Summary
Phosphatidyl inositol 3-kinase (PI3K) signalling in the hypothalamus has been implicated in the regulation of energy homeostasis, but the critical brain sites where this intracellular signal integrates various metabolic cues to regulate food intake and energy expenditure are unknown. Here we show that mice with reduced PI3K activity in the ventromedial hypothalamic nucleus (VMH) are more sensitive to high fat diet-induced obesity due to reduced energy expenditure. In addition, inhibition of PI3K in the VMH impaired the ability to alter energy expenditure in response to acute high fat diet feeding and food deprivation. Furthermore, the acute anorexigenic effects induced by exogenous leptin were blunted in the mutant mice. Collectively, our results indicate that PI3K activity in VMH neurons plays a physiologically relevant role in the regulation of energy expenditure.
Introduction
Obesity is now recognized as a global crisis because of its increasing prevalence and serious comorbidities, including type-2 diabetes, cancer and cardiovascular diseases (Reilly and Rader, 2003). The central nervous system regulates body weight homeostasis by maintaining a balance between energy intake and expenditure. Obesity occurs when the homeostatic networks fail to adapt to excess energy intake, as occurs with exposure to high fat diet (HFD). The ventromedial hypothalamic nucleus (VMH, also known as the VMN) has long been thought to be an important component of the neural circuits responsible for the homeostatic regulation of body weight and food intake. For example, numerous studies showed that lesions of the VMH produce obesity due to both increased food intake and decreased energy expenditure (see (King, 2006)). However, many of these results were questioned because the electrolytic insults and knife cuts used to lesion the VMH likely damaged the surrounding regions (such as the arcuate nucleus) as well as neuronal fibers passing through the VMH (Gold, 1973). Recent mouse genetic studies have begun to circumvent these issues by performing deletion of key genes specifically in VMH neurons. These studies were based on the finding that a transcription factor, steroidogenic factor-1 (SF1), is expressed exclusively in the VMH neurons within the brain (Ikeda et al., 1995). Deletion of SF1 in mice disrupts VMH structure (Dellovade et al., 2000) and leads to obesity (Majdic et al., 2002). These findings supported the model that VMH neurons are physiological regulators of body weight homeostasis.
Multiple metabolic signals have been demonstrated to regulate energy homeostasis via actions in the VMH. For example, leptin directly activates SF1 neurons in the VMH and selective deletion of leptin receptors from SF1 neurons produces obesity (Bingham et al., 2008; Dhillon et al., 2006). Estrogen acts on the estrogen receptor α (ERα) expressed by VMH neurons to regulate energy expenditure as animals with ERα knocked down in the VMH develop obesity due to reduced energy expenditure (Musatov et al., 2007). Likewise, knock-down of brain-derived neurotrophic factor (BDNF) in the VMH and dorsomedial hypothalamic nucleus produces hyperphagic obesity (Unger et al., 2007).
Interestingly, all of the aforementioned hormonal and neural signals have been shown to activate the phosphatidyl inositol 3-kinase (PI3K) signalling pathway in neurons. For example, leptin activates the PI3K pathway in the hypothalamus (Niswender et al., 2001; Zhao et al., 2002), and pharmacological evidence indicates that leptin’s effects on feeding (Zhao et al., 2002) and lipolysis (Buettner et al., 2008) require intact PI3K activity in the hypothalamus. Similarly, estrogen regulates expression of PI3K subunits in the hypothalamus including the VMH and the PI3K/Akt cascade mediates estrogen’s actions in hypothalamic neurons (Malyala et al., 2008). Furthermore, BDNF activates the PI3K pathway in neurons to promote synaptic formation (Yoshii and Constantine-Paton, 2007). Therefore, PI3K signalling in VMH neurons may be a common pathway that integrates metabolic cues to provide a coordinated control of energy homeostasis. In the present study, we generated a mouse model with reduced PI3K signalling specifically in the VMH to assess the physiological relevance of PI3K in VMH neurons in the regulation of energy balance.
Results and Discussions
Generation of mice lacking PI3K in VMH neurons
PI3K consists of an 85kDa regulatory subunit (p85) and a 110kDa catalytic subunit (p110) (Cantley, 2002). As the primary insulin responsive isoform of PI3K, p110α is selectively activated by the insulin receptor substrate (IRS) signaling complex and is required for IRS-associated PI3K activity in the hypothalamus (Foukas et al., 2006; Knight et al., 2006). Thus, we crossed mice carrying loxP flanked p110α alleles (p110αlox/lox mice) (Zhao et al., 2006) with transgenic SF1-Cre mice which express Cre-recombinase driven by SF1 regulatory elements (Dhillon et al., 2006). These crosses produced mice lacking p110α only in SF1 neurons (p110αlox/lox/SF1-Cre mice) and mice bearing p110αlox/lox alleles alone. The latter group of littermates served as controls in all experiments.
