltammetry (FSCV) to directly measure the extracellular glutamate and adenosine levels during HFS, respectively.27-31 There are two potential sources of glutamate and adenosine in the brain: astrocytes32 and neurons. There are large pools of glutamate and adenosine32,33 stored in astrocytes that can be released by mechanical stimulation.34,35 In addition, astrocytes release glutamate and adenosine through a Ca2+-dependent mechanism, establishing an important reciprocal interaction with neurons.36-38 Despite these results, HFS mediated neurotransmitter release is assumed to be of neuronal origin, and the possibility that the neurotransmitter may come from astrocytes has thus far been ignored. Here, we test the hypotheses that HFS results in glutamate and adenosine release, that this neurotransmitter release has the functional consequence of abolishing neural network oscillations, and that the neurotransmitter release may, at least in part, be of astrocytic origin.
Methods
In vitro thalamic slice preparation
The following experiments were approved by Institutional Animal Care and Use Committees in accordance with National Institute of Health guidelines for use of animals in teaching and research. For the preparation of slices, 3-4 month old male ferrets (Mustela putorious furo; Marshall Farms; North Rose, New York) were deeply anesthetized with sodium pentobarbital (30-40mg/kg) and killed by decapitation. The forebrain was rapidly removed, and the hemispheres were separated with a midline incision. Four hundred micron thick slices were cut using a vibratome (Ted Pella, Inc.) in the sagittal plane. During the preparation of slices, the tissue was placed in a solution (5° C) in which NaCl was replaced with sucrose while maintaining an osmolarity of 307mOsm to increase tissue viability.39 Slices were placed in an interface style recording chamber (Fine Sciences Tools) maintained at 34 ± 1° C and allowed at least two hours to recover. The bath was perfused with artificial cerebrospinal fluid (aCSF) which contained (126mM NaCl; 2.5mM KCl; 1.2mM MgSO4; 1.25mM NaH2PO4; 2mM CaCl2; 26mM NaHCO3; 10mM dextrose and was aerated with 95% O2, 5% CO2 to a final pH of 7.4. For the first 20 minutes of perfusion, the bathing medium contained an equal mixture of aCSF and the sucrose-substituted solution. Glutamate electrochemistry in slices were performed using glutamate biosensors (Pinnacle Technology Inc., Lawrence, KS), as described below.
Electrophysiology
Electrical stimulation was achieved through placement of a bipolar stimulating electrode (FHC, Inc., Bowdoinham, ME, USA) that delivered the stimulation (100μs duration; 10-500μA amplitude; 100 - 120Hz frequency). Multi-unit extracellular neuronal action potential activity was obtained using tungsten micro electrodes (FHC, Inc., Bowdoinham, ME, USA). High frequency stimulation (10s duration, 100Hz, 100μs pulse width, 300μA) of the purified astrocytes was also performed with the stimulating and recording electrodes within ∼100 μM of each other. Mean values are given ± SEM. The data were analyzed using Chart (eDaq) on a Pentium style computer and figures were drawn using CorelDRAW (Corel).
Glutamate electrochemistry
Glutamate biosensors (Pinnacle Technology Inc., Lawrence, KS) were made of lengths of Teflon-coated platinum iridium (7%) wire (Pt-Ir, 0.25o.d., Medwire, Mount Vernon, NY).40 An anodized 0.05 mm Ag wire was wrapped on the Teflon coated Pt-Ir electrode to create an Ag/AgCl reference counter electrode. The sensing cavity was formed by stripping the Teflon coating from one end, revealing the bare Pt-Ir electrode (0.35mm and 1.0mm lengths). An interferent screening inner-membrane coated the bare Pt-Ir electrode, and the enzyme layer was formed over the inner-membrane by co-immobilizing glutamate oxidase and ascorbate oxidase with glutaraldehyde and bovine serum albumin (BSA). Glutamate biosensors were tested in 0.1M phosphate-buffered saline (PBS pH=7.4) for a minimum glutamate sensitivity of 300pA/uM and for insensitivity to ascorbate (response to 250uM ascorbate less than 0.5nA). Sensors that did not meet these criteria were rejected. Sensor length used with brain slices had a sensing region of ∼350um.
Adenosine Electrochemistry
Adenosine measurements were made using Wireless Instantaneous Neurotransmitter Concentration System (WINCS) which utilize fast-scan cyclic voltammetry (FSCV) at a polyacrylonitrile-based (T-650) carbon-fiber microelectrode (CFM).31 The method for using FSCV for adenosine measurement has previously been described in detail.29 The pseudo-color plot exhibits three-dimensional information; each x-, y-axis, and color gradient indicates time, potential waveform applied at the CFM and currents detected from the CFM, respectively. The potential waveform applied was from -0.4V to +1.5V and back at 400V/s every 100ms.
Primary astrocyte culture
Astrocyte cultures were prepared from the cortices of neonatal rats (1-3 day old) using the Worthington Papain Dissociation System (Worthington Biochemical Corporation, Lakewood, NJ) as per the method of Huettner and Baughman.41 Briefly, cortices of neonatal rats were dissected, treated with papain (20U/ml), dissociated by trituration and plated in 75cm2 flasks in Dulbecco's modified Eagle's medium supplemented with 10% charcoal-stripped FBS and 1% penicillin/streptomycin (100U/ml penicillin, 100μg/ml streptomycin). Cells were fed twice weekly until they reached confluence (Day 10-12 in vitro) at which point they were mechanically shaken for 1 hr on an orbital shaker to remove any remaining oligodendrocytes and microglia. Subsequently, cultures were treated with trypsin for 30 min at 37°C and re-plated into 24-well culture dishes. Resultant cultures were greater than 98% astrocytic as determined previously by flow cytometry.42 The cells were washed twice in PBS prior to inserting the stimulating and glutamate recording electrodes into the well containing ∼1.0 × 106 cells. Glutamate electrochemistry in astrocyte cultures were performed using glutamate biosensors (Pinnacle Technology Inc., Lawrence, KS), as described above.
Immunocytochemistry
Astrocytes on coverslips were washed three times in PBS and then fixed in 4% paraformaldehyde for 10min at room temperature. After rinsing again in PBS, coverslips were blocked in 10% normal goat serum for 30min followed by overnight incubation at 4°C with primary antibody (mouse anti-rat GFAP, 1:500, GA5 clone, Sigma). The following day, slides were washed in PBS and secondary antibody was applied for 2 hours (goat anti-mouse Alexa Fluor 555, 1:250, Molecular Probes). After a final wash, cells were post-fixed in acid-alcohol (95% ethanol, 5% glacial acetic acid) for 10 min, rinsed and mounted with VectaShield (Vector Laboratories), examined with an Olympus fluorescence microscope, and images were captured with a Q-Fire cooled camera.
