Abstract
Patients with the genomic instability syndrome Fanconi anemia (FA) commonly develop progressive bone marrow failure and have high risk of cancer. Certain manifestations of the disease suggest that the FA immune system is dysfunctional and may contribute to the pathogenesis of both bone marrow failure and malignancies. Here we have investigated inflammation and innate immunity in FA hematopoietic cells using mice deficient in Fanconi complementation group C gene (Fancc). We demonstrate that Fancc-deficient mice exhibit enhanced inflammatory response and are hypersensitive to lipopolysaccharide-induced septic shock as a result of hematopoietic suppression. This exacerbated inflammatory phenotype is intrinsic to the hematopoietic system and can be corrected by the re-expression of a wild-type FANCC gene, suggesting a potential role of the FANCC protein in innate immunity. Lipopolysaccharide-mediated hematopoietic suppression requires two major inflammatory agents, tumor necrosis factor-α and reactive oxygen species. In addition, lipopolysaccharide-induced excessive accumulation of reactive oxygen species in Fancc−/− bone marrow cells overactivates the stress kinase p38 and requires prolonged activation of the c-Jun N-terminal kinase. Our data implicate a role of inflammation in pathogenesis of Fanconi anemia and bone marrow failure diseases in general.
Keywords: Apoptosis, Cytokines, Fanconi anemia, Hematopoiesis, Inflammation
Introduction
Fanconi anemia (FA) is a genetic disorder characterized by progressive bone marrow failure and cancer predisposition (1, 2). Somatic cell fusion studies show that FA is genetically heterogeneous, with at least 12 complementation groups identified thus far (3). The genes encoding the groups A (FANCA), B (FANCB), C (FANCC), D1 (FANCD1/BRCA2), D2 (FANCD2), E (FANCE), F (FANCF), G (FANCG/XRCC9), J (BACH1/BRIP1), L (FANCL/PHF9), M (FANCM/Hef), and N (PALB2) have been cloned (4–17). Studies on the function of these FA proteins have shown that they function to protect against genotoxic stress by forming complexes with each other (18–20) and that they protect hematopoietic cells from apoptotic cues by both suppressing apoptotic signaling pathways and enhancing survival signaling pathways (21–26). Functional inactivation of any of these proteins leads to clinical phenotypes of FA and cellular phenotype of genomic instability (1, 2).
The most important clinical features of FA are hematological, as the progressive bone marrow failure represents the hallmark of the disease and leading cause of patient death (1). It has been proposed that bone marrow failure in aplastic anemia including FA results from hematopoietic stem cell depletion (27). Since FA hematopoietic progenitor and stem cells have high rates of stress-induced apoptosis and reduced repopulating ability (21, 28, 29), the FA proteins are believed to play important roles in the maintenance of hematopoiesis. Indeed, consistent with the observations that the cells derived from FA patients are intolerant of oxidative stress, it has been reported that FA proteins, particularly the complementation group C (FANCC) protein, play a crucial role in oxidative stress signaling in a variety of cell types including hematopoietic cells (30–36). More recently, cytokine hypersensitivity of FA hematopoietic cells to apoptotic cues has been proposed as a major factor in the pathogenesis of bone marrow failure in three FA mouse models (Fanca−/−, Fancc−/−, and Fancg−/−) (37, 38).
The cytokine tumor necrosis factor α (TNF-α) is a vital member of the multifunctional TNF superfamily and has important roles in immunity and cellular remodeling as well as influencing apoptosis and cell survival (reviewed in 37). The biological activities of TNF-α are mediated by two structurally related but functionally distinct receptors, designated the p55 and p75 TNF-α receptors. The activation TNF-α receptor triggers a complex array of signaling events, giving rise to the pleiotropic effects of TNF-α on cells (39). TNF-α is a major mediator of inflammation and plays a key role in the pathogenesis of such inflammatory diseases as rheumatoid arthritis, Crohn’s disease, and psoriasis, as demonstrated by the successful treatment of such conditions with antibodies to TNF-α or with a soluble TNF-α receptor fusion protein (40). With respect to abnormal hematopoiesis, it is well recognized that TNF-α is involved in many disease situations including anemia, myelodysplasia, and leukemia (41). TNF-α exerts many of its biological effects through the activation of the MAPK stress signaling cascade including JNK, p38MAPK, and ERK (42), as well as the NF-κB transcription factor (43). Signal transduction triggered by TNF-α also induces an increase in intracellular reactive oxygen species (ROS). It is established that TNF-α-induced ROS production involves the JNK and NF-κB pathways (44, 45).
Patients with FA have abnormally high levels of proinflammatory TNF-α, low levels of natural killer cell activity and reduced lymphocyte counts, and are highly susceptible to bacterial infection (46–57). While these clinical manifestations suggest that the FA immune system may be dysfunctional, the mechanism(s) underlying these abnormalities and the signaling pathways involved in FA innate immunity have not been elucidated. In this study we used mice deficient in FA complementation group C gene (Fancc) to investigate inflammation and innate immunity in Fancc−/− hematopoietic cells. We demonstrate that Fancc-deficient mice exhibits enhanced inflammatory response and are extremely sensitive to lipopolysaccharide (LPS)-induced septic shock as a result of hematopoietic suppression. Our data implicate a role of inflammation in pathogenesis of FA and bone marrow failure diseases in general.
Material and Methods
Mice and treatments
WT and Fancc−/− mice were generated by interbreeding the heterozygous Fancc+/− mice (provided by Dr. Manuel Buchwald, University of Toronto; 58). The genetic background of the mice is C57BL/6 (CD45.2+). Fancc−/−Tnfa−/− mice were generated by mating Fancc+/− with Tnfa−/− (Jackson Laboratory, Bar Harbor, ME), followed by mating of F1 heterozygous siblings. All of the mice were used at approximately 10–14 weeks of age. For septic shock studies, mice were injected intraperitoneally (i.p.) with a single dose of 25 mg/kg lipopolysaccharide (LPS; Sigma Chemical, St. Louis, MO). For other studies, LPS was administered at a dose of 1 mg/kg. When indicated, N-acetyl-L-cysteine (NAC; 100 mg/kg; Sigma Chemical, St. Louis, MO), p38 inhibitor SB203580 (20 mg/kg; Calbiochem, San Diego, CA), or JNK inhibitor SP600125 (15 mg/kg; Calbiochem, San Diego, CA) was administered by i.p. injection 30 min before and after LPS injection. For anti-TNF-α treatment, TNF-α-treatment mice were injected with 20 μg of an anti-mouse TNF-α neutralizing antibody or control IgG (R&D Systems) 30 min after LPS injection. All experimental procedures conducted in this study were approved by the Institutional Animal Care and Use Committee of Cincinnati Children’s Hospital Medical Center.
Flow cytometric analysis of HSC and lineage differentiation
Low-density BM mononuclear cells were suspended in FACS buffer (0.1% FCS in 0.02% sodium azide) and incubated with the indicated antibodies on ice for 30 min, followed by two washes. Data were collected on a FACSCalibur (BD Biosciences). Antibodies used were (Miltenyi Biotec Inc.), Sca-1-PE, c-kit-APC, B220/CD3e, Gr-1/CD11b, Ter119 (all from BD PharMingen, San Diego, CA).
Retroviral vectors and infection
The full-length human FANCC cDNA (GeneBank sequence accession number NM000136) was amplified by polymerase chain reaction (PCR), using Pfu DNA polymerase (Stratagene) and subcloned into the NotI site of retroviral vector SFβ91 (a gift from Dr. Christopher Baum, Cincinnati Children’s Hospital Medical Center) to create SFβ91-FANCC. The retroviral vectors HA-MKK6-KM and Flag-MKK7-KM have been reported elsewhere (42). Retroviral supernatant was collected at 36 hours, 48 hours and 60 hours respectively after transfection. BM mononuclear cells were plated onto Retronectin (Takara-Shuzo)-coated non-tissue culture 6- or 12-well plates and pre-stimulated for 2 days in IMDM medium containing 20% FCS, 100 ng/mL SCF, 20 ng/mL IL-6, and 50 ng/mL Flt-3L (Peprotech). Cells were then exposed to the retroviral supernatant for 3 hours at 37°C in the presence of 4 μg/mL polybrene (Sigma Chemical, St. Louis, MO). Cells were centrifuged at 600 ×g for 45 minutes. Infection was repeated 2 times and infection efficiency was assessed by the detection of green fluorescent protein (GFP)-positive cells by flow cytometry.
Clonogenic progenitor assays
BM progenitor cells were cultured in a 35 mm tissue culture dish in 4 ml of semisolid medium containing 3 ml of MethoCult M 3134 (Stem Cell Technologies) and the following growth factors: 100 ng/mL SCF, 10 ng/mL IL-3, 100 ng/mL granulocyte colony-stimulating factor (G-CSF), and 4 U/mL erythropoietin (Peprotech). On day 10 after plating, the colony number was counted and photographed. Colony growth results were expressed as mean (of triplicate plates) ± S.D of three independent experiments.
BM transplantation
Age-matched congenic B6.SJL-PtrcaPep3b/BoyJ (B6.BoyJ; CD45.1+) mice (Jackson Laboratories, Bar Harbor, ME) were used as transplant recipients. These mice were lethally irradiated (9.5 Gy, 110 cGy/min, 137Cs gamma rays) and injected intravenously with 2 × 106 test BM mononuclear cells (CD45.2+), mixed with 1 × 106 competitor cells (BoyJ; CD45.1+). Donor-derived repopulation in recipients was assessed by the proportion of leukocytes in peripheral blood that expressed the CD45.2 marker by flow cytometry. Short- and long-term engraftment and multi-lineage repopulation analysis of donor cells were performed at 4- and 16-week post-transplantation, respectively.
Apoptosis Assay
Cells were stained with annexin V and 7-AAD using BD ApoAlert Annexin V kit (BD PharMingen) in accordance with the manufacturer’s instructions. Apoptosis was analyzed by quantification of Annexin V positive cell population by flow cytometry.
Determination of ROS production
BM cells were incubated with CM-H2DCFDA (Molecular Probe) in the dark for 15 min at 37°C. After washing, the cells were analyzed by flow cytometry using a FACSCalibur (BD Biosciences). Data were analyzed by using the CellQuest program (BD Biosciences).