We first validated the selective deletion of p110α in SF1 cells in p110αlox/lox/SF1-Cre mice. We found that the p110α alleles were deleted from the genome in tissues that express SF1, including the hypothalamus, pituitary, adrenal gland and testis (Zhao et al., 2001), whereas the p110α allele remained intact in tissues that do not express SF1 (e.g. the cortex and brainstem) (Supple Fig. 1a). Further, we found that p110α mRNA was significantly reduced in the VMH from p110αlox/lox/SF1-Cre mice, while expression of p110β, another p110 isoform present in the hypothalamus (Cantley, 2002), was not changed (Supple Fig. 1b). Finally, we crossed a FoxO1GFP reporter allele (Fukuda et al., 2008) to the mice lacking p110α in SF-1 neurons. This allowed assessment of PI3K activity specifically in SF1 neurons by monitoring FoxO1GFP translocation between the nucleus and cytoplasm (Fukuda et al., 2008). We found that while FoxO1GFP was localized in both the cytoplasm and nucleus of the SF1 neurons cultured from FoxO1GFP/SF1-Cre mice (control mice). In contrast FoxO1GFP was significantly more concentrated in the nucleus of the SF1 neurons from the p110αlox/lox/FoxO1GFP/SF1-Cre mice (Supple Fig. 1c). Collectively, these results demonstrate that PI3K and its downstream signalling pathway are disrupted in SF1 neurons in p110αlox/lox/SF1-Cre mice.
We then assessed whether deletion of p110α affects the viability of SF1 neurons in the VMH as assessed by qPCR and in situ hybridization for SF1 mRNA. We found that SF1 mRNA was not significantly changed in the VMH of p110αlox/lox/SF1-Cre mice compared to the controls (Supple Figs. 1b and 1d).
SF-1 is also expressed in the pituitary, adrenal glands and gonads, thus we assessed levels of glucocorticoids and gonadotropins in control and experimental mice. We found no changes in the serum corticosterone, testosterone, FSH or LH in p110αlox/lox/SF1-Cre mice (Supple Table 1). Therefore, the metabolic phenotypes outlined below are not likely due to impaired PI3K signalling in the pituitary, adrenal gland or gonads, but rather to altered PI3K activity in SF1 VMH neurons.
Mice lacking PI3K in VMH SF1 neurons develop obesity on HFD
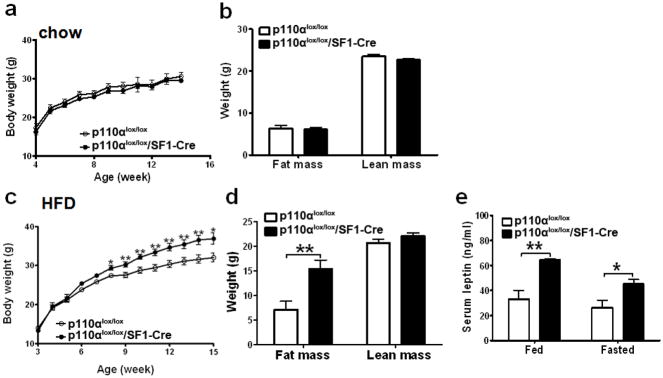
Male p110αlox/lox/SF1-Cre mice on regular chow showed comparable body weight and body composition as their p110αlox/lox littermates (Figs. 1a and 1b). However, male mice lacking p110α in SF1 neurons displayed increased sensitivity to diet-induced obesity. Specifically, p110αlox/lox/SF1-Cre males fed with HFD showed significantly increased body weight and adiposity compared to their controls (Figs. 1c and 1d). Elevated circulating leptin levels were observed in these mice at both fed and fasted conditions (Fig. 1e). The obese phenotype was not due to the presence of the SF1-Cre transgene per se, as mice bearing SF1-Cre alone have been shown to have the same body weight gain as wildtype mice (Dhillon et al., 2006). Consistent with our observations, VMH lesions have been shown to increase the susceptibility to diet-induced obesity (Inoue et al., 1977; Oku et al., 1984). Our results suggest that PI3K signalling in VMH neurons may play an important role in resisting diet-induced obesity. However, we did not formally examine if the obese phenotypes seen in HFD-fed p110αlox/lox/SF1-Cre mice can be blunted by switching from HFD back to regular chow, which is characteristically seen in diet-induced obesity (Enriori et al., 2007; Shi et al., 2009).
Fig. 1.