Measurement of intracellular Ca2+
Calcium measurements were performed in order to monitor the ability of the electrical stimulation to evoke a wave of elevated calcium in astrocytes. [Ca2+] was measured by monitoring the fluorescence of the Ca2+ indicator fluo-4. This indicator was loaded into cells by incubation for 30min at room temperature in the AM ester derivative of the dye. Fluorescent excitation was provided using a 480DF10 band-pass filter (Omega Optical). For time-lapse image acquisition, the epifluorescence microscope was equipped with a cooled digital camera. For quantitative studies, the temporal dynamics in fluorescence were expressed as background-subtracted dF/Fo. Images presented in the figures have been spatially filtered with a low-pass filter.
Statistical analysis
Glutamate release data in Figure 6 is represented as mean ± SEM. Data was analyzed by one-way analysis of variance as appropriate. Individual group differences were ascertained by Tukey's Multiple Comparison Test. In all cases, p < 0.05 was considered significant.
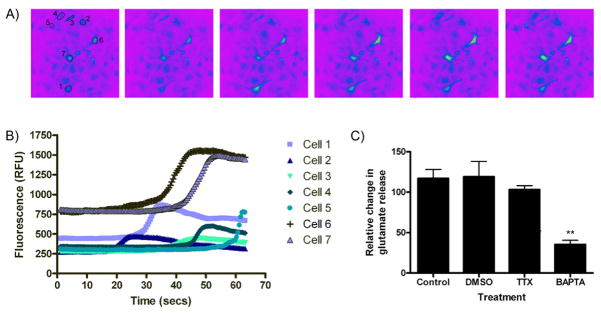
Figure 6.
High frequency stimulation induces Ca2+ release from cultures astrocytes. (A) High frequency stimulation induced calcium increases as indicated by the fluorescent dye, fluo-4. (B) The fluorescence intensity for several individual cells, designated in (A), was monitored following HFS and demonstrates transient increases in a unique cell-specific pattern. (C) Mean calcium increase monitored from multiple cells. Note HFS was applied for 10s beginning at 15s with the following parameters: 100Hz, 100μs pulse width, 300μA. (D) HFS-induced glutamate release was significantly decreased (**P<0.01 vs. control) following chelation of calcium with BAPTA-AM (50μM, 45min) suggesting a calcium-dependent mechanism of release.
Results
High Frequency Stimulation Abolishes Network Oscillations
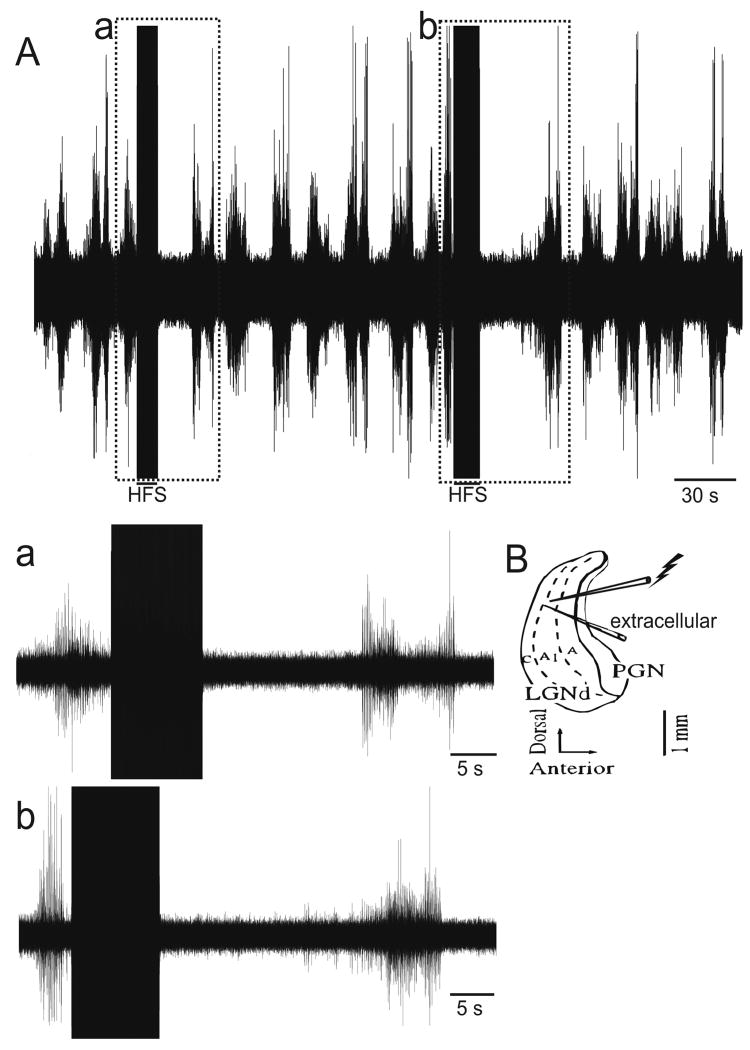
Extracellular recordings were made in the ferret perigeniculate nucleus (PGN) in vitro slice preparation, which generates spontaneous spindle waves (Figure 1; n=21). As demonstrated previously,23 extracellular recording revealed spontaneous activity (Figure 1A) due to spindle wave generation. HFS was applied by a stimulating electrode positioned within ∼100μm of the extracellular recording electrode (Figure 1B). In the immediate post-stimulation period, spindle wave activity was abolished as noted by the lack of neuronal activity (Figure 1a and b). The spindling returned in 20-25s, indicating that the neurons were not lesioned or damaged. Taken together, these results confirm that HFS abolishes spontaneous network oscillations in a reduced preparation such as the ferret thalamic slice.
Figure 1.
High Frequency Stimulation (HFS) abolishes network oscillations in the ferret thalamus (A) Spontaneous spindle oscillations in a ferret thalamic slice preparation. Boxed areas indicate segments during which HFS was applied (a,b). Enlargements of segments from the top trace during HFS (duration ∼3s, frequency 100Hz, intensity 300μA, pulse width 100μs) showing absence of spindle wave following HFS. Spontaneous spindle waves return 20-25s following HFS. (B) Schema of thalamic slice preparation demonstrating placement of stimulating and extracellular recording electrodes in the ferret lateral geniculate nucleus (LGN), within ∼100μm of each other.
