Abstract
The formation of mammalian secondary palate requires a series of developmental events such as growth, elevation and fusion. Despite recent advances in the field of palate development, the process of palate elevation remains poorly understood. The current consensus on palate elevation is that the distal end of the vertical palatal shelf corresponds to the medial edge of the elevated horizontal palatal shelf. We provide evidence suggesting that the prospective medial edge of the vertical palate is located toward the interior side (the side adjacent to the tongue), instead of the distal end, of the vertical palatal shelf and that the horizontal palatal axis is generated through palatal outgrowth from the side of the vertical palatal shelf rather than rotating the pre-existing vertical axis orthogonally. Since palate elevation represents a classical example of embryonic tissue re-orientation, our findings here may also shed light on the process of tissue re-orientation in general.
Keywords: palate development, palate elevation, tissue re-orientation
Introduction
The formation of mammalian secondary palate involves multiple developmental events, including growth, elevation and fusion (Ferguson, 1988; Hilliard et al., 2005; Chai and Maxson, 2006; Gritli-Linde, 2007). During mouse development, the two developing palatal shelves first grow vertically along the lateral sides of the tongue on embryonic days 12.5 and 13.5 (E12.5 and E13.5) (Ferguson, 1988; Murray and Schutte, 2004; Gritli-Linde, 2007). On E14.5, however, the two vertical palatal shelves re-orientate to a horizontal position above the dorsal side of the tongue, a process termed palate elevation (Ferguson, 1988; Gritli-Linde, 2007). The two elevated palatal shelves then grow horizontally towards each other to meet along the facial midline and form the medial edge epithelial (MEE) seam. The MEE seam then undergoes degeneration giving rise to a fused palate with mesenchymal confluence, a process referred to as palate fusion (Ferguson, 1988; Hilliard et al., 2005).
Significant efforts have been made to understand the cellular mechanisms of palate fusion, especially the degeneration of the MEE seam. A number of lineage tracing studies have suggested that MEE seam degeneration is mediated by epithelial-mesemchymal transition (EMT)(Griffith and Hay, 1992), cell migration (Carette and Ferguson, 1992), apoptotic cell death (Cuervo and Covarrubias, 2004; Vaziri Sani et al., 2005), or a combination of all three (Jin and Ding, 2006a). At the molecular level, Smad2 mediated TGF-β3 signaling has been demonstrated to be essential for palate fusion (Kaartinen et al., 1995; Proetzel et al., 1995; Cui et al., 2005). Consistently, epithelial- specific deletion of the TGF-β type II receptor gene also causes cleft palate in mouse embryos (Xu et al., 2006).Conversely, Jagged2 (Jag2) expression may prevent epithelial fusion because Jag2 mutant embryos display premature fusion of the palatal shelves with the tongue, probably due to precocious differentiation of periderm cells (Jiang et al., 1998; Casey et al., 2006). It has been reported that Msx1 and BMP4 are responsible for anterior palate growth (Zhang et al., 2002). Other regulatory factors involved in mouse palatal growth include the BMP receptor 1A (Alk3) (Liu et al., 2005), the growth factor PDGF-C (Ding et al., 2004) and the transcription factor Osr2 (Lan et al., 2004).
In contrast to palatal growth and fusion, the mechanism of palate elevation is poorly understood and little progress has been made in the past decades (Gritli-Linde, 2007). The current consensus on palate elevation is that the distal end of the vertical palatal shelf corresponds to the medial edge region in the horizontal palatal shelf (Gritli-Linde, 2007). In this model, the palatal shelves must overcome the physical obstruction imposed by the tongue during elevation. Previous studies have suggested that the forward displacement of the mandible and the tongue lowers the level of the tongue during palate elevation (Seegmiller and Fraser, 1977; Lavrin et al., 2001). However, this forward displacement of the tongue may not be sufficient to position the palatal shelf above the tongue. In fact, palate re-orientation does not require the dorsal level of the tongue to be below the distal end of the palatal shelf. It has been observed that the two palatal shelves do not elevate in unison, but sequentially in a very short time frame (Greene and Kochhar, 1973; Luke, 1984). Often one shelf is completely horizontal, whilst the other remains vertical with its distal end far below the dorsal level of the tongue (Greene and Kochhar, 1973; Luke, 1984), indicating that the palatal shelves can re-orient without moving above the dorsal level of the tongue. This observation also suggests that palate elevation involve complicated movements beyond a simple rotation. Consistent with this notion, previous histological studies observed that the palate shelves bulge out medially and squeeze into the space above the tongue, and it has been speculated that that palate shelves may “flow” over the tongue during elevation (Greene and Kochhar, 1973; Diewert and Tait, 1979). In addition, a recent in vitro study using carbon labeling suggested tissue remodeling in the anterior and posterior palate development (Chou et al., 2004). The mechanism driving the palate elevation is still poorly understood. However, a correlation between hyaluronic acid synthesis and palate elevation has been observed although a causative relationship has not been established (Ferguson, 1988). In addition, it has been recently reported that loss-of-function mutations in Snai1,2 and Wnt5A resulted in vertical palate shelves (Murray et al., 2007; He et al., 2008), indicating that the functions of these genes are involved in palate elevation.
We previously reported that Zfhx1a mutant mice displayed delayed palate elevation by 24 to 48 hours (Jin et al., 2008). In this study, we used Zfhx1a and Jag2 mutant mice to investigate the process of palate elevation and found that the prospective medial edge region is not located in the distal end of the vertical palatal shelf and the horizontal palatal axis is generated by palatal outgrowth from the side rather than rotating the pre-existing vertical axis orthogonally.
