Abstract
In a prospective randomized, controlled trial of ART during TB treatment versus TB treatment alone in Ugandan patients with CD4 >350 cells/μL, ART did not accelerate microbiologic, radiographic or clinical responses to TB therapy. 18% of participants had positive sputum smears after 5 months of TB therapy despite negative cultures.
Keywords: Tuberculosis, HIV, antiretroviral therapy
INTRODUCTION
Recent World Health Organization (WHO) antiretroviral therapy (ART) guidelines recommend ART in all persons with active TB regardless of CD4 cell count to delay HIV disease progression [1]. We sought to determine if ART during TB therapy accelerates clinical, radiographic and microbiologic TB treatment outcomes compared to TB therapy alone in HIV-infected pulmonary TB cases with CD4 counts >350 cells/μL in a prospective, randomized controlled trial in Uganda. We also examined cases with sputum smears positive for acid fast bacilli (AFB) at month five of TB therapy to determine if these patients were at higher risk of TB treatment failure or recurrence, defined by culture results. In the absence of TB culture, WHO guidelines recommend classifying patients with positive AFB smears during the fifth month of TB therapy as treatment failures [2].
METHODS
Study design
HIV-infected, ART-naïve adults with CD4 >350 cells/μL and newly diagnosed pulmonary TB at the National Tuberculosis and Leprosy Programme clinic in Kampala, were eligible for the Punctuated Antiretroviral Therapy (PART) randomized, controlled trial. All participants received isoniazid, rifampicin, ethambutol and pyrazinamide for two months, then isoniazid and rifampicin for four months. The intervention arm initiated six months of ART (abacavir/zidovudine/lamivudine) two to four weeks after starting TB therapy, while control arm subjects initiated ART if CD4 was <250 cells/μL during follow-up. Institutional review boards in San Francisco and Kampala approved this study.
Diagnostics
Three sputum specimens were obtained for AFB smear and Mycobacterium tuberculosis (MTB) culture at baseline, and 1, 2, 5, 12 and 18 months after initiating TB therapy. Smear and culture negative subjects were indicated if radiography and symptoms were consistent with TB.
Study outcomes
TB treatment outcomes included time to conversion of smears and cultures to negative, treatment failure, recurrence, paradoxical reactions as defined by Narita, et al [3], radiographic changes read by a blinded study radiologist at 1, 6, 12 and 18 months after TB treatment initiation, and symptoms recorded during regular follow-up visits over 48 months. Treatment failure was defined as MTB growth in sputum culture during the fifth month of TB therapy, independent of smear results [2]. Recurrence was defined as symptomatic or radiographic worsening, and new MTB growth in sputum culture in a patient with clinical improvement and negative cultures after completing TB treatment [2]. We hypothesized that ART would accelerate microbiologic, radiographic and clinical TB treatment response, and reduce TB treatment failures and recurrences.
RESULTS
The intervention (n=109) and control arms (n=114) had similar baseline characteristics, aside from mediastinal adenopathy (Table). Median duration of follow-up was similar in both arms (22 months, IQR: 14–34).
Table.
Baseline clinical, microbiologic and radiographic characteristics by study arm.
| Control Arm N=114 (%) | Intervention Arm N=109 (%) | p1 | |
|---|---|---|---|
| Baseline Clinical Characteristics | |||
| Age (mean) | 33 | 31 | 0.222 |
| Male sex | 70 (61) | 56 (51) | 0.13 |
| CD4 [cells/μL] (median) | 535 | 516 | 0.513 |
| Viral Load [log] (median) | 4.6 | 4.6 | 0.983 |
| Cough | 114 (100) | 107 (98) | 0.24 |
| Purulent Sputum | 81 (71) | 83 (76) | 0.39 |
| Hemoptysis | 11 (10) | 11 (10) | 0.91 |
| Sweats | 85 (75) | 69 (63) | 0.07 |
| Fever | 84 (74) | 79 (73) | 0.84 |
| Weight Loss | 91 (80) | 81 (74) | 0.33 |
| Baseline TB Microbiology | |||
| MTB Culture Positive | 98/113 (87) | 98/108 (91) | 0.35 |
| AFB Smear Positive | 101/113 (89) | 103/108 (95) | 0.13 |
| Isoniazid Resistance | 4/92 (4) | 3/95 (3) | 0.67 |
| Multi-drug Resistance | 0 | 0 | |
| Baseline Radiographic Findings | |||
| Upper Lobe Infiltrate | 93 (82) | 89 (82) | 0.99 |
| Lower Lobe Infiltrate | 84 (74) | 83 (76) | 0.67 |
| Fibrosis | 14 (12) | 9 (8) | 0.32 |
| Cavitary Disease | 63 (55) | 73 (67) | 0.07 |
| Miliary Disease | 4 (4) | 3 (3) | 1 |
| Adenopathy | 6 (5) | 0 | 0.03 |
| Effusion | 11 (10) | 4 (4) | 0.08 |
| Pleural Thickening | 5 (4) | 3 (3) | 0.51 |
| Extent of lung disease | 0.39 | ||
| Normal | 5 (4) | 2 (2) | |
| Minimal | 19 (17) | 18 (16) | |
| Moderate | 36 (32) | 27 (25) | |
| Advanced | 54 (47) | 62 (57) | |
χ2 test unless otherwise indicated.