High Frequency Stimulation Results in Glutamate and Adenosine Release
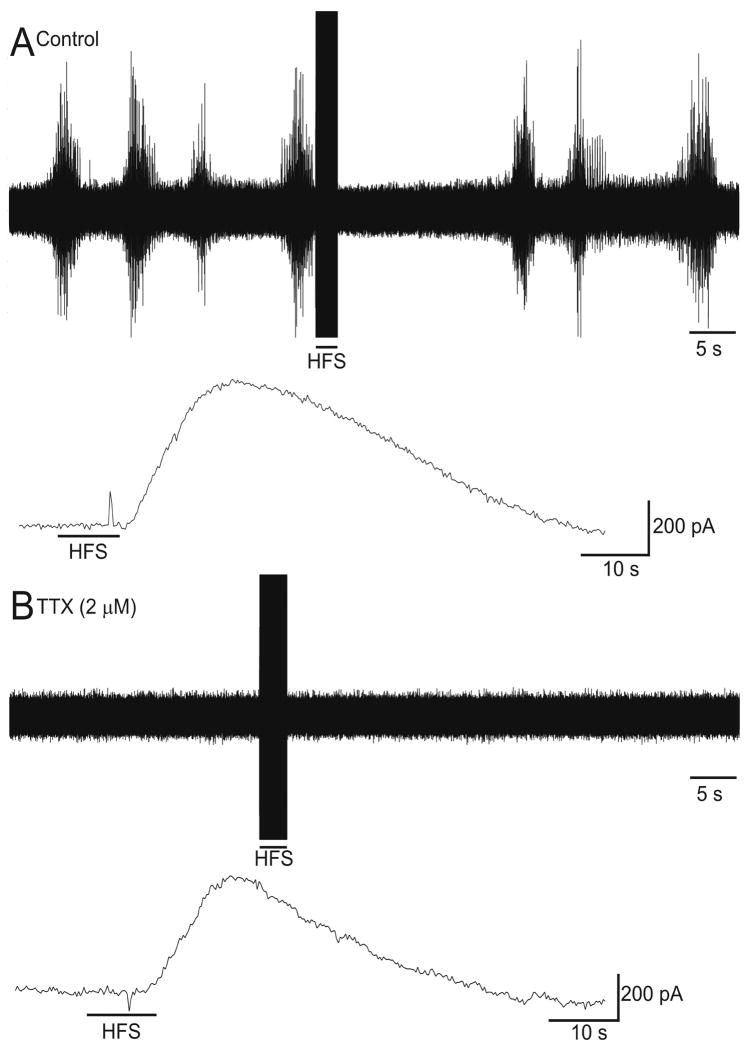
In order to determine the temporal relationship between the suppression of spindle waves and glutamate release, we measured extracellular activity and glutamate levels concurrently in ferret thalamic slices in vitro. As shown in Figure 2A, HFS (100Hz, 100μs pulse width, 300μA) abolished spontaneous spindle oscillations. Simultaneously, an increase in extracellular glutamate levels was measured (Figure 2A, lower trace; n=10). In order to determine if the glutamate release was of neuronal origin, we treated slices with the Na+ channel blocker tetrodotoxin, which abolished spindle waves in the extracellular recording as would be expected (Figure 2B, upper trace). Tetrodotoxin inhibits sodium channel-dependent axonal activity and should inhibit axonal-dependent exocytosis at the synapse, but TTX failed to block the majority of glutamate release induced by HFS (Figure 2B, lower trace), suggesting that either the glutamate is derived from a non-neuronal source (i.e. astrocytes) or glutamate is released independently of axonally driven synaptic glutamate release.
Figure 2.
HFS in the ferret thalamic slice results in glutamate release that is not blocked by the classic neuronal exocytosis inhibitor tetrodotoxin. HFS of the LGN (100Hz, 100μs pulse width, 300μA) for 10s in the in vitro ferret thalamic slice abolished spindles waves in the post-stimulation period (A, upper trace) and resulted in a concurrent increase in extracellular glutamate as measured by an enzyme-linked glutamate sensor (A, lower trace). After tetrodotoxin (TTX, 2μM) was applied to the bath, neuronal activity was abolished (B, upper trace); however, an increase in extracellular glutamate was still recorded by the glutamate sensor (B, lower trace). The stimulating electrode and the glutamate sensor electrode were positioned within ∼100μm of each other.
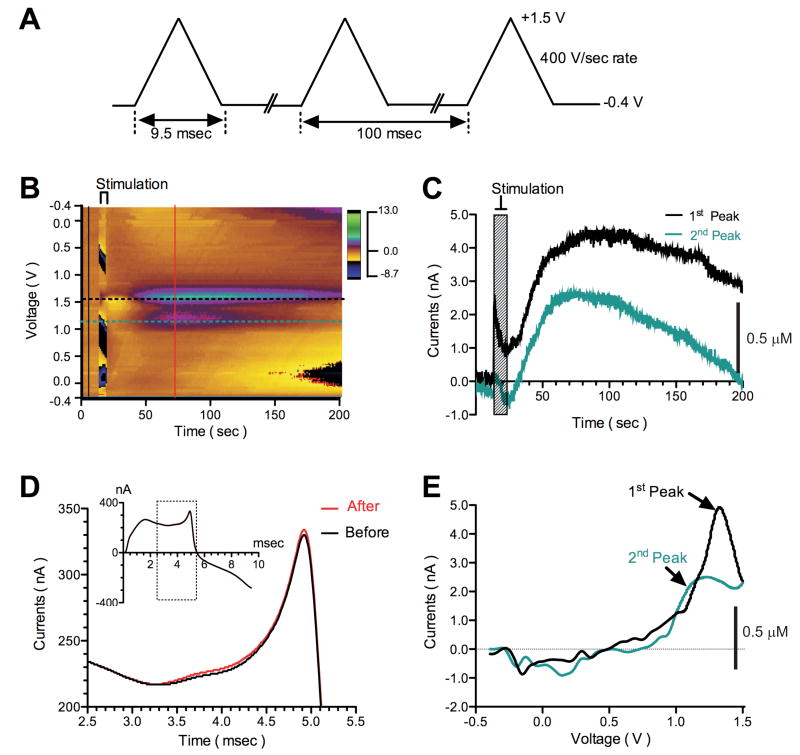
To test whether electrical stimulation could also result in adenosine efflux, we placed a stimulating electrode (125Hz, 100-200μA, 100μs pulse width) and the carbon-fiber microelectrode (CFM) within 200μm of the stimulating electrode in the ferret thalamic slice (n=3). Figure 3A demonstrates the voltage waveform for measuring adenosine, where the potential waveform applied was from -0.4V to +1.5V and back at 400V/s every 100ms. As seen in Figure 3B, the electrical stimulation-induced adenosine efflux was measurable using FSCV. The pseudo-color plot shows adenosine efflux results by HFS delivered for 5s. During FSCV, a large background current is present at the CFM by the rapid scanning of the potential (Figure 3B). Black and red solid vertical lines in Figure 3D demonstrate to the relationship between background charging currents (before and after HFS, respectively) and applied voltages. HFS increased this background current only slightly (red solid line, see also inset). The HFS evoked adenosine efflux exhibited unique voltammetric oxidation peaks, where the green oval surrounded by the purple ring (at approximately +1.5V) and the purple oval (at approximately +1.0V) represent the 1st and 2nd oxidation peaks of adenosine, respectively (Figures 3C and 3E). Cyclic-voltammograms were obtained by the background subtraction. As seen in Figure 3E, a representative cyclic voltammogram shows the 1st and 2nd oxidation peaks of adenosine at +1.5 and +1.0V, respectively.