Results and Discussion
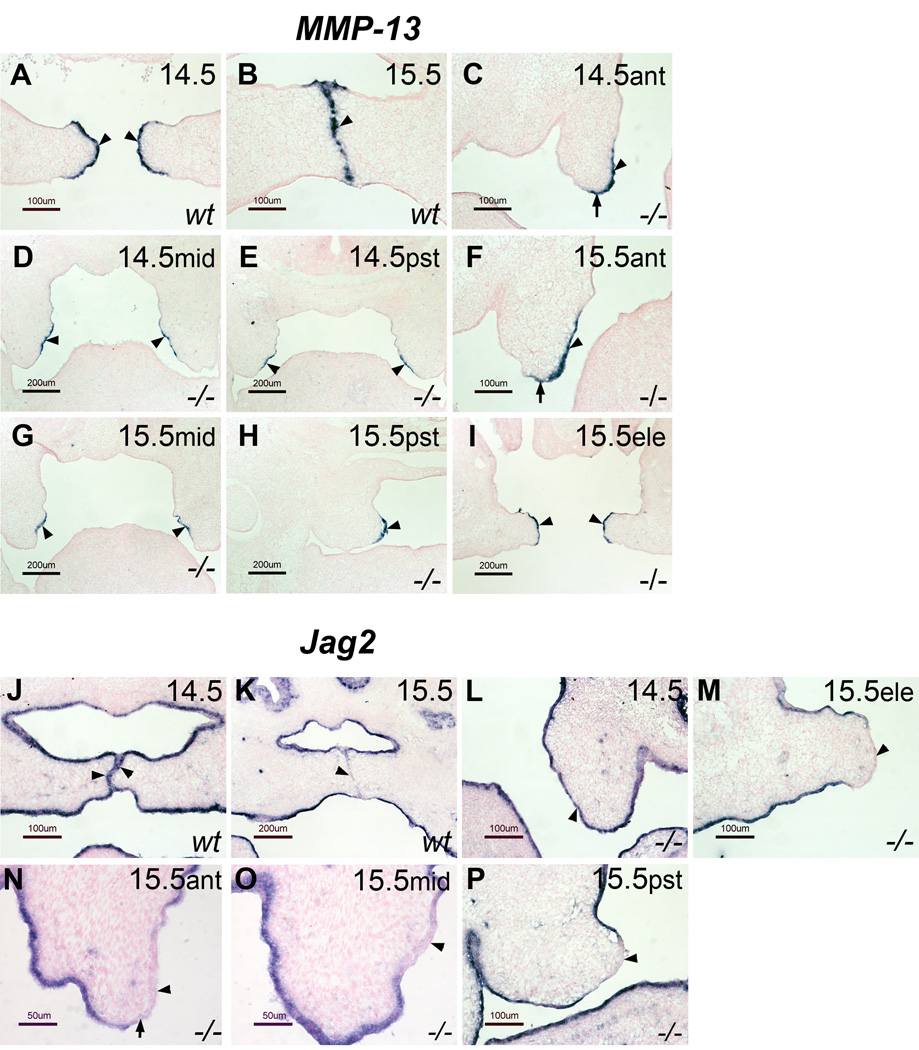
As mentioned above, the current model for palate elevation assumes that the distal end of the vertical palatal shelf corresponds to the medial edge region of the horizontal palatal shelf (see supplementary Fig. 1A and B). However, several observations prompted us to suspect that this assumption may not be correct. For example, in the posterior region of the vertical E13.5 palate, the prospective nasal surface is longer than the prospective oral surface. After palate re-orientation to a horizontal position on E14.5, however, the definitive nasal surface in the posterior region is shorter than the oral surface (Supplementary Fig.1C and D). This phenomenon is difficult to explain if the distal end of the vertical palate corresponds to the medial edge area of the horizontal palate. Similarly, it was reported before that the TGF-β3 expression domain on E14.5 is mainly located in the medial edge epithelium with equal extension to the oral and nasal surfaces, whereas on E13.5, when the palatal shelves are vertical, the TGF-β3 expression domain is mainly located within the prospective nasal epithelium, the surface facing the tongue (Fitzpatrick et al., 1990; Pelton et al., 1990). We therefore hypothesized that the prospective medial edge region of the vertical palate is located on the interior side, the side facing the tongue, rather than on the distal end, and that palate re-orientation is through horizontal outgrowth from the interior side of the vertical shelf towards the midline. To test this hypothesis, we used Zfhx-1a (also known as ZEB-1 and δEF1) mutant mice (Takagi et al., 1998; Postigo, 2003). Palate re-orientation occurs very rapidly within a short time window in wild type mice (Gritli-Linde, 2007), making it difficult to study the sequential events during re-orientation in detail. In our previous study, we reported that Zfhx-1a mutant embryos were delayed in palate elevation by 24–48 hours (Jin et al., 2008). Briefly, on E14.5, 100% of palatal shelves from Zfhx-1a mutants remain vertical along the entire length from anterior to posterior. On E15.5, 75% of the Zfhx-1a mutant palate shelves are vertical in the anterior and middle regions, but the extreme posterior region was proceeding to a horizontal position, while the other 25% were fully horizontal from anterior to posterior (Jin et al., 2008). Interestingly, regional specification of the medial edge epithelium occurred normally, as determined by both marker analysis and an in vitro fusion assay (Jin et al., 2008). Thus, the Zfhx-1a mutant line provides a model to examine medial edge epithelium (MEE) markers in an E14.5 palatal shelf that is still vertical. If our hypothesis is correct, the MEE specific markers of horizontal palates should be located on the interior side, rather than on the distal end, of E14.5 Zfhx-1a mutant vertical palates. In wild type embryos, MMP13 is not expressed in the vertical palate prior to E13.75 (data not shown), however, once the palatal shelves become horizontal on E14.5, MMP13 is expressed exclusively in the MEE and this MEE specific expression continues until early E15.5 before the MEE degenerates (Fig.1A and B), consistent with previous reports (Blavier et al., 2001; Jin et al., 2008). We therefore examined MMP13 expression in the vertical Zfhx-1a mutant palate on E14.5 and E15.5 to determine whether the expression is on the interior side or distal end of the mutant vertical palate. As shown in figure 1, the expression of MMP13 is exclusively located on the interior side of the E14.5 Zfhx-1a mutant vertical palatal shelves in the middle and posterior regions (Fig. 1D and E). In the most anterior region, the expression resides primarily within the interior side and covers only a portion of the distal tip (Fig. 1C). As mentioned previously, by E15.5, 25% of Zfhx-1a mutant palatal shelves were fully horizontal (Jin et