Deletion of p110α in SF1 neurons increases sensitivity to diet-induced obesity. (a) Weekly body weight was measured in group housed male mice weaned on regular chow (n=12/genotype). (b) Body composition was measured in 15-week old male mice fed with regular chow (n=12/genotype). (c) Weekly body weight was measured in group housed male mice weaned on HFD (n=16 or 23/genotype). (d) Body composition was measured in 18-week old male mice fed with HFD (n=10/genotype). (e) Serum leptin levels were measured in 7-month old male mice at both fed and fasted conditions (n=6/genotype). Data are presented as mean ± SEM, and * P<0.05 and **P<0.01 between p110αlox/lox/SF1-Cre mice and p110αlox/lox mice.
Inhibition of PI3K in VMH SF1 neurons disrupts regulation of energy expenditure
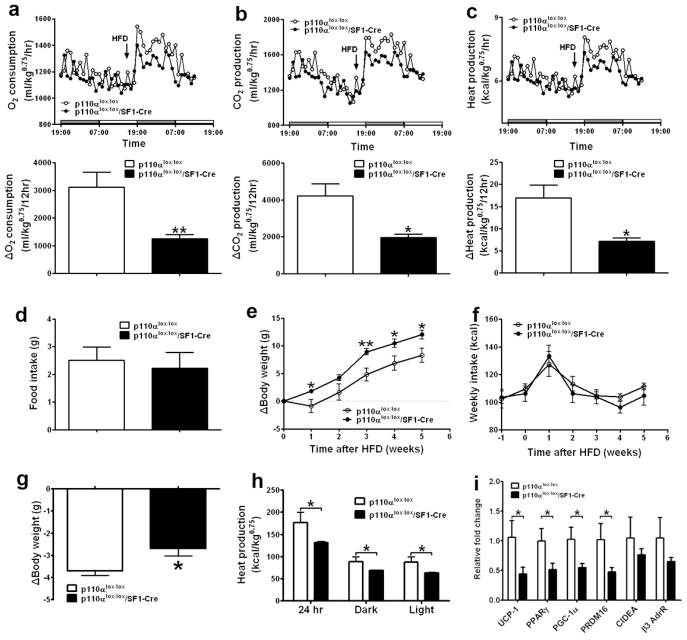
The development of obesity in SF1 knockout mice has been attributed primarily to deficient regulation of energy expenditure (Majdic et al., 2002). Similar to SF1 knockout mice, we found that mice lacking p110α in SF1 neurons are not hyperphagic, as body weight-matched young mice consumed similar amount of energy when fed with either chow or HFD (Figs. 2a and 2b). No significant difference in energy expenditure was detected between chow-fed p110αlox/lox/SF1-Cre males and their controls (data not shown). However, HFD-fed p110αlox/lox/SF1-Cre mice showed significantly reduced energy expenditure (O2 consumption, CO2 production and heat production) compared to their body weight-matched p110αlox/lox littermates (Figs. 2c–2e). Components of total energy expenditure include energy required for physical activities, basal metabolism, and thermogenesis evoked by stimuli such as food intake (Butler and Kozak, 2010; Castaneda et al., 2005). Since neither ambulatory movements nor rearing activities were significantly changed in p110αlox/lox/SF1-Cre mice (Figs. 2f and 2g), we conclude that the decreases in energy expenditure observed in p110αlox/lox/SF1-Cre mice are due to reduced basal metabolic rate and/or diet-induced thermogenesis.
Fig. 2.
Deletion of p110α in SF1 neurons reduces energy expenditure. (a–b) Daily food intake was measured in 7-week old male mice with comparable body weight fed with regular chow (a) or with HFD (b) (n=11–15/genotype). (c–g) Seven-week old chow-fed male mice (n=11 or 16/genotype) were fed with HFD for 2 weeks and matched for body weight (p110αlox/lox: 23.8±0.7 g vs p110αlox/lox/SF1-Cre: 24.9±0.7 g, P=0.31), followed by metabolic analyses using the TSE metabolic chambers. Data are presented as mean ± SEM, and * P<0.05 and **P<0.01 between p110αlox/lox/SF1-Cre mice and p110αlox/lox mice.
The VMH has been implicated in the regulation of diet-induced thermogenesis, as VMH lesions interrupt the signal that activates thermogenesis in response to excess energy intake (Vander Tuig et al., 1985). Thus, we examined whether PI3K activity in VMH neurons is required for appropriate thermogenic responses to excess nutrition. When exposed to HFD feeding, body weight-matched p110αlox/lox/SF1-Cre mice and controls increased O2 consumption, CO2 production and heat production. However, the increase in energy expenditure in p110αlox/lox/SF1-Cre mice was significantly less than that in controls (Figs. 3a–3c). The impaired thermogenic responses to HFD feeding in p110αlox/lox/SF1-Cre mice were not due to reductions in food intake, as food intake after being switched to HFD was comparable between p110αlox/lox/SF1-Cre mice and p110αlox/lox mice (Fig. 3d). Further, 3 weeks after HFD feeding, the body weight of p110αlox/lox/SF1-Cre mice started to diverge compared to p110αlox/lox mice (Fig. 3e), while energy intake of these mice was not significantly different (Fig. 3f). Thus, we conclude that reduced PI3K signalling in VMH neurons disrupts the adaptation to excess caloric intake, which may contribute to HFD-induced obesity.
