Abstract
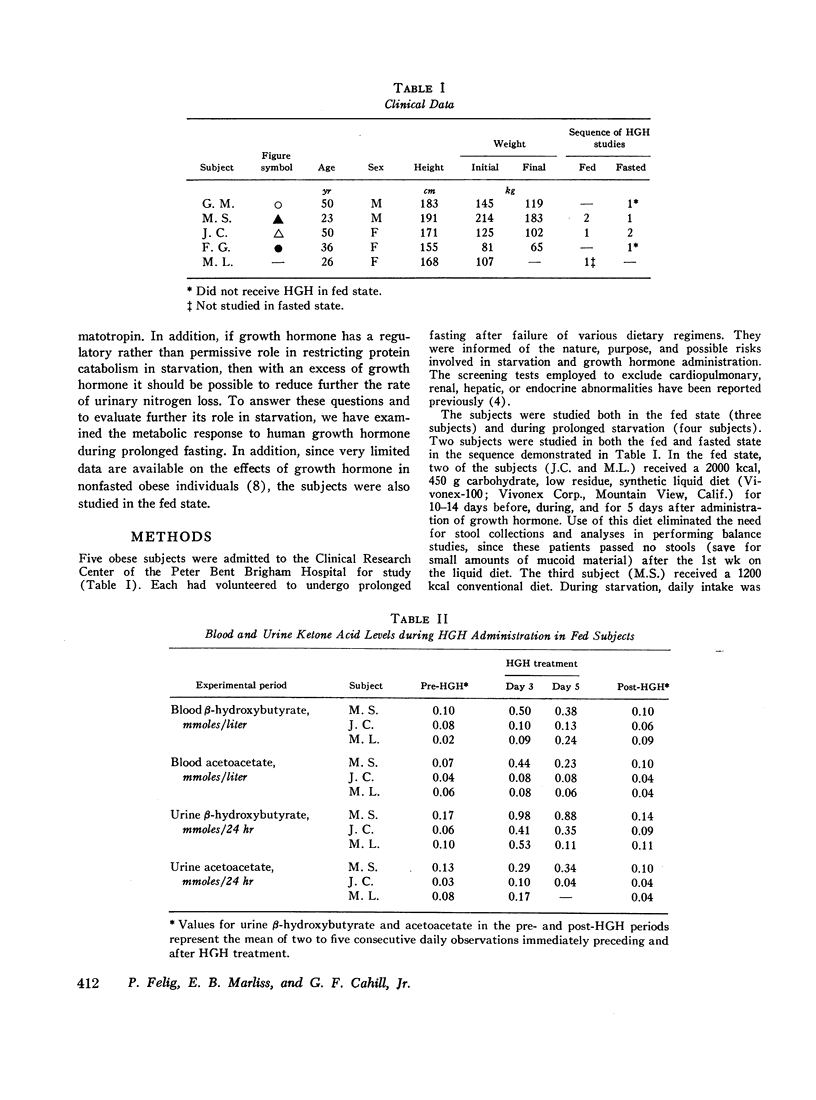
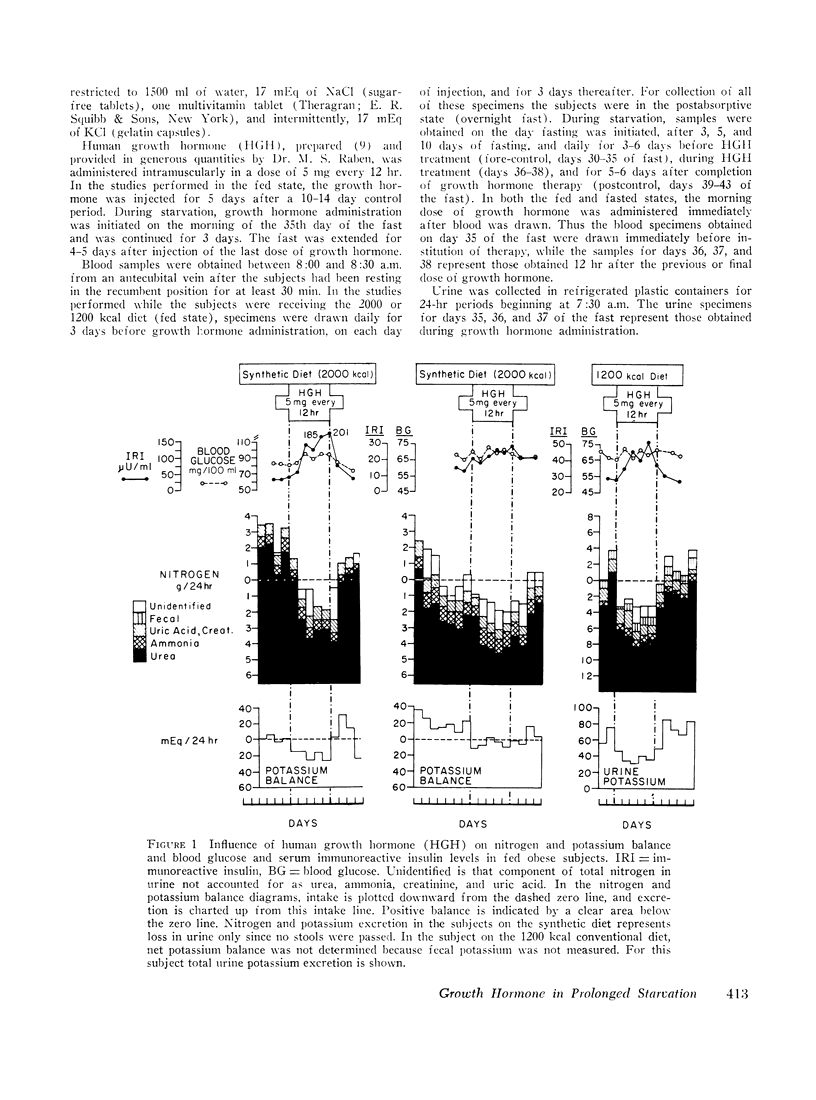
The metabolic response to human growth hormone (HGH) was studied in five obese subjects in the fed state and during prolonged (5-6 