al., 2008). In these embryos, MMP13 was exclusively expressed in the MEE region from anterior to posterior (Fig.1I). The other 75% remained vertical in the anterior and middle regions. In these mutants, MMP13 expression was exclusively located on the interior side in the middle vertical region (Fig. 1G), and in the most anterior region, the expression was primarily located on the interior side with slight extension to the distal end (Fig. 1F). The posterior region of these palates was proceeding to be horizontal and MMP13 expression was located in a region corresponding to the MEE (Fig. 1H). These results indicate that the prospective medial edge epithelium is located mainly on the interior side of the vertical palate, except for the most anterior region, where a portion of the distal region contributes to the future medial edge region. To further confirm this result, we also examined the expression of Jag2. In wild type embryos, the Jag2 expression domain covers the entire palate epithelium up to E14.5 (Casey et al., 2006; Jin et al., 2008). However, the expression is specifically down-regulated in the MEE around E15.0–E15.25 when palate fusion occurs (Casey et al., 2006; Jin et al., 2008). This down-regulation may be essential for palate fusion because loss of Jag2 promotes epithelial cell fusion (Jiang et al., 1998). We found that the down-regulation of Jag2 expression in the MEE region is independent of palate contact, since it occurs in the MEE region of elevated, but not touching, E15.5 Zfhx-1a mutant palates (Jin et al., 2008). Importantly, Jag2 down-regulation also occurs in those unelevated E15.5 Zfhx-1a mutant palates. Consistent with the MMP13 expression in Zfhx-1a mutant palates, a Jag2–free domain resides exclusively on the interior side in the middle region of the vertical palate (Fig. 1O). In the most anterior region of the vertical palates, Jag2 down-regulation was located mostly on the interior side with limited extension into the distal region (Fig.1N). The posterior region of these E15.5 mutant palates are proceeding to be horizontal, and the down-regulation of Jag2 occurs in the MEE region (Fig.1P). Based on these results, MMP13 expression and Jag2 down-regulation, we conclude that during palate development, the prospective MEE region is located on the interior side of the vertical palate before palate elevation/re-orientation, with the exception of the most anterior region where a portion of the distal region contributes to the MEE of horizontal palatal shelf. The identification of the prospective medial edge epithelium on the interior side, rather than the distal end, of the vertical palatal shelf raises the possibility that palate re-orientation results from outgrowth from the side of palatal shelf rather than a rotation of the vertical palatal axis. This question is particularly interesting because previous studies suggested that the shelves may “flow” over the tongue (Greene and Kochhar, 1973; Diewert and Tait, 1979). However, due to the lack of MEE markers at that time, these studies were unable to address the relationship between palate re-orientation and the prospective medial edge. We further examined MMP13 expression in Zfhx-1a mutant palates between E15.5 and E16.5 to determine the region of the developing MEE in relation to the outgrowth zone, and found the future MEE, marked by MMP13 expression, is immediately underneath the outgrowth tip (Supplementary Fig. 2A). We also observed “outgrowth tip/zone” in histological sections of embryos that undergo normal palate re-orientation (Supplementary Fig.2B and C), consistent with the observation from a previous study suggesting that the palate shelves may “flow” over the tongue during elevation (Greene and Kochhar, 1973).
Figure 1. MMP13 expression (A–I) and Jag2 down-regulation (J–P) in Zfhx-1a−/− embryos showing that the prospective MEE is located on the interior side of the vertical palate shelf.