Fig. 3.
Deletion of p110α in SF1 neurons disrupts the thermogenic regulation in response to HFD feeding and fasting. (a–d) Six-month old chow-fed male mice (n=6/genotype) were matched for body weight (p110αlox/lox: 37.7±1.4 g vs p110αlox/lox/SF1-Cre: 37.4±1.0 g, P=0.90) and adapted to the TSE metabolic chambers. The mice were provided with HFD at 17:00 (2 hr prior to dark cycle), and metabolic parameters were monitored from 24 hr before the HFD feeding till 24 hr afterwards using the TSE metabolic chambers. Upper panels: temporal levels of O2 consumption (a), CO2 production (b) and heat production (c). The arrow indicates the beginning of HFD feeding. Lower panels: changes in O2 consumption (a), CO2 production (b) and heat production (c) between the 12-hr dark cycle before HFD feeding and the 12-hr dark cycle afterwards in p110αlox/lox/SF1-Cre and p110αlox/lox mice. (d) HFD intake during the 12-hr dark cycle in the TSE chambers. (e–f) The mice were maintained on HFD for 6 weeks and weekly body weight gain (e) and energy intake (f) were recorded. (g–h) Five-month old chow-fed male mice (n=6/genotype) were matched for body weight (p110αlox/lox: 31.5±0.6 g vs p110αlox/lox/SF1-Cre: 32.4±1.1 g, P=0.51) and fasted for 24 hr. The body weight loss was measured (g) and heat production was recorded using the TSE chambers (h). (i) Fourteen-week old chow-fed mice were fed with HFD for 3 weeks (mean body weight: p110αlox/lox: 33.1±3.4 g vs p110αlox/lox/SF1-Cre: 38.8±2.6 g, P=0.26), and BAT were collected after euthanasia. Messenger RNA levels of indicated BAT genes were quantified with real-time PCR (n=6 or 7/genotype). Data are presented as mean ± SEM, and * P<0.05 and **P<0.01 between p110αlox/lox/SF1-Cre mice and p110αlox/lox mice.
We also assessed the adaptability of p110αlox/lox/SF1-Cre mice to caloric deprivation. Chow-fed mice with matched body weight were fasted for 24 hours. p110αlox/lox/SF1-Cre mice showed significantly less weight loss than p110αlox/lox mice (Fig. 3g). In addition, we found that the heat production during fasting period was significantly lower in p110αlox/lox/SF1-Cre mice than that in p110αlox/lox mice (Fig. 3h). These findings suggest that intact PI3K signalling in the VMH is also required for the regulation of energy expenditure in response to food deprivation.
Brown adipose tissue (BAT) plays crucial roles in regulating energy expenditure (Dulloo, 2002; Lowell and Bachman, 2003). We therefore examined the expression of the thermogenic genes in BAT from male mice that had been fed with HFD for 3 weeks. Uncoupling protein 1 (UCP1) expressed in BAT generates heat by uncoupling the oxidation of fuel from adenosine triphosphate production (Niijima et al., 1984; Sell et al., 2004). We found that UCP1 mRNA levels in p110αlox/lox/SF1-Cre BAT were significantly reduced compared to controls (Fig. 3i), which may, at least in part, account for the impaired diet-induced thermogenesis in the p110αlox/lox/SF1-Cre mice. In addition, expression levels of peroxisome proliferator-activated receptor γ (PPARγ), PPARγ co-activator-1α (PGC-1α) and PRDM16, factors that stimulate UCP1 expression (Seale et al., 2007; Sell et al., 2004), were significantly lower in p110αlox/lox/SF1-Cre BAT. In contrast, the levels of CIDEA and β3 adrenergic receptor in BAT were not altered (Fig. 3i). Taken together, these findings demonstrate that reduced PI3K activity in VMH neurons leads to a reduction in the thermogenic functions of BAT by suppressing UCP1 expression. While previous studies indicated the involvement of VMH in BAT-mediated thermogenesis (Kageyama et al., 2003; Niijima et al., 1984; Saito et al., 1989; Sakaguchi et al., 1988), our findings provide a link that couples altered PI3K signalling in VMH neurons to BAT thermogenesis.