wk) starvation. In the fed state (three subjects), HGH induced an elevation in basal serum insulin concentration, a minimal increase in blood and urine ketone levels, and a marked reduction in urinary nitrogen and potassium excretion resulting in positive nitrogen and potassium balance.
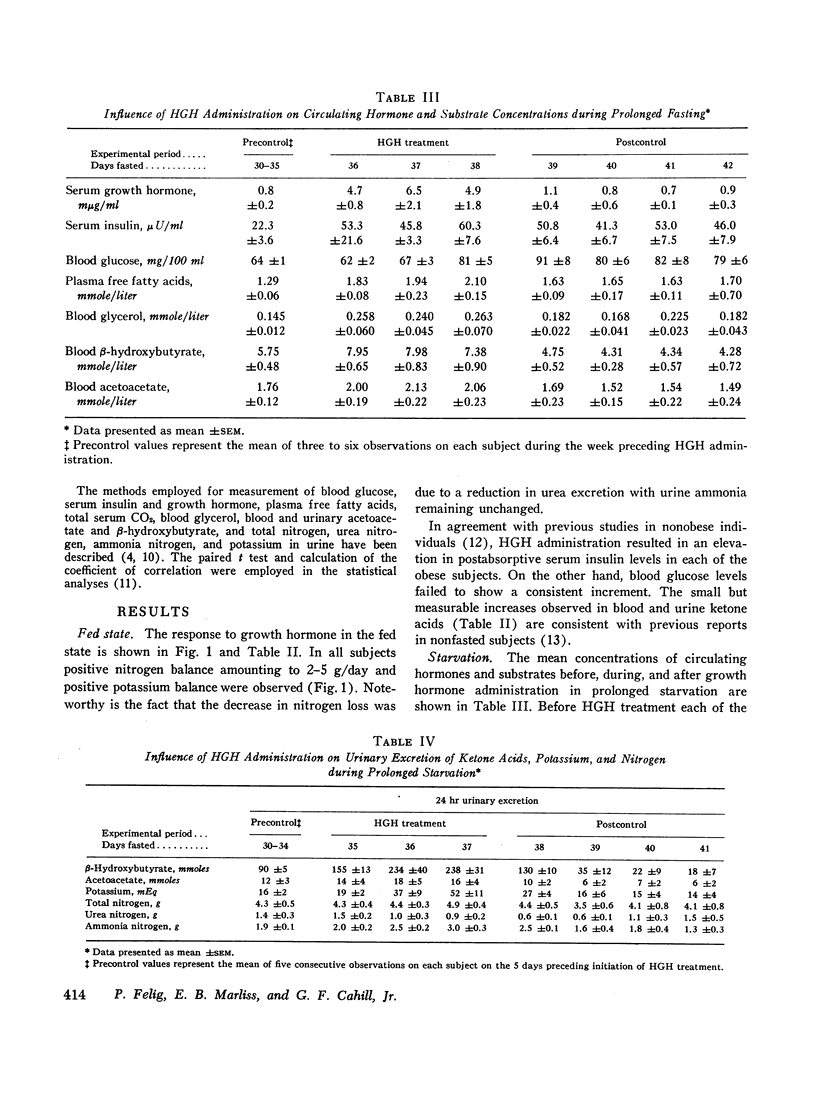
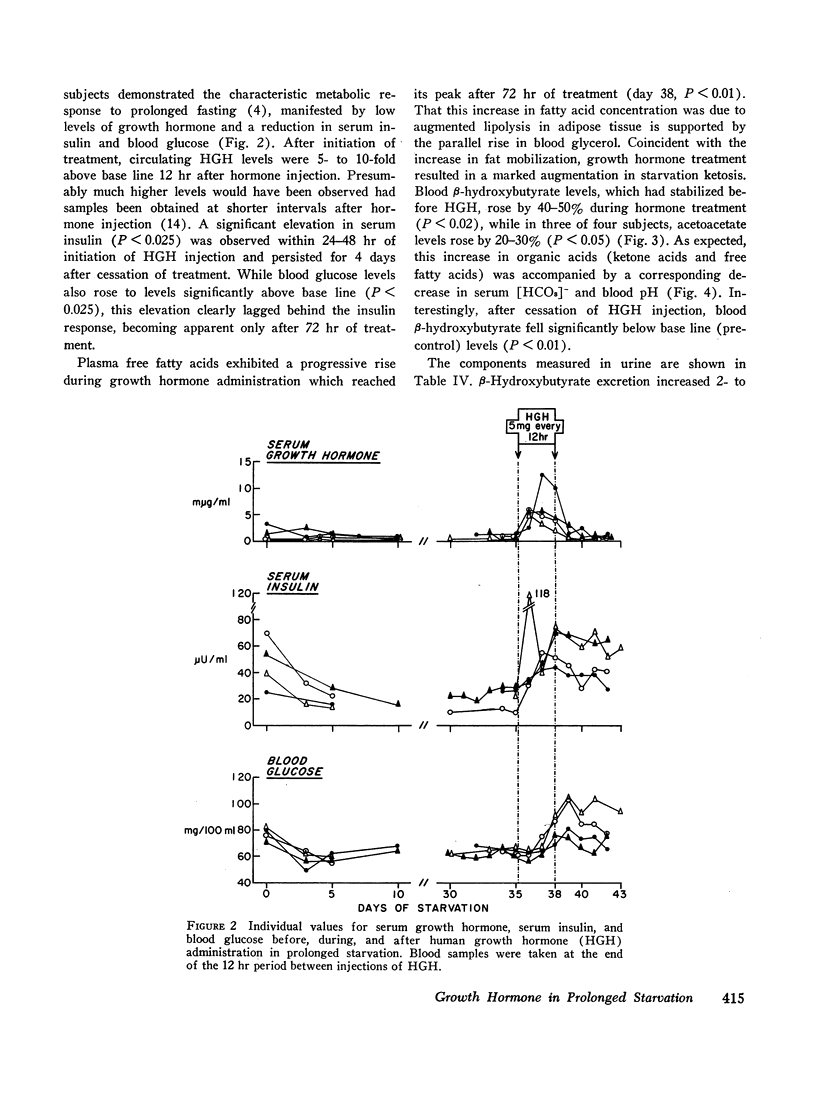
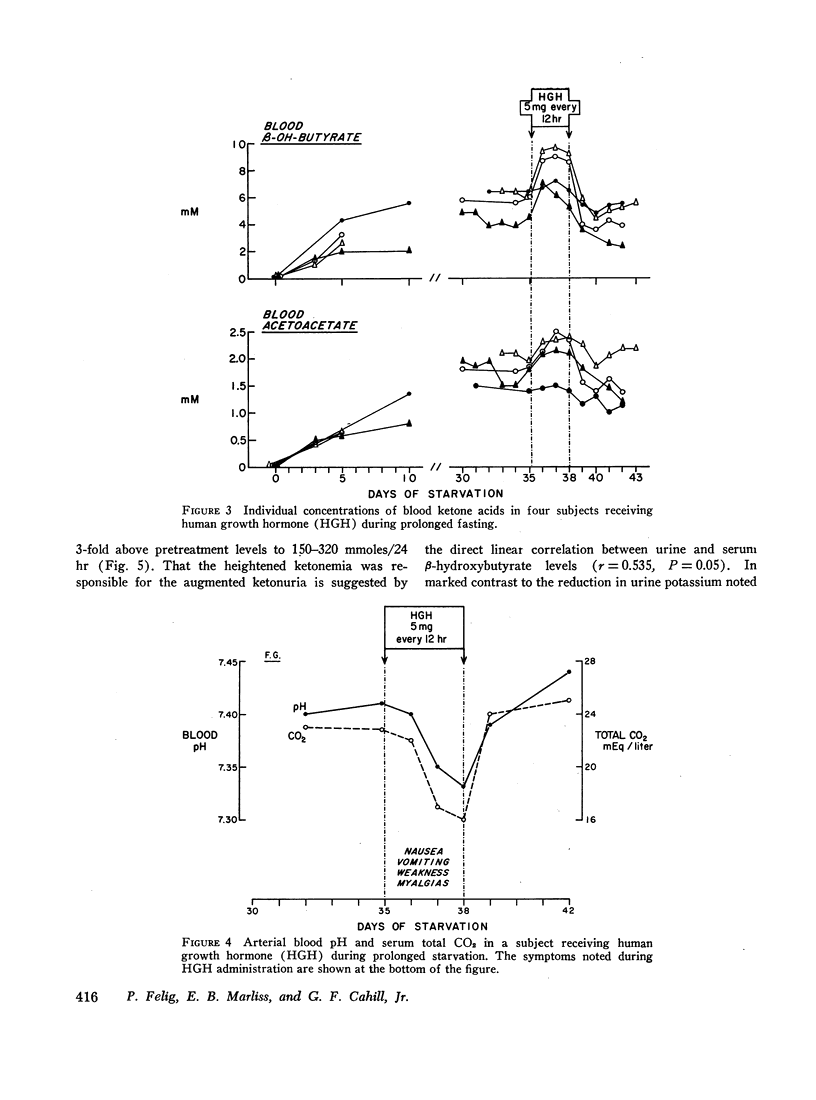
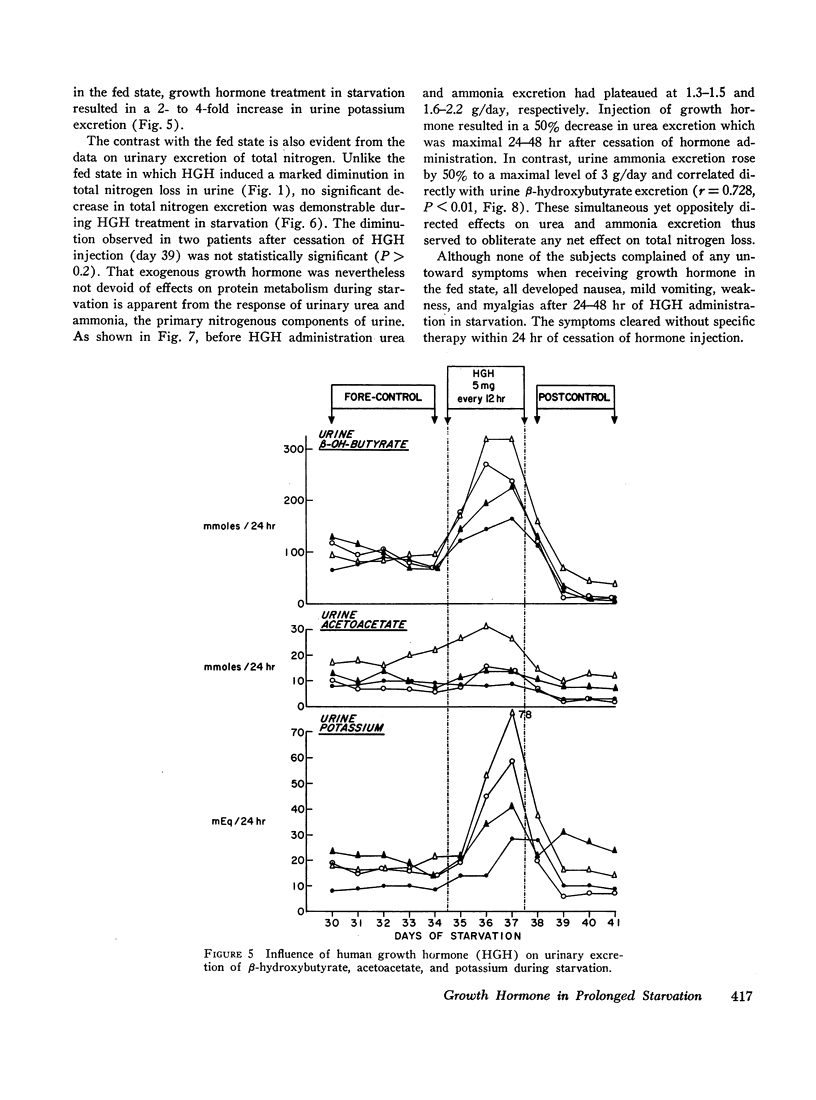