Figure 3.
HFS induces adenosine efflux in the in vitro ferret thalamic slice. (A) Voltage waveform for measuring adenosine. (B) The pseudo-color plot shows adenosine efflux by HFS (125Hz, 200μA, for 5s, 100μs pulse width), detected with WINCS-based FSCV at a CFM. The axes and color gradient indicate the time, the potential waveform applied at the CFM, and the currents detected from the CFM, respectively. Adenosine exhibits unique voltammetric oxidation peaks; the green oval surrounded by the purple ring (at approximately +1.5V) and the purple oval (at approximately +1.0V) represent the 1st and 2nd oxidation peaks of adenosine, respectively. (C) Black and green dotted horizontal lines in (B) indicate 1st and 2nd adenosine oxidation peak currents versus time. This current-time plot clearly shows adenosine efflux induced by HFS. (D) Black and red solid vertical lines in (B) refer to the relationship between background charging currents (before and after HFS, respectively) and applied voltages. A large background current is present at the CFM (Black solid line, see also inset). HFS increased this background current only slightly (red solid line, see also inset). Black-lined box indicates the source of the data shown in the expanded area. (E) A cyclic-voltammogram was obtained by background subtraction (subtracted black line-indicated currents from red line-indicated currents in (D)). A representative cyclic voltammogram shows the 1st and 2nd oxidation peaks at +1.5 and +1.0V, respectively. The green line indicates the differential oxidation peak obtained by forward-going potential from -0.4 to +1.5V, and black line by reverse-going potential from +1.5 to -0.4V.
Possible astrocytic origin of HFS-induced neurotransmitter release
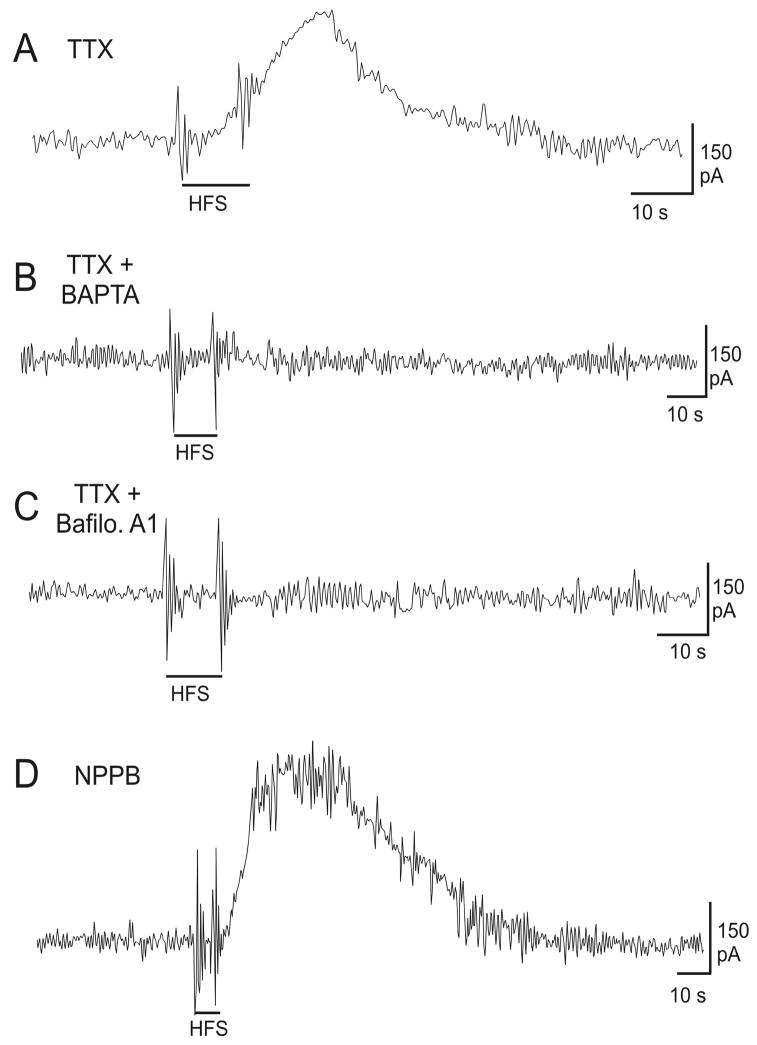
In order to determine if the HFS-induced glutamate release was originating from astrocytes, we treated slices simultaneously with TTX (to block neuronal activity, Figure 4A, n=6) and several compounds previously shown to inhibit astrocytic glutamate release. In addition, to establish whether the observed vesicular glutamate release was calcium-dependent, slices were treated with the calcium chelator 1,2-bis(2-aminophenoxy)-ethane-N,N,N′,N′-tetraacetic acid tetrakis acetoxymethyl ester (BAPTA-AM, 50μM, 1h). When slices were perfused with BAPTA-AM and TTX, HFS-induced glutamate release was completely eliminated suggesting a calcium-dependent mechanism (Figure 4B, n=5). Next, we employed Bafilomycin A1 (1μM, 45min), an inhibitor of vesicular glutamate transport, in the ferret slice. Figure 4C shows that Bafilomycin A1 also abolished glutamate release following HFS (n=5). Finally, in order to establish whether glutamate release may also occur through volume sensitive ion channels, we treated slices with NPPB (100μM, 10min). This treatment did not block HFS-induced glutamate release in our paradigm (Figure 4D, n=3). Overall, these results suggest that astocytes may contribute significantly to vesicular glutamate release following HFS in thalamic slices through a calcium-dependent mechanism.