Mice lacking PI3K in VMH SF1 neurons show blunted responses to acute leptin
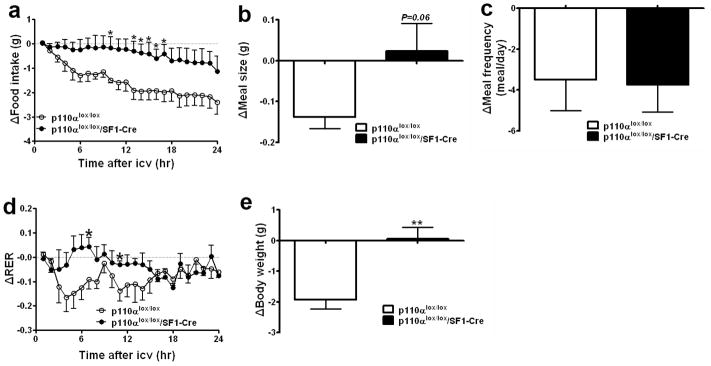
Specific deletion of PIP3 phosphatase (Pten) in leptin receptor-expressing neurons, which over-activates the PI3K pathway, causes increased energy expenditure and decreased body weight (Plum et al., 2007), suggesting that the PI3K pathway may be downstream of leptin in the regulation of energy homeostasis. However, the critical brain sites where the PI3K pathway mediates leptin signal to regulate energy homeostasis have not been fully explored. We found that in p110αlox/lox control mice, intracerebroventricular (i.c.v.) injections of leptin significantly inhibited food intake by decreasing both meal size and meal frequency over a 24 hr period (Figs. 4a–4c). In contrast, in p110αlox/lox/SF1-Cre mice leptin-induced inhibition of food intake was significantly blunted (Fig. 4a). Leptin-induced reduction in meal size treaded to be blunted (Fig. 4b), while effects of leptin on meal frequency remained unchanged (Fig. 4c). Administration of leptin also promoted fat oxidation in control mice, demonstrated by decreased respiratory exchange rate (RER), while this effect was significantly blunted in p110αlox/lox/SF1-Cre mice (Fig. 4d). Body weight of p110αlox/lox mice was significantly reduced 24 hr after leptin injections, whereas acute leptin administration did not reduce body weight of p110αlox/lox/SF1-Cre mice (Fig. 4e). These results indicate that PI3K signalling in VMH neurons is required for mediating acute effects of exogenous leptin on energy homeostasis.
Fig. 4.
Deletion of p110α in SF1 neurons blunts the anorexigenic effects of central leptin. Five-month old chow-fed male mice (n=4 or 6/genotype) were matched for body weight, and received saline (1 μl, i.c.v.) at 16:00 followed by leptin (6 μg in 1 μl saline, i.c.v.) 24 hr later. Leptin-induced reductions in food intake (a), meal size (b), meal frequency (c) and RER (d) were monitored using the TSE metabolic chambers. (e) Leptin-induced weight loss was measured. Data are presented as mean ± SEM, and * P<0.05 and **P<0.01 between p110αlox/lox/SF1-Cre mice and p110αlox/lox mice.
We have identified PI3K-p110α as a mediator downstream of leptin receptor activation in SF1 neurons. However, it is notable that we observed differences between mice lacking PI3K in SF1 neurons compared to mice lacking leptin receptors in these neurons. For instance, p110αlox/lox/SF1-Cre mice do not show hyperphagia, although the acute appetite-suppressing responses to exogenous leptin at a pharmacological dose are blunted in these mice, suggesting that other signaling pathways may exist, which compensate for impaired PI3K signaling under resting conditions. While mice lacking leptin receptors in SF1 neurons develop obesity on both regular chow and HFD (Bingham et al., 2008; Dhillon et al., 2006), p110αlox/lox/SF1-Cre mice show increased sensitivity to diet-induced obesity but maintain normal body weight when fed with regular chow. Moreover, SF1-specific deletion of leptin receptors causes insulin resistance before onset of obesity (Bingham et al., 2008), whereas p110αlox/lox/SF1-Cre mice do not show deficits in glucose homeostasis (Supple Fig. 2). Therefore, it is likely that leptin actions in SF1 neurons are also partly mediated by other signalling pathways, such as the Jak-Stat3 or ERK pathways (Robertson et al., 2008). Indeed, SF1-specific deletion of suppressor of cytokine signaling-3 (Socs-3), a potent feedback inhibitor of the leptin-induced Jak-Stat3 pathway (Bjorbaek et al., 1998; Howard et al., 2004), leads to improved glucose homeostasis (Zhang et al., 2008), supporting the hypothesis that the Jak-Stat3 pathway may mediate leptin actions in the SF1 neurons to control glycemic balance. In summary, our results indicate that PI3K activity in VMH neurons plays a physiologically relevant role in the regulation of energy expenditure which may play a key role in the physiological response to excess intake of calories. Moreover, it will be interesting to assess whether this response is altered during the development of obesity.