Histology and Immunohistochemistry
During necropsy, organs were removed, preserved in formalin, and then embedded in paraffin blocks. Sections were stained with hematoxylin and eosin (H&E). For immunohistochemistry, paraffin sections were deparaffinized, rehydrated, incubated in 0.1 mM sodium citrate (pH 6.0), washed and incubated with peroxidase blocking reagent (Vector Laboratories, VectaStain Elite ABC kit). After washing in PBS, the slides were incubated with the primary antibody HNE (11-S; Alpha Diagnostic International, San Antonio, TX) or myeloperoxidase (MPO; Cat. # RB-373-A0; LAB Vision Corporation, Fremout, CA). Following three PBS washes, slides were incubated with secondary antibody and then detected with the VectaStain Elite ABC reagents.
NF-κB activation
Nuclear protein extracts were prepared from BM cells using a Transfactor Extraction kit (BD Biosciences). Nuclear extracts were incubated with DNA specific for the NF-κB consensus sequence, and the DNA binding activity of NF-κB was measured using a Transfactor kit (BD Biosciences).
Immunoblotting
Cells were solubilized in RIPA lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 1% Nonidet NP-40) containing a cocktail of protease inhibitors (Calbiochem, San Diego, CA). Equal amounts of protein were separated on a 10% SDS-polyacrylamide gel electrophoresis gel, transferred to a nitrocellulose membrane, and blotted with antibodies against p65 or IκBα (Santa Cruz Biotechnology), phosphorylated p38, phosphorylated JNK, the pan kinases p38 and JNK (all from Cell Signaling), and β-actin (Sigma).
Real-time PCR analysis
Total RNA was prepared with RNeasy kit (Qiagen) following the manufacturer’s procedure. Following treatment with RNase-free DNase, RNA was reverse transcribed using Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA). Real-time PCR was performed on a ABI PRISM 7700 sequence detection system (Applied Biosystems, Foster City, CA) with SYBR green PCR master mix (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions. Samples were normalized to the level of GAPDH mRNA, and the relative expression levels were determined by the standard curve method. Primer sequences are available upon request.
Serum levels of cytokines
The serum levels of inflammatory cytokines were measured using the enzyme linked immunoadsorbent assays (ELISA) kits from R & D Systems.
Statistics
Data were analyzed statistically using a two-tail Student’s t test or Kaplan-Meier survival analysis. The level of statistical significance stated in the text was based on the p values. p<0.05 was considered statistically significant.
Results
LPS suppresses hematopoietic functions
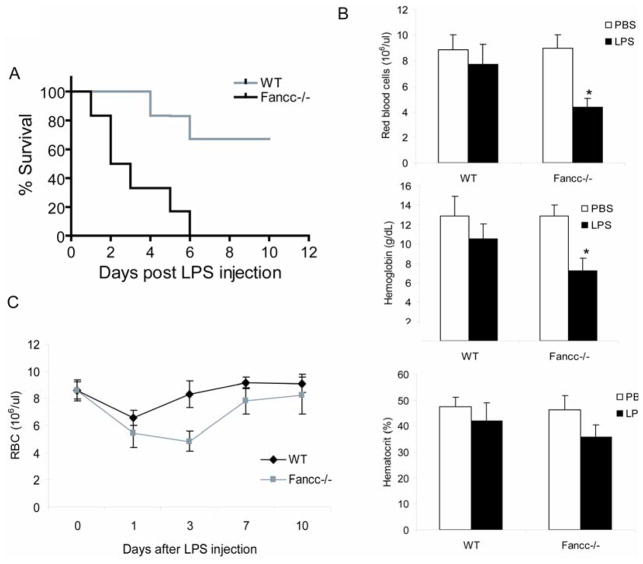
Since mutations in the FA complementation group C gene FANCC repress clonal growth of hematopoietic progenitor cells and disruption of the Fancc gene, in mice, renders hematopoietic progenitor cells hypersensitive to the pro-apoptotic effect of IFN-γ and TNF-α (21–23, 26, 37, 38, 46, 49), we studied innate immune response in mice deficient for the Fancc gene. Fancc−/− mice were extremely sensitive to septic shock by lipopolysaccharide (LPS), an immunological endotoxin from Gram-negative bacteria (Fig 1A). LPS-treated Fancc−/− mice exhibited cytopenia, as evidenced by decrease in red cell counts, hemoglobin, and hematocrit values (Fig. 1B). Consistent with this, analysis of BM of LPS-treated Fancc−/− mice revealed a decrease in BM cellularity accompanied by extensive areas of necrosis (data not shown). In another set of experiments we analyzed hematopoietic recovery in mice injected with LPS. We found that Fancc−/− mice recovered from hemo-suppression slowly, taking as much twice the time as WT mice to reach the pre-treatment level (Fig. 1C).
Figure 1.
Impaired hematopoietic recovery and hypersensitivity to septic shock in LPS-treated Fancc-deficient mice. (A) Fancc−/− mice are hypersensitive to LPS-induced septic shock. Kaplan-Meier survival curves are shown for a single dose (25mg/kg) of intraperitoneally injected LPS. Experiments were repeated three times, each with 6 animals (total 18 mice) for Wild-type (WT) or Fancc−/− mice (10–14 weeks old). The log rank test indicated a statistically significant difference (p < 0.01) in survival between the two genotype groups. (B) LPS reduces blood counts in Fancc-deficient mice. WT or Fancc−/− mice were injected intraperitoneally (i.p.) with LPS in PBS at a single dose of 1 mg/kg. Number of red blood cells and concentrations of hemoglobin and hematocrit in peripheral blood were determined on day 3 after the last dose of LPS. (C) Fancc-deficiency compromises hematopoietic recovery in vivo following LPS treatment. WT or Fancc−/− Mice were injected i.p. with LPS (1 mg/kg). Red blood cell counts were conducted for 10 days after injection. Data are expressed as mean ± SD of two independent experiments, each with 6 animals (total 12 mice).
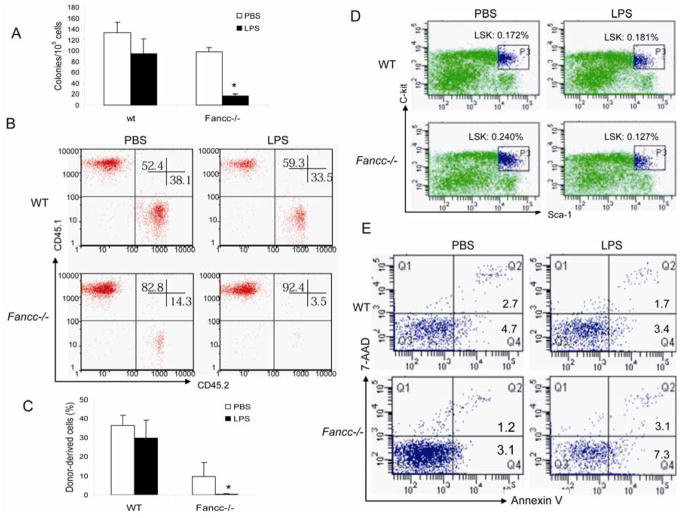
We next asked if LPS suppressed hematopoietic progenitor activity. As shown in Fig. 2A, total number of colonies formed by BM cells from LPS–treated Fancc−/− mice was more than 3-fold lower than that of WT mice. We then performed BM transplantation to evaluate the in vivo effect of LPS on hematopoietic reconstitution. Analysis of short-term (4-week) engraftment demonstrated that LPS significantly compromised the BM repopulating ability of BM cells isolated from Fancc−/− mice injected with a single dose of the endotoxin (Fig. 2B). More dramatically, LPS almost completely disabled long-term hematopoietic reconstitution of Fancc−/− BM cells with few donor-derived cells detected in the peripheral blood cells of the recipients at 16 weeks after transplantation (Fig. 2C). However, analysis of the composition of lymphoid (B220/CD3e), myeloid (Gr-1/CD11b) and erythroid (Ter119) showed that LPS did not compromise multiple lineage reconstitution (data not shown). We next asked whether LPS reduced stem cell pool or induced excessive cell death in Fancc−/− hematopoietic progenitor cells. While LPS did not cause notable change stem cell pool in WT mice, approximately 2-fold reduction of BM stem/progenitor (lineage-negative, Sca-1-positive, c-kit-positive; LSK) cell frequencies was observed in LPS-treated Fancc−/− mice compared to untreated littermate controls (Fig. 2D). In addition, BM stem/progenitor cells of LPS-treated Fancc−/− mice showed increase in apoptosis (7-AADlowAnnexin V+) and necrosis (7-AADhighAnnexin V+) (Fig. 2E). Collectively, these results indicate that LPS suppresses hematopoietic reconstitution in Fancc−/− mice, at least in part, through induction of stem/progenitor cell death.
Figure 2.
LPS suppresses HSC/progenitor activity. (A) LPS inhibits colony-forming activity of Fancc−/− BM progenitor cells. WT or Fancc−/− Mice were injected intraperitoneally (i.p.) with PBS or LPS at a single dose of 1 mg/kg. 24 h later, low-density BM mononuclear cells were isolated and subjected to clonogenic assay. Data shown represent the number (mean ± SD) of total colonies from three independent experiments. *Statistical significance between PBS-injected and LPS-injected samples at P < .05. (B) LPS impairs repopulating ability of Fancc-deficient BM cells. 2 × 106 BM mononuclear cells isolated from control (PBS-injected) or LPS-injected mice (CD45.2+) were transplanted, along with 1 × 106 competitor cells from B6.BoyJ mice (CD45.1+), into lethally irradiated recipient (B6.BoyJ) mice and short-term engraftment was evaluated at 4 weeks post-transplantation. Shown is representative flow cytometric presentation of three independent experiments, each with 6 animals (total 18 mice). Numbers in the corners indicate percent of events in that quadrant. (C) LPS impairs self-renewal capacity of Fancc−/− HSCs. Long-term engraftment was evaluated at 16 weeks post-transplantation. Data represent mean ± SD of three independent experiments, each with 6 animals (total 18 mice). *Statistical significance between PBS-injected and LPS-injected groups at P < .05. (D) Effect of LPS on hematopoietic stem cell frequency. WT or Fancc−/− Mice were injected i.p. with PBS or LPS in PBS at a dose of 1 mg/kg. The mice were then sacrificed 24 h later and BM mononuclear cells were prepared and stained with antibodies against lineage markers (Lin) and Sca-1-PE and c-kit-APC. The cells were then analyzed by flow cytometry to obtain fractions representing HSCs. The frequencies of LSK cells as a percentage of total BM mononuclear cells are indicated. (E) LPS-induced BM LSK cell death of Fancc-deficient mice. BM cells from PBS- or LPS-treated WT and Fancc−/− mice were stained with lineage maker antibodies along with Sca-1-PE and c-kit-APC antibodies, and then with annexin V. Percentages of apoptosis in the LSK population were analyzed by flow cytometry. Numbers in the quadrants indicate percent of cells labeled for 7-AADlowAnnexin V+ or 7-AADhighAnnexin V+.