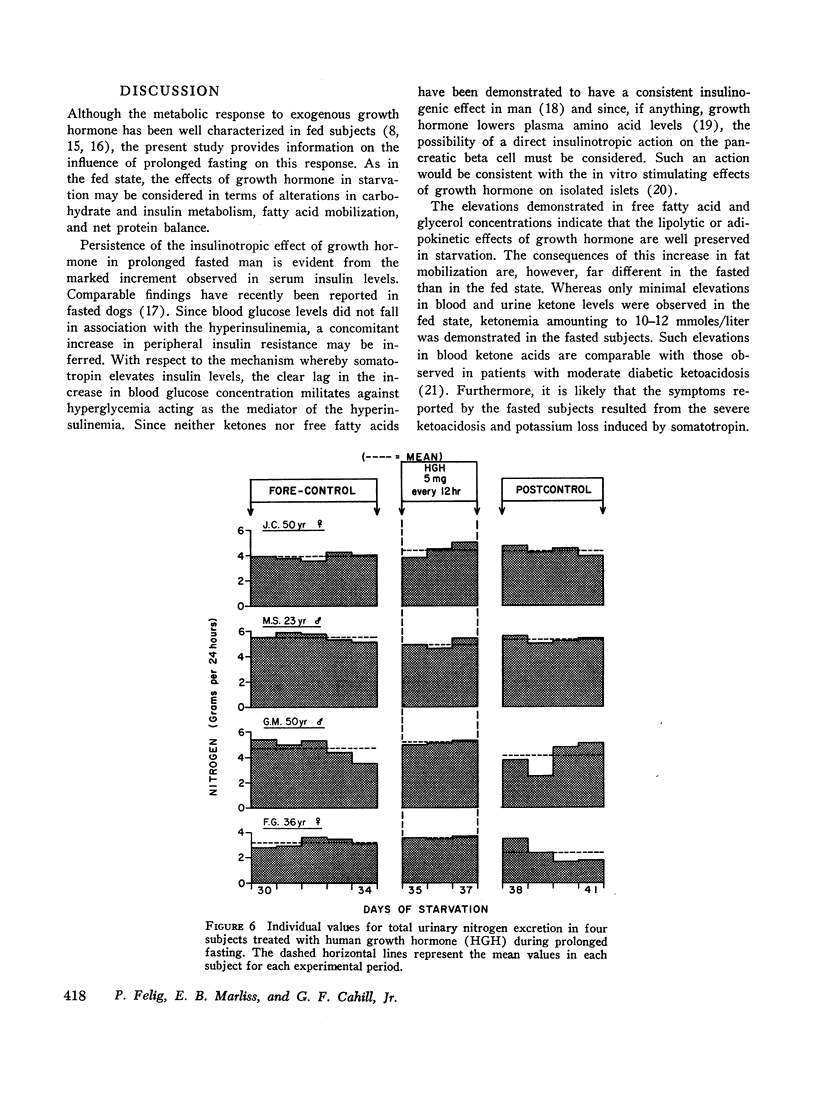
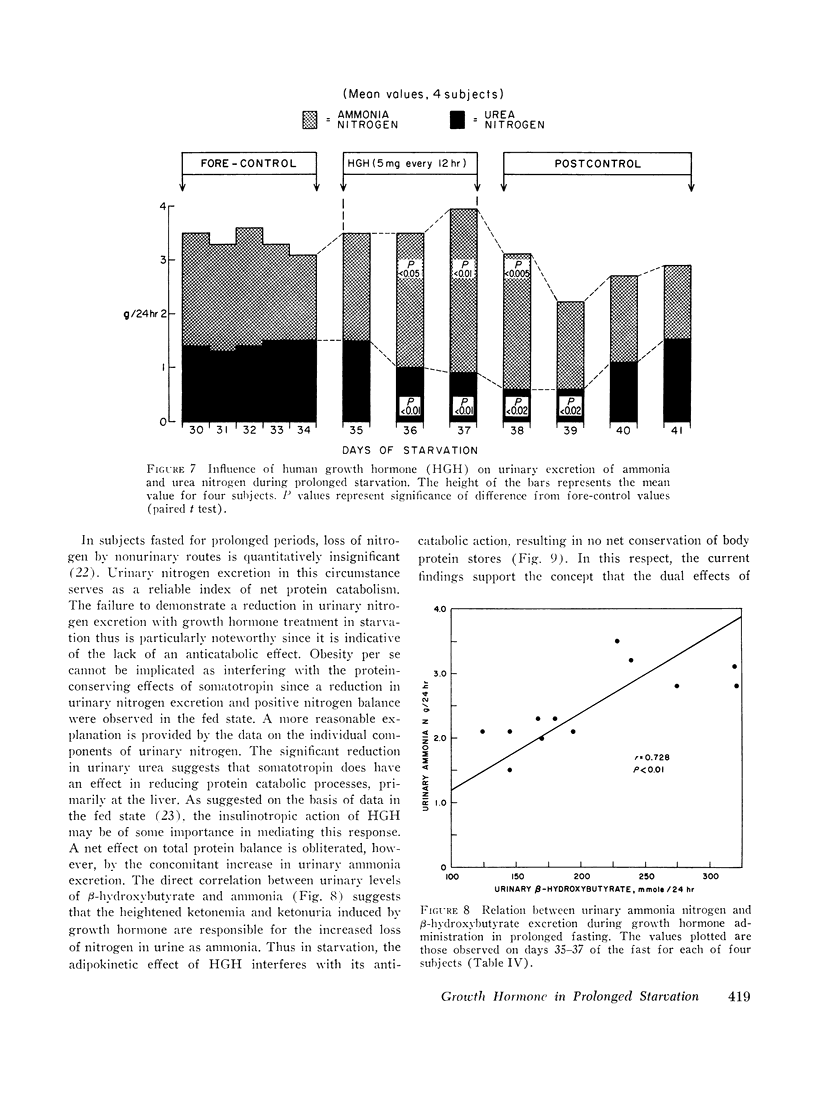
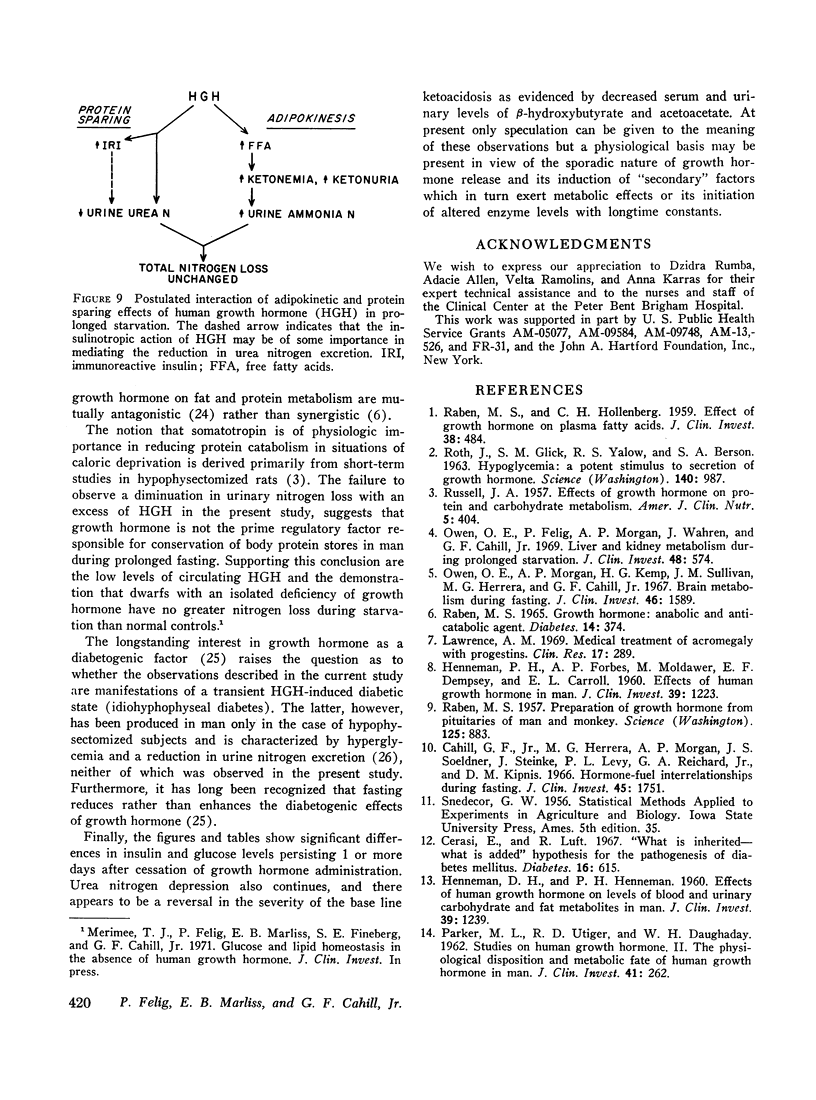
In prolonged fasting (four subjects), HGH administration resulted in a 2- to 3-fold increase in serum insulin which preceded a 50% elevation in blood glucose. Persistence of the lipolytic effects of HGH was indicated by a rise in free fatty acids and glycerol. The response differed markedly from the fed state in that blood β-hydroxybutyrate and acetoacetate levels rose by 20-40%, resulting in total blood ketone acid concentrations of 10-12 mmoles/liter, ketonuria of 150-320 mmoles/day, and increased urinary potassium loss. The subjects complained of nausea, vomiting, weakness, and myalgias. Despite a 50% reduction in urea excretion during HGH administration, total nitrogen loss remained unchanged as urinary ammonia excretion rose by 50% and correlated directly with the degree of ketonuria.