(A) MMP 13 is expressed exclusively in the MEE of E14.5 wild type mouse palatal shelves (arrowheads). (B) MMP13 expression remains in the MEE of wild type palates on E15.5 (arrowhead). (C) In the anterior region of E14.5 Zfhx-1a−/− palates, MMP13 expression is located primarily on the interior side of the vertical palate (arrowhead), with limited extension to a portion of the distal end (arrow). (D and E) In the middle (D) and posterior (E) regions of E14.5 Zfhx-1a−/− palate, MMP13 expression is located exclusively on the interior surface (arrowheads). (F) In the anterior region of unelevated E15.5 Zfhx-1a mutant palates, MMP13 expression is located primarily on the interior surface of the vertical palate (arrowhead) with minimal extension to the distal end (arrow). (G) In the middle region of unelevated E15.5 Zfhx-1a mutant palates, MMP13 expression is found exclusively on the interior side of the vertical palate (arrowheads). (H) The posterior region of unelevated E15.5 Zfhx-1a mutant palate is proceeding to a horizontal position and MMP13 expression is located in the region corresponding to the MEE area (arrowhead). (I) MMP13 expression is located exclusively in the MEE area of elevated E15.5 Zfhx-1a mutant palate (arrowheads). (J) Jag2 is expressed in the epithelium surrounding the entire wild type palate shelf on E14.5 (arrowheads). (K) Jag2 expression is specifically down-regulated in the MEE region in wild type palate around E15.5 (arrowhead). (L) Jag2 expression surrounds the entire Zfhx-1a−/− palate shelf on E14.5 (arrowhead). (M) In the elevated E15.5 Zfhx-1a−/− palate, Jag2 is also specifically down-regulated in the MEE area (arrowhead). (N) In the anterior region of the unelevated E15.5 Zfhx-1a−/− palate, Jag2 is down-regulated on the interior side (arrowhead) and a portion of the distal tip (arrow). (O) In the middle region of unelevated E15.5 Zfhx-1a−/− palates, Jag2 down-regulation occurs exclusively on the interior side of the vertical palatal shelf (arrowhead). (P) The posterior region of unelevated E15.5 Zfhx-1a−/− palatal shelf is nearing a horizontal position and Jag2 down-regulation is found in the region corresponding to the MEE area (arrowhead). Ant: anterior; Mid: middle; Pst: posterior; Ele: elevated.
The outgrowth that converts the vertical shelf to the horizontal shelf should, in principle, be driven by the mesenchymal cells. We therefore examined the expression of goosecoid, a homeobox gene expressed specifically in the medial edge mesenchyme of the horizontal palatal shelves (Jin and Ding, 2006b). As shown in figure 2, goosecoid-expressing cells are organized as a condensed block adjacent to the MEE on E15.5 and E14.5 (Fig. 2A and B). This condensed mesenchyme cell block is visible by HE staining after E16.5 (Fig. 2E). However, the goosecoid-expressing cells present on E13.5 are diffuse (Fig. 2D), and become gradually restricted to the interior side of the vertical shelf on E13.75 (Fig. 2C). Thus, it appears that palate re-orientation is accompanied by mesenchymal cell condensation, and this process is delayed in Zfhx-1a mutant embryos (Fig. 2F, G and H). Cell movement during mesenchyme cell condensation can, in principle, trigger palatal outgrowth. However, it is also possible that the observed mesenchyme cell condensation is a result of palate re-orientation. To rule out this possibility, we examined mesenchyme cell condensation in Jag2 mutant embryos. Previous reports demonstrated that loss of Jag2 leads to abnormal fusion between the palate and tongue, physically preventing palate elevation (Jiang et al., 1998). If mesenchyme cell condensation is the consequence of palate re-orientation, goosecoid-expressing cells should not form a condensed block in the palates of Jag2 mutant embryos. Conversely, if it is a trigger of the outgrowth, mesenchyme cell condensation in Jag2 mutants may still occur. As shown in figure 2, the condensed mesenchyme cell block is present in Jag2 mutant palatal shelves on E15.5 and E16.5 as visualized by both goosecoid in situ hybridization and HE staining (Fig. 2I and J). Consistent with our hypothesis, the condensed cell block is located on the interior side of the vertical Jag2−/− palatal shelves on E15.5 and E16.5 (Fig. 2I and J)., In addition, Jag2 mutant palatal shelves bulged out toward the tongue on E15.5 and E16.5 (see arrowheads in Fig. 2I and J), indicating that palatal outgrowth indeed occurs. Moreover, in certain areas of Jag2 mutant embryos on E17.5, the palate shelves were almost horizontal with a medial edge-like region fused with the tongue (Fig. 2L and M), confirming that the interior side, rather than distal end, becomes the medial edge because the distal palate is never in contact with the tongue (Fig. 2K). Also, the formation of a horizontal-like palate in the Jag2 mutant embryos on E17.5 provides additional support for our hypothesis that the horizontal palate axis is generated by palate outgrowth from the side, since it would be impossible to rotate the palatal shelves in Jag2 mutants.
Figure 2. Mesenchymal cell condensation and palatal outgrowth during palate re-orientation.
(A and B) Goosecoid-expressing cells in the horizontal palates of wild type E15.5 (A) and E14.5 embryos (B) are organized as a condensed block in the medial edge area (arrows). (C) Goosecoid expressing cells are formed as a band restricted to the interior side of wild type palate on E13.75 (arrows). (D) Goosecoid-expressing cells in E13.5 wild type palates are more widely distributed (arrows). (E) HE staining showing the high density of cells in the medial edge area (arrows). (F) Goosecoid-expressing cells are organized as a condensed block in the elevated E15.5 Zfhx-1a−/− mutant palates (arrows). (G and H) Goosecoid-expressing cells in unelevated E15.5 (G) and E14.5 (H) Zfhx-1a mutant palates are confined in a strip restricted to interior side, similar to E13.75 wild type palates. (I) Goosecoid expression showing condensed cells (arrow) formed on the interior side of E15.5 Jag2−/− palatal shelves. (J) HE staining showing the formation of a condensed cell block (arrow) on the interior side of E16.5 Jag2−/− palates. (K) Jag2−/− palates on E13.5 showing that the distal end is not in contact with the tongue (arrow). (L–N) Whole view (L) and high power views (M and N) of HE stained E17.5 Jag2−/− palates showing a horizontal-like palate (arrows in L) with the MEE region fused with the tongue (arrow in M) and a condensed cell block located on the interior side (N).