LPS-induced inflammation is exacerbated in Fancc−/− mice
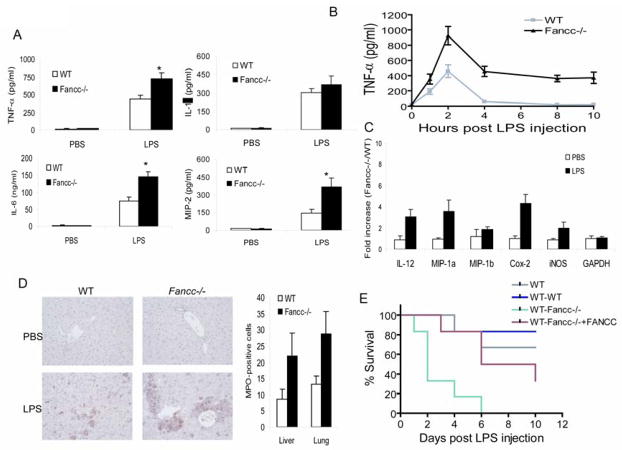
The hypersensitivity of Fancc−/− mice to LPS-induced septic shock prompted us to investigate whether these mice display an enhanced inflammatory response. LPS activates hematopoietic innate immune cells such as macrophages and dendritic cells to produce large amounts of TNF-α, IL-1β and IL-6, which then mediate a cascade of inflammatory responses leading to endotoxic shock (59). We thus determined the levels of these inflammatory cytokines in serum from mice treated with LPS. Fancc−/− mice showed significant increase in the levels of TNF-α, IL-6 and macrophage inflammatory protein 2 (MIP-2) compared to WT littermate controls (Fig 3A). Albeit statistically insignificant, increased serum levels of IL-1β were also observed in treated Fancc−/− mice. Importantly, TNF-α levels in serum of LPS-treated Fancc−/− mice peaked at 2 h and were maintained at high levels for 10 h post LPS injection (Fig 3B). This was in contrast to WT littermate controls, where TNF-α levels dropped off markedly after peaking at 2h. In addition, real-time quantitative PCR analysis of the Fancc−/− BM cells cultured in the presence of LPS showed prolonged high expression of other known pro-inflammatory genes, including macrophage inflammatory protein (MIP)-1α, cyclooxygenase 2 (COX2), and inducible nitric oxide synthase (iNOS) (Fig. 3C). Consistent with this, liver and lung tissues of LPS-treated Fancc−/− mice showed increased immunoreactivity for myeloperoxidase (MPO), a major marker of inflammation, suggesting enhanced neutrophil recruitment and local inflammation (Fig 3D). These results suggest that long-lasting high levels of proinflammatory cytokines may mediate prolonged and exacerbated inflammatory response in Fancc−/− mice, which might account for the hypersensitivity of these animals to LPS- induced septic shock.
Figure 3.
Exacerbated inflammatory responses in hematopoietic cells of LPS-treated Fancc−/− mice. (A) Fancc-deficient mice release significantly more proinflammatory cytokines than WT animals following LPS challenge. WT or Fancc−/− Mice were injected i.p. with PBS or LPS in PBS at a dose of 1 mg/kg. The mice were then sacrificed 2 h later and serum was assessed for the indicated cytokines by ELISA. Data are expressed as mean ± SD of two independent experiments with n=3 animals per group for each experiment. *Statistical significance between PBS-injected and LPS-injected samples at P < 0.05. (B) Kinetics of circulating TNF-α release induced by LPS. WT or Fancc−/− Mice were injected i.p. with LPS (1 mg/kg), and serum samples were collected at the indicated time points. TNF-α concentration was determined by ELISA. (C) Expression of genes encoding inflammatory proteins was examined in BM cells of LPS-treated mice. WT or Fancc−/− Mice were injected i.p. with PBS or LPS in PBS at a dose of 1 mg/kg. The mice were then sacrificed 2 h later and low-density BM mononuclear cells were isolated, total RNA was prepared, and gene expression was analyzed by real-time PCR. Data are presented as the fold increase in mRNA expression in Fancc−/− BM cells relative to WT cells, which was given an arbitrary level of 1.0 for each gene. Results are means ± SD of three independent experiments. (D) Paraffin-embedded liver sections of PBS- or LPS-treated WT and Fancc−/− mice were stained with antibody against the neutrophil marker myeloperoxidase (MPO) (40×). Right panel: MPO-positive cell counts in liver and lung of LPS-treated mice. Numbers are represented as mean ± SD of three experiments. (E) Kaplan-Meier survival curves for radiation chimera mice. WT mice were lethally irradiated and reconstituted with WT (WT-WT), Fancc−/− (WT-Fancc−/−), or FANCC-transduced Fancc−/− (WT-Fancc−/−+FANCC) BM cells. Ten weeks posttransplantation, recipient mice were injected with a single dose (25mg/kg) of LPS. Experiments were repeated two times, each with 6 recipients (total 12 mice per group).
To determine whether the exacerbated inflammatory phenotype observed in LPS-treated Fancc−/− mice was intrinsic to the hematopoietic system, we reconstituted lethally irradiated congenic C57BL/6 mice with bone marrow from Fancc−/− mice or WT littermate controls. Bone marrow transplantation was also performed with Fancc−/− bone marrow cells that had been functionally corrected with a DNA repair-proficient FANCC gene, as tested by mitomycin C sensitivity assay (1, 2). Mice receiving Fancc−/− bone marrow were significantly more susceptible to LPS-induced septic shock than those transplanted with WT marrow (Fig. 3E). These Fancc−/− bone marrow transplanted recipients also displayed abnormal high levels of serum TNF-α, IL-6 and MIP-2 (Table 1). Remarkably, complementation of Fancc−/− bone marrow cells with the DNA repair-proficient FANCC gene significantly mitigated these deregulated innate immune responses. Thus, the exacerbated inflammatory phenotype seen in Fancc−/− mice is due to an intrinsic defect in the hematopoietic system.
Table 1.
Inflammatory cytokines in recipient mice
| Donor | Treatment | Serum cytokines | ||
|---|---|---|---|---|
| TNF-α | IL-6 | MIP-2 | ||
| WT | PBS | 41.2±4.1 | 9.3±2.5 | 31.2±5.7 |
| LPS | 303±52.6 | 49.3±7.4 | 134±36.8 | |
| Fancc−/− | PBS | 28.8±4.4 | 11.3±3.2 | 34.8±3.8 |
| LPS | 485±62.8 | 76.4±23.6 | 225±38.3 | |
| Fancc−/− +FANCC | PBS | 29.6±6.4 | 14.3±4.1 | 39.2±7.3 |
| LPS | 342±47.6 | 55.4±18.6 | 168±29.0 | |
TNF-α and MIP-2, pg/ml; IL-6, ng/ml
Hematopoietic suppression by LPS is mostly mediated by TNF-α
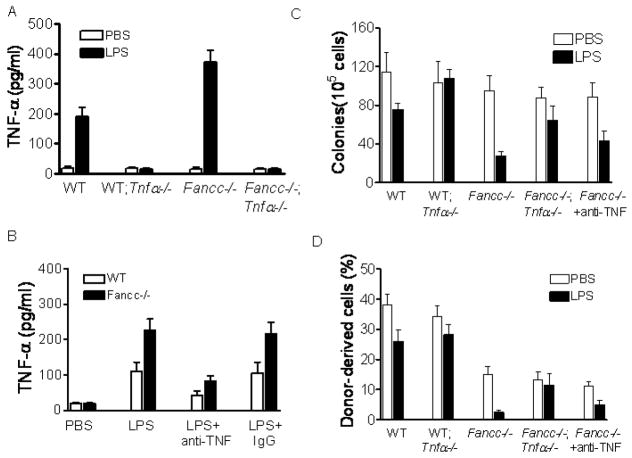
TNF-α is a major pro-inflammatory cytokine whose production is strongly induced by LPS (39, 59). We proposed that LPS-induced TNF-α was responsible in part for the observed hematopoietic suppression. LPS administration to WT and Fancc−/− mice resulted in robust TNF-α induction, whereas this induction was ablated in mice deficient for the Tnfa gene (Fig. 4A). Administration of an anti-TNF-α antibody 30 min after LPS injection effectively neutralized most of the circulating TNF-α (Fig. 4B). To determine whether LPS-mediated hematopoietic suppression required TNF-α, we examined the proliferative potential of hematopoietic progenitors using two established assays: clonogenic progenitor assay and competitive hematopoietic repopulation. Indeed, LPS mediated progenitor growth inhibition through TNF-α, as ablation of TNF-α production in WT (Tnfa−/−) or Fancc−/− (Fancc−/−Tnfa−/−) mice rescued progenitor growth (Fig. 4C). Neutralization of circulating TNF-α with the anti-TNF-α antibody also reduced, albeit less profound, the inhibitory effect of LPS in progenitor growth (Fig. 4C). Similar results were obtained with BM reconstitution assay, in which cells from LPS-injected WT Tnfa−/− mice were able to reconstitute irradiated BM as efficiently as those from untreated counterparts (Fig. 4D). Again, Tnfa deficiency in Fancc−/− mice also abrogated the negative effect of LPS on BM repopulation (Fig. 4D). We conclude that TNF-α is an important mediator of LPS-induced hematopoietic suppression.