t-test.
Wilcoxon rank sum test.
Microbiologic Outcomes
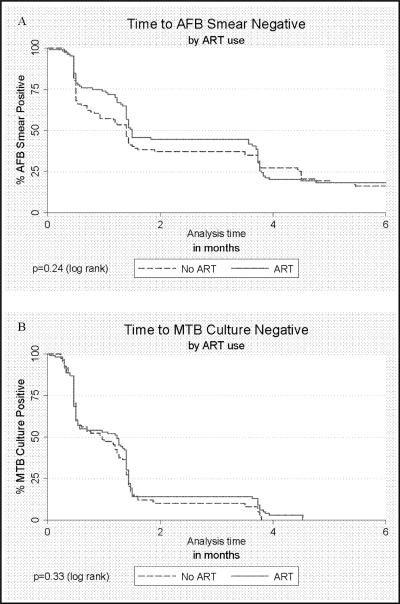
High rates of HIV RNA suppression were achieved in the intervention arm after 6 months of ART, with 89 of 103 (86.4%) achieving a viral load <400 copies/mL. Median time to conversion to negative MTB culture did not differ significantly in the intervention (38 days) vs. control arm (29 days; log rank p=0.37). Similarly, median time to conversion to negative AFB smears did not differ significantly in the intervention (43 days) vs. control arms (42 days, log rank p=0.27; Figure). No treatment failures occurred in either arm. There were three recurrences in the intervention arm and four in the control arm (p=0.5).
Figure 1.
Time to acid fastbacilli (AFB) smear (A) and M. tuberculosis (MTB) culture (B) conversion to negative among smear and culture positive HIV-infectedpulmonary TB cases with CD4>350 cells/μL according to antiretroviral therapy (ART) use during TB therapy.
Radiographic Outcomes
At TB treatment completion, chest x-rays were similar in both arms, though a greater proportion of lower lobe infiltrates was seen in the intervention vs. control arm (8% vs. 0%, p=0.03). This latter finding did not persist at month 12.
Clinical Outcomes
At month 6, the control arm had more cough (43% vs. 25%, p=0.007), productive sputum (21% vs. 9%, p=0.02), dyspnea (6% vs. 0%, p=0.02) and rash (13% vs. 3%, p=0.014) than the intervention arm. The most common causes of respiratory symptoms (41%) in the control arm were non-TB pneumonia and upper respiratory infections. By month 12, there were no significant symptomatic differences between study arms. No paradoxical reactions were observed.
Persistently positive AFB smears
30 of 165 (18%) participants in whom sputum specimens were obtained at month five were AFB smear positive (control: 14/79 (18%) and intervention arm: 16/86 (19%), [p=0.88]); all were culture negative. All 30 participants were smear positive at baseline; 83% had 3+ bacilli on baseline smear, whereas by month five, 3% had 3+ and 63% had 1+ bacilli. None had baseline INH resistance. Of the 28 with smears performed at month 12 or 18, 25 were smear negative and 3 had recurrent TB. Compared to participants who converted their smears to negative by month 5, persistently smear positive cases were more likely to be culture positive at baseline (100% vs. 87%, p=0.02), to have baseline x-rays with cavitation (83% vs. 60%, p=0.01), pleural thickening (13% vs. 2%, p=0.02) and upper lobe infiltrates (97% vs. 80%, p=0.02), to be male (73% vs. 48%, p=0.01) and to have ever smoked tobacco (50% vs. 26%, p=0.009) on univariate analysis. On multivariate analysis, only cavitation (OR: 3.3, 95% CI: 1.1–10.1, p=0.03) and pleural thickening (OR: 7.2, 95% CI: 1.5–34.6, p=0.01) were independently associated with persistent smear positivity. There were no significant differences in culture conversion time, mean baseline CD4 count, or baseline symptoms between month 5 smear positive and negative groups. TB treatment failure and recurrence rates were similar between the groups.