Experimental Procedures
Animal Care
Care of all animals and procedures were approved by the UT Southwestern Medical Center. Mice were housed in a temperature-controlled environment in groups of two to four at 22°C–24°C using a 12 hr light/12 hr dark cycle. The mice were fed either standard chow (4% fat, #7001, Harlan-Teklad, Madison, WI) or HFD (42% fat, #88137, Harlan Teklad) and water was provided ad libitum. Study animals were male offspring of crosses between p110αlox/lox/SF1-Cre mice and p110αlox/lox mice. All the mice were on a mixed C57BL/6J;129S6/SvEv background.
Body weight, food intake and body composition
Body weight was measured weekly from group housed mice. Daily food intake was measured in individually housed mice. Body composition was determined using quantitative magnetic resonance (QMR) (Bruker’s Minispec MQ10, Houston, TX).
Metabolic Chambers
As described before (Xu et al., 2008), meal pattern, physical activity and energy expenditure were monitored using a combined indirect calorimetry system (TSE Systems GmbH, Bad Homburg, Germany) in young chow-fed or HFD-fed mice. Mice were housed individually at room temperature (22°C) under an alternating 12:12-h light-dark cycle. After adaptation for 6 days, physical activity was determined for 5 days using a multi-dimensional infrared light beam system with beams installed on cage bottom and cage top levels. Ambulatory movement was defined as breaks of any two different light beams at cage bottom level, while rearing was recorded when the mouse broke any light beam at the top levels. Simultaneously, O2 consumption, CO2 production and heat production were measured and normalized by metabolic body size (body weight0.75) to determine the energy expenditure. In addition, meal patterns were determined continuously by integration of weighing sensors fixed at the top of the cage from which the food containers have been suspended into the sealed cage environment. Meals were defined as food intake events with a minimum duration of 60 seconds, and a break of 300 second between food intake events.
To assess diet-induced thermogenesis, 6-month old chow-fed mice with matched body weight were acclimatized in the TSE metabolic chambers as described above, followed by continuous monitoring of energy expenditure for 3 days. Chow was provided from day 1, and replaced by HFD at 17:00 of day 2 (2 hr prior to the start of dark cycle). Body weight was measured at 17:00 on the 3 successive days.
To assess the thermogenic responses to food deprivation, 5-month old mice with matched body weight were acclimatized in the TSE metabolic chambers, followed by continuous monitoring of energy expenditure for 3 days. Chow was provided from day 1, and removed at 17:00 of day 2. Body weight was measured at 17:00 on the 3 successive days.
Responses to i.c.v. leptin
Stainless steel cannulae were inserted into the lateral ventricles (0.34 mm caudal and 1 mm lateral from bregma; depth, 2.3 mm) of anesthetized mice (100 mg/kg ketamine hydrochloride and 6 mg/kg Xylazine i.p.). Mice were allowed to recover for at least 1 week before i.c.v. injection and were not used until they regained their pre-surgery weights. Cannulation placement was confirmed by demonstration of increased thirst after administration of angiotensin (10 ng).
Cannulated mice were acclimatized in the TSE metabolic chambers provided with chow, followed by continuous monitoring of energy expenditure and food intake for 4 days. Saline (1 μl) was i.c.v. injected at 16:00 of day 2; leptin (6 μg in 1 μl saline) was i.c.v. injected at 16:00 of day 3. Body weight was measured at 16:00 of day 2 to day 4 (The dark cycle started at 18:00 at the time of experiment).
Statistics
The data are presented as mean ± SEM. Statistical analyses were performed using SigmaStat 2.03. After confirming normal distribution of data, comparisons between two genotypes were made by the unpaired two-tailed Student’s t-test; repeated-measures ANOVA were used to compare changes over time between two genotypes. P < 0.05 was considered to be statistically significant.