Figure 4.
TNF-α is the mediator of LPS-induced hematopoietic suppression. (A) WT, Fancc−/− mice or their littermates deficient for Tnfa were injected with PBS or LPS (1 mg/kg). The mice were then sacrificed 2 h later and serum was assessed for the indicated cytokines by ELISA. Data are expressed as mean ± SD of two independent experiments with n=3 animals per group for each experiment. (B) Neutralization of circulating TNF-α. WT or Fancc−/− mice were injected with PBS or LPS (1 mg/kg), and 30 min later the mice were injected with 20 μg of an anti-mouse TNF-α neutralizing antibody (LPS+anti-TNF) or control IgG (LPS+IgG). The mice were then sacrificed 2 h later and serum was assessed for TNF-α by ELISA. Data are expressed as mean ± SD of two independent experiments with 3 animals per group for each experiment. (C) Neutralization or ablation of LPS-induced TNF-α rescues progenitor growth. WT, Fancc−/− mice or their littermates deficient for Tnfa were injected with PBS or a single dose of LPS (1 mg/kg). Mice were injected with 20 μg of a TNF-α neutralizing antibody 30 min after LPS injection. 24 h after LPS injection, BM cells were isolated and subjected to clonogenic assay. Data shown represent the number (mean ± SD) of total number of colonies from three independent experiments. (D) Neutralization or ablation of LPS-induced TNF-α restores HSC self-renewal ability. 2 × 106 BM mononuclear cells isolated from the mice described in (C) were transplanted, along with 1 × 106 competitor cells from B6.BoyJ mice (CD45.1+), into lethally irradiated recipient mice and long-term engraftment was evaluated at 16 weeks post-transplantation. Data represent mean ± SD of three independent experiments with n=3 recipients per group for each experiment.
The role of TNF-α-induced ROS in hematopoietic suppression by LPS
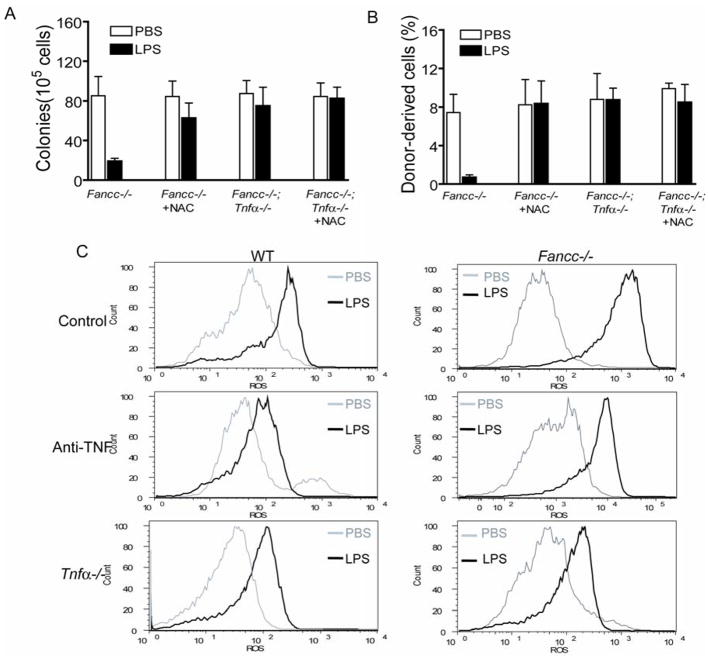
One mechanism by which LPS mediates inflammatory effect is to increase the cellular oxidative stress (60), which has been known to be very harmful to hematopoietic cells particularly to those from Fanconi patients (1). We suspected that TNF-α-induced ROS was the source of LPS-generated cellular oxidative stress responsible in part for the observed hematopoietic suppression. To test this notion, we pretreated the LPS–injected mice with the ROS scavenger N-acetyl-L-cysteine (NAC). NAC rescued both progenitor growth (Fig 5A) and repopulating ability (Fig. 5B) of the BM cells from LPS-injected WT and Fancc−/− mice. Notably, NAC did not have any effect on progenitor growth or hematopoietic reconstitution of BM cells from those mice that could not produce TNF-α (Fig 5A, B), indicating that TNF-α is the source of LPS-generated oxidative stress.
Figure 5.
TNF-α-induced ROS production is responsible for hematopoietic suppression by LPS. (A) ROS scavenger NAC or ablation of LPS-induced TNF-α rescues progenitor growth. WT, Fancc−/− mice or their littermates deficient for Tnfa were injected with PBS or a single dose of LPS (1 mg/kg). Mice were injected with N-acetyl-L-cysteine (NAC; 100 mg/kg) 30 min before and after LPS injection. 24 h after LPS injection, BM cells were isolated and subjected to clonogenic assay. Data shown represent the number (mean ± SD) of total number of colonies from three independent experiments. (B) Anti-oxidant NAC or ablation of LPS-induced TNF-α restores HSC self-renewal ability. 2 × 106 Fancc−/− BM mononuclear cells isolated from the mice described in (A) were transplanted, along with 1 × 106 competitor cells from B6.BoyJ mice (CD45.1+), into lethally irradiated recipient mice and long-term engraftment was evaluated at 16 weeks post-transplantation. Data represent mean ± SD of three independent experiments with 3 recipients per group for each experiment. (C) ROS production. WT, Fancc−/− mice or their littermates deficient for Tnfa were injected with PBS or a single dose of LPS (1 mg/kg). Mice were injected with 20 μg of a TNF-α neutralizing antibody 30 min after LPS injection. 24 h after LPS injection, BM cells were isolated and labeled with CM-H2DCFDA followed by flow cytometry.
To directly ask whether LPS-generated ROS required TNF-α, we stained BM cells freshly isolated from LPS-injected mice with CM-H2 DCFDA, a cell-permeable fluorescence dye that reacts to a broad spectrum of ROS. LPS induced substantially more ROS in BM of Fancc−/− mice than in WT mice (Fig. 5C). TNF-α was required for this ROS production, as administration of the neutralizing anti-TNF-α antibody or deletion of the Tnfa gene in these mice significantly reduced ROS accumulation (Fig. 5C).
Excessive ROS accumulation in Fancc−/− BM cells overactivates p38 and requires prolonged JNK activation
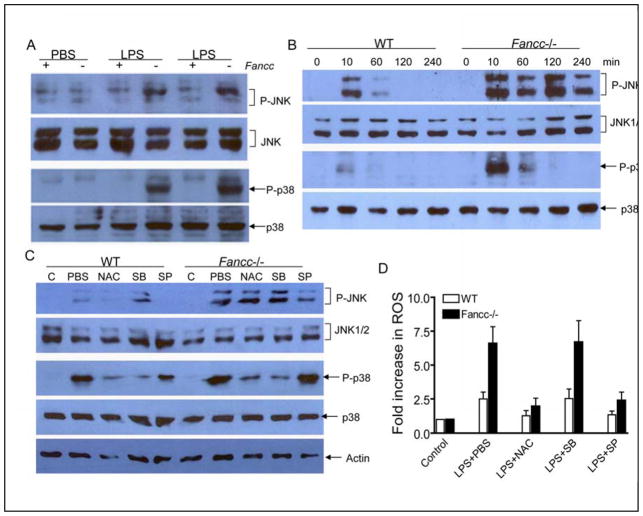
We further investigated the molecular mechanism that leads to excessive ROS production in Fancc−/− BM cells. It has been reported that activation of the transcription factor NF-κB inhibits TNF-α-induced ROS production (43, 44). We wondered if NF-κB activity might be defective in Fancc−/− BM cells. LPS-induced NF-κB activation was indistinguishable in BM cells freshly isolated from LPS-injected WT and Fancc−/− mice, as assessed by the degradation of NF-κB inhibitor IκBα and the DNA-binding activity of NF-κB (data not shown). However, we observed overactivation of the p38 kinase in BM cells isolated from Fancc−/− mice sacrificed 1 h after LPS injection (Fig. 6A). Strikingly, LPS-induced JNK activation was persistent in BM cells isolated from LPS-injected Fancc−/− mice at this time point but disappeared in their WT counterparts (Fig. 6A). Kinetics study with in vitro culture of isolated BM cells in the presence of LPS further demonstrated enhanced p38 and prolonged JNK activation in Fancc−/− cells (Fig. 6B). Thus, LPS-induced p38 overactivation or/and prolonged JNK activation may be responsible for the difference in oxidative stress conditions in WT and Fancc−/− mice.
Figure 6.
Deregulated activation of stress kinases p38 and JNK by LPS-induced ROS in Fancc-deficient BM cells. (A) WT or Fancc−/− mice were injected with PBS or a single dose of LPS (1 mg/kg). The mice were then sacrificed 1 h after LPS injection, and protein extracts were prepared from low-density BM mononuclear cells and analyzed by immunoblotting with anti-phospho-JNK, anti-JNK, anti-phospho-p38, and anti-p38 antibodies. Results with one pair of PBS- and two pairs of LPS-treatment mice are shown. (B) Fancc-deficient BM cells exhibit higher p38 and prolonged JNK activation induced by LPS. Low-density BM mononuclear cells from WT or Fancc−/− mice were cultured in the presence of LPS (100 μg/ml) for the indicated time periods, and protein extracts were prepared from low-density BM mononuclear cells and analyzed by immunoblotting with anti-phospho-JNK, anti-JNK, anti-phospho-p38, and anti-p38 antibodies. (C) ROS activates p38 but not JNK kinase. WT or Fancc−/− mice were injected with a single dose of LPS (1 mg/kg). NAC (100 mg/kg), p38 inhibitor SB203580 (20 mg/kg), JNK inhibitor SP600125 (15 mg/kg) or PBS was administered to LPS-treated mice by i.p. injection 30 min before and after LPS injection. Control (C) groups received PBS only. The mice were then sacrificed 1 h after LPS injection, and protein extracts were prepared from low-density BM mononuclear cells and analyzed by immunoblotting with anti-phospho-JNK, anti-JNK, anti-phospho-p38, anti-p38, and anti-actin antibodies. (D) Prolonged JNK activation by LPS in Fancc-deficient BM cells increases ROS production. WT or Fancc−/− mice were injected with a single dose of LPS (1 mg/kg). NAC (100 mg/kg), p38 inhibitor SB203580 (20 mg/kg), JNK inhibitor SP600125 (15 mg/kg), or PBS was administered to LPS-treated mice by i.p. injection 30 min before and after LPS injection. Control (C) groups received PBS only. The mice were then sacrificed 1 h after LPS injection, and BM cells were isolated and labeled with CM-H2DCFDA followed by flow cytometry. Data represent mean ± SD of two independent experiments.