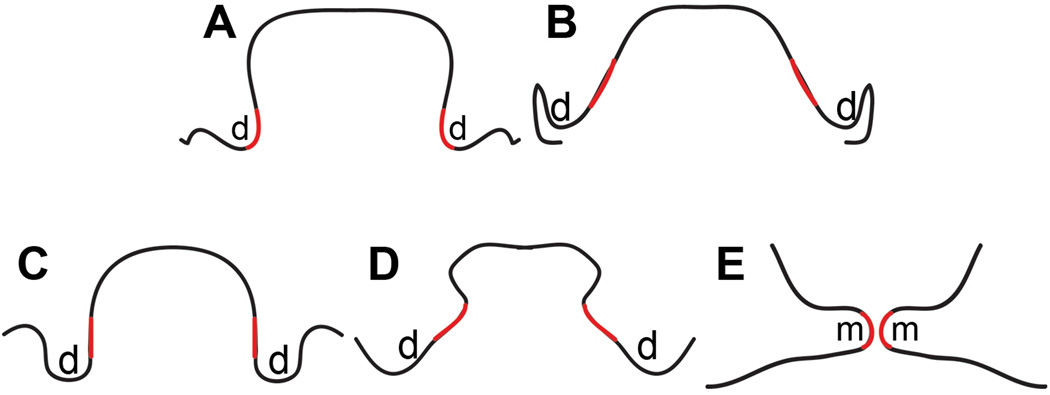
Based on the above results, including the MEE marker analysis in two different manners (presence of MMP13 expression and down-regulation of Jag2 expression), cell condensation on the interior side of Jag2 mutant vertical palate shelves and the formation of a horizontal-like palate in Jag2 mutants with a medial edge fused with the tongue, we propose that the prospective medial edge is located on the interior side of the vertical palatal shelf and the horizontal palate shelf is formed by outgrowth from the side of the vertical palate shelf rather than by rotating the vertical axis (Fig.3). Because goosecoid expression (the medial edge mesenchymal cell marker used here) is absent in the extreme anterior region (data not shown), we were not able to visualize the mesenchymal cell condensation in this area. However, we believe that a similar mesenchymal re-modeling also occurs in that region. The prospective MEE marker in the very anterior region is mainly expressed on the interior surface facing the tongue with extension covering only a portion of the distal region, suggesting that the majority of the medial edge in the anterior region is also derived from the interior surface rather than the distal tip. In addition, previous carbon labeling experiments also suggested tissue re-modeling in the anterior palate shelf (Chou et al., 2004).
Figure 3. A proposed model for palate re-orientation.
The prospective and definitive MEE is shown in red. (A–C) Anterior (A), posterior (B) and middle (C) regions of E13.5 palate. (D) The middle region of palate at an intermediate stage between E13.75 and E14.5. (E) The middle region of E14.5 palate. d: distal end; m: medial edge area.
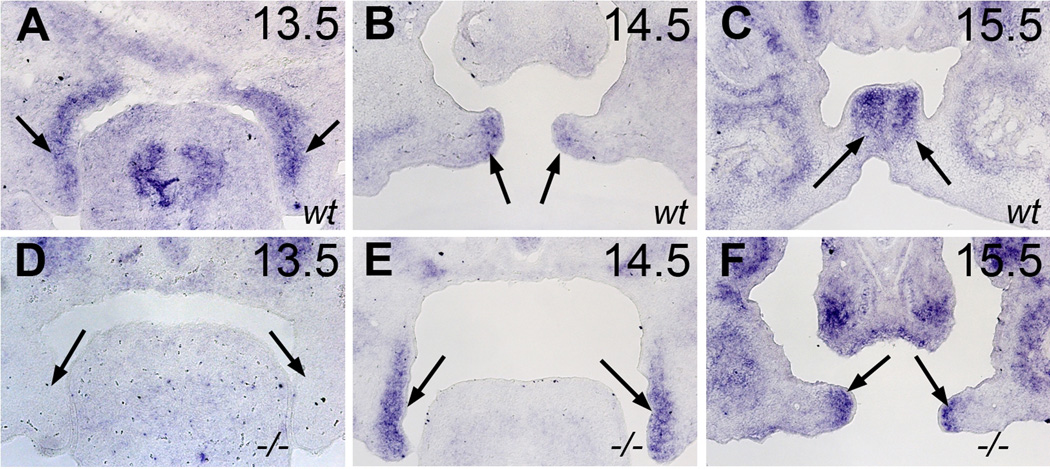
We then attempted to identify the candidate genes responsible for this outgrowth and Snai1 came to our attention. Snai1 has been reported to be expressed in the medial edge mesenchyme cells in the horizontal palatal shelves and its expression is regulated by TGF-β (Martinez-Alvarez et al., 2004; Pungchanchaikul et al., 2005), a signaling pathway that also interacts with Zfhx-1a (Nishimura et al., 2006). Recently, a study using Zebrafish embryos demonstrated that Snai1 function can affect axial mesoendoderm cell migration during gastrulation (Blanco et al., 2007). Palatal outgrowth can be viewed as mesenchyme cell migration or movement. We therefore tested if Snai1 expression is delayed in the development of Zfhx-1a mutant palates. As shown in figure 4, Snai1 expression in wild type palates begins on E13.5, whereas Zfhx-1a mutant palates did not express Snai1 until E14.5, 24 hours later than wild type embryos (Fig.4). Once Snai1 expression initiates in Zfhx-1a mutant palates on E14.5, the intensity of expression is similar to wild type palates (Fig.4), consistent with a recent study reporting that Snai1 expression level is not changed in Zfhx-1a mutant palate as measured by quantitative RT-PCR (Liu et al., 2008). Importantly, neural crest cell-specific inactivation of the Snai1 gene on a Snai2−/− mutant background leads to cleft palate due to failure of palate elevation without abnormal fusion between palate shelf and the tongue or other tissues, demonstrating the function of Snail genes in palate re-orientation (Murray et al., 2007). A recent study revealed Wnt5A mediated palate mesenchymal cell migration and loss of Wnt5A leads to the failure of palate re-orientation in the posterior region, demonstrating the requirement of mesenchymal cell migration palate re-orientation (He et al., 2008).