DISCUSSION
In a prospective, randomized controlled trial, we found that triple nucleoside ART does not accelerate microbiologic, clinical or radiographic improvement during TB therapy in HIV/TB patients with high CD4 counts. We found a high proportion of patients with persistent AFB in sputum smear late in TB therapy despite sputum culture conversion; a finding associated with cavitation and pleural thickening on baseline chest x-ray but not ART use. Smear positive, culture negative status at month 5 was not associated with TB treatment failure or recurrence.
Few studies have evaluated the impact of ART on TB treatment response among co-infected patients with high CD4 counts. Given the central role of T cells in containing TB infection and developing TB-specific immunity [4], we hypothesized that ART-induced immune maintenance and recovery would accelerate the clinical, radiographic and microbiologic responses to TB treatment. As new guidelines recommend ART in all TB/HIV patients, the role of ART in TB treatment response in patients with high CD4 counts is particularly relevant [1].
Comparisons by HIV status in the pre-ART era suggested that HIV does not affect the likelihood of responding to TB treatment [5, 6], but were limited by the competing risk of non-TB death at low CD4 counts. In one post-ART era study, ART was associated with more rapid culture and smear conversion [7], although this observational study may have been subject to selection bias and residual confounding on factors associated with ART use. Our randomized, controlled trial found no impact of ART on TB treatment response. Explanations of our findings include the possibility that the efficacy and speed of response to TB therapy are not dependent upon T cell-mediated immunity. Alternatively, ART-induced T cell recovery with subsequent TB-specific immunity may take longer than 6 months to develop. It is possible that ART containing non-nucleoside reverse transcriptase inhibitors or protease inhibitors (regimens that are more virologically potent than triple nucleoside ART [8]) might accelerate response to TB therapy, though this seems unlikely given the high rates of virologic suppression achieved in the intervention arm. Though we found no benefit to ART in terms of TB treatment outcomes at high CD4 counts, multiple advantages to early ART initiation have been found including decreased TB incidence with CD4 cell recovery to >500 [9] and reduced all-cause mortality [10].
AFB smear positivity late in TB therapy occurred commonly in our study, despite TB culture clearance in all study participants and high rates of TB cure. TB/HIV patients with high CD4 counts tend to have a higher pulmonary bacillary load at diagnosis than patients with low CD4 counts (<350) [11] and may be at higher risk for this phenomenon. Smear positivity with negative culture late in therapy is well-described in HIV-uninfected patients and has been attributed to the persistence of non-viable MTB, or to colonization with non-tuberculous mycobacteria (NTM) [12]. Patients in this study were not colonized with NTM suggesting persistence of non-viable MTB. Similar to studies of HIV-uninfected TB, we found persistent smear positivity to be associated with cavitary disease, likely reflecting slow clearance of MTB bacilli from the airway due to a high baseline bacillary burden [13]. Pleural thickening was also associated with late smear positivity, possibly due to greater parenchymal involvement in disease extending to the pleura.
In settings lacking MTB culture, WHO guidelines recommend classifying patients with positive AFB smears during the fifth month of TB therapy as treatment failures [2]. Our results suggest that in settings where smear alone is used for follow-up during TB treatment, or where PCR-based testing may supplant culture during follow-up, there is significant potential for misclassification of patients as treatment failures despite a high likelihood of future cure. Patients in this study received a standard 6 month course of TB therapy guided by negative cultures. TB therapy was not extended even in patients with positive smears. There was no evidence of excess treatment failure or recurrence in these subjects who had close follow-up although our study was underpowered to detect small differences in the groups. Further studies are needed to determine how to utilize current TB diagnostics to monitor treatment response in settings with limited access to TB culture.