Supplementary Material
Acknowledgments
We would like to thank the Mouse Metabolic Phenotyping Core at UTSW Medical Center (supported by NIH PL1 DK081182 and UL1 RR024923). This work was supported by grants from Canadian Institute of Health Research (YX) and from the NIH (K99 DK085330 to YX, 1F32DK066972 to JWH, R01DK071051 to BBL, RL1 DK081185, R37DK53301 and R01DK071320 to JKE, and R01CA134502-01 to JJZ), by UTSW Medical Center Disease-Oriented Clinical Scholars Program (JMZ), by the V Foundation (JJZ), by an ADA research grant (1-07-RA-41 to JKE and LG), and by an ADA Smith Family Foundation Pinnacle Program Award to JKE.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bingham NC, Anderson KK, Reuter AL, Stallings NR, Parker KL. Selective loss of leptin receptors in the ventromedial hypothalamic nucleus results in increased adiposity and a metabolic syndrome. Endocrinology. 2008;149:2138–2148. doi: 10.1210/en.2007-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1:619–625. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- Buettner C, Muse ED, Cheng A, Chen L, Scherer T, Pocai A, Su K, Cheng B, Li X, Harvey-White J, Schwartz GJ, Kunos G, Rossetti L, Buettner C. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med. 2008;14:667–675. doi: 10.1038/nm1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AA, Kozak LP. A recurring problem with the analysis of energy expenditure in genetic models expressing lean and obese phenotypes. Diabetes. 2010;59:323–329. doi: 10.2337/db09-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Castaneda TR, Jurgens H, Wiedmer P, Pfluger P, Diano S, Horvath TL, Tang-Christensen M, Tschop MH. Obesity and the neuroendocrine control of energy homeostasis: the role of spontaneous locomotor activity. J Nutr. 2005;135:1314–1319. doi: 10.1093/jn/135.5.1314. [DOI] [PubMed] [Google Scholar]
- Dellovade TL, Young M, Ross EP, Henderson R, Caron K, Parker K, Tobet SA. Disruption of the gene encoding SF-1 alters the distribution of hypothalamic neuronal phenotypes. J Comp Neurol. 2000;423:579–589. doi: 10.1002/1096-9861(20000807)423:4<579::aid-cne4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S, Jr, Elmquist JK, Lowell BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Dulloo AG. Biomedicine. A sympathetic defense against obesity. Science. 2002;297:780–781. doi: 10.1126/science.1074923. [DOI] [PubMed] [Google Scholar]
- Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, Grove KL, Cowley MA. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 2007;5:181–194. doi: 10.1016/j.cmet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Foukas LC, Claret M, Pearce W, Okkenhaug K, Meek S, Peskett E, Sancho S, Smith AJ, Withers DJ, Vanhaesebroeck B. Critical role for the p110alpha phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature. 2006;441:366–370. doi: 10.1038/nature04694. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Jones JE, Olson D, Hill J, Lee CE, Gautron L, Choi M, Zigman JM, Lowell BB, Elmquist JK. Monitoring FoxO1 localization in chemically identified neurons. J Neurosci. 2008;28:13640–13648. doi: 10.1523/JNEUROSCI.4023-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold RM. Hypothalamic obesity: the myth of the ventromedial nucleus. Science. 1973;182:488–490. doi: 10.1126/science.182.4111.488. [DOI] [PubMed] [Google Scholar]
- Howard JK, Cave BJ, Oksanen LJ, Tzameli I, Bjorbaek C, Flier JS. Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat Med. 2004;10:734–738. doi: 10.1038/nm1072. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Luo X, Abbud R, Nilson JH, Parker KL. The nuclear receptor steroidogenic factor 1 is essential for the formation of the ventromedial hypothalamic nucleus. Mol Endocrinol. 1995;9:478–486. doi: 10.1210/mend.9.4.7659091. [DOI] [PubMed] [Google Scholar]
- Inoue S, Campfield LA, Bray GA. Comparison of metabolic alterations in hypothalamic and high fat diet-induced obesity. Am J Physiol. 1977;233:R162–168. doi: 10.1152/ajpregu.1977.233.3.R162. [DOI] [PubMed] [Google Scholar]
- Kageyama H, Osaka T, Kageyama A, Kawada T, Hirano T, Oka J, Miura M, Namba Y, Ricquier D, Shioda S, Inoue S. Fasting increases gene expressions of uncoupling proteins and peroxisome proliferator-activated receptor-gamma in brown adipose tissue of ventromedial hypothalamus-lesioned rats. Life Sci. 2003;72:3035–3046. doi: 10.1016/s0024-3205(03)00225-x. [DOI] [PubMed] [Google Scholar]
- King BM. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol Behav. 2006;87:221–244. doi: 10.1016/j.physbeh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Knight ZA, Gonzalez B, Feldman ME, Zunder ER, Goldenberg DD, Williams O, Loewith R, Stokoe D, Balla A, Toth B, Balla T, Weiss WA, Williams RL, Shokat KM. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell. 2006;125:733–747. doi: 10.1016/j.cell.2006.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell BB, Bachman ES. Beta-Adrenergic receptors, diet-induced thermogenesis, and obesity. J Biol Chem. 2003;278:29385–29388. doi: 10.1074/jbc.R300011200. [DOI] [PubMed] [Google Scholar]