Figure 4.
Glutamate measurements made in the in vitro ferret thalamic slice demonstrate that HFS-induced glutamate release is vesicular and calcium-dependent. (A) Tetrodotoxin (TTX) treatment has no effect on glutamate release. (B) HFS-induced glutamate release is suppressed by treatment with the calcium chelator, BAPTA-AM (50μM, 1h; n=3). (C) Bath application of Bafilomycin (1μM, 45min; n=9), an inhibitor of vacuolar type H+-ATPase, in addition to TTX, resulted in inhibition of glutamate release. (D) The anion-channel blocker NPPB (100μM, 10min), does not inhibit HFS-induced glutamate release in the thalamic slice. The stimulating electrode and the glutamate sensor electrode were positioned within ∼100μm of each other.
Response of Primary Astrocytes to HFS
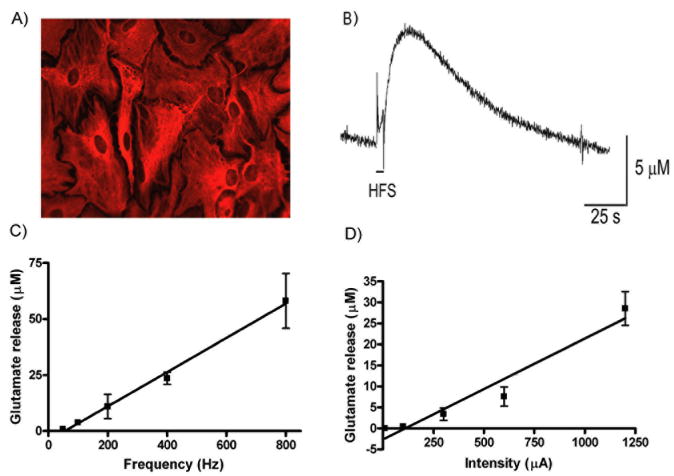
In order to determine if HFS leads to astrocytic glutamate release, we utilized a primary astrocytic culture that we have previously characterized by flow cytometry to show > 98% purity.42 Staining for glial fibrillary acidic protein (GFAP), a marker for astrocytes, is shown in Figure 5A. High frequency stimulation (10s duration, 100Hz, 100μs pulse width, 300μA) of the purified astrocytes increased extracellular glutamate (Figure 5B, n=5) and the glutamate level decreased to baseline upon cessation of stimulation. Of note, the HFS evoked glutamate release profile was similar to the glutamate release profile observed in the rat in vivo43 and in the ferret thalamic slices in vitro. Furthermore, this glutamate release was frequency (Figure 5C) and intensity-dependent (Figure 5D) suggesting it could be specifically modulated by varying stimulation parameters.
Figure 5.
HFS induces glutamate release in primary astrocyte cultures. (A) Immunocytochemistry of a primary astrocytic culture revealed that > 98% of the cells were astrocytes as shown by GFAP staining. (B) High frequency stimulation (HFS) of the astrocytic culture (100Hz, 100μs pulse width, 300μA) for 10 seconds resulted in an increase in extracellular glutamate as measured by an enzyme-linked glutamate sensor. Astrocytic glutamate release was both frequency (C) and intensity (D) dependent. The stimulating electrode and the glutamate sensor electrode were positioned within ∼100μm of each other.
To determine if HFS led to calcium-dependent glutamate release in astrocytic cultures, we monitored [Ca2+]i. As shown in Figure 6A, HFS led to a rise in astrocytic [Ca2+]i, that was stimulus-time locked. Figure 6B depicts individual cell alterations in intracellular calcium and highlights the fact that each astrocyte responds in a unique fashion to the electrical stimulation. While each cell displayed a specific temporal and maximal response, there was a time-dependent generalized increase in astrocytic intracellular calcium after HFS applied to the culture (Figure 6C). Finally, while TTX treatment had no effect on HFS-induced glutamate release in astrocytes (Figure 6D), BAPTA-AM (50μM, 45min) completely abolished it (n=7; **P<0.01 vs. control), suggesting that HFS induces glutamate release in a calcium-dependent fashion.
Discussion
The results of these experiments demonstrate that HFS elevates extracellular glutamate and adenosine levels in the ferret thalamic slice that was not inhibited by treatments that block axonally dependent exocytotic release of neurotransmitter from neurons. These results indicate that the neurotransmitter release evoked by DBS may be derived in part from a non-neuronal source. HFS of isolated astrocytes resulted in glutamate release that was similar to the profiles observed in vitro and in vivo previously,43 suggesting that astrocytic release may contribute to the increase in extracellular glutamate levels seen in these settings. Furthermore, the inhibition of HFS mediated glutamate release by BAPTA and Bafilomycin A1 in the presence of TTX suggests that the release of glutamate occurred from astrocytes through a Ca2+-dependent vesicular mechanism as suggested in previous studies.44 Whether similar mechanisms exist for release of adenosine from astrocytes requires further study. Functionally, HFS increased glutamate and adenosine and abolished synchronized oscillations in the ferret thalamic slices. Taken together, these results suggest that astrocytes may be an important target for the therapeutic effect of deep brain stimulation.
Functional consequences of HFS-mediated neurotransmitter release
We demonstrated that HFS applied to the thalamus results in both glutamate and adenosine release. Our results suggest that a component of the neurotransmitter release is of non-neuronal origin and is capable of abolishing spindle oscillations. Both tremor and absence seizures appear to involve abnormal oscillatory activity in the thalamus. The frequency of tremor in Parkinson's disease is 3-6Hz18 and the frequency of spike-wave discharges in absence seizures is 3Hz. Interestingly, HFS applied to the area containing tremor cells leads to immediate tremor arrest and a rapid reversal when stimulation ceases.19 Similarly, HFS applied to the thalamus in our experiments led to immediate glutamate and adenosine release, which decreased to pre-stimulation levels when stimulation ceased. Thus, the HFS-mediated glutamate and adenosine release may be important in the ability of DBS to abolish synchronized neural network oscillations such as those seen in tremor and seizures. Importantly, Bekar et al.26 have previously shown that thalamic DBS is associated with a marked increase in the local efflux of adenosine triphosphate (ATP) and extracellular accumulation of the neuromodulator catabolic product, adenosine. According to previous work,45 adenosine inhibits neuronal activities in the LGN and PGN thalamus by hyperpolarizing the thalamic neurons through the activation of potassium channels. In contrast to this, our previous work25 showed that glutamate antagonists reversed the HFS-induced reduction of spindles. This means glutamate, an excitatory neurotransmitter, reduces the spindles. These things strongly suggest that even though adenosine co-releases with glutamate, the adenosine effect would be masked by the glutamate effect. Overall, this implies that glutamate plays a more prominent role than adenosine in reducing spindles in this system. As a result, we further focused our studies on the characterization of glutamate release from astrocytes. It should be note, however, that the role of each of these neurotransmitter systems in the clinical efficacy of DBS in human patients still requires further investigation.