The excessive levels of oxidative stress observed in BM cells from LPS-injected Fancc−/− mice may be resulted from p38 overactivation or prolonged JNK activation. To distinguish between these possibilities, we treated LPS-injected mice with antioxidant NAC, p38 inhibitor SB203580, or JNK inhibitor SP600125. Administration of SB203580 effectively abrogated LPS-induced p38 activation in both WT and Fancc−/− mice (Fig. 6C). Notably, NAC significantly reduced p38 activity in LPS-injected Fancc−/− mice. LPS-induced activation of p38 was also reduced by NAC treatment in WT mice, albeit less dramatic. However, p38 inhibitor did not affect LPS-induced ROS production in these mice (Fig. 6D). In contrast, the JNK inhibitor, which suppressed LPS-induced JNK activation in both WT and Fancc−/− mice (Fig. 6C), dramatically reduced LPS-induced ROS production in Fancc−/− mice and, to a less degree, in WT mice (Fig. 6D). Interestingly, NAC administration to LPS-injected mice had no effect on JNK activation (Fig. 6C).
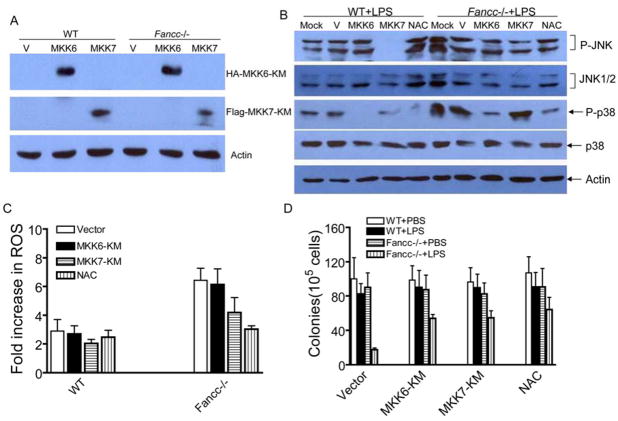
To unambiguously define the contribution of p38 and JNK to LPS-induced ROS production and hematopoietic suppression, we inhibited specifically the kinases using dominant-negative mutants of their upstream activators, MKK6-KM (for p38) and MKK7-KM (for JNK). We expressed these mutants in BM mononuclear cells by retroviral gene transfer. Expression of the mutant proteins was verified by western blotting (Fig. 7A). The functionality of the dominant-negative mutants was demonstrated by their ability to inhibit the activity of the endogenous kinases (Fig. 7B). Consistent with the in vivo results (Fig. 6), inhibition of JNK by MKK7-KM but not p38 by MKK6-KM reduced LPS-induced ROS production (Fig. 7C). Clonogenic progenitor assay demonstrated that inhibition of either p38 or JNK was sufficient to rescue progenitor growth (Fig. 7D). Collectively, these results suggest that the MAPK kinases p38 and JNK play distinct roles in LPS-mediated hematopoietic suppression in Fancc−/− cells: prolonged JNK activation increases ROS accumulation leading to p38 overactivation.
Figure 7.
p38 and JNK play distinct roles in LPS-mediated hematopoietic suppression in Fancc-deficient BM cells. (A) Low-density BM cells from WT or Fancc−/− mice were transduced with retroviruses carrying vector alone, MKK6-KM, or MKK7-KM, and protein extracts were prepared and analyzed by immunoblotting with anti-HA (for MKK6-KM), anti-Flag (for MKK7-KM), and anti-actin antibodies. (B) Inhibition of LPS-induced p38 and JNK activation by overexpression of dominant negative MKK6 and MKK7 in BM cells. Cells described in (A) were cultured in the presence of LPS (100 μg/ml) for 10 min, and protein extracts were prepared and analyzed by immunoblotting with anti-phospho-JNK, anti-JNK, anti-phospho-p38, anti-p38, and anti-actin antibodies. NAC (100 μM) was added to the culture before LPS treatment. (C) Inhibition of JNK but not p38 reduced LPS-induced ROS production. Cells described in (A) were cultured in the presence of LPS (100 μg/ml) for 10 min, and labeled with CM-H2DCFDA followed by flow cytometry. NAC (100 μM) was added to the culture before LPS treatment. Data represent mean ± SD of three independent experiments. (D) Inhibition of LPS-induced JNK or p38 activation partially rescues progenitor growth. Cells described in (A) were cultured in the presence of LPS (100 μg/ml) for 10 min, and subjected to clonogenic assay described in Materials and Methods. NAC (100 μM) was added to the culture before LPS treatment. Data represent mean ± SD of three independent experiments.
Discussion
The present study demonstrates that mice deficient in the Fanconi gene Fancc exhibited enhanced inflammatory response and were extremely sensitive to LPS-induced septic shock. Inflammation as a consequence of the activation of innate immune system is essential for host survival yet has the potential for devastating consequences if not precisely controlled or resolved. The fact that patients with FA frequently show overproduced TNF-α in their serum and plasma (46–49) suggest that these patients may consistently be subjected to inflammatory cues. LPS-treated Fancc-deficient mice not only exhibit elevated levels of TNF-α secreting into serum, but the production of ROS is also enhanced. Overproduction of these two major inflammatory agents results in deregulation of the stress kinases p38 and JNK, leading to hematopoietic suppression, heightened septic shock and animal mortality. Our study thus implicates a functional deficiency in FA innate immunity.
The observed prolonged induction of TNF-α in Fancc-deficient mice suggested that the inflammatory cytokine is responsible in part for LPS-induced hematopoietic suppression and subsequent septic shock. Indeed, the in vivo findings presented here clearly demonstrate that deletion of Tnfa gene or neutralization of TNF-α in LPS-treated Fancc−/− mice effectively rescued progenitor growth and hematopoietic reconstitution. This finding underscores pathogenic roles of TNF-α in clinical manifestations in bone marrow failure-related diseases including FA (39–41). Bone marrow cells from FA patients show overproduction of TNF-α (46–49). Overproduction of TNF-α has also been implicated in other pathological conditions related to chronic inflammation, cancer, and aging (39–41, 61). Our finding also highlights a regulatory role of TNF-α in hematopoiesis. TNF-α has been shown to decrease cytokine-driven hematopoietic stem cell (HSC) expansion, interferes with HSC self-renewal, and compromises the ability of HSC to reconstitute hematopoiesis (62–66). Therefore, overproduction of TNF-α may play a pivotal role in pathogenesis of certain bone marrow failure diseases through inhibiting hematopoiesis.
We demonstrated that ROS, another primary inflammatory agent, was overproduced in LPS-treated Fancc−/− mice, which was dependent on TNF-α and largely responsible for LPS-induced hematopoietic suppression. Direct, compelling evidence has suggested that TNF-α-mediated cytotoxicity is due to the induced ROS production in a variety of cell types (44, 45). Since FA cells including BM hematopoietic cells are hypersensitive to oxidative stress (1, 30–36), it is conceivable that ROS-mediated inflammation contributes to the observed hematopoietic suppression and toxicity in LPS-treated Fancc−/− mice. Alternatively, ROS can cause oxidative DNA damage. Recent studies have shown that the production of ROS by TNF-α at inflammatory sites causes DNA damage (39, 67–69). We believe that in FA cells, in which the repair of oxidative DNA damage may be deficient or whose DNA may be susceptible to oxidative attack, the ability of TNF-α-induced ROS to damage DNA is a potential mechanism through which ROS mediate their effects on the inflammatory process.
An extensive body of evidence has suggested that FA cells are in an in vivo pro-oxidant state (36) and that the FA proteins play important roles in cellular responses to oxidative stress. For example, the FANCC protein has been found to interact with NADPH cytochrome P450 reductase and glutathione S-transferase P1-1 (30, 31), two enzymes involved in either triggering or detoxifying reactive intermediates including ROS. In addition, mice with combined deficiencies of the anti-oxidative enzyme, Cu/Zn superoxide dismutase and Fancc genes demonstrated a defective hematopoiesis (32). Another FA protein, FANCG, interacts with cytochrome P450 2E1 (33) and mitochondrial peroxiredoxin-3 (70), suggesting a possible role of FANCG in protection against oxidative DNA damage. Significantly, Saadatzadeh et al. (34) recently showed that oxidant hypersensitivity of Fancc−/− cells was due to an altered redox regulation and ASK1 hyperactivation. Moreover, oxidative stress induces complex formation by two major FA proteins, FANCA and FANCG (36). Our present finding that LPS/TNF-α-generated ROS induces hematopoietic suppression in Fancc−/− mice corroborates a critical role for oxidative stress in FA phenotype and disease progression.
Perhaps more importantly, inflammatory ROS may contribute to the progression of cancer-related bone marrow failure diseases like FA. ROS have been associated with the initiation or aggravation of diverse pathological states including cancers (71–76). In FA, the disease typically progresses from anemia to myelodysplasia (MDS) then to acute myeloid leukemia (AML) (1, 2). High levels of ROS production in patients with anemia would be consistent with the observation implicating ROS production as a mechanism of TNF-α-induced cell death (1, 30–36). ROS are known to cause carcinogenic mutations, which may promote clonal evolution in MDS and leukemic transformation.