It is concluded that in prolonged starvation (a) HGH may have a direct insulinotropic effect on the beta cell independent of alterations in blood glucose concentration, (b) persistence of the lipolytic action of HGH results in severe exaggeration of starvation ketosis and interferes with its anticatabolic action by necessitating increased urinary ammonia loss, and (c) failure of HGH to reduce net protein catabolism in starvation suggests that this hormone does not have a prime regulatory role in conserving body protein stores during prolonged fasting.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECK J. C., McGARRY E. E., DYRENFURTH I., VENNING E. H. The metabolic effects of human and monkey growth hormone in man. Ann Intern Med. 1958 Nov;49(5):1090–1105. doi: 10.7326/0003-4819-49-5-1090. [DOI] [PubMed] [Google Scholar]
- Cahill G. F., Jr, Herrera M. G., Morgan A. P., Soeldner J. S., Steinke J., Levy P. L., Reichard G. A., Jr, Kipnis D. M. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966 Nov;45(11):1751–1769. doi: 10.1172/JCI105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerasi E., Luft R. "What is inherited--what is added" hypothesis for the pathogenesis of diabetes mellitus. Diabetes. 1967 Sep;16(9):615–627. doi: 10.2337/diab.16.9.615. [DOI] [PubMed] [Google Scholar]
- HENNEMAN D. H., HENNEMAN P. H. Effects of human growth hormone on levels of blood urinary carbohydrate and fat metabolites in man. J Clin Invest. 1960 Aug;39:1239–1245. doi: 10.1172/JCI104139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENNEMAN P. H., FORBES A. P., MOLDAWER M., DEMPSEY E. F., CARROLL E. L. Effects of human growth hormone in man. J Clin Invest. 1960 Aug;39:1223–1238. doi: 10.1172/JCI104138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE R., LUFT R. THE RELATION BETWEEN THE GROWTH AND DIABETOGENIC EFFECTS OF THE SO-CALLED GROWTH HORMONE OF THE ANTERIOR PITUITARY. Diabetes. 1964 Nov-Dec;13:651–655. doi: 10.2337/diab.13.6.651. [DOI] [PubMed] [Google Scholar]
- Martin J. M., Gagliardino J. J. Effect of growth hormone on the isolated pancreatic islets of rat in vitro. Nature. 1967 Feb 11;213(5076):630–631. doi: 10.1038/213630a0. [DOI] [PubMed] [Google Scholar]
- Owen O. E., Felig P., Morgan A. P., Wahren J., Cahill G. F., Jr Liver and kidney metabolism during prolonged starvation. J Clin Invest. 1969 Mar;48(3):574–583. doi: 10.1172/JCI106016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen O. E., Morgan A. P., Kemp H. G., Sullivan J. M., Herrera M. G., Cahill G. F., Jr Brain metabolism during fasting. J Clin Invest. 1967 Oct;46(10):1589–1595. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARKER M. L., UTIGER R. D., DAUGHADAY W. H. Studies on human growth hormone. II. The physiological disposition and metabolic fate of human growth hormone in man. J Clin Invest. 1962 Feb;41:262–268. doi: 10.1172/JCI104479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABEM M. S. GROWTH HORMONE: ANABOLIC AND ANTICATABOLIC AGENT. Diabetes. 1965 Jun;14:374–374. doi: 10.2337/diab.14.6.374. [DOI] [PubMed] [Google Scholar]
- RABEN M. S., HOLLENBERG C. H. Effect of growth hormone on plasma fatty acids. J Clin Invest. 1959 Mar;38(3):484–488. doi: 10.1172/JCI103824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABEN M. S. Preparation of growth hormone from pituitaries of man and monkey. Science. 1957 May 3;125(3253):883–884. doi: 10.1126/science.125.3253.883. [DOI] [PubMed] [Google Scholar]
- ROTH J., GLICK S. M., YALOW R. S., BERSONSA Hypoglycemia: a potent stimulus to secretion of growth hormone. Science. 1963 May 31;140(3570):987–988. doi: 10.1126/science.140.3570.987. [DOI] [PubMed] [Google Scholar]
- RUSSELL J. A. Effects of growth hormone on protein and carbohydrate metabolism. Am J Clin Nutr. 1957 Jul-Aug;5(4):404–416. doi: 10.1093/ajcn/5.4.404. [DOI] [PubMed] [Google Scholar]
- RUSSELL J. A. Hormonal control of amino acid metabolism. Fed Proc. 1955 Sep;14(3):696-705; discussion, 705-6. [PubMed] [Google Scholar]
- Rabinowitz D., Merimee T. J., Burgess J. A. Growth hormone-insulin interaction. Fact and speculation. Diabetes. 1966 Dec;15(12):905–910. doi: 10.2337/diab.15.12.905. [DOI] [PubMed] [Google Scholar]
- Rathgeb I., Steele R., Winkler B., Altszuler N. Influence of fasting on changes in glucose metabolism induced by growth hormone injection in the normal dog. Diabetes. 1970 Jul;19(7):487–491. doi: 10.2337/diab.19.7.487. [DOI] [PubMed] [Google Scholar]
- Ruderman N. B., Toews C. J., Shafrir E. Role of free fatty acids in glucose homeostasis. Arch Intern Med. 1969 Mar;123(3):299–313. [PubMed] [Google Scholar]