ACKNOWLEDGMENTS
The authors thank the study participants, as well as Mike Odie, Pam Murnane and the PART study team. This work was supported in part by the NIH/NIAID (K-24 A151982 [Havlir]), the Traineeships in AIDS Prevention Studies, NIMH (T32 MH-19105-21 [Kegeles]), and The PART study (Grant Number: AI051219-0607 [Whalen]).
Footnotes
The clinical trials registration number for the study described in this manuscript is: NCT00078247.
The authors do not have any conflicts of interest.
REFERENCES
- 1.World Health Organization Rapid advice: antiretroviral therapy for HIV infection in adults and adolescents. 2009 November;:1–25. [Google Scholar]
- 2.Revised international definitions in tuberculosis control. Int J Tuberc Lung Dis. 2001 Mar;5(3):213–5. [PubMed] [Google Scholar]
- 3.Narita M, Ashkin D, Hollender ES, Pitchenik AE. Paradoxical worsening of tuberculosis following antiretroviral therapy in patients with AIDS. American journal of respiratory and critical care medicine. 1998 Jul;158(1):157–61. doi: 10.1164/ajrccm.158.1.9712001. [DOI] [PubMed] [Google Scholar]
- 4.Boom WH, Canaday D, Fulton S, Gehring A, Rojas R, Torres M. Human Immunity to M. Tuberculosis: T cell subsets and antigen processing. Tuberculosis. 2003;83:98–106. doi: 10.1016/s1472-9792(02)00054-9. [DOI] [PubMed] [Google Scholar]
- 5.Murray J, Sonnenberg P, Shearer SC, Godfrey-Faussett P. Human immunodeficiency virus and the outcome of treatment for new and recurrent pulmonary tuberculosis in African patients. American journal of respiratory and critical care medicine. 1999 Mar;159(3):733–40. doi: 10.1164/ajrccm.159.3.9804147. [DOI] [PubMed] [Google Scholar]
- 6.Perriens JH, St Louis ME, Mukadi YB, et al. Pulmonary tuberculosis in HIV-infected patients in Zaire. A controlled trial of treatment for either 6 or 12 months. The New England journal of medicine. 1995 Mar 23;332(12):779–84. doi: 10.1056/NEJM199503233321204. [DOI] [PubMed] [Google Scholar]
- 7.Nahid P, Gonzalez LC, Rudoy I, et al. Treatment outcomes of patients with HIV and tuberculosis. American journal of respiratory and critical care medicine. 2007 Jun 1;175(11):1199–206. doi: 10.1164/rccm.200509-1529OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gulick RM, Ribaudo HJ, Shikuma CM, et al. Triple-nucleoside regimens versus efavirenz-containing regimens for the initial treatment of HIV-1 infection. The New England journal of medicine. 2004 Apr 29;350(18):1850–61. doi: 10.1056/NEJMoa031772. [DOI] [PubMed] [Google Scholar]
- 9.Lawn SD, Myer L, Edwards D, Bekker LG, Wood R. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS (London, England) 2009 Aug 24;23(13):1717–25. doi: 10.1097/QAD.0b013e32832d3b6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. The New England journal of medicine. 2009 Apr 30;360(18):1815–26. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mugusi F, Villamor E, Urassa W, Saathoff E, Bosch RJ, Fawzi WW. HIV co-infection, CD4 cell counts and clinical correlates of bacillary density in pulmonary tuberculosis. Int J Tuberc Lung Dis. 2006 Jun;10(6):663–9. [PubMed] [Google Scholar]
- 12.Vidal R, Martin-Casabona N, Juan A, Falgueras T, Miravitlles M. Incidence and significance of acid-fast bacilli in sputum smears at the end of antituberculous treatment. Chest. 1996 Jun;109(6):1562–5. doi: 10.1378/chest.109.6.1562. [DOI] [PubMed] [Google Scholar]
- 13.Palaci M, Dietze R, Hadad DJ, et al. Cavitary disease and quantitative sputum bacillary load in cases of pulmonary tuberculosis. Journal of clinical microbiology. 2007 Dec;45(12):4064–6. doi: 10.1128/JCM.01780-07. [DOI] [PMC free article] [PubMed] [Google Scholar]