The Role of Astrocytic Neurotransmitter Release
Recent studies from various fields have highlighted the theory that astrocytes are active participants in synaptic communication.46 In order for efficient excitatory signaling to take place in the nervous system, the levels of synaptic glutamate need to be exquisitely controlled. Astrocytes are thought to perform the majority of this function by clearing synaptic glutamate via specific sodium-dependent glutamate transporters.47 Astrocytes play a more extensive and integral role in the regulation of glutamate than previously suspected.48 Most importantly, astrocytes respond to neuronal activity by exhibiting calcium waves,40,49 which have been shown to further lead to glutamate release.38 In addition, several studies have now established that astrocytes contain vesicular glutamate stores that can be triggered to undergo exocytosis by mechanical stimulation,50 activation of ionotropic glutamate receptors44 or activation metabotropic glutamate receptors.51 Finally, previous studies have also demonstrated that high frequency stimulation of hippocampal slices or astrocyte cultures can elicit astrocytic calcium waves.52,53 Of note, earlier work by Hassinger et al.49 showed that calcium responses to mechanical and electrical stimulation were not abolished by tetrodotoxin treatment suggesting that activation of neuronal Na+ channels was not required for astrocytic calcium responses. Similarly, in the current study TTX did not attenuate glutamate release in the ferret thalamic slice or in astrocyte cultures. These findings suggest that inhibition of neuronal activity alone was not sufficient to block astrocytic responses; however, treatment with the calcium chelator BAPTA or the vesicular pump inhibitor Bafilomycin was. In addition to a vesicular mechanism, astrocytes also release glutamate through volume sensitive channels after ATP stimulation.54 We did not observe an effect of the anion channel blocker, NPPB, on glutamate levels in thalamic slices after HFS. Therefore, we attribute the persistence of glutamate release during HFS after TTX treatment to a direct effect of HFS on astrocytes. It is, however, possible that HFS directly depolarizes axonal terminals and elicits neuronal vesicular release of glutamate even when axonal transmission has been inhibited by TTX. Were this the case, we would have expected much reduced glutamate release during HFS with TTX present (the more local effect of HFS directly on synaptic release of glutamate should be smaller than this local effect plus axonally driven vesicular release of glutamate). The persistence of almost identical patterns of glutamate release in the thalamic slice before and after TTX treatment favors a non-neuronal source for glutamate, and the bafilomycin sensitivity of HFS-dependent glutamate release from cultured astrocytes is consistent with this interpretation. Overall, we infer from these results that specific stimuli may recruit different gliotransmitter release mechanisms that may modify the spatio-temporal characteristics of subsequent neuronal responses.
Through the coordinated release of molecules such as glutamate and ATP, astrocytes orchestrate the excitation and inhibition of synaptic networks55 and therefore play a key role in neurological pathophysiology. For example, astrocytes are implicated in the excitation underlying seizures.56 In an elegant study by Tian et al.57 astrocytic calcium waves and glutamate release were linked to the neuronal depolarization underlying epileptic discharges and several classic antiepileptic agents were shown to directly inhibit astrocytic calcium signaling. Previous work by the same laboratory58 demonstrated an activity-dependent potentiation of inhibitory synaptic transmission that was dependent on astrocytic calcium signaling. In addition, Newman and Zahs59 determined, in a retinal eyecup preparation, that neuronal spike frequency decreased in a manner dependent on astrocytic calcium because the effect was blocked when intracellular calcium stores were depleted with thapsigargin. These authors further hypothesized that calcium-induced glial glutamate excited inhibitory interneurons to decrease ganglion cell firing via GABA and glycine release. Similarly, in the rat thalamus, glutamate antagonists blocked the ability of HFS to modulate spindle oscillations.24 The prime location of astrocytes at the tripartite synapse allows them to synchronize neuronal responses, an effect which has been linked to activation of extrasynaptic NMDA receptors.60 Taken together, these studies suggest that glutamate release from astrocytes can modulate synaptic transmission in a variety of ways that are likely dependent on the nature and intensity of the inducing stimulus as well as CNS location, among other factors.
The potential mechanism of deep brain stimulation in the treatment of various disorders remains an area of active research. Herein, we present evidence consistent with a role of astrocytic glutamate release in the modulation of neuronal responses important to the cessation of abnormal oscillatory activity. These findings are important because they underscore the role of bi-directional glial-synaptic signaling in the central nervous system during normal and pathological conditions. Future therapies aimed specifically at glia in addition to neurons, may therefore be useful in the treatment of complex neurological diseases.
Acknowledgments
The authors wish to thank Katarina Kristic and Anthony Paravati for their help with the in vitro studies.
Financial and Material Support: This work was supported by: NIH (K08 NS 52232 award to KHL), Mayo Foundation (2008-2010 Research Early Career Development Award for Clinician Scientists award to KHL).