- Majdic G, Young M, Gomez-Sanchez E, Anderson P, Szczepaniak LS, Dobbins RL, McGarry JD, Parker KL. Knockout mice lacking steroidogenic factor 1 are a novel genetic model of hypothalamic obesity. Endocrinology. 2002;143:607–614. doi: 10.1210/endo.143.2.8652. [DOI] [PubMed] [Google Scholar]
- Malyala A, Zhang C, Bryant DN, Kelly MJ, Ronnekleiv OK. PI3K signaling effects in hypothalamic neurons mediated by estrogen. J Comp Neurol. 2008;506:895–911. doi: 10.1002/cne.21584. [DOI] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, Kaplitt MG, Ogawa S. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci U S A. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niijima A, Rohner-Jeanrenaud F, Jeanrenaud B. Role of ventromedial hypothalamus on sympathetic efferents of brown adipose tissue. Am J Physiol. 1984;247:R650–654. doi: 10.1152/ajpregu.1984.247.4.R650. [DOI] [PubMed] [Google Scholar]
- Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Jr, Schwartz MW. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413:794–795. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- Oku J, Bray GA, Fisler JS, Schemmel R. Ventromedial hypothalamic knife-cut lesions in rats resistant to dietary obesity. Am J Physiol. 1984;246:R943–948. doi: 10.1152/ajpregu.1984.246.6.R943. [DOI] [PubMed] [Google Scholar]
- Plum L, Rother E, Munzberg H, Wunderlich FT, Morgan DA, Hampel B, Shanabrough M, Janoschek R, Konner AC, Alber J, Suzuki A, Krone W, Horvath TL, Rahmouni K, Bruning JC. Enhanced leptin-stimulated Pi3k activation in the CNS promotes white adipose tissue transdifferentiation. Cell Metab. 2007;6:431–445. doi: 10.1016/j.cmet.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Reilly MP, Rader DJ. The metabolic syndrome: more than the sum of its parts? Circulation. 2003;108:1546–1551. doi: 10.1161/01.CIR.0000088846.10655.E0. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Leinninger GM, Myers MG., Jr Molecular and neural mediators of leptin action. Physiol Behav. 2008;94:637–642. doi: 10.1016/j.physbeh.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Minokoshi Y, Shimazu T. Accelerated norepinephrine turnover in peripheral tissues after ventromedial hypothalamic stimulation in rats. Brain Res. 1989;481:298–303. doi: 10.1016/0006-8993(89)90806-8. [DOI] [PubMed] [Google Scholar]
- Sakaguchi T, Bray GA, Eddlestone G. Sympathetic activity following paraventricular or ventromedial hypothalamic lesions in rats. Brain Res Bull. 1988;20:461–465. doi: 10.1016/0361-9230(88)90135-9. [DOI] [PubMed] [Google Scholar]
- Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6:38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell H, Deshaies Y, Richard D. The brown adipocyte: update on its metabolic role. Int J Biochem Cell Biol. 2004;36:2098–2104. doi: 10.1016/j.biocel.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Shi H, Akunuru S, Bierman JC, Hodge KM, Mitchell MC, Foster MT, Seeley RJ, Reizes O. Diet-induced obese mice are leptin insufficient after weight reduction. Obesity (Silver Spring) 2009;17:1702–1709. doi: 10.1038/oby.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger TJ, Calderon GA, Bradley LC, Sena-Esteves M, Rios M. Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J Neurosci. 2007;27:14265–14274. doi: 10.1523/JNEUROSCI.3308-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Tuig JG, Kerner J, Romsos DR. Hypothalamic obesity, brown adipose tissue, and sympathoadrenal activity in rats. Am J Physiol. 1985;248:E607–617. doi: 10.1152/ajpendo.1985.248.5.E607. [DOI] [PubMed] [Google Scholar]
- Xu Y, Jones JE, Kohno D, Williams KW, Lee CE, Choi MJ, Anderson JG, Heisler LK, Zigman JM, Lowell BB, Elmquist JK. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron. 2008;60:582–589. doi: 10.1016/j.neuron.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii A, Constantine-Paton M. BDNF induces transport of PSD-95 to dendrites through PI3K-AKT signaling after NMDA receptor activation. Nat Neurosci. 2007;10:702–711. doi: 10.1038/nn1903. [DOI] [PubMed] [Google Scholar]
- Zhang R, Dhillon H, Yin H, Yoshimura A, Lowell BB, Maratos-Flier E, Flier JS. Selective inactivation of Socs3 in SF1 neurons improves glucose homeostasis without affecting body weight. Endocrinology. 2008;149:5654–5661. doi: 10.1210/en.2008-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao AZ, Huan JN, Gupta S, Pal R, Sahu A. A phosphatidylinositol 3-kinase phosphodiesterase 3B-cyclic AMP pathway in hypothalamic action of leptin on feeding. Nat Neurosci. 2002;5:727–728. doi: 10.1038/nn885. [DOI] [PubMed] [Google Scholar]
- Zhao JJ, Cheng H, Jia S, Wang L, Gjoerup OV, Mikami A, Roberts TM. The p110alpha isoform of PI3K is essential for proper growth factor signaling and oncogenic transformation. Proc Natl Acad Sci U S A. 2006;103:16296–16300. doi: 10.1073/pnas.0607899103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Bakke M, Krimkevich Y, Cushman LJ, Parlow AF, Camper SA, Parker KL. Steroidogenic factor 1 (SF1) is essential for pituitary gonadotrope function. Development. 2001;128:147–154. doi: 10.1242/dev.128.2.147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.