Intriguingly, our study indicates that LPS induced sustained activation of JNK and p38 overactivation in Fancc−/− mice. This deregulation of the stress kinases required LPS-induced ROS production and sustained JNK activation correlated with high ROS production in bone marrow cells from LPS-treated Fancc−/− mice. It has recently reported that TNF-α induced a high level of p38 activation in FA cells (26, 77). Consistent with previous observations that abnormal p38 and JNK activation is predominantly pro-apoptotic (78–80), we showed that LPS-induced activation of these two stress kinases in Fancc−/− mice inhibited progenitor proliferation. Notably, we found that LPS-induced ROS could activate p38 but required prolonged JNK activation. Simultaneous and quantitatively balanced induction of JNK/p38 and NF-κB pathways by TNF-α has been demonstrated in different cell types (81, 82). Disruption of this balance (e.g. by inhibition of the NF-κB or JNK pathway) may tip the cell towards apoptosis or proliferation. It is established that the production of inflammatory ROS involves the JNK and NF-κB pathways (43–45). For instance, TNF-α-induced ROS activate JNK, which in turn leads to more ROS production and exacerbated inflammation (45). In certain disease situations where inflammation plays a pathogenic role, ROS production stimulates NF-κB activation (83, 84). While we did not see a diminished NF-κB activation in the bone marrow cells from LPS-treated Fancc−/− mice, it would be intriguing to know whether NF-κB activation is necessary for the emergence of abnormal cell clones and the subsequent progression of FA to MDS and AML. Nevertheless, our results provide new insight into the role of p38 and JNK in FA innate immunity.
The results presented here suggest that antagonizing proinflammatory TNF-α and/or ROS may have therapeutic benefit in patients with FA. The elimination of TNF-α by either neutralizing antibody or deleting the Tnfa gene not only abrogated the negative effect of LPS on progenitor proliferation but also restored the ability of the progenitor cells to reconstitute irradiated bone marrow. Likewise, inhibition of ROS production rescued hematopoietic function otherwise suppressed by LPS. Therefore, a pharmacological ablation of TNF-α and/or ROS will potentially limit the severity of inflammatory phenotype by transiently controlling these primary proinflammatory signals. These findings may be extended to other bone marrow failure disease such as aplastic anemia and MDS.
Acknowledgments
We thank Dr. Manuel Buchwald (Hospital for Sick Children, University of Toronto) for the Fancc+/− mice, Dr. Christopher Baum (Cincinnati Children’s Hospital Medical Center) for the retroviral vector SFβ91, Jeff Bailey and Victoria Summey for bone marrow transplantation, and the Vector Core of the Cincinnati Children’s Research Foundation (Cincinnati Children’s Hospital Medical Center) for the preparation of retroviruses. Q.P. thanks Dr. Grover Bagby (Oregon Health Science University) for continued support.
Financial support information: This work was supported in part by a Leukemia Research Foundation grant, a Trustee grant, and NIH grants R01 CA109641 and R01 HL076712.
Abbreviations
- FA
Fanconi anemia
- HSC
hematopoietic stem cells
- IL
interleukin
- JNK
c-Jun N-terminal kinase
- LPS
lipopolysaccharide
- LSK
lineage-negative, Sca-1-positive, c-kit-positive
- ROS
reactive oxygen species
- SCF
stem cell factor
- TNF-α
tumor necrosis factor-α
References
- 1.Bagby GC., Jr Genetic basis of Fanconi anemia. Curr Opin Hematol. 2003;10:68–76. doi: 10.1097/00062752-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy RD, D’Andrea AD. The Fanconi Anemia/BRCA pathway: new faces in the crowd. Genes Dev. 2005;19:2925–2940. doi: 10.1101/gad.1370505. [DOI] [PubMed] [Google Scholar]
- 3.Levitus M, Rooimans MA, Steltenpool J, Cool NF, Oostra AB, Mathew CG, Hoatlin ME, Waisfisz Q, Arwert F, De Winter JP, Joenje H. Heterogeneity in Fanconi anemia: evidence for two new genetic subtypes. Blood. 2003;103:2498–2503. doi: 10.1182/blood-2003-08-2915. [DOI] [PubMed] [Google Scholar]
- 4.Timmers C, Taniguchi T, Hejna J, Reifsteck C, Lucas L, Bruun D, Thayer M, Cox B, Olson S, D’Andrea AD, Moses R, Grompe M. Positional cloning of a novel Fanconi anemia gene, FANCD2. Mol Cell. 2001;7:241–248. doi: 10.1016/s1097-2765(01)00172-1. [DOI] [PubMed] [Google Scholar]
- 5.Strathdee CA, Gavish H, Shannon WR, Buchwald M. Cloning of cDNAs for Fanconi’s anaemia by functional complementation. Nature. 1992;356:763–767. doi: 10.1038/356763a0. [DOI] [PubMed] [Google Scholar]
- 6.Lo Ten Foe JR, Rooimans MA, Bosnoyan-Collins L, Alon N, Wijker M, Parker L, Lightfoot J, Carreau M, Callen DF, Savoia A, Cheng NC, van Berkel CG, Strunk MH, Gille JJ, Pals G, Kruyt FA, Pronk JC, Arwert F, Buchwald M, Joenje H. Expression cloning of a cDNA for the major Fanconi anaemia gene, FAA. Nature Genet. 1996;14:320–323. doi: 10.1038/ng1196-320. [DOI] [PubMed] [Google Scholar]
- 7.de Winter JP, Waisfisz Q, Rooimans MA, van Berkel CG, Bosnoyan-Collins L, Alon N, Carreau M, Bender O, Demuth I, Schindler D, Pronk JC, Arwert F, Hoehn H, Digweed M, Buchwald M, Joenje H. The Fanconi anaemia group G gene FANCG is identical with XRCC9. Nat Genet. 1998;20:281–283. doi: 10.1038/3093. [DOI] [PubMed] [Google Scholar]
- 8.de Winter JP, Rooimans MA, van Der WL, van Berkel CG, Alon N, Bosnoyan-Collins L, de Groot J, Zhi Y, Waisfisz Q, Pronk JC, Arwert F, Mathew CG, Scheper RJ, Hoatlin ME, Buchwald M, Joenje H. The Fanconi anaemia gene FANCF encodes a novel protein with homology to ROM. Nat Genet. 2000;24:15–16. doi: 10.1038/71626. [DOI] [PubMed] [Google Scholar]
- 9.de Winter JP, Leveille F, van Berkel CG, Rooimans MA, van Der WL, Steltenpool J, Demuth INV, Morgan, Alon N, Bosnoyan-Collins L, Lightfoot J, Leegwater PA, Waisfisz Q, Komatsu K, Arwert F, Pronk JC, Mathew CG, Digweed M, Buchwald M, Joenje H. Isolation of a cDNA Representing the Fanconi Anemia Complementation Group E gene. Am J Hum Genet. 2000;67:1306–1308. doi: 10.1016/s0002-9297(07)62959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howlett NG, Taniguchi T, Olson S, Cox B, Waisfisz Q, De Die-Smulders C, Persky N, Grompe M, Joenje H, Pals G, Ikeda H, Fox EA, D’Andrea AD. Biallelic inactivation of BRCA2 in Fanconi anemia. Science. 2002;297:606–609. doi: 10.1126/science.1073834. [DOI] [PubMed] [Google Scholar]
- 11.Meetei AR, de Winter JP, Medhurst AL, Wallisch M, Waisfisz Q, van de Vrugt HJ, Oostra AB, Yan Z, Ling C, Bishop CE, Hoatlin ME, Joenje H, Wang W. A novel ubiquitin ligase is deficient in Fanconi anemia. Nat Genet. 2003;35:165–170. doi: 10.1038/ng1241. [DOI] [PubMed] [Google Scholar]
- 12.Meetei AR, Levitus M, Xue Y, Medhurst AL, Zwaan M, Ling C, Rooimans MA, Bier P, Hoatlin M, Pals G, de Winter JP, Wang W, Joenje H. X-linked inheritance of Fanconi anemia complementation group B. Nat Genet. 2004;36:1219–1224. doi: 10.1038/ng1458. [DOI] [PubMed] [Google Scholar]
- 13.Meetei AR, Medhurst AL, Ling C, Xue Y, Singh TR, Bier P, Steltenpool J, Stone S, Dokal I, Mathew CG, Hoatlin M, Joenje H, de Winter JP, Wang W. A human ortholog of archael DNA repair protein HEF is defective in Fanconi anemia complementation group M. Nat Genet. 2005;37:958–963. doi: 10.1038/ng1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levran O, Attwooll C, Henry RT, Milton KL, Neveling K, Rio P, Batish SD, Kalb R, Velleuer E, Barral S, Ott J, Petrini J, Schindler D, Hanenberg H, Auerbach AD. The BRCA1-interacting helicase BRIP1 is deficient in Fanconi anemia. Nat Genet. 2005;37:931–933. doi: 10.1038/ng1624. [DOI] [PubMed] [Google Scholar]
- 15.Levitus M, Waisfisz Q, Godthelp BC, de Vries Y, Hussain S, Wiegant WW, Elghalbzouri-Maghrani E, Steltenpool J, Rooimans MA, Pals G, Arwert F, Mathew CG, Zdzienicka MZ, Hiom K, de Winter JP, Joenje H. The DNA helicase BRIP1 is defective in Fanconi anemia complementation group J. Nat Genet. 2005;37:934–935. doi: 10.1038/ng1625. [DOI] [PubMed] [Google Scholar]