References
- 1.Krack P, Batir A, Van Blercom N, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 2003 Nov 13;349(20):1925–1934. doi: 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]
- 2.Bronte-Stewart H. Surgical therapy for dystonia. Curr Neurol Neurosci Rep. 2003 Jul;3(4):296–305. doi: 10.1007/s11910-003-0006-0. [DOI] [PubMed] [Google Scholar]
- 3.Bereznai B, Steude U, Seelos K, Botzel K. Chronic high-frequency globus pallidus internus stimulation in different types of dystonia: a clinical, video, and MRI report of six patients presenting with segmental, cervical, and generalized dystonia. Mov Disord. 2002 Jan;17(1):138–144. doi: 10.1002/mds.1250. [DOI] [PubMed] [Google Scholar]
- 4.Kupsch A, Benecke R, Muller J, et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med. 2006 Nov 9;355(19):1978–1990. doi: 10.1056/NEJMoa063618. [DOI] [PubMed] [Google Scholar]
- 5.Koller WC, Pahwa PR, Lyons KE, Wilkinson SB. Deep brain stimulation of the Vim nucleus of the thalamus for the treatment of tremor. Neurology. 2000;55(12 Suppl 6):S29–33. [PubMed] [Google Scholar]
- 6.Lozano AM. Vim thalamic stimulation for tremor. Arch Med Res. 2000 May-Jun;31(3):266–269. doi: 10.1016/s0188-4409(00)00081-3. [DOI] [PubMed] [Google Scholar]
- 7.Hodaie M, Wennberg RA, Dostrovsky JO, Lozano AM. Chronic anterior thalamus stimulation for intractable epilepsy. Epilepsia. 2002 Jun;43(6):603–608. doi: 10.1046/j.1528-1157.2002.26001.x. [DOI] [PubMed] [Google Scholar]
- 8.Vonck K, Boon P, Goossens L, et al. Neurostimulation for refractory epilepsy. Acta Neurol Belg. 2003 Dec;103(4):213–217. [PubMed] [Google Scholar]
- 9.Gybels JM, Sweet WH. Neurosurgical treatment of persistent pain. Physiological and pathological mechanisms of human pain. Pain Headache. 1989;11:1–402. [PubMed] [Google Scholar]
- 10.Maciunas RJ, Maddux BN, Riley DE, et al. Prospective randomized double-blind trial of bilateral thalamic deep brain stimulation in adults with Tourette syndrome. J Neurosurg. 2007 Nov;107(5):1004–1014. doi: 10.3171/JNS-07/11/1004. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg BD, Malone DA, Friehs GM, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006 Nov;31(11):2384–2393. doi: 10.1038/sj.npp.1301165. [DOI] [PubMed] [Google Scholar]
- 12.Gabriels L, Cosyns P, Nuttin B, Demeulemeester H, Gybels J. Deep brain stimulation for treatment-refractory obsessive-compulsive disorder: psychopathological and neuropsychological outcome in three cases. Acta Psychiatr Scand. 2003 Apr;107(4):275–282. [PubMed] [Google Scholar]
- 13.Lipsman N, Neimat JS, Lozano AM. Deep brain stimulation for treatment-refractory obsessive-compulsive disorder: the search for a valid target. Neurosurgery. 2007 Jul;61(1):1–11. doi: 10.1227/01.neu.0000279719.75403.f7. discussion 11-13. [DOI] [PubMed] [Google Scholar]
- 14.Malone DA, Jr, Dougherty DD, Rezai AR, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009 Feb 15;65(4):267–275. doi: 10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005 Mar 3;45(5):651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Schlaepfer TE, Cohen MX, Frick C, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008 Jan;33(2):368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- 17.Deep-Brain Stimulation for Parkinson's Disease Study Group. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson's disease. N Engl J Med. 2001 Sep 27;345(13):956–963. doi: 10.1056/NEJMoa000827. [DOI] [PubMed] [Google Scholar]
- 18.Lenz FA, Normand SL, Kwan HC, et al. Statistical prediction of the optimal site for thalamotomy in parkinsonian tremor. Mov Disord. 1995 May;10(3):318–328. doi: 10.1002/mds.870100315. [DOI] [PubMed] [Google Scholar]
- 19.Benabid AL, Pollak P, Gao D, et al. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J Neurosurg. 1996 Feb;84(2):203–214. doi: 10.3171/jns.1996.84.2.0203. [DOI] [PubMed] [Google Scholar]
- 20.McCormick DA, Contreras D. On the cellular and network bases of epileptic seizures. Annu Rev Physiol. 2001;63:815–846. doi: 10.1146/annurev.physiol.63.1.815. [DOI] [PubMed] [Google Scholar]
- 21.von Krosigk M, Bal T, McCormick DA. Cellular mechanisms of a synchronized oscillation in the thalamus. Science. 1993 Jul 16;261(5119):361–364. doi: 10.1126/science.8392750. [DOI] [PubMed] [Google Scholar]
- 22.Lee KH, Blaha CD, Harris BT, et al. Dopamine efflux in the rat striatum evoked by electrical stimulation of the subthalamic nucleus: potential mechanism of action in Parkinson's disease. Eur J Neurosci. 2006 Feb;23(4):1005–1014. doi: 10.1111/j.1460-9568.2006.04638.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee KH, Hitti FL, Shalinsky MH, Kim U, Leiter JC, Roberts DW. Abolition of spindle oscillations and 3-Hz absence seizurelike activity in the thalamus by using high-frequency stimulation: potential mechanism of action. J Neurosurg. 2005 Sep;103(3):538–545. doi: 10.3171/jns.2005.103.3.0538. [DOI] [PubMed] [Google Scholar]
- 24.Anderson T, Hu B, Pittman Q, Kiss ZH. Mechanisms of deep brain stimulation: an intracellular study in rat thalamus. J Physiol. 2004 Aug 15;559(Pt 1):301–313. doi: 10.1113/jphysiol.2004.064998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee KH, Chang SY, Roberts DW, Kim U. Neurotransmitter release from high-frequency stimulation of the subthalamic nucleus. J Neurosurg. 2004 Sep;101(3):511–517. doi: 10.3171/jns.2004.101.3.0511. [DOI] [PubMed] [Google Scholar]
- 26.Bekar L, Libionka W, Tian GF, et al. Adenosine is crucial for deep brain stimulation-mediated attenuation of tremor. Nat Med. 2008 Jan;14(1):75–80. doi: 10.1038/nm1693. [DOI] [PubMed] [Google Scholar]
- 27.Kohno T, Asai S, Iribe Y, Hosoi I, Shibata K, Ishikawa K. An improved method for the detection of changes in brain extracellular glutamate levels. J Neurosci Methods. 1998 Jun 1;81(1-2):199–205. doi: 10.1016/s0165-0270(98)00041-7. [DOI] [PubMed] [Google Scholar]
- 28.Oldenziel WH, Beukema W, Westerink BH. Improving the reproducibility of hydrogel-coated glutamate microsensors by using an automated dipcoater. J Neurosci Methods. 2004 Dec 30;140(1-2):117–126. doi: 10.1016/j.jneumeth.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 29.Swamy BE, Venton BJ. Subsecond detection of physiological adenosine concentrations using fast-scan cyclic voltammetry. Anal Chem. 2007 Jan 15;79(2):744–750. doi: 10.1021/ac061820i. [DOI] [PubMed] [Google Scholar]