Figure 4. In situ hybridization showing the expression of Snai1 in Zfhx-1a−/− palates is delayed by 24 hours compared wild type palates.
Snai1 expression is detected in wild type palatal mesenchymal cells from E13.5 through E15.5 (A–C). Snai1 expression is not found in E13.5 Zfhx-1a−/− palates, but is present in Zfhx-1a−/− palates on E14.5 and E15.5.
As mentioned previously, palate re-orientation is the least understood process in palate development (Gritli-Linde, 2007). The assumption that the distal end of the vertical palatal shelf corresponds to the medial edge of the horizontal palatal shelf may have played a negative role in the study of palate re-orientation. In addition, our model explains how palate shelves overcome the physical obstruction of the tongue during re-orientation. Because it is an outgrowth from the side of the vertical shelf, it is not necessary to move the distal end above the dorsal level of the tongue. This is not to say that the forward displacement of the tongue is not important. We think that a combination of our model and the forward displacement of the tongue allow the palatal shelves to overcome the physical obstruction of the tongue during palatal re-orientation.
Experimental Procedures
Zfhx-1a and Jag2 mutant mouse lines have been described previously (Jiang et al., 1998; Takagi et al., 1998). Mouse embryonic heads were dissected on gestation days 13.5 through 17.5 post coitum (the day when vaginal plugs were observed was designated as day 0.5 post coitum), placed in cold PBS and processed for HE staining or section in situ hybridization using digoxygenin-labeled antisense riboprobes as described by Shen (Shen, 2001).
Supplementary Material
(A and B) Schematics showing mouse palatal shelves (arrows) before elevation on E13.5 (A) and after elevation on E14.5 (B). (C and D) According to the current model, the interior and lateral surfaces of the vertical palatal shelves correspond to the future nasal and oral surfaces of the horizontal palate shelves, respectively. However, in the posterior region of E13.5 vertical palatal shelves, the interior surface (black line in C) is much longer than the lateral surface (green line in C), whereas the definitive nasal surface in the horizontal palate (black line in D) is much shorter than the oral surface (green line in D). This phenomenon can not be explained by the current model. d: distal end; m: medial edge area; T: tongue.
(A) MMP13 expression in Zfhx-1a−/− palate at a intermediate stage between E15.5 to E16.5 showing the prospective MEE, marked by MMP13 expression, in relation to the palate outgrowth zone. (B and C) HE staining of a normal mouse palate around E14.0 showing the stage at which the palate is outgrowing. Arrows indicate the outgrowth tip.
Acknowledgements
Correspondence and reprints should be addressed to J Ding (j0ding03@gwise.louisville.edu). We thank Dr. Rulang Jiang at the University of Rochester, Dr. Mary B. Goldring at Harvard Medical School and Dr. Zi-Jian Lan at University of Louisville for providing in situ probes. This work was supported by research grants from National Institutes of Health, USA (COBRE program of the National Center for Research Resource P20RR017702 to the University of Louisville Birth Defects Center, EY017869 to Doug Darling; DE015565 and DE016845 to Jixiang Ding.) and by Grant-in-Aid for Scientific Research (C) (No. 18570198) to Yujiro Higashi from The Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- Blanco MJ, Barrallo-Gimeno A, Acloque H, Reyes AE, Tada M, Allende ML, Mayor R, Nieto MA. Snail1a and Snail1b cooperate in the anterior migration of the axial mesendoderm in the zebrafish embryo. Development. 2007;134:4073–4081. doi: 10.1242/dev.006858. [DOI] [PubMed] [Google Scholar]
- Blavier L, Lazaryev A, Groffen J, Heisterkamp N, DeClerck YA, Kaartinen V. TGF-beta3-induced palatogenesis requires matrix metalloproteinases. Mol Biol Cell. 2001;12:1457–1466. doi: 10.1091/mbc.12.5.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carette MJ, Ferguson MW. The fate of medial edge epithelial cells during palatal fusion in vitro: an analysis by DiI labelling and confocal microscopy. Development. 1992;114:379–388. doi: 10.1242/dev.114.2.379. [DOI] [PubMed] [Google Scholar]
- Casey LM, Lan Y, Cho ES, Maltby KM, Gridley T, Jiang R. Jag2-Notch1 signaling regulates oral epithelial differentiation and palate development. Dev Dyn. 2006;235:1830–1844. doi: 10.1002/dvdy.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Y, Maxson RE., Jr Recent advances in craniofacial morphogenesis. Dev Dyn. 2006;235:2353–2375. doi: 10.1002/dvdy.20833. [DOI] [PubMed] [Google Scholar]
- Chou MJ, Kosazuma T, Takigawa T, Yamada S, Takahara S, Shiota K. Palatal shelf movement during palatogenesis: a fate map of the fetal mouse palate cultured in vitro. Anat Embryol (Berl) 2004;208:19–25. doi: 10.1007/s00429-004-0379-0. [DOI] [PubMed] [Google Scholar]
- Cuervo R, Covarrubias L. Death is the major fate of medial edge epithelial cells and the cause of basal lamina degradation during palatogenesis. Development. 2004;131:15–24. doi: 10.1242/dev.00907. [DOI] [PubMed] [Google Scholar]
- Cui XM, Shiomi N, Chen J, Saito T, Yamamoto T, Ito Y, Bringas P, Chai Y, Shuler CF. Overexpression of Smad2 in Tgf-beta3-null mutant mice rescues cleft palate. Dev Biol. 2005;278:193–202. doi: 10.1016/j.ydbio.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Diewert VM, Tait B. Palatal process movement in the rat as demonstrated in frozen sections. J Anat. 1979;128:609–618. [PMC free article] [PubMed] [Google Scholar]
- Ding H, Wu X, Bostrom H, Kim I, Wong N, Tsoi B, O'Rourke M, Koh GY, Soriano P, Betsholtz C, Hart TC, Marazita ML, Field LL, Tam PP, Nagy A. A specific requirement for PDGF-C in palate formation and PDGFR-alpha signaling. Nat Genet. 2004;36:1111–1116. doi: 10.1038/ng1415. [DOI] [PubMed] [Google Scholar]
- Ferguson MW. Palate development. Development. 1988;103 Suppl:41–60. doi: 10.1242/dev.103.Supplement.41. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick DR, Denhez F, Kondaiah P, Akhurst RJ. Differential expression of TGF beta isoforms in murine palatogenesis. Development. 1990;109:585–595. doi: 10.1242/dev.109.3.585. [DOI] [PubMed] [Google Scholar]
- Greene RM, Kochhar DM. Spatial relations in the oral cavity of cortisone-treated mouse fetuses during the time of secondary palate closure. Teratology. 1973;8:153–161. doi: 10.1002/tera.1420080207. [DOI] [PubMed] [Google Scholar]
- Griffith CM, Hay ED. Epithelial-mesenchymal transformation during palatal fusion: carboxyfluorescein traces cells at light and electron microscopic levels. Development. 1992;116:1087–1099. doi: 10.1242/dev.116.4.1087. [DOI] [PubMed] [Google Scholar]
- Gritli-Linde A. Molecular control of secondary palate development. Dev Biol. 2007;301:309–326. doi: 10.1016/j.ydbio.2006.07.042. [DOI] [PubMed] [Google Scholar]
- He F, Xiong W, Yu X, Espinoza-Lewis R, Liu C, Gu S, Nishita M, Suzuki K, Yamada G, Minami Y, Chen Y. Wnt5a regulates directional cell migration and cell proliferation via Ror2-mediated noncanonical pathway in mammalian palate development. Development. 2008;135:3871–3879. doi: 10.1242/dev.025767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard SA, Yu L, Gu S, Zhang Z, Chen YP. Regional regulation of palatal growth and patterning along the anterior-posterior axis in mice. J Anat. 2005;207:655–667. doi: 10.1111/j.1469-7580.2005.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang R, Lan Y, Chapman HD, Shawber C, Norton CR, Serreze DV, Weinmaster G, Gridley T. Defects in limb, craniofacial, and thymic development in Jagged2 mutant mice. Genes Dev. 1998;12:1046–1057. doi: 10.1101/gad.12.7.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin JZ, Ding J. Analysis of cell migration, transdifferentiation and apoptosis during mouse secondary palate fusion. Development. 2006a;133:3341–3347. doi: 10.1242/dev.02520. [DOI] [PubMed] [Google Scholar]
- Jin JZ, Ding J. Analysis of Meox-2 mutant mice reveals a novel postfusion-based cleft palate. Dev Dyn. 2006b;235:539–546. doi: 10.1002/dvdy.20641. [DOI] [PubMed] [Google Scholar]
- Jin JZ, Li Q, Higashi Y, Darling DS, Ding J. Analysis of Zfhx1a mutant mice reveals palatal shelf contact-independent medial edge epithelial differentiation during palate fusion. Cell Tissue Res. 2008;333:29–38. doi: 10.1007/s00441-008-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J. Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial-mesenchymal interaction. Nat Genet. 1995;11:415–421. doi: 10.1038/ng1295-415. [DOI] [PubMed] [Google Scholar]
- Lan Y, Ovitt CE, Cho ES, Maltby KM, Wang Q, Jiang R. Odd-skipped related 2 (Osr2) encodes a key intrinsic regulator of secondary palate growth and morphogenesis. Development. 2004;131:3207–3216. doi: 10.1242/dev.01175. [DOI] [PubMed] [Google Scholar]
- Lavrin IO, McLean W, Seegmiller RE, Olsen BR, Hay ED. The mechanism of palatal clefting in the Col11a1 mutant mouse. Arch Oral Biol. 2001;46:865–869. doi: 10.1016/s0003-9969(01)00044-9. [DOI] [PubMed] [Google Scholar]
- Liu W, Sun X, Braut A, Mishina Y, Behringer RR, Mina M, Martin JF. Distinct functions for Bmp signaling in lip and palate fusion in mice. Development. 2005;132:1453–1461. doi: 10.1242/dev.01676. [DOI] [PubMed] [Google Scholar]
- Liu Y, El-Naggar S, Darling DS, Higashi Y, Dean DC. Zeb1 links epithelial-mesenchymal transition and cellular senescence. Development. 2008;135:579–588. doi: 10.1242/dev.007047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke DA. Epithelial proliferation and development of rugae in relation to palatal shelf elevation in the mouse. J Anat 138. 1984;(Pt 2):251–258. [PMC free article] [PubMed] [Google Scholar]
- Martinez-Alvarez C, Blanco MJ, Perez R, Rabadan MA, Aparicio M, Resel E, Martinez T, Nieto MA. Snail family members and cell survival in physiological and pathological cleft palates. Dev Biol. 2004;265:207–218. doi: 10.1016/j.ydbio.2003.09.022. [DOI] [PubMed] [Google Scholar]
- Murray JC, Schutte BC. Cleft palate: players, pathways, and pursuits. J Clin Invest. 2004;113:1676–1678. doi: 10.1172/JCI22154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray SA, Oram KF, Gridley T. Multiple functions of Snail family genes during palate development in mice. Development. 2007;134:1789–1797. doi: 10.1242/dev.02837. [DOI] [PubMed] [Google Scholar]
- Nishimura G, Manabe I, Tsushima K, Fujiu K, Oishi Y, Imai Y, Maemura K, Miyagishi M, Higashi Y, Kondoh H, Nagai R. DeltaEF1 mediates TGF-beta signaling in vascular smooth muscle cell differentiation. Dev Cell. 2006;11:93–104. doi: 10.1016/j.devcel.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Pelton RW, Hogan BL, Miller DA, Moses HL. Differential expression of genes encoding TGFs beta 1, beta 2, and beta 3 during murine palate formation. Dev Biol. 1990;141:456–460. doi: 10.1016/0012-1606(90)90401-4. [DOI] [PubMed] [Google Scholar]
- Postigo AA. Opposing functions of ZEB proteins in the regulation of the TGFbeta/BMP signaling pathway. Embo J. 2003;22:2443–2452. doi: 10.1093/emboj/cdg225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proetzel G, Pawlowski SA, Wiles MV, Yin M, Boivin GP, Howles PN, Ding J, Ferguson MW, Doetschman T. Transforming growth factor-beta 3 is required for secondary palate fusion. Nat Genet. 1995;11:409–414. doi: 10.1038/ng1295-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pungchanchaikul P, Gelbier M, Ferretti P, Bloch-Zupan A. Gene expression during palate fusion in vivo and in vitro. J Dent Res. 2005;84:526–531. doi: 10.1177/154405910508400608. [DOI] [PubMed] [Google Scholar]
- Seegmiller RE, Fraser FC. Mandibular growth retardation as a cause of cleft palate in mice homozygous for the chondrodysplasia gene. J Embryol Exp Morphol. 1977;38:227–238. [PubMed] [Google Scholar]
- Shen MM. Identification of differentially expressed genes in mouse development using differential display and in situ hybridization. Methods. 2001;24:15–27. doi: 10.1006/meth.2001.1152. [DOI] [PubMed] [Google Scholar]
- Takagi T, Moribe H, Kondoh H, Higashi Y. DeltaEF1, a zinc finger and homeodomain transcription factor, is required for skeleton patterning in multiple lineages. Development. 1998;125:21–31. doi: 10.1242/dev.125.1.21. [DOI] [PubMed] [Google Scholar]
- Vaziri Sani F, Hallberg K, Harfe BD, McMahon AP, Linde A, Gritli-Linde A. Fate-mapping of the epithelial seam during palatal fusion rules out epithelial-mesenchymal transformation. Dev Biol. 2005;285:490–495. doi: 10.1016/j.ydbio.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Xu X, Han J, Ito Y, Bringas P, Jr, Urata MM, Chai Y. Cell autonomous requirement for Tgfbr2 in the disappearance of medial edge epithelium during palatal fusion. Dev Biol. 2006;297:238–248. doi: 10.1016/j.ydbio.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Song Y, Zhao X, Zhang X, Fermin C, Chen Y. Rescue of cleft palate in Msx1-deficient mice by transgenic Bmp4 reveals a network of BMP and Shh signaling in the regulation of mammalian palatogenesis. Development. 2002;129:4135–4146. doi: 10.1242/dev.129.17.4135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A and B) Schematics showing mouse palatal shelves (arrows) before elevation on E13.5 (A) and after elevation on E14.5 (B). (C and D) According to the current model, the interior and lateral surfaces of the vertical palatal shelves correspond to the future nasal and oral surfaces of the horizontal palate shelves, respectively. However, in the posterior region of E13.5 vertical palatal shelves, the interior surface (black line in C) is much longer than the lateral surface (green line in C), whereas the definitive nasal surface in the horizontal palate (black line in D) is much shorter than the oral surface (green line in D). This phenomenon can not be explained by the current model. d: distal end; m: medial edge area; T: tongue.
(A) MMP13 expression in Zfhx-1a−/− palate at a intermediate stage between E15.5 to E16.5 showing the prospective MEE, marked by MMP13 expression, in relation to the palate outgrowth zone. (B and C) HE staining of a normal mouse palate around E14.0 showing the stage at which the palate is outgrowing. Arrows indicate the outgrowth tip.