- 16.Xia B, Dorsman JC, Ameziane N, de Vries Y, Rooimans MA, Sheng Q, Pals G, Errami A, Gluckman E, Llera J, Wang W, Livingston DM, Joenje H, de Winter JP. Fanconi anemia is associated with a defect in the BRCA2 partner PALB2. Nat Genet. 2006 Dec 31; doi: 10.1038/ng1942. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Reid S, Schindler D, Hanenberg H, Barker K, Hanks S, Kalb R, Neveling K, Kelly P, Seal S, Freund M, Wurm M, Batish SD, Lach FP, Yetgin S, Neitzel H, Ariffin H, Tischkowitz M, Mathew CG, Auerbach AD, Rahman N. Biallelic mutations in PALB2 cause Fanconi anemia subtype FA-N and predispose to childhood cancer. Nat Genet. 2006 Dec 31; doi: 10.1038/ng1947. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.de Winter JP, van Der WL, De Groot J, Stone S, Waisfisz Q, Arwert F, Scheper RJ, Kruyt FA, Hoatlin ME, Joenje H. The Fanconi anemia protein FANCF forms a nuclear complex with FANCA, FANCC and FANCG. Hum Mol Genet. 2000;9:2665–2674. doi: 10.1093/hmg/9.18.2665. [DOI] [PubMed] [Google Scholar]
- 19.Medhurst AL, Huber PA, Waisfisz Q, de Winter JP, Mathew CG. Direct interactions of the five known Fanconi anaemia proteins suggest a common functional pathway. Hum Mol Genet. 2001;10:423–429. doi: 10.1093/hmg/10.4.423. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Higuera, Taniguchi IT, Ganesan S, Meyn MS, Timmers C, Hejna J, Grompe M, D’Andrea A. Interaction of the Fanconi anemia proteins and BRCA1 in a common pathway. Mol Cell. 2001;7:249–262. doi: 10.1016/s1097-2765(01)00173-3. [DOI] [PubMed] [Google Scholar]
- 21.Haneline LS, Broxmeyer HE, Cooper S, Hangoc G, Carreau M, Buchwald M, Clapp DW. Multiple inhibitory cytokines induce deregulated progenitor growth and apoptosis in hematopoietic cells from Fac−/− mice. Blood. 1998;91:4092–4098. [PubMed] [Google Scholar]
- 22.Pang Q, Fagerlie S, Christianson TA, Keeble W, Faulkner G, Diaz J, Rathbun RK, Bagby GC. The Fanconi Anemia Protein FANCC Binds to and Facilitates the Activation of STAT1 by Gamma Interferon and Hematopoietic Growth Factors. Mol Cell Biol. 2000;20:4724–4735. doi: 10.1128/mcb.20.13.4724-4735.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fagerlie SR, Diaz J, Christianson TA, McCartan K, Keeble W, Faulkner GR, Bagby GC. Functional correction of FA-C cells with FANCC suppresses the expression of interferon g-inducible genes. Blood. 2001;97:3017–3024. doi: 10.1182/blood.v97.10.3017. [DOI] [PubMed] [Google Scholar]
- 24.Pang Q, Keeble W, Christianson TA, Faulkner GR, Bagby GC. FANCC interacts with hsp70 to protect hematopoietic cells from IFNg/TNFa- mediated cytotoxicity. EMBO J. 2001;20:4478–4489. doi: 10.1093/emboj/20.16.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pang Q, Christianson TA, Keeble W, Koretsky T, Bagby GC. The anti-apoptotic function of Hsp70 in the interferon-inducible double-stranded RNA-dependent protein kinase-mediated death signaling pathway requires the Fanconi anemia protein, FANCC. J Biol Chem. 2002;277:49638–49643. doi: 10.1074/jbc.M209386200. [DOI] [PubMed] [Google Scholar]
- 26.Bijangi-Vishehsaraei K, Saadatzadeh MR, Werne A, McKenzie KA, Kapur R, Ichijo H, Haneline LS. Enhanced TNF-a-induced apoptosis in Fanconi anemia type C-deficient cells is dependent on apoptosis signal-regulating kinase 1. Blood. 2005;106:4124–4130. doi: 10.1182/blood-2005-05-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maciejewski JP, Risitano A. Hematopoietic stem cells in aplastic anemia. Arch Med Res. 2003;34:520–527. doi: 10.1016/j.arcmed.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Haneline LS, Gobbett TA, Ramani R, Carreau M, Buchwald M, Yoder MC, Clapp DW. Loss of FancC function results in decreased hematopoietic stem cell repopulating ability. Blood. 1999;94:1–8. [PubMed] [Google Scholar]
- 29.Haneline LS, Li X, Ciccone SL, Hong P, Yang Y, Broxmeyer HE, Lee SH, Orazi A, Srour EF, Clapp DW. Retroviral-mediated expression of recombinant Fancc enhances the repopulating ability of Fancc −/− hematopoietic stem cells and decreases the risk of clonal evolution. Blood. 2003;101:1299–1307. doi: 10.1182/blood-2002-08-2404. [DOI] [PubMed] [Google Scholar]
- 30.Kruyt FA, Hoshino T, Liu JM, Joseph P, Jaiswal AK, Youssoufian H. Abnormal microsomal detoxification implicated in Fanconi anemia group C by interaction of the FAC protein with NADPH cytochrome P450 reductase. Blood. 1998;92:3050–3056. [PubMed] [Google Scholar]
- 31.Cumming RC, Lightfoot J, Beard K, Youssoufian H, O’Brien PJ, Buchwald M. Fanconi anemia group C protein prevents apoptosis in hematopoietic cells through redox regulation of GSTP1. Nat Med. 2001;7:814–820. doi: 10.1038/89937. [DOI] [PubMed] [Google Scholar]
- 32.Hadjur S, Ung K, Wadsworth L, Dimmick J, Rajcan-Separovic E, Scott RW, Buchwald M, Jirik FR. Defective hematopoiesis and hepatic steatosis in mice with combined deficiencies of the genes encoding Fancc and Cu/Zn superoxide dismutase. Blood. 2001;98:1003–1011. doi: 10.1182/blood.v98.4.1003. [DOI] [PubMed] [Google Scholar]
- 33.Futaki M, Igarashi T, Watanabe S, Kajigaya S, Tatsuguchi A, Wang J, Liu JM. The FANCG Fanconi anemia protein interacts with CYP2E1: possible role in protection against oxidative DNA damage. Carcinogenesis. 2002;23:67–72. doi: 10.1093/carcin/23.1.67. [DOI] [PubMed] [Google Scholar]
- 34.Saadatzadeh MR, Bijangi-Vishehsaraei K, Hong P, Bergmann H, Haneline LS. Oxidant hypersensitivity of Fanconi anemia type C-deficient cells is dependent on a redox-regulated apoptotic pathway. J Biol Chem. 2004;279:16805–16812. doi: 10.1074/jbc.M313721200. [DOI] [PubMed] [Google Scholar]
- 35.Park SJ, Ciccone SL, Beck BD, Hwang B, Freie B, Clapp DW, Lee SH. Oxidative stress/damage induces multimerization and interaction of Fanconi anemia proteins. J Biol Chem. 2004;279:30053–30059. doi: 10.1074/jbc.M403527200. [DOI] [PubMed] [Google Scholar]
- 36.Pagano G, Degan P, d’Ischia M, Kelly FJ, Nobili B, Pallardó FV, Youssoufian H, Zatterale A. Oxidative stress as a multiple effector in Fanconi anaemia clinical phenotype. Eur J Haematol. 2005;75:93–100. doi: 10.1111/j.1600-0609.2005.00507.x. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Yang Y, Yuan J, Hong P, Freie B, Orazi A, Haneline LS, Clapp DW. Continuous in vivo infusion of interferon-gamma (IFN-gamma) preferentially reduces myeloid progenitor numbers and enhances engraftment of syngeneic wild-type cells in Fancc−/− mice. Blood. 2004;104:1204–1209. doi: 10.1182/blood-2004-03-1094. [DOI] [PubMed] [Google Scholar]
- 38.Si Y, Ciccone S, Yang FC, Yuan J, Zeng D, Chen S, van de Vrugt H, Critser J, Arwert F, Haneli LS, Clapp DW. Continuous in vivo infusion of interferon-gamma (IFN-{gamma}) enhances engraftment of syngeneic wild-type cells in Fanca−/− and Fancg−/− mice. Blood. 2006;108:4283–4287. doi: 10.1182/blood-2006-03-007997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aggarwal BB. Signalling pathways of the TNF supperfamily: a double-edged sword. Nature Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 40.Maini RN, Taylor PC. Anti-cytokine therapy for rheumatoid arthritis. Annu Rev Med. 2000;51:207–229. doi: 10.1146/annurev.med.51.1.207. [DOI] [PubMed] [Google Scholar]
- 41.Young NS. Hematopoietic cell destruction by immune mechanisms in acquired aplastic anemia. Semin Hematol. 2000;37:3–14. doi: 10.1016/s0037-1963(00)90026-x. [DOI] [PubMed] [Google Scholar]
- 42.Varfolomeev EE, Ashkenazi A. Tumor necrosis factor: an apoptosis JuNKie? Cell. 2004;116:491–497. doi: 10.1016/s0092-8674(04)00166-7. [DOI] [PubMed] [Google Scholar]
- 43.Tang G, Minemoto Y, Dibling B, Purcell NH, Li Z, Karin M, Lin A. Inhibition of JNK activation through NF-kappaB target genes. Nature. 2001;414:313–317. doi: 10.1038/35104568. [DOI] [PubMed] [Google Scholar]
- 44.Sakon S, Xue X, Takekawa M, Sasazuki T, Okazaki T, Kojima Y, Piao JH, Yagita H, Okumura K, Doi T, Nakano H. NF- B inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J. 2003;22:3898–3909. doi: 10.1093/emboj/cdg379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ventura JJ, Cogswell P, Flavell RA, Baldwin AS, Jr, Davis RJ. JNK potentiates TNF-stimulated necrosis by increasing the production of cytotoxic reactive oxygen species. Genes Dev. 2004;18:2905–2915. doi: 10.1101/gad.1223004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dufour C, Corcione A, Svahn J, Haupt R, Poggi V, Beka’ssy AN, Scime R, Pistorio A, Pistoia V. TNF-alpha and IFN-gamma are overexpressed in the bone marrow of Fanconi anemia patients and TNF-alpha suppresses erythropoiesis in vitro. Blood. 2003;102:2053–2059. doi: 10.1182/blood-2003-01-0114. [DOI] [PubMed] [Google Scholar]
- 47.Schultz JC, Shahidi NT. Tumor necrosis factor-alpha overproduction in Fanconi’s anemia. Am J Hematol. 1993;42:196–201. doi: 10.1002/ajh.2830420211. [DOI] [PubMed] [Google Scholar]
- 48.Rosselli F, Sanceau J, Gluckman E, Wietzerbin J, Moustacchi E. Abnormal lymphokine production: a novel feature of the genetic disease Fanconi anemia. II. In vitro and in vivo spontaneous overproduction of tumor necrosis factor alpha. Blood. 1994;83:1216–1225. [PubMed] [Google Scholar]
- 49.Fagerlie SR, Bagby GC. Immune defects in fanconi anemia. Crit Rev Immunol. 2006;26:81–96. doi: 10.1615/critrevimmunol.v26.i1.40. [DOI] [PubMed] [Google Scholar]
- 50.MacMillan ML, Auerbach AD, Davies SM, Defor TE, Gillio A, Giller R, Harris R, Cairo M, Dusenbery K, Hirsch B, Ramsay NK, Weisdorf DJ, Wagner JE. Haematopoietic cell transplantation in patients with Fanconi anaemia using alternate donors: results of a total body irradiation dose escalation trial. Br J Haematol. 2000;109:121–129. doi: 10.1046/j.1365-2141.2000.01955.x. [DOI] [PubMed] [Google Scholar]
- 51.Froom P, Aghai E, Dobinsky JB, Quitt M, Lahat N. Reduced natural killer activity in patients with Fanconi’s anemia and in family members. Leuk Res. 1987;11:197–199. doi: 10.1016/0145-2126(87)90026-9. [DOI] [PubMed] [Google Scholar]
- 52.Hersey P, Edwards A, Lewis R, Kemp A, McInnes J. Deficient natural killer cell activity in a patient with Fanconi’s anaemia and squamous cell carcinoma. Association with defect in interferon release. Clin Exp Immunol. 1982;48:205–212. [PMC free article] [PubMed] [Google Scholar]
- 53.Lebbe C, Pinquier L, Rybojad M, Chomienne C, Ochonisky S, Miclea JM, Gluckman E, Morel P. Fanconi’s anaemia associated with multicentric Bowen’s disease and decreased NK cytotoxicity. Br J Dermatol. 1993;129:615–618. doi: 10.1111/j.1365-2133.1993.tb00497.x. [DOI] [PubMed] [Google Scholar]
- 54.Castello G, Gallo C, Napolitano M, Ascierto PA. Immunological phenotype analysis of patients with Fanconi’s anaemia and their family members. Acta Haematol. 1998;100:39–43. doi: 10.1159/000040861. [DOI] [PubMed] [Google Scholar]
- 55.Perussia B, Kobayashi M, Rossi ME, Anegon I, Trinchieri G. Immune interferon enhances functional properties of human granulocytes: role of Fc receptors and effect of lymphotoxin, tumor necrosis factor, and granulocyte-macrophage colony-stimulating factor. J Immunol. 1987;138:765–774. [PubMed] [Google Scholar]
- 56.Pedersen FK, Hertz H, Lundsteen C, Platz P, Thomsen M. Indication of primary immune deficiency in Fanconi’s anemia. Acta Paediatr Scand. 1977;66:745–751. doi: 10.1111/j.1651-2227.1977.tb07983.x. [DOI] [PubMed] [Google Scholar]
- 57.Fagerlie SR, Koretsky T, Torok-Storb B, Bagby GC. Impaired type I IFN-induced Jak/STAT signaling in FA-C cells and abnormal CD4+ Th cell subsets in Fancc−/− mice. J Immunol. 2004;173:3863–3870. doi: 10.4049/jimmunol.173.6.3863. [DOI] [PubMed] [Google Scholar]
- 58.Chen M, Tomkins D, Auerbach W, McKerlie C, Youssoufian H, Liu L, Gan O, Carreau M, Auerbach A, Groves T, Guidos C, Freedman M, Cross J, Percy D, Dick J, Joyner A, Buchwald M. Inactivation of Fac in mice produces inducible chromosomal instability and reduced fertility reminiscent of Fanconi anaemia. Nature Genet. 1996;12:448–451. doi: 10.1038/ng0496-448. [DOI] [PubMed] [Google Scholar]
- 59.Lopez-Bojorquez LN, Dehesa AZ, Reyes-Teran G. Molecular mechanisms involved in the pathogenesis of septic shock. Arch Med Res. 2004;35:465–479. doi: 10.1016/j.arcmed.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 60.Pattanaik U, Prasad K. Endotoxemia and oxidative stress. Ann N Y Acad Sci. 1996;793:506–510. doi: 10.1111/j.1749-6632.1996.tb33551.x. [DOI] [PubMed] [Google Scholar]
- 61.Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature. 2004;431:405–406. doi: 10.1038/431405a. [DOI] [PubMed] [Google Scholar]
- 62.Bryder D, Ramsfjell V, Dybedal I, Theilgaard-Monch K, Hogerkorp CM, Adolfsson J, Borge OJ, Jacobsen SE. Self-renewal of multipotent long-term repopulating hematopoietic stem cells is negatively regulated by Fas and tumor necrosis factor receptor activation. J Exp Med. 2001;194:941–952. doi: 10.1084/jem.194.7.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dybedal I, Bryder D, Fossum A, Rusten LS, Jacobsen SE. Tumor necrosis factor (TNF)-mediated activation of the p55 TNF receptor negatively regulates maintenance of cycling reconstituting human hematopoietic stem cells. Blood. 2001;98:1782–1791. doi: 10.1182/blood.v98.6.1782. [DOI] [PubMed] [Google Scholar]
- 64.Rosenfeld C, List A. A hypothesis for the pathogenesis of myelodysplastic syndromes: implications for new therapies. Leukemia. 2000;14:2–8. doi: 10.1038/sj.leu.2401618. [DOI] [PubMed] [Google Scholar]
- 65.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 66.Liesveld JL, Jordan CT, Phillips GL. The hematopoietic stem cell in myelodysplasia. Stem Cells. 2004;22:590–599. doi: 10.1634/stemcells.22-4-590. [DOI] [PubMed] [Google Scholar]
- 67.Goossens V, De Vos K, Vercammen D, Steemans M, Vancompernolle K, Fiers W, Vandenabeele P, Grooten J. Redox regulation of TNF signaling. Biofactors. 1999;10:145–156. doi: 10.1002/biof.5520100210. [DOI] [PubMed] [Google Scholar]
- 68.Suematsu N, Tsutsui H, Wen J, Kang D, Ikeuchi M, Ide T, Hayashidani S, Shiomi T, Kubota T, Hamasaki N, Takeshita A. Oxidative stress mediates tumor necrosis factor-alpha-induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation. 2003;107:1418–1423. doi: 10.1161/01.cir.0000055318.09997.1f. [DOI] [PubMed] [Google Scholar]
- 69.Wheelhouse NM, Chan YS, Gillies SE, Caldwell H, Ross JA, Harrison DJ, Prost S. TNF-alpha induced DNA damage in primary murine hepatocytes. Int J Mol Med. 2003;12:889–894. [PubMed] [Google Scholar]
- 70.Mukhopadhyay SS, Leung KS, Hicks MJ, Hastings PJ, Youssoufian H, Plon SE. Defective mitochondrial peroxiredoxin-3 results in sensitivity to oxidative stress in Fanconi anemia. J Cell Biol. 2006;175:225–235. doi: 10.1083/jcb.200607061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dakubo GD, Parr RL, Costello LC, Franklin RB, Thayer RE. Altered metabolism and mitochondrial genome in prostate cancer. J Clin Pathol. 2006;59:10–16. doi: 10.1136/jcp.2005.027664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005;11:1306–1313. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maccio A, Madeddu C, Massa D, Mudu MC, Lusso MR, Gramignano G, Serpe R, Melis GB, Mantovani G. Hemoglobin levels correlate with interleukin-6 levels in patients with advanced untreated epithelial ovarian cancer: role of inflammation in cancer-related anemia. Blood. 2005;106:362–367. doi: 10.1182/blood-2005-01-0160. [DOI] [PubMed] [Google Scholar]
- 75.Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 76.Mori K, Shibanuma M, Nose K. Invasive potential induced under long-term oxidative stress in mammary epithelial cells. Cancer Res. 2004;64:7464–7472. doi: 10.1158/0008-5472.CAN-04-1725. [DOI] [PubMed] [Google Scholar]
- 77.Pearl-Yafe M, Halperin D, Scheuerman O, Fabian I. The p38 pathway partially mediates caspase-3 activation induced by reactive oxygen species in Fanconi anemia C cells. Biochem Pharmacol. 2004;67:539–546. doi: 10.1016/j.bcp.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 78.Liu ZG. Adding facets to TNF signaling. The JNK angle. Mol Cell. 2003;12:795–796. doi: 10.1016/s1097-2765(03)00399-x. [DOI] [PubMed] [Google Scholar]
- 79.Varfolomeev EE, Ashkenazi A. Tumor necrosis factor: an apoptosis JuNKie? Cell. 2004;116:491–497. doi: 10.1016/s0092-8674(04)00166-7. [DOI] [PubMed] [Google Scholar]
- 80.Deng Y, Ren X, Yang L, Lin Y, Wu X. A JNK-dependent pathway is required for TNFalpha-induced apoptosis. Cell. 2003;115:61–70. doi: 10.1016/s0092-8674(03)00757-8. [DOI] [PubMed] [Google Scholar]
- 81.Tucker SJ, Rae C, Littlejohn AF, Paul A, MacEwan DJ. Switching leukemia cell phenotype between life and death. Proc Natl Acad Sci U S A. 2004;101:12940–12945. doi: 10.1073/pnas.0400949101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kriehuber E, Bauer W, Charbonnier AS, Winter D, Amatschek S, Tamandl D, Schweifer N, Stingl G, Maurer D. The balance between NF-{kappa}B and JNK/AP-1 activity controls dendritic cell life and death. Blood. 2005;106:175–183. doi: 10.1182/blood-2004-08-3072. [DOI] [PubMed] [Google Scholar]
- 83.Sarkar D, Lebedeva IV, Emdad L, Kang DC, Baldwin AS, Jr, Fisher PB. Human polynucleotide phosphorylase (hPNPaseold-35): a potential link between aging and inflammation. Cancer Res. 2004;64:7473–7478. doi: 10.1158/0008-5472.CAN-04-1772. [DOI] [PubMed] [Google Scholar]
- 84.Rahman I, Marwick J, Kirkham P. Redox modulation of chromatin remodeling: impact on histone acetylation and deacetylation, NF-kappaB and pro-inflammatory gene expression. Biochem Pharmacol. 2004;68:1255–1267. doi: 10.1016/j.bcp.2004.05.042. [DOI] [PubMed] [Google Scholar]