- 30.Cechova S, Venton BJ. Transient adenosine efflux in the rat caudate-putamen. J Neurochem. 2008 May;105(4):1253–1263. doi: 10.1111/j.1471-4159.2008.05223.x. [DOI] [PubMed] [Google Scholar]
- 31.Agnesi F, Tye SJ, Bledsoe JM, et al. Wireless Instantaneous Neurotransmitter Concentration System-based amperometric detection of dopamine, adenosine, and glutamate for intraoperative neurochemical monitoring. J Neurosurg. 2009 Oct;111(4):701–711. doi: 10.3171/2009.3.JNS0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bezzi P, Vesce S, Panzarasa P, Volterra A. Astrocytes as active participants of glutamatergic function and regulators of its homeostasis. Adv Exp Med Biol. 1999;468:69–80. doi: 10.1007/978-1-4615-4685-6_6. [DOI] [PubMed] [Google Scholar]
- 33.Schousboe A, Fosmark H, Hertz L. High content of glutamate and of ATP in astrocytes cultured from rat brain hemispheres: effect of serum withdrawal and of cyclic AMP. J Neurochem. 1975 Dec;25(6):909–911. doi: 10.1111/j.1471-4159.1975.tb04429.x. [DOI] [PubMed] [Google Scholar]
- 34.Halassa MM, Florian C, Fellin T, et al. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009 Jan 29;61(2):213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Innocenti B, Parpura V, Haydon PG. Imaging extracellular waves of glutamate during calcium signaling in cultured astrocytes. J Neurosci. 2000 Mar 1;20(5):1800–1808. doi: 10.1523/JNEUROSCI.20-05-01800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cotrina ML, Lin JH, Lopez-Garcia JC, Naus CC, Nedergaard M. ATP-mediated glia signaling. J Neurosci. 2000 Apr 15;20(8):2835–2844. doi: 10.1523/JNEUROSCI.20-08-02835.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perea G, Araque A. Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes. J Neurosci. 2005 Mar 2;25(9):2192–2203. doi: 10.1523/JNEUROSCI.3965-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bezzi P, Carmignoto G, Pasti L, et al. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998 Jan 15;391(6664):281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- 39.Aghajanian GK, Rasmussen K. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse. 1989;3(4):331–338. doi: 10.1002/syn.890030406. [DOI] [PubMed] [Google Scholar]
- 40.Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994 Jun 30;369(6483):744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- 41.Huettner JE, Baughman RW. Primary culture of identified neurons from the visual cortex of postnatal rats. J Neurosci. 1986 Oct;6(10):3044–3060. doi: 10.1523/JNEUROSCI.06-10-03044.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tawfik VL, Lacroix-Fralish ML, Bercury KK, Nutile-McMenemy N, Harris BT, Deleo JA. Induction of astrocyte differentiation by propentofylline increases glutamate transporter expression in vitro: heterogeneity of the quiescent phenotype. Glia. 2006 Aug 15;54(3):193–203. doi: 10.1002/glia.20365. [DOI] [PubMed] [Google Scholar]
- 43.Lee KH, Kristic K, van Hoff R, et al. High-frequency stimulation of the subthalamic nucleus increases glutamate in the subthalamic nucleus of rats as demonstrated by in vivo enzyme-linked glutamate sensor. Brain Res. 2007 Aug 8;1162:121–129. doi: 10.1016/j.brainres.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 44.Pasti L, Zonta M, Pozzan T, Vicini S, Carmignoto G. Cytosolic calcium oscillations in astrocytes may regulate exocytotic release of glutamate. J Neurosci. 2001 Jan 15;21(2):477–484. doi: 10.1523/JNEUROSCI.21-02-00477.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pape HC. Adenosine promotes burst activity in guinea-pig geniculocortical neurones through two different ionic mechanisms. J Physiol. 1992 Feb;447:729–753. doi: 10.1113/jphysiol.1992.sp019026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bezzi P, Volterra A. A neuron-glia signalling network in the active brain. Curr Opin Neurobiol. 2001 Jun;11(3):387–394. doi: 10.1016/s0959-4388(00)00223-3. [DOI] [PubMed] [Google Scholar]
- 47.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001 Sep;65(1):1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 48.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999 May;22(5):208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 49.Hassinger TD, Atkinson PB, Strecker GJ, et al. Evidence for glutamate-mediated activation of hippocampal neurons by glial calcium waves. J Neurobiol. 1995 Oct;28(2):159–170. doi: 10.1002/neu.480280204. [DOI] [PubMed] [Google Scholar]
- 50.Araque A, Li N, Doyle RT, Haydon PG. SNARE protein-dependent glutamate release from astrocytes. J Neurosci. 2000 Jan 15;20(2):666–673. doi: 10.1523/JNEUROSCI.20-02-00666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bezzi P, Gundersen V, Galbete JL, et al. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci. 2004 Jun;7(6):613–620. doi: 10.1038/nn1246. [DOI] [PubMed] [Google Scholar]
- 52.Porter JT, McCarthy KD. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J Neurosci. 1996 Aug 15;16(16):5073–5081. doi: 10.1523/JNEUROSCI.16-16-05073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dani JW, Chernjavsky A, Smith SJ. Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron. 1992 Mar;8(3):429–440. doi: 10.1016/0896-6273(92)90271-e. [DOI] [PubMed] [Google Scholar]
- 54.Takano T, Kang J, Jaiswal JK, et al. Receptor-mediated glutamate release from volume sensitive channels in astrocytes. Proc Natl Acad Sci U S A. 2005 Nov 8;102(45):16466–16471. doi: 10.1073/pnas.0506382102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fellin T, Pascual O, Haydon PG. Astrocytes coordinate synaptic networks: balanced excitation and inhibition. Physiology (Bethesda) 2006 Jun;21:208–215. doi: 10.1152/physiol.00161.2005. [DOI] [PubMed] [Google Scholar]
- 56.Fellin T, Haydon PG. Do astrocytes contribute to excitation underlying seizures? Trends Mol Med. 2005 Dec;11(12):530–533. doi: 10.1016/j.molmed.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 57.Tian GF, Azmi H, Takano T, et al. An astrocytic basis of epilepsy. Nat Med. 2005 Sep;11(9):973–981. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998 Dec;1(8):683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- 59.Newman EA, Zahs KR. Modulation of neuronal activity by glial cells in the retina. J Neurosci. 1998 Jun 1;18(11):4022–4028. doi: 10.1523/JNEUROSCI.18-11-04022.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004 Sep 2;43(5):